Abstract
Objective:
Negative affect is a precipitant for binge eating in bulimia nervosa (BN). The purpose of the current study was to examine the effect of negative affect on food choices on a more granular level among individuals with BN using a computerized Food Choice Task.
Method:
Individuals with BN (n=25) and healthy controls (HC, n=21) participated in a computerized Food Choice Task following negative and neutral affect inductions, across two study sessions. During the task participants rated high and low-fat food items for Healthiness and Tastiness. Individuals then made a series of choices between a neutral-rated food and high and low-fat foods and were then given a snack based upon these choices.
Results:
Overall negative affect score increased significantly for both the BN and HC groups following the negative affect induction. The group of individuals with BN, relative to the HC group, was less likely to choose high-fat foods (z=−2.763, p=0.006), and these choices were not impacted by affect condition. Health ratings influenced food choices significantly more among individuals with BN than HC (z=2.55, p=0.01).
Discussion:
Induction of negative affect was successful, yet was not related to an increase in proportion of high-fat food choices in the group of individuals with BN. The Food Choice Task captured dietary restriction in individuals with BN and results highlight the utility of this task as a probe to examine how the values of healthiness and tastiness impact food choice in individuals with BN.
Keywords: Bulimia Nervosa, Negative Affect, Dietary Restriction, Eating Disorders, Food Choice
1. INTRODUCTION
Negative affect has been identified as a precipitant for binge eating in bulimia nervosa (BN) across self-report, ecological momentary assessment (EMA), and experimental methodologies [1–4]. Overall severity of negative affect and a trajectory of increasing negative affect predict the occurrence of binge eating episodes [5–7]. These binge eating episodes are characterized by a higher caloric content and higher proportion of calories from fat than non-binge eating episodes [8]. It has been hypothesized, and there is evidence to suggest, that individuals with BN exhibit difficulty regulating emotions and may act impulsively when experiencing these negative emotional states [9–12]. Thus, when individuals with BN experience negative affect, a tendency towards impulsivity and difficulties with emotion regulation may be vulnerabilities which lead to binge eating. However, the mechanism by which negative affect leads to binge eating in BN is not clear, including how the valuation of particular foods may shift under negative affect, potentially leading to binge eating.
To date, experimental manipulations of affect and subsequent measurement of eating in individuals with BN have generally examined overall caloric intake or frequency of binge eating episodes. Computer tasks that ask participants to make choices about food potentially allow for examination of decision making on a more granular level. A food choice task has previously [13, 14] been found to capture the eating patterns commonly seen among individuals with anorexia nervosa (AN)- e.g., restrictive eating and avoidance of high-fat food. Proportion of high fat food choices made during this task significantly correlates with actual eating [15]. In the task, individuals rate a series of food images across two sets of values: healthiness and tastiness. From these ratings, a food that is considered “neutral” in both health and taste is selected and individuals are asked to make a series of choices between this neutral food and the other foods. This task has the advantage of calibrating choices to individualized food preferences. Additionally, this task allows examination of the extent to which values of health and taste influence food choice across different populations. In addition, task outcomes are reliable over time [16]
The purpose of the current study was to examine the effect of induced negative affect on choices made about food among individuals with BN. As individuals with BN may binge eat in response to negative affect, and binge eating is often characterized by an increase in proportion of calories from fat, we hypothesized that individuals with BN would make more high-fat food choices on the Food Choice Task following a negative affect induction as compared to a neutral affect induction. We hypothesized we would see no change in high-fat food choices in healthy controls (HC) across neutral and negative affect induction conditions. We also hypothesized that individuals with BN would be less likely to employ “self-control” when making choices where there was a conflict between health and taste ratings (e.g., a food is rated as healthy but not tasty, or tasty but not healthy) following negative affect induction compared to following the neutral affect induction, with no such effect found in HC. Finally, we conducted exploratory analyses to examine the health and taste ratings of individuals with BN and HC and their influence on food choice.
2. MATERIALS AND METHODS
2.1. Participants:
Participants were 25 individuals who met DSM-5 [17] diagnostic criteria for BN and 21 HC, group-matched for age and BMI. Diagnosis of BN was made via the Structured Clinical Interview for DSM-IV (SCID) [18] and Eating Disorders Examination (EDE) [19] and confirmed by a doctoral level clinician (PhD or MD). All participants were female, between the ages of 18 and 40 years. Exclusion criteria for the group of individuals with BN included bipolar disorder, any psychotic disorder, acute suicidality, alcohol or substance abuse within the past 3 months, major medical illness, use of medications known to affect appetite or cognition, and allergies to foods that would interfere with participation in the task or religious dietary practices that could affect decisions on the Food Choice Task. For the HC group, current or past psychiatric illness was an additional exclusion criterion. Patients with BN received either inpatient or outpatient treatment at the Eating Disorders Research Clinic at the New York State Psychiatric Institute/Columbia University Medical Center. For those receiving outpatient treatment, all study procedures were completed prior to the initiation of treatment, and for inpatients, study procedures were completed within the first week following admission. HC called the clinic to participate in research in response to advertisements and were paid for their participation. The New York State Psychiatric Institute Institutional Review Board reviewed and approved this study and all participants provided written informed consent.
