Abstract
Objective:
To determine the impact of policy changes for oxygen saturation (SpO2) alarm limits on neonatal mortality and morbidity among very preterm infants.
Study design:
Retrospective cohort study of very preterm infants in the NICHD Neonatal Research Network. Infants were classified based on treatment at a hospital with an SpO2 alarm policy change and study epoch (before vs after policy change). We used a generalized linear mixed model to determine the effect of hospital group and epoch on the primary outcomes of mortality and severe retinopathy of prematurity (ROP) and secondary outcomes of necrotizing enterocolitis, bronchopulmonary dysplasia, and any ROP.
Results:
There were 3,809 infants in 10 hospitals with an SpO2 alarm policy change and 3,685 infants in 9 hospitals without a policy change. The nature of most policy changes was to narrow the SpO2 alarm settings. Mortality was lower in hospitals without a policy change (adjusted Odds Ratio [aOR] 0.63; 95% Confidence Interval 0.50, 0.80) but did not differ between epochs in policy change hospitals. The odds of bronchopulmonary dysplasia were higher for hospitals with a policy change (aOR 1.65; 95% Confidence Interval 1.36, 2.00) but did not differ for hospitals without a policy change. Severe ROP and necrotizing enterocolitis did not differ between epochs for either group. The adjusted odds of any ROP were lower in recent years in both hospital groups.
Conclusion:
Changing SpO2 alarm policies was not associated with reduced mortality or increased severe ROP among very preterm infants.
Keywords: Preterm, oxygen saturation, mortality, retinopathy of prematurity
Oxygen is commonly used in the treatment of extremely preterm infants. Like many interventions, oxygen has a therapeutic window. Clinicians must titrate supplemental oxygen to provide adequate oxygen delivery to tissues while avoiding oxygen-related injury to developing organs. The optimal target pulse oximetry saturation (SpO2) range to achieve this balance remains undefined.
Five large international randomized trials were undertaken to determine the impact of lower (85-89%) vs. higher (91-95%) SpO2 target ranges on mortality and morbidity in extremely preterm infants.(1–3) None of these individual trials demonstrated superiority for either SpO2 target with respect to the composite primary outcome of death or neurodevelopmental disability. However, the individual trials and pooled analysis of these trials suggest that there is a tradeoff in secondary outcomes for either SpO2 target.(4,5) Assignment to the higher SpO2 target reduced the incidence of death and necrotizing enterocolitis, and assignment to the lower SpO2 target reduced the incidence of severe retinopathy of prematurity (ROP).
These findings have led to continued debate regarding optimal SpO2 targets in extremely preterm infants, (6) with many NICUs implementing changes to their SpO2 targets.(7) Previous authors have reported higher rates of ROP following an increase in SpO2 targets, (8) but this finding is not consistent.(9) Further, secular trends in infant demographics and clinical practice may influence clinical outcomes in a before/after study design, particularly in a single-site setting.
We designed the current multi-site study within the National Institute of Child Health and Human Development Neonatal Research Network (NRN) to investigate the interaction between changes in SpO2 alarm limit policies in NRN hospitals and time (before/after policy changes). Our objective was to identify the association between changes in SpO2 alarm limit policies on neonatal mortality and morbidity and supplemental oxygen exposure among very preterm infants.
Methods
This was a retrospective cohort study using prospectively collected data in the NRN Generic Database (GDB). We included infants in the GDB who were born between 1/1/2006-12/31/2014 with birth weight 401-1000 grams or gestational age <29 weeks and who were treated at a hospital that participated in the NRN continuously from 2006-2014. We excluded infants who were born during the study washout period (see Study Epoch Definition, below), infants who died within the first 12 hours of life (as they were not included in the GDB), infants with major congenital anomalies, infants who were born at referring hospitals and transferred to an NRN hospital (as these infants were not consistently enrolled in the GDB throughout the study period), and infants who had been identified as likely to be eligible for enrollment in SUPPORT(1) (Surfactant Positive Pressure and Pulse Oximetry Randomized Trial).
Each enrolled infant was classified on the basis of 2 exposures: treatment at a hospital with an SpO2 alarm policy change during the study period, and the study epoch in which they were born (before or after policy change).
Policy Change Definition
We administered a questionnaire to NRN site principal investigators in October 2016 to identify hospitals that changed their SpO2 alarm setting policy between 2006 and 2014. SpO2 policies, including alarm settings, had to be clearly documented (such as within a practice standard). Because SpO2 alarm settings may match or slightly exceed the extremes of the desired SpO2 targets,(6) we characterized policies based on the presence of change in the SpO2 alarm settings, not the specified SpO2 targets. Hospitals with an SpO2 alarm setting policy change during the study period were designated “policy change.” Hospitals without a policy change during the study period were classified “no policy change.”
Study Epoch Definition
For hospitals with a policy change, we defined the study epochs based on the date of the policy change for each individual hospital. We designated a 6-month period before and after the policy change as the “washout” period. For each of these hospitals, Epoch 1 was defined as 1/1/06 until 6 months prior to that hospital’s policy change, and Epoch 2 was defined as the interval starting 6 months after the hospital’s policy change until 12/31/14. Infants born during the 1-year washout period were not included in this analysis.
For hospitals without a policy change, we designated the calendar year 2010 (the year SUPPORT results became available) as the transition between Epoch 1 and 2. For those hospitals, we defined Epoch 1 as 1/1/06-12/31/09, and Epoch 2 as 1/1/11-12/31/14. Infants born during the 1-year washout period 1/1/10-12/31/10 were not included in this analysis.
Clinical Outcomes
The primary outcomes were mortality before hospital discharge, transfer, or 120 days of life for infants with longer hospitalization; severe ROP, defined as ROP treatment or retinal detachment in either eye. These were selected because of the observed tradeoff in the risks of these outcomes in the oxygen targeting randomized trials. Infants who were diagnosed with severe ROP prior to death were considered to have both primary outcomes. Each primary outcome was reported separately. Secondary outcomes included necrotizing enterocolitis ≥ stage 2 (10), any ROP, moderate/severe bronchopulmonary dysplasia (BPD) (NIH consensus definition (11)), supplemental oxygen use after discharge, and cumulative days on supplemental oxygen during the hospitalization among infants who survived to discharge.
Information on the highest FiO2 level at pre-specified time points is recorded in the GDB. We examined the highest FiO2 recorded on the following days: 24 hours, 3 days, 7 days, 14 days, and 28 days. We also assessed the highest FiO2 across these time points; this analysis was restricted to infants who survived to 28 days to reduce bias introduced by early death.
Statistical Analyses
Our first objective was to assess the relationship between changes in SpO2 alarm setting policies and changes in the primary and secondary outcomes between the study epochs. We used a generalized linear mixed model to explore the effect of instituting a change in hospital policy on the proportion of infants with each outcome between Epoch 1 and 2. Models included the hospital-level effect of policy change (yes/no), epoch, and the interaction between policy change and epoch. A significant interaction term would indicate that the difference in outcomes between Epoch 1 and 2 varied based on the hospital group (policy change or no policy change). We adjusted this analysis for the following infant-level characteristics: gestational age, birth weight, multiple gestation, antenatal steroid exposure, sex, race, ethnicity, intubation for resuscitation, small for gestational age status(12), and admission temperature(13). Although different infants were present during the two epochs, a random effect for hospital was included in the models to account for the fact that infants treated at the same hospital may have more similar outcomes.
Our second objective was to examine the relationship between instituting a change in SpO2 alarm settings, epoch, and supplemental oxygen exposure in extremely preterm infants. Because the highest FiO2 variable was highly skewed, with a large number of infants whose highest FiO2 was 0.21, we modeled a dichotomous variable, highest FiO2>0.21, based on hospital groupings and epochs. This analysis used a similar generalized linear mixed model and adjusted for the same covariates. P values < .05 were considered statistically significant, and hospital policy change (yes/no) and epoch interaction terms with p-values <0.05 were considered evidence of an epoch effect that differed between the two hospital groupings. No adjustment was made for multiple comparisons. Only non-missing data were included in analysis; statistical modeling methods assumed missing data were missing at random. All analyses were performed using SAS 9.4.