2.2. Materials and Procedure
2.2.1. Overview:
Participants completed the study tasks on two separate study days (mean time between study days = 3.0 ± 2.3 days, range: 1-9 days) and each day’s participation lasted approximately four hours. Participants were told that they would be asked to write about life experiences they had had while listening to selections of music and would also be asked to participate in a computerized task in which they made decisions about food and received a snack based upon those choices. On study days, pre-procedure intake was standardized (granola bar, yogurt, piece of fruit, and water) and participants were instructed to have nothing to eat or drink, except water, between the standardized meal and the day’s research participation two hours later. On one study day, participants underwent a negative affect induction and on the other study day they underwent a neutral affect induction, in counterbalanced and randomized order. Following each affect induction, participants took part in the Food Choice Task in an incentive-compatible design (i.e., following the task, participants received an actual snack based on a randomly selected choice trial).
2.2.2. Food Choice Task (Figure 1):
Figure 1. Food Choice Task.
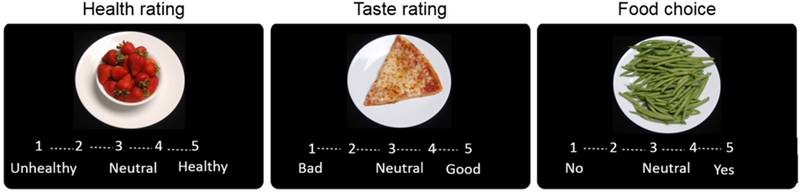
Participants rate 43 food items during three phases. In the Health and Taste phases participants rate each food on a 5-point Likert scale ranging from Unhealthy to Healthy in the Health phase and Bad to Good in the Taste phase. In the Choice phase, participants indicate the strength of their preference for the food item, compared with their own individually rated neutral reference item. “No” indicates selection of the neutral reference item, which was visible next to the computer screen, and “Yes” indicates selection of the item on that trial.
The Food Choice Task [13] is comprised of 3 phases: the Health Rating phase and Taste Rating phase (in counterbalanced order), followed by the Choice phase. During each of these phases, participants were shown images of the same 43 food items, 25 of which were categorized as “low-fat” (<30% kcals from fat) and 18 of which were categorized as “high-fat” (>30% kcals from fat[20]). During the Health Rating phase, participants were asked to rate the healthiness of each of the 43 food items on a 5-point scale where “1” was “unhealthy”, “3” was “neutral”, and “5” was “healthy”. During the Taste Rating phase, participants were asked to rate the tastiness of the 43 food items on a 5-point scale where “1” was “bad”, “3” was neutral”, and “5” was “good”. At the conclusion of the Health and Taste rating phases, one food item that was rated as neutral for both Health and Taste was selected as a neutral Reference Item to be used in the Choice phase of the task. If no item was rated as neutral on both Health and Taste, an item that had been rated a “3” on Health and “4” or above on Taste was used as the Reference Item, following the same algorithm used previously [13, 15]. During the Choice phase of the task, participants were asked to make a series of choices between the neutral Reference Item (which was visible to the participant via a printed image of the food and did not change throughout the task) and the other food items presented on the computer screen. They were instructed that one of their choices would be given to them as a snack at the conclusion of the task, to increase the likelihood that participants’ choices would reflect what they actually wanted to eat. After completion of the task, participants were served a snack-sized portion of one of their choices. The Choice phase includes low-fat and high-fat trials, as well as trials that present a conflict between healthiness and tastiness ratings. Choosing healthy but non-tasty foods or not choosing tasty but unhealthy foods is assumed to indicate use of “self-control” [14]. Task outcome variables included: 1) proportion of high-fat food items chosen over the reference item, 2) proportion of self-control choices that were made, 3) health and taste ratings for high and low-fat food groups.