Results
There were 19 NRN hospitals with continuous participation in the GDB between 2006-2014. Of these, 10 changed the policy for SpO2 alarm settings, and 9 did not change the policy during the study period. Among hospitals with a SpO2 policy change, the median SpO2 alarm limits transitioned from 85% (lower) and 96% (upper) to revised median limits of 89% (lower) and 95% (upper) (Figure 1). Among hospitals without a SpO2 alarm policy change, the median SpO2 alarm limits were 88% (lower) and 95% (upper).
Figure 1:
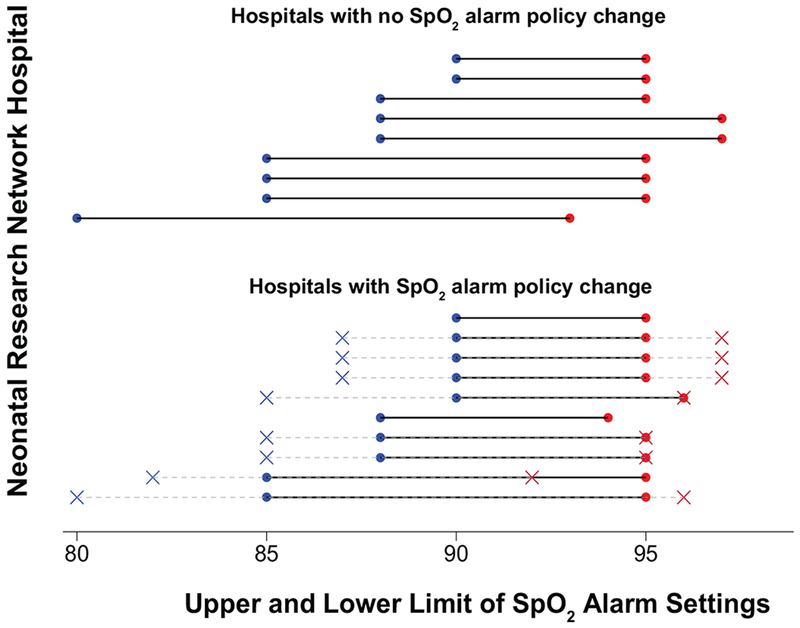
Oxygen saturation (SpO2) alarm settings for hospitals with and without a policy change. For hospitals without a SpO2 alarm policy change, median alarm limits were 88% (lower limit) and 95% (upper limit). For hospitals with a policy change, original alarm settings, shown in X marks, had median values of 85% (lower limit) and 96% (upper limit). The revised alarm settings, shown in circles, had median values of 89% (lower limit) and 95% (upper limit). Original alarm settings are not shown for 2 hospitals in the SpO2 alarm policy change group: 1 hospital transitioned from no policy to an SpO2 alarm policy, and 1 hospital did not have record of the original SpO2 alarm settings.
Of 7,494 infants included in this study (Figure 2; available at www.jpeds.com), there were 3,809 infants in hospitals with a SpO2 alarm policy change and 3,685 infants in hospitals without a policy change. Differences in demographic characteristics between epochs for each group of hospitals are shown in Table I. Mortality did not significantly differ between epochs for infants in hospitals with a SpO2 alarm policy change, and mortality was significantly lower in Epoch 2 for infants in hospitals without a SpO2 alarm policy change (Table 2). Severe ROP did not significantly differ between epochs for either group.
Figure 2.