2.2.3. Affect Induction[21].
Induction was comprised of a combination of music and writing about autobiographic experiences. During the negative affect induction, participants were asked to write about a recent negative experience while listening to the musical selection “Adagio for Strings” by Samuel Barber for eight minutes, which has been shown to induce negative mood [21]. During the neutral affect induction participants were asked to write about the route they had taken to get to the study site that day while listening to the neutral jazz musical piece “Dancing with the Sun” by Celia Felix for eight minutes. The Profile of Mood States (POMS; [22]) was administered immediately before and after the affect inductions to assess momentary changes in affect. The POMS is comprised of 65 items wherein participants are asked to indicate how strongly they are experiencing, at that moment, a particular feeling state on a 5-point scale from “not at all” to “extremely.” The POMS yields five subscale scores measuring negative affect: Anger, Confusion, Depression, Fatigue, and Tension, and one measuring positive affect: Vigor. Per standard practice, we calculated a POMS overall negative affect score by summing these five negative affect subscale scores and subtracting the Vigor score. Higher scores indicate higher levels of negative affect.
2.2.4. Additional Measures:
Severity of eating disorder psychopathology was assessed via the Eating Disorder Examination Questionnaire (EDE-Q; [23]); emotion regulation difficulties were assessed using the Difficulties in Emotion Regulation Scale (DERS; [24]); negative urgency- e.g., the tendency to respond to negative affect with rash action, was assessed via the Urgency, Premeditation, Perseverance, Sensation Seeking, and Positive Urgency Behavior Scale-Negative Urgency subscale (UPPS-P Negative Urgency [25]).
2.3. Data Analysis
Demographic variables were compared between diagnostic groups (BN vs HC) using Chi square analysis for nominal variables and independent sample t-tests for continuous variables. Change in POMS overall negative affect scores following affect induction was analyzed using mixed ANOVA (2 [Affect Condition: Neutral/Negative] × 2 [Time: Pre/Post Affect induction] × 2 [Group: HC/BN]).
2.3.1. Choice and rating analyses:
The main analysis approach was to use multilevel regression models to analyze trial-by-trial data from the choice and rating phases. These models account for the unbalanced data (the task has more lower fat than higher fat items) and minimize the influence of outliers. For the Choice phase, responses on the 5-point scale were converted to binary yes or no responses indicating preferences for the trial-unique food item over the constant neutral Reference Item (responses indifferent between the two options were omitted). The binomial choice data were modeled with multilevel logistic regression and the continuous rating phase data (1 to 5) were modeled using multilevel linear regression. For analyses testing the associations between ratings and the influence of ratings on choices, ratings entered as independent variables were z-scored. The repeated-measures nature of the data was taken into account by entering all within-subject factors (the intercept, main effects of Food type, Affect condition, and their interaction) as random by participant [26, 27].
More specifically, analyses were implemented in R[28] using the glmer function of the lme4 package [29] for binomial choice data and the lmer function of the lme4 package [29] for continuous rating data. Food choices (yes/no coded 1/0) and health and taste ratings (ratings from 1 to 5) were examined by entering Food type (low-fat/high-fat coded −1/1), Affect Condition (Neutral/Negative coded −1/1), and Group (HC/BN coded −1/+1) as independent variables.
To examine the influence of Health vs. Taste valuations on food decisions in the Choice block (yes/no coded 1/0), we entered ratings (z-scored Health and Taste ratings), Affect Condition (Neutral/Negative coded −1/1), and group (HC/BN coded as −1/+1) as independent variables in a multilevel logistic regression. The association between Health and Taste ratings was examined by entering Taste ratings as the dependent variable (ratings from 1 to 5) and Health ratings (z-scored) and group (HC/BN coded as −1/+1) as independent variables.
For analyses of continuous outcome data the significance of the partial correlation coefficients was assessed by χ2 statistics, and accompanying p values were derived for the estimates from type-III analysis of variance tables from the ANOVA function in the car package for R [30]. The esticon function in the doBy package was used when contrasting regression parameters.
2.3.2. Self-control analyses:
Trials presenting a conflict between health and taste ratings provide an opportunity to implement self-control [14] and choosing healthy non-tasty foods or not choosing tasty unhealthy foods is taken to indicate application of self-control. The number of trials with opportunity for self-control was analyzed using mixed ANOVA (2 [Affect Condition: Neutral/Negative] × 2 [Group: HC/BN]). Use vs. non-use of self-control (coded 1/0) was modeled with multilevel logistic regression, entering Affect Condition (Neutral/Negative coded −1/1), and Group (HC/BN coded −1/+1) as independent variables.
2.3.3. Associations between food choice and clinical measures:
Associations between eating pathology (EDE-Q Global), difficulties with emotion regulation (DERS Total), negative urgency (UPPS-P), and proportion of high-fat foods chosen and self-control during the Food Choice Task were assessed via Pearson correlation. For these exploratory analyses, Bonferroni correction for multiple comparisons results in an alpha level of 0.008 (0.05/6).