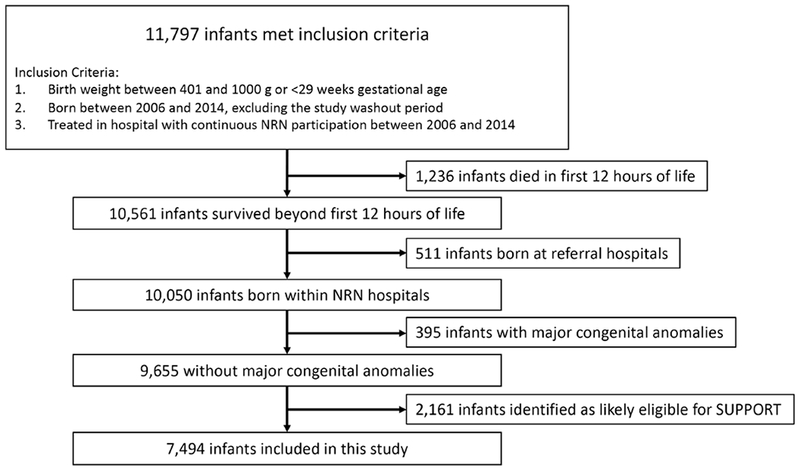
Study Flow Diagram.
NRN: Neonatal research network; SUPPORT: Surfactant Positive Pressure and Pulse Oximetry Randomized Trial
Table 1:
Baseline maternal and infant characteristics
| Infants in hospitals with SpO2 alarm policy change (n=3809) | Infants in hospitals with no SpO2 alarm policy change (n=3685) | |||||
|---|---|---|---|---|---|---|
| Characteristic | Epoch 1 n=1981 | Epoch 2 n=1828 | P value | Epoch 1 n=1620 | Epoch 2 n=2065 | P-value |
| Antenatal Steroids | 1599/1977 (80.9%) | 1676/1826 (91.8%) | <0.001 | 1387/1605 (86.4%) | 1892/2063 (91.7%) | <0.001 |
| Race | 0.002 | 0.13 | ||||
| Black | 816/1949 (41.9%) | 816/1756 (46.5%) | 724/1598 (45.3%) | 867/2053 (42.2%) | ||
| White | 1046/1949 (53.7%) | 830/1756 (47.3%) | 746/1598 (46.7%) | 1043/2053 (50.8%) | ||
| Other | 87/1949 (4.5%) | 110/1756 (6.3%) | 128/1598 (8.0%) | 143/2053 (7.0%) | ||
| Hispanic | 424/1959 (21.6%) | 276/1823 (15.1%) | <0.001 | 195/1464 (13.3%) | 266/2035 (13.1%) | 0.83 |
| Multiple gestation | 451/1981 (22.8%) | 442/1828 (24.2%) | 0.30 | 445/1620 (27.5%) | 576/2065 (27.9%) | 0.78 |
| Gestational age, weeks; mean (SD) | 26.7 (2.1) | 26.2 (2.0) | <0.001 | 26.8 (2.0) | 26.3 (1.9) | <0.001 |
| Birth weight, grams; mean (SD) | 891 (245) | 840 (237) | <0.001 | 906 (240) | 870 (233) | <0.001 |
| Male sex | 995/1981 (50.2%) | 944/1828 (51.6%) | 0.38 | 811/1620 (50.1%) | 1064/2065 (51.5%) | 0.38 |
| SGA | 337/1981 (17.0%) | 294/1828 (16.1%) | 0.44 | 246/1620 (15.2%) | 282/2065 (13.7%) | 0.19 |
| Delivery room intubation | 1158/1981 (58.5%) | 1040/1827 (56.9%) | 0.34 | 997/1620 (61.5%) | 1286/2065 (62.3%) | 0.65 |
| Admission temperature, °F; mean (SD) | 97.5 (1.6) | 97.7 (1.3) | <0.001 | 96.9 (1.9) | 97.6 (1.6) | <0.001 |
Abbreviations: F: Fahrenheit; SD: standard deviation; SGA: small for gestational age; SpO2: oxygen saturation
Table 2:
Changes in outcomes between epochs for infants in hospitals with and without a SpO2 alarm policy change.