3. RESULTS
Among individuals with BN, 5 patients were receiving inpatient treatment and 20 received outpatient treatment. Clinical characteristics are shown in Table 1. The groups were well matched for age and BMI, as well as for ethnicity with 72% (n=15) of HC and 68% (n=17) of the group of individuals with BN reporting that they are white, (χ2(1, n=46)= 0,06, p=0.80). In the group of individuals with BN, 4 identified as Asian and 2 as “other”, and in the HC group 3 identified as Asian, 3 as African American, and 1 as “other”. Seven individuals with BN identified as Hispanic and Caucasian while none in the HC group identified as Hispanic.
Table 1.
Clinical Characteristics of Patients with Bulimia Nervosa and Healthy Controls.
| HC | BN | ||||||
|---|---|---|---|---|---|---|---|
| (n=21) | (n=25) | ||||||
| Mean | SD | Mean | SD | t | df | p | |
| Age (years) | 26.1 | 4.7 | 25.0 | 4.9 | −0.7 | 45 | 0.46 |
| BMI (kg/m2) | 22.4 | 3.5 | 22.8 | 3.9 | −0.4 | 44 | 0.70 |
| EDE-Q Global | 0.2 | 0.2 | 3.8 | 1.3 | −12.4 | 44 | <.001 |
| DERS | 54.2 | 11.2 | 114.9 | 25.1 | −9.8 | 40 | <.001 |
| UPPS-P: Negative | 1.7 | 0.5 | 2.8 | 0.5 | −7.7 | 43 | <.001 |
Note. BMI= Body Mass Index; BN= Bulimia Nervosa; DERS= Difficulties with Emotion Regulation Scale; EDE-Q Global= Eating Disorder Examination-Questionnaire Global Score; HC= Healthy Control; UPPS-P: Negative Urgency= Urgency, Premeditation, Perseverance, Sensation Seeking, and Positive Urgency Behavior Scale- Negative Urgency subscale
3.1. Change in POMS with Affect Induction:
There was a significant two-way interaction of affect condition (negative or neutral) by time (pre- or post-affect induction), such that the POMS overall negative affect score increased significantly for both the BN and HC groups following the negative affect induction but not the neutral affect induction (F1,44= 28.76, partial η2=0,39, p<.001; see Figure 2). The three-way interaction between affect condition, time, and group (BN or HC) was not significant, indicating that the affect induction did not differentially affect the two groups (F1,44= 0.70, partial η2=0.02, p= 0.41). There was, however, a significant between-subjects effect of group, such that the group of individuals with BN had significantly higher ratings on the POMS overall negative affect score than the HC group, regardless of affect condition or time (F1,44= 28.36, partial η2=0.39, p<.001).
Figure 2. Effectiveness of affect induction.
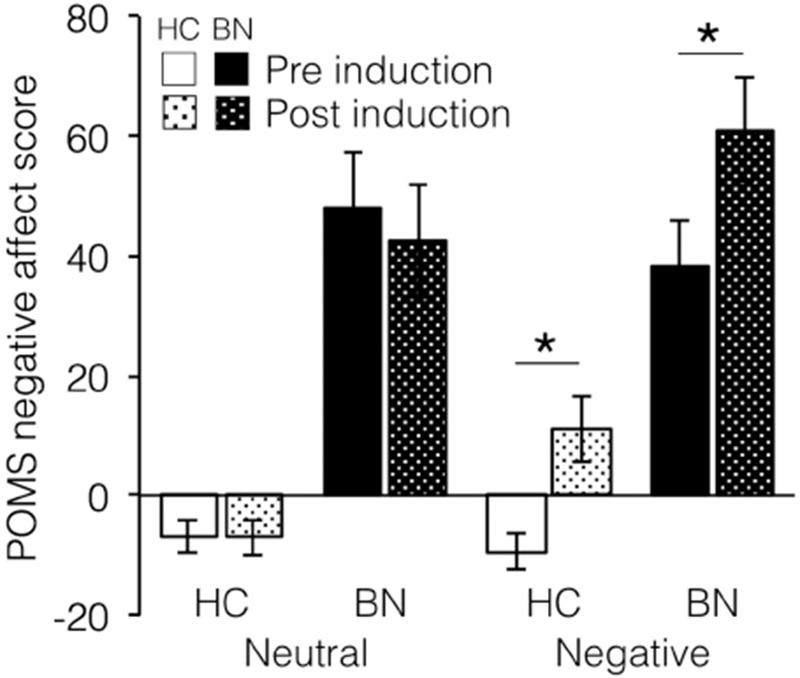
Negative affect increased in both groups with the negative affect induction and showed no change with the neutral affect induction. * indicates p<0.05, HC = healthy controls, BN = bulimia nervosa.
3.2. Food Choices by Fat Content:
High-fat foods were less likely to be chosen than the neutral reference item relative to low-fat foods chosen over the neutral reference item (z=− 4.66, p < 0.0001) in both groups. The group of individuals with BN was more likely than the HC group to choose the neutral reference item than the other foods presented (z=−2.65, p=0.01). Additionally, there was a Group × Food type interaction (z=−1.99, p=0.046, see Figure 3c) indicating that the group of individuals with BN, relative to the HC group, was less likely to choose high-fat foods (z=−2.763, p=0.006), but not low-fat foods (z=−1.25, p=0.21).
Figure 3. Food Choice Task behavior collapsed across affect conditions.
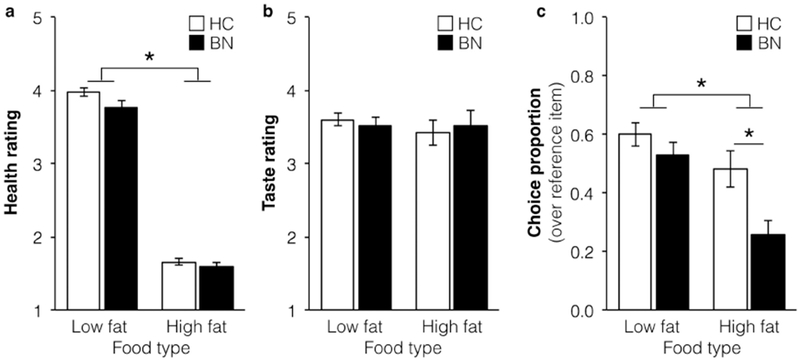
(a) Health ratings were significantly higher for low-fat foods (and did not differ between groups or affect conditions, (b) Taste ratings were similar across groups, food types, and affect conditions, (c) In the Choice phase, the BN group was less likely than the HC group to choose high-fat foods in particular. * indicates p<0.05, HC = healthy controls, BN = bulimia nervosa.
There was no main effect of Affect Condition (z=−0.54, p=0.59) or interaction between Food type and Affect Condition (p=0.28) or Group and Affect Condition (p=0.34). The three-way interaction between Food type, Affect Condition and Group was not significant (z=−1.77, p=0.077).
3.3. Food Choices and Self-Control:
The mean number of trials with an opportunity for self-control was 11.90 ±6.17 for individuals with BN and 11.33 ±6.04 for HC. There were no significant differences between groups (F1,44=0.10, p=0.76) or affect conditions (F1,44=0.97, p=0.33), and no interaction effects (F1,44=0.03, p=0.88) regarding number of trials with an opportunity for self-control. On trials that presented opportunities to make a self-control choice, the group of individuals with BN used self-control 54.70% ±28.23 of trials, compared to 25.68% ±29.60 for HC (z=3.77, p<0.001). There was no main effect of Affect Condition on self-control use (z=0.07, p=0.95), and no interaction between Affect Condition and Group (z=−0.14, p=0.89).
3.4. Health and Taste Ratings:
For the Health ratings, both groups rated high-fat foods as less healthy than low-fat foods (Estimate: =−1.12, χ2 = 1773, p<0.0001; see Figure 3). There were no main effects of Group (Estimate= −0.07, χ2 = 2.55, p=0.11), Affect Condition (Estimate=0.01, χ2 = 0.39, p=0.53). All interactions were nonsignificant (p>0.05).
For the Taste ratings, there were no significant main effects or interactions (p>0.05).
3.5. Associations between Health, Taste, and Choice:
Both Health (z=7.11, p<0.0001) and Taste (z=11.50, p<0.0001) ratings influenced food choices (see Figure 4a). Health ratings influenced choice significantly more among individuals with BN relative to HC (z=2.55, p=0.01), and there was a trend for Taste ratings to influence choice more among HC relative to individuals with BN (z=−1.76, p=0.08). A comparison of the influence of taste and health ratings within the HC group revealed that choices were significantly more influenced by taste ratings (χ2= 22.86, p< 0.00001). In contrast, choices among individuals with BN were equally influenced by taste and health ratings (χ2= 0.001, p=0.97). There was an interaction between Affect Condition and the influence of Taste on Choice such that Taste was slightly less influential over Choice in the negative affect condition versus the neutral affect condition, regardless of Group (z=−2.23, p=0.03).
Figure 4. Relationships between health ratings, taste ratings, and food choice.