| Infants in hospitals with SpO2 alarm policy change (n=3809) | Infants in hospitals with no SpO2 alarm policy change (n=3685) | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Epoch 1 n=1981 | Epoch 2 n=1828 | aOR or Mean Difference (95% CI)* | Epoch 1 n=1620 | Epoch 2 n=2065 | aOR or Mean Difference (95% CI)* | Adjusted Interaction P-value |
| Mortality | 297/1979 (15.0%) | 324/1828 (17.7%) | 0.94 (0.75, 1.18) | 269/1615 (16.7%) | 280/2061 (13.6%) | 0.63 (0.50, 0.80) | 0.01 |
| Severe ROP | 127/1703 (7.5%) | 126/1535 (8.2%) | 1.09 (0.78, 1.52) | 88/1300 (6.8%) | 127/1746 (7.3%) | 0.91 (0.64, 1.29) | 0.46 |
| Necrotizing enterocolitis | 245/1980 (12.4%) | 250/1827 (13.7%) | 0.96 (0.78, 1.20) | 210/1620 (13.0%) | 250/2065 (12.1%) | 0.83 (0.67, 1.04) | 0.35 |
| BPD | 592/1722 (34.4%) | 700/1541 (45.4%) | 1.65 (1.36, 2.00) | 443/1343 (33.0%) | 794/1797 (44.2%) | 1.21 (0.99, 1.48) | 0.03 |
| Any ROP | 829/1703 (48.7%) | 655/1535 (42.7%) | 0.71 (0.59, 0.86) | 706/1300 (54.3%) | 935/1746 (53.6%) | 0.57 (0.47, 0.69) | 0.11 |
| Cumulative days on supplemental O2 (days), mean (SD)** | 43.5 (39.8) | 53.2 (42.5) | 3.87 (1.80, 5.94) | 39.5 (37.2) | 49.8 (40.3) | 1.70 (−.038, 3.79) | 0.14 |
| Discharged home on O2** | 161/1395 (11.5%) | 201/1113 (18.1%) | 1.47 (1.09, 1.99) | 223/1183 (18.9%) | 331/1357 (24.4%) | 1.12 (0.86, 1.45) | 0.17 |
Abbreviations: aOR: adjusted Odds Ratio; BPD: bronchopulmonary dysplasia; CI: confidence interval; ROP: retinopathy of prematurity; SD: standard deviation; SpO2: oxygen saturation
Analyses adjusted for gestational age, birth weight, multiple gestation, antenatal steroids, sex, race, ethnicity, delivery room intubation, small for gestational age status, and admission temperature
Among infants who survived to discharge
For infants in hospitals with a SpO2 alarm policy change, the adjusted odds of BPD were significantly higher in Epoch 2. There was no difference in BPD between epochs among infants in hospitals without a SpO2 alarm policy change. Necrotizing enterocolitis did not differ between epochs for either group. There was a reduction in the adjusted odds of any ROP in Epoch 2 for both groups of hospitals. The interaction term between epoch and hospital group was not significant for this outcome, indicating that the reduction in ROP between study epochs did not vary based on hospital group.
There was no significant interaction between hospital group and epoch for the outcomes of cumulative oxygen exposure or supplemental oxygen use after discharge. Averaged across both groups of hospitals, infants born in Epoch 2 who survived to discharge had longer exposure to supplemental oxygen (mean difference 2.79 days; 95% Confidence Interval [CI] 1.30, 4.28; p-value <0.001) and were more likely to be discharged home on supplemental oxygen (adjusted Odds Ratio 1.28; 95% CI 1.05, 1.57; p-value=0.015).
For both groups of hospitals, the adjusted odds of highest FiO2 >0.21 at 14 and 28 days of life were higher in Epoch 2. There was no significant interaction between epoch and hospital group for those individual time points. However, there was a significant interaction between hospital and epoch for the combined variable of highest FiO2>0.21 across the first 28 days of life (Table 3).