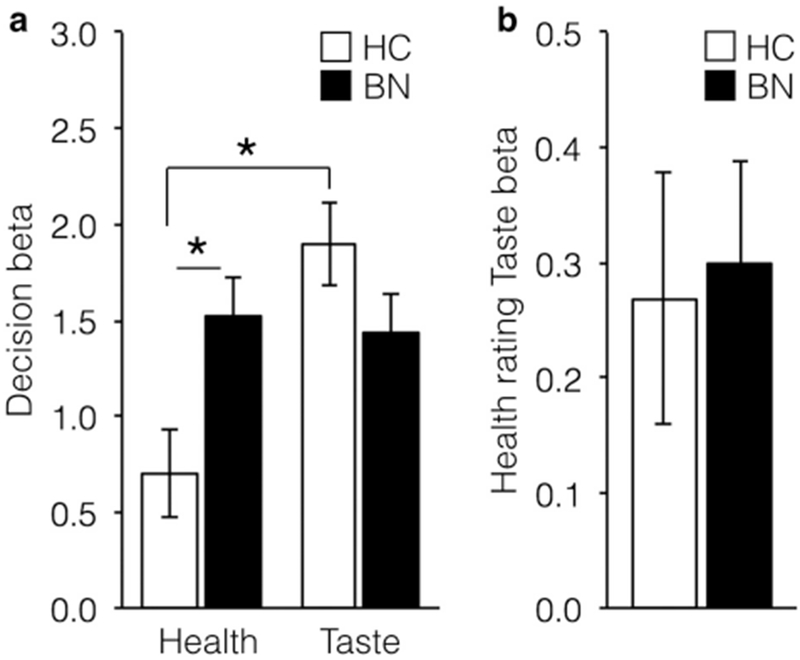
(a) Health ratings influenced food choice significantly more in the BN group relative to HC group (χ2=0.001, p=0.97). Among HC, taste ratings influenced choice more than did health ratings (χ2=22.86, p< 0.00001). (b) Health ratings were significantly related to Taste ratings in BN and HC groups (χ2= 16.64, p<0.0001). * indicates p<0.05, HC = healthy controls, BN = bulimia nervosa.
Health ratings were significantly associated with Taste ratings overall (χ2= 16.64, p<0.0001) and did not differ between groups (χ2= 0.004, p=0.95, Figure 4b) or affect conditions (χ2= 0.12, p=0.73).
3.6. Associations between food choice and clinical measures:
Within the group of individuals with BN, proportion of high-fat foods chosen across affect induction conditions was not significantly associated with the EDE-Q Global Score (r=0.15, p=0.46), DERS (r=0.15, p=0.49), or UPPS-P Negative Urgency (r=0.25, p=0.23). Use of self-control was also not significantly associated with EDE-Q Global Score (r=−0.06, p=0.78), DERS (r=−0.04 p=0.85), or UPPS-P Negative Urgency (r=0.02, p=0.93). Similarly, in the HC group, proportion of high-fat foods chosen was not significantly associated with the DERS (r=−0.04, p=0.87), EDE-Q Global Score (r=−0.35, p=0.13), or UPPS-P Negative Urgency (r=−0.17, p=0.47), nor was use of self-control associated with the DERS (r=0.07, p=0.79), EDE-Q Global Score (r=0.41, p=0.06), or UPPS-P Negative Urgency (r=0.20, p=0.38).
4. DISCUSSION
During a computerized Food Choice Task, individuals with BN chose high-fat foods significantly less often, and made choices consistent with use of self-control significantly more often, than HC. Induction of negative affect was successful, but was not related to an increase in selection of high-fat foods or use of self-control in the group of individuals with BN. These data did not support our hypothesis that negative affect would increase high-fat food choices and decrease self-control use among individuals with BN. These results align with other studies which have also successfully induced negative mood but failed to produce a meaningful change in intake in a clinical population [31, 32].
On the other hand, these results dramatically illustrate that outside of binge episodes, individuals with BN restrict dietary intake. Specifically, individuals with BN eat less than HC in laboratory settings [33–35] and consume a lower percentage of calories from fat than HC in non-binge meals [8]. This pattern of dietary restriction may play a role in maintaining binge eating behavior in individuals with BN, as ecological momentary assessment (EMA) data show that binge eating is predicted by caloric restriction the day prior [36]. The current results, then, may capture a pattern of eating that is a known behavioral precursor of subsequent binge eating, and highlight that this restrictive eating is undertaken despite individuals with BN rating foods to be just as tasty as HC, which is in contrast to findings in anorexia nervosa [13, 15].
Previous studies using this task found that individuals with AN rated all food, regardless of fat content, as less healthy and less tasty than HC [13, 15], suggesting a difference between AN and BN. However, both individuals with AN and BN made more choices using self-control than HC did. A possible interpretation of these findings is that among individuals with BN the choice of what to eat relies on the continuous employment of self-control and the suppression of tastiness in favor of healthiness. Self-control has been shown to be an exhaustible asset [37, 38]. The depletion of self-control by a range of stresses including emotional stress and continued use of effortful cognitive control, may set the stage for disinhibited eating [8, 37–39].