Table 3:
Change in supplemental oxygen exposure at specified time points between epochs for infants in hospitals with and without an oxygen saturation alarm policy change.
| Infants in hospitals with SpO2 alarm policy change (n=3809) | Infants in hospitals with no SpO2 alarm policy change (n=3685) | ||||||
|---|---|---|---|---|---|---|---|
| FiO2 >0.21 at time point | Epoch 1 n=1981 | Epoch 2 n=1828 | aOR (95% CI)* | Epoch 1 n=1620 | Epoch 2 n=2065 | aOR (95% CI)* | Adjusted Interaction P-value |
| 24 hours | 1193/1952 (61.1%) | 1199/1802 (66.5%) | 1.00 (0.85, 1.17) | 845/1582 (53.4%) | 1133/2031 (55.8%) | 1.08 (0.92, 1.27) | 0.475 |
| Day 3 | 1381/1923 (71.8%) | 1403/1788 (78.5%) | 1.15 (0.96, 1.39) | 1010/1553 (65.0%) | 1410/2020 (69.8%) | 1.17 (0.99, 1.40) | 0.881 |
| Day 7 | 1010/1835 (55.0%) | 1103/1718 (64.2%) | 1.16 (0.96, 1.40) | 694/1454 (47.7%) | 1061/1956 (54.2%) | 0.98 (0.81, 1.18) | 0.215 |
| Day 14 | 1005/1681 (59.8%) | 1131/1647 (68.7%) | 1.25 (1.01, 1.54) | 697/1313 (53.1%) | 1282/1889 (67.9%) | 1.60 (1.30, 1.96) | 0.096 |
| Day 28 | 878/1570 (55.9%) | 1042/1577 (66.1%) | 1.28 (1.04, 1.59) | 556/1165 (47.7%) | 1163/1819 (63.9%) | 1.68 (1.35, 2.09) | 0.083 |
| Across the first 28 days** | 1408/1767 (79.7%) | 1380/1593 (86.6%) | 1.29 (1.02, 1.63) | 1051/1416 (74.2%) | 1584/1850 (85.6%) | 1.79 (1.44, 2.23) | 0.045 |
Abbreviation: aOR: adjusted odds ratio; CI: confidence interval; FiO2: fraction of inspired oxygen; SpO2: oxygen saturation
Analyses adjusted for gestational age, birth weight, multiple gestation, antenatal steroids, sex, race, ethnicity, delivery room intubation, small for gestational age status, and admission temperature
among infants who survived to 28 days of life
Discussion
The target SpO2 values to optimize outcomes for preterm infants remains a topic of active debate, with some authors uniformly advocating a higher SpO2 target range.(14) Given the wide variation in SpO2 targets employed across neonatal intensive care units,(15) reflexively implementing these higher targets would imply a change in oxygen targeting policies for many hospitals. We sought to determine the impact of changing SpO2 alarm settings for preterm infants on neonatal mortality and morbidity. In the post SUPPORT era, half of NRN hospitals revised their policy for SpO2 alarm settings and the other half made no changes. Among NRN hospitals that revised their oxygen saturation policy, the nature of most of these changes was to narrow the range of SpO2 alarm settings, consistent with other reports.(7) We found no evidence that modifying the SpO2 alarm setting policy reduced mortality or increased severe ROP. Supplemental oxygen exposure was higher in Epoch 2 in both groups of hospitals, but this finding was not significantly associated with a policy change in SpO2 alarm settings.
Manley et al reported their single-center experience of 346 preterm infants after increasing SpO2 targets from 88-92% to 91-95%. ROP was significantly more frequent among infants born after the SpO2 target change, and mortality rates did not significantly differ.(8) Other authors have not observed significant differences in neonatal morbidity following changes to SpO2 target policies.(9) The impact of changing SpO2 alarm limits on clinical outcomes in a given setting likely depends on many factors, such as local baseline outcome rates.
Secular trends in infant demographics and clinical practice make it difficult to isolate the impact of a given change in practice on clinical outcomes in a single site before/after study. Due to our multisite study design, we were able to assess the interaction between epochs and hospital grouping in order to better account for concurrent secular trends. Previous authors have described decreasing mortality over time among extremely preterm infants.(16–18) Similarly, we observed a reduction in mortality in Epoch 2 among hospitals without a hospital policy change - where a wider range of acceptable SpO2 alarm limits was retained. Conversely, the adjusted odds of BPD were significantly higher in Epoch 2 for hospitals where SpO2 alarm settings were revised. The interaction between instituting a policy change and epoch was significant for both of these outcomes. We speculate that additional unmeasured differences in infant demographics and hospital practice may have contributed to these study findings. Nonetheless, our results do not suggest that changing SpO2 alarm settings alone led to a significant benefit in neonatal outcomes.
Use of supplemental oxygen was assessed in multiple ways. More infants were exposed to FiO2 >0.21 at 14 and 28 days of life in Epoch 2 in both hospital groups. In addition, the cumulative duration of oxygen exposure and use of supplemental oxygen after discharge were both increased in Epoch 2 for both groups of hospitals. Although we adjusted for changes in important baseline characteristics, other unmeasured differences in patient demographics may have contributed to increased oxygen use in Epoch 2. In addition, we speculate that the lower mortality rate in Epoch 2 within hospitals without an SpO2 alarm policy change may have led to increased supplemental oxygen use among survivors. Despite the fact that supplemental oxygen use increased, we did not find any evidence of increased rates of severe ROP in Epoch 2 for infants born in either group of hospitals, and rates of any ROP were lower in Epoch 2 for both groups.
This analysis was restricted to SpO2 policies in the neonatal intensive care unit setting. We did not account for changes in delivery room oxygen management following changes to neonatal resuscitation treatment recommendations in 2010.(19) Reassuringly, a meta-analysis of randomized trials comparing high versus low initial FiO2 for delivery room resuscitation of infants <28 weeks gestation found no significant differences in clinical outcomes of death, ROP, or BPD.(20)
Study limitations include the observational study design. Although we accounted for important baseline demographic characteristics and interventions that changed between epochs, it is possible that other secular trends in practice at participating hospitals influenced the study results. In addition, we recognize that hospitals may vary in terms of how strictly the alarm policies were followed (15) or how tightly infants’ SpO2 levels were maintained within set alarm limits.(21) Finally, we classified hospitals based on a change to the SpO2 alarm settings and not the absolute values of the alarm limits. Our objective was not to determine the impact of specific SpO2 targets on patient outcomes. This question, addressed in the pooled analysis of 5 RCTs in the NeOProM collaboration, is unlikely to be answered in an observational study.
In conclusion, we did not find evidence that narrowing SpO2 alarm limits had a significant impact on neonatal mortality or severe ROP among more than 7,000 extremely preterm infants in the NICHD NRN. These results suggest that changing policies for oxygen saturation alarm settings alone may not confer a significant benefit on preterm infants’ outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr Abhik Das (DCC Principal Investigator), Dr Marie Gantz (DCC Statistician) and Mr Benjamin Carper (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Supported by the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (K23HD084727 to EEF). In addition, the National Institutes of Health, the NICHD, the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Generic Database Study through cooperative agreements (U10 HD27904, U10 HD21364, M01 RR80, U10 HD27853, M01 RR8084, U10 HD40492, UL1 TR1117, M01 RR30, UL1 TR1111, U10 HD27851, M01 RR39, U10 HD27856, M01 RR750, U10 HD21373, U10 HD36790, U10 HD27880, M01 RR70, U10 HD34216, M01 RR32, U10 HD53109, M01 RR59, U10 HD53089, M01 RR997, U10 HD40689, M01 RR633, U10 HD21385). Although the NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
Abbreviations:
- BPD:
bronchopulmonary dysplasia
- CI:
confidence interval
- FiO2:
fraction of inspired oxygen
- GDB:
Generic Database
- NRN:
Neonatal Research Network
- ROP:
retinopathy of prematurity
- SpO2:
pulse oximetry oxygen saturation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented as an abstract at the Pediatric Academic Societies annual meeting, May 6-9, 2017, San Francisco, California.