4.1. Limitations.
The lack of effect of negative mood on eating is surprising, but may be related to experimental design. It may be that dietary disinhibition in individuals with BN is not elicited in this study because the task emphasizes receipt of a snack-sized, rather than meal-sized or larger, portion of food. Additionally, the length of time between the standardized lunch and Food Choice Task and snack may have been insufficiently long to produce hunger-induced impulsive responding on the task. Of note, there is evidence to suggest that in a naturalistic setting certain facets of negative affect may be more related to the onset of binge eating episodes in individuals with BN than others, namely feelings of guilt relative to feelings of fear, hostility, and sadness [7]. During the negative affect induction in the current study participants were not prompted to write about experiences that would elicit a particular facet of negative affect nor does the assessment tool used to measure momentary affect in the current study, the POMS, have a subscale measuring feelings of guilt. While affect induction did not impact behavior in this small study, this may not generalize beyond study conditions. It is also possible that individuals with BN may have chosen healthier foods during this task than they would have in a naturalistic setting because they were being observed and binge eating is most often secretive in nature. Participants were not assessed for the presence of personality disorders, and individuals with BN with alcohol or substance abuse in the previous three months were also excluded from the current sample, which may have led to the exclusion of individuals with especially heightened levels of impulsivity and emotion regulation difficulties who may have been more reactive to the effects of a negative mood induction. Additionally, it should be noted that the sample was small, not ethnically diverse, and did not include men, therefore generalizability of results is limited.
4.2. Conclusion.
Ratings of health and taste were similar in the BN and HC groups. However, healthiness and tastiness influenced decisions about what foods to eat in a different way among individuals with BN than among HC. Individuals with BN prioritized the healthiness more than did HC, who prioritized the tastiness over the healthiness of food items when making choices. Thus, these results impart important information about the values that inform how individuals with BN make decisions about what to eat, and suggest a potentially cognitively taxing approach, given their reliance on the employment of self-control. Neuroimaging during active food choice to better understand the neural mechanisms of dietary self-control in individuals with BN may be able to illuminate how this behavior is maintained in BN, how it might be effectively targeted in treatment, and how and why the treatment responses of individuals with AN and BN differ so dramatically.
Highlights:
Affect induction and food choice tasks were completed in bulimia nervosa and healthy control groups.
Patients with bulimia nervosa made fewer high fat food choices than controls.
Patients with bulimia nervosa made more self-control choices than healthy controls.
Affect induction did not impact food choices in individuals with bulimia nervosa or controls.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- 1.Agras WS and Telch CF, The effects of caloric deprivation and negative affect on binge eating in obese binge-eating disordered women. Behavior Therapy, 1998. 29(3): p. 491–503. [Google Scholar]
- 2.Chua JL, Touyz S, and Hill A, Negative mood-induced overeating in obese binge eaters: An experimental study. International Journal of Obesity, 2004. 28(4): p. 606–610. [DOI] [PubMed] [Google Scholar]
- 3.Haedt-Matt AA and Keel PK, Revisiting the affect regulation model of binge eating: A meta-analysis of studies using ecological momentary assessment. Psychological Bulletin, 2011. 137(4): p. 660–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polivy J and Herman CP, Etiology of binge eating: Psychological mechanisms, in Binge eating: Nature, assessment, and treatment, Fairburn CG and Wilson GT, Editors. 1993, Guilford Press: New York, NY: p. 173–205. [Google Scholar]
- 5.Smyth JM, et al. , Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. Journal of Consulting and Clinical Psychology, 2007. 75(4): p. 629–638. [DOI] [PubMed] [Google Scholar]
- 6.Goldschmidt AB, et al. , Ecological momentary assessment of stressful events and negative affect in bulimia nervosa. Journal of Consulting and Clinical Psychology, 2014. 82(1): p. 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg KC, et al. , Facets of negative affect prior to and ollowing binge-only, purge-only, and binge/purge events in women with bulimia nervosa. Journal of Abnormal Psychology, 2013. 122(1): p. 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sysko R, et al. , Impulsivity and test meal intake among women with bulimia nervosa. Appetite, 2017. 112: p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer S, et al. , The role of negative urgency and expectancies in problem drinking and disordered eating: Testing a model of comorbidity in pathological and at-risk samples. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors, 2012. 26(1): p. 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer S, Peterson CM, and McCarthy D, A prospective test of the influence of negative urgency and expectancies on binge eating and purging. Psychology of Addictive Behaviors, 2013. 27(1): p. 294–300. [DOI] [PubMed] [Google Scholar]
- 11.Whiteside U, et al. , Difficulties regulating emotions: Do binge eaters have fewer strategies to modulate and tolerate negative affect? Eating Behaviors, 2007. 8(2): p. 162–169. [DOI] [PubMed] [Google Scholar]
- 12.Svaldi J, et al. , Emotion regulation deficits in eating disorders: A marker of eating pathology or general psychopathology? Psychiatry Research, 2012. 197(1): p. 103–111. [DOI] [PubMed] [Google Scholar]
- 13.Steinglass J, et al. , Restrictive food intake as a choice-A paradigm for study. International Journal of Eating Disorders, 2015. 48(1): p. 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hare TA, Camerer CF, and Rangel A, Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 2009. 324(5927): p. 646–8. [DOI] [PubMed] [Google Scholar]
- 15.Foerde K, et al. , Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nature Neuroscience, 2015. 18(11): p. 1571–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foerde K, et al. , Assessment of test-retest reliability of a food choice task among healthy individuals. Appetite, 2018. 123: p. 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric, A. Diagnostic and statistical manual of mental disorders : DSM-5. 2013; Available from: http://dsm.psychiatryonline.org/book.aspx?bookid=556.
- 18.First MB, et al. , Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P, 1/2007 revision). 2007, New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- 19.Cooper Z and Fairburn C, The eating disorder examination: A semi-structured interview for the assessment of the specific psychopathology of eating disorders. International Journal of Eating Disorders, 1987. 6(1): p. 1–8. [Google Scholar]
- 20.Expert Panel on Detection, E., Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Journal of the American Medical Association, 2001. 285(19): p. 2486. [DOI] [PubMed] [Google Scholar]
- 21.Werthmann J, et al. , Looking at food in sad mood: Do attention biases lead emotional eaters into overeating after a negative mood induction? Eating Behaviors, 2014. 15(2): p. 230–236. [DOI] [PubMed] [Google Scholar]
- 22.McNair DM, Droppleman LF, and Lorr M, Edits manual for the profile of mood states: POMS. 1992: Edits. [Google Scholar]
- 23.Fairburn CG and Beglin SJ, Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders, 1994. 16(4): p. 363–370. [PubMed] [Google Scholar]
- 24.Gratz KL and Roemer L, Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment, 2004. 26(1): p. 41–54. [Google Scholar]
- 25.Lynam DR, et al. , The UPPS-P: Assessing five personality pathways to impulsive behavior. West Lafayette, IN: Purdue University, 2006. [Google Scholar]
- 26.Schielzeth H and Forstmeier W, Conclusions beyond support: Overconfident estimates in mixed models. Behavioral Ecology, 2008. 20(2): p. 416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barr DJ, et al. , Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 2013. 68(3): p. 255–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Team RC, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Austria, 2015. 2018, ISBN 3-900051-07-0: URL http://www.R-project.org.
- 29.Bates D and Maechler M, lme4. linear mixed-effects models using S4 classes. R package version 0.999375-31;[cited 2010 August 12] 2009. [Google Scholar]
- 30.Fox J, Weisberg s (2011) an r companion to applied regression. R package version 2.0-10, 2011. [Google Scholar]
- 31.Aubie CD and Jarry JL, Weight-related teasing increases eating in binge eaters. Journal of Social and Clinical Psychology, 2009. 28(7): p. 909–936. [Google Scholar]
- 32.Laessle RG and Schulz S, Stress-induced laboratory eating behavior in obese women with binge eating disorder. International Journal of Eating Disorders, 2009. 42(6): p. 505–510. [DOI] [PubMed] [Google Scholar]
- 33.Rosen JC, et al. , Standardized test meals in assessment of eating behavior in bulimia nervosa: Consumption of feared foods when vomiting is prevented. International Journal of Eating Disorders, 1985. 4(1): p. 59–70. [Google Scholar]
- 34.Kissileff HR, et al. , Laboratory studies of eating behavior in women with bulimia. Physiology & Behavior, 1986. 38(4): p. 563–570. [DOI] [PubMed] [Google Scholar]
- 35.Walsh BT, Kissileff HR, and Hadigan CM, Eating behavior in bulimia. Annals of the New York Academy of Sciences, 1989. 575: p. 446–54. [DOI] [PubMed] [Google Scholar]
- 36.Zunker C, et al. , Ecological momentary assessment of bulimia nervosa: Does dietary restriction predict binge eating? Behaviour Research and Therapy, 2011. 49(10): p. 714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vohs KD and Heatherton TF, Self-regulatory failure: A resource-depletion approach. Psychological Science, 2000. 11(3): p. 249–254. [DOI] [PubMed] [Google Scholar]
- 38.Heatherton TF and Wagner DD, Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences, 2011. 15(3): p. 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward A and Mann T, Don’t mind if I do: Disinhibited eating under cognitive load. Journal of Personality and Social Psychology, 2000. 78(4): p. 753–763. [DOI] [PubMed] [Google Scholar]