References
- 1.Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Target Ranges of Oxygen Saturation in Extremely Preterm Infants. New Eng J Med. 2010;362:1959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenson BJ, Tarnow-Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368:2094–104. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, et al. Effects of Targeting Higher vs Lower Arterial Oxygen Saturations on Death or Disability in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA. 2013;309:2111–20. [DOI] [PubMed] [Google Scholar]
- 4.Askie LM, Darlow BA, Davis PG, Finer N, Stenson B, Vento M, et al. Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants. Cochrane Database Syst Rev. 2017; 4:CD011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA. 2018;319:2190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings JJ, Polin RA, COMMITTEE ON FETUS AND NEWBORN. Oxygen Targeting in Extremely Low Birth Weight Infants. Pediatrics. 2016;138:e20162904–e20162904. [DOI] [PubMed] [Google Scholar]
- 7.Huizing MJ, Villamor-Martínez E, Vento M, Villamor E. Pulse oximeter saturation target limits for preterm infants: a survey among European neonatal intensive care units. Eur J Pediatr. 2017;176:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manley BJ, Kuschel CA, Elder JE, Doyle LW, Davis PG. Higher Rates of Retinopathy of Prematurity after Increasing Oxygen Saturation Targets for Very Preterm Infants: Experience in a Single Center. J Pediatr. 2016;168:242–4. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren P, Hård A-L, Wilde Å, Löfqvist C, Smith LEH, Hellström A. Implementing higher oxygen saturation targets reduced the impact of poor weight gain as a predictor for retinopathy of prematurity. Acta Paediatrica. 2018;107: 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal Necrotizing Enterocolitis. Ann Surg. 1978;187:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jobe AH, Bancalari E. Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. [DOI] [PubMed] [Google Scholar]
- 12.Walsh MC, Di Fiore JM, Martin RJ, Gantz M, Carlo WA, Finer N. Association of Oxygen Target and Growth Status With Increased Mortality in Small for Gestational Age Infants: Further Analysis of the Surfactant, Positive Pressure and Pulse Oximetry Randomized Trial. JAMA Pediatr. 2016;170:292–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laptook AR, Salhab W, Bhaskar B. Admission Temperature of Low Birth Weight Infants: Predictors and Associated Morbidities. Pediatrics. 2007;119:e643–9. [DOI] [PubMed] [Google Scholar]
- 14.Stenson BJ. Oxygen Saturation Targets for Extremely Preterm Infants after the NeOProM Trials. Neonatology. 2016;109:352–8. [DOI] [PubMed] [Google Scholar]
- 15.Hagadorn JI, Sink DW, Buus-Frank ME, Edwards EM, Morrow KA, Horbar JD, et al. Alarm safety and oxygen saturation targets in the Vermont Oxford Network iNICQ 2015 collaborative. J Perinatol. 2017;37:270–6. [DOI] [PubMed] [Google Scholar]
- 16.Ancel P-Y, Goffinet F, Kuhn P, Langer B, Matis J, Hernandorena X, et al. Survival and Morbidity of Preterm Children Born at 22 Through 34 Weeks’ Gestation in France in 2011: Results of the EPIPAGE-2 Cohort Study. JAMA Pediatr. 2015;169:230–8. [DOI] [PubMed] [Google Scholar]
- 17.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo KT, Lee QY, Quek WS, Wang YA, Bolisetty S, Lui K, et al. Trends in Morbidity and Mortality of Extremely Preterm Multiple Gestation Newborns. Pediatrics. 2015;136:263–71. [DOI] [PubMed] [Google Scholar]
- 19.Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S516–38. [DOI] [PubMed] [Google Scholar]
- 20.Oei JL, Vento M, Rabi Y, Wright I, Finer N, Rich W, et al. Higher or lower oxygen for delivery room resuscitation of preterm infants below 28 completed weeks gestation: a meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017;102: F24–30. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt B, Roberts RS, Whyte RK, Asztalos EV, Poets C, Rabi Y, et al. Impact of Study Oximeter Masking Algorithm on Titration of Oxygen Therapy in the Canadian Oxygen Trial. J Pediatr. 2014;165:666–671.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


