Abstract
Background
We characterized the performance characteristics of guideline-recommended invasive mediastinal staging for lung cancer and developed a prediction model for nodal disease as a potential alternative approach to staging.
Methods
We conducted a prospective cohort study of adults with suspected/ confirmed non-small cell lung cancer without evidence of distant metastatic disease (by computed tomography/positron emission tomography) who underwent nodal evaluation by invasive mediastinal staging and/or at the time of resection. The true-positive rate (TPR) was the proportion of patients with true nodal disease selected to undergo invasive mediastinal staging based on guideline recommendations, and the false-positive rate (FPR) was the proportion of patients without true nodal disease selected to undergo invasive mediastinal staging. Logistic regression was used to predict nodal disease using radiographic predictors.
Results
Among 123 eligible subjects, 31 (25%) had pathologically confirmed nodal disease. A guideline-recommended invasive staging strategy had a TPR and FPR of 100% and 65%, respectively. The prediction model fit the data well (goodness-of-fit test p=0.55) and had excellent discrimination (optimism corrected c-statistic 0.78, 95% confidence interval 0.72–0.89). Exploratory analysis revealed that use of the prediction model could achieve a FPR of 44% at a TPR of 97%.
Conclusions
A guideline-recommended strategy for invasive mediastinal staging selects all patients with true nodal disease and a majority of patients without nodal disease for invasive mediastinal staging. Our prediction model appears to maintain (within a margin of error) the sensitivity of a guideline-recommended invasive staging strategy and has the potential to reduce the use of invasive procedures.
INTRODUCTION
In the current era of value-driven health care[1], it is important to consider novel approaches to maintaining or improving the accuracy of lung cancer staging while decreasing use of diagnostic tests. Practice guidelines recommend computed tomography (CT) and positron emission tomography (PET) for non-invasive staging of patients with suspected/confirmed non-small cell lung cancer (NSCLC)[2], [3]. For patients without distant metastases, guidelines recommend invasive mediastinal staging (IMS) for those with radiographic findings predictive of nodal disease[2], [3]. Reducing the use of invasive tests is a particularly attractive target for value-optimization because these tests impose a burden upon patients (e.g., discomfort, anxiety), have rare but potentially life-threatening risks[4]–[5], and are costly.
One way to improve patient selection for IMS is to better predict the presence of nodal disease. As a first step towards achieving this goal, we characterized the performance characteristics of a guideline-recommended IMS strategy. Second, we developed and cross-validated a prediction model for nodal disease based on previously-described radiographic risk factors[6]–[14]. Finally, we tested whether the addition of a plasma-based biomarker for nodal disease—vascular endothelial growth factor-C (VEGF-C)[15]–[20]—improves prediction.
METHODS
Study Design and Population
We conducted a prospective study of adults with suspected/confirmed NSCLC staged by CT/ PET, without distant metastases, and who underwent nodal evaluation by IMS and/or intraoperatively during pulmonary resection (sampling or dissection). Nodal evaluation was at the discretion of treating providers in this observational, pragmatic study. IMS refers to mediastinoscopy, endobronchial-ultrasound, or both. Staging was based on the 7th-edition of the American Joint Committee on Cancer. Ineligible subjects included those with synchronous, metachronous, or recurrent lung cancer, or who underwent chemotherapy without confirmation of nodal disease. We studied patients referred to the Seattle Cancer Care Alliance Lung Cancer Early-Detection and Prevention Clinic (January 2014-December 2016) and the University of Washington Thoracic Surgery Clinic (October 2014-December 2016) who consented to participate in an observational lung biorepository approved by the Fred Hutchinson Cancer Research Center Institutional Review Board (file#6663, protocol#2242). The review board approved a modification (M100214) to pursue our study. Our study complies with the Reporting Recommendations for Tumor Marker Prognostic Studies[21].
Clinical Variables
Trained chart abstractors ascertained study variables from the electronic medical record. Two clinicians (FCV, FF) verified the accuracy of predictor and outcome variables. We recorded tumor size, maximum standardized uptake (SUVMax) of the primary tumor, lymphadenopathy (documented as such or ≥1cm in short axis), and fluorodeoxygluose (FDG)-uptake within lymph nodes, and other radiographic variables (e-Appendix A) based on the radiology report. A board-certified thoracic surgeon (FF) reviewed CT images to determine central tumor location (tumor not amenable to wedge resection). We also measured tumor location based on definitions provided by American College of Chest Physicians (ACCP)[2] and National Comprehensive Cancer Network (NCCN)[3].
We collected blood using a previously-reported standardized protocol[22] and measured plasma VEGF-C levels using a commercially-available enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
Performance Characteristics of Guideline-Recommended IMS Staging
The true-positive rate (TPR) represents the proportion of patients with true (pathologically-confirmed) nodal disease selected for IMS. The false-positive rate (FPR) represents the proportion of patients without pathologically-confirmed nodal disease selected for IMS. More specifically, “positive” and “negative” refer to whether guidelines would or would not select a patient for IMS; “positive” and “negative” do not refer to the presence or absence of clinically positive nodes. Pathologic evaluation of lymph nodes obtained from IMS and/or at the time of pulmonary resection was the gold-standard for determining nodal status. Both ACCP and NCCN recommend IMS for tumors >3cm, central location, hilar/mediastinal lymphadenopathy, or nodal FDG-uptake[2], [3]. Guidelines do not recommend against IMS in the absence of these findings; therefore, a guideline-recommended strategy refers to the minimum criteria for selecting patients for IMS.
Model Development
We performed a comprehensive literature review resulting in an a priori set of radiographic predictors of nodal disease (e-Appendix A). Based on an empirically-supported heuristic for powering regression analyses[23], we constructed a logistic-regression model to estimate the probability of nodal disease and limited it to six degrees-of-freedom to mitigate overfitting. We selected variables and parameterizations to maximize discrimination. We considered interaction terms and non-linear parameterization of continuous predictors using fractional polynomial regression[24]. For variables with <10% missing data, we imputed values using median and modal values for continuous and categorical predictors, respectively. We did not consider variables with >10% missing data for inclusion in the model. We constructed another model including radiographic factors from the “final” model and log-transformed values of VEGF-C[22].
Statistical Analysis
We characterized the uncertainty of TPR and FPR estimates for a guideline-recommended staging strategy using binomial-exact 95% confidence intervals (CIs). We evaluated model performance using discrimination and calibration. To internally cross-validate our model, we used bootstrapping (600-replicates) to estimate an optimism-corrected c-statistic[25], [26]. We used nested non-parametric bootstrapping (6000-outer replicates and 600-inner replicates) to calculate corresponding 95%CIs, with the number of replicates based on convergence of upper (97.5%) and lower (2.5%) percentiles evaluated by simulation[25], [27]. To test whether VEGF-C improved prediction, we compared models with and without VEGF-C using the likelihood ratio test[28], defining significance as p-value<0.05. We conducted pre-specified sensitivity analyses varying the definition of central location, using a different dependent variable (N2/N3), and repeating our primary analysis in subgroups with confirmed NSCLC and ≥3 sampled mediastinal nodal stations. Finally, we conducted an exploratory analysis to evaluate the potential implications of using our model in practice by using a threshold probability of nodal disease above which patients would be classified as high-risk (i.e., selected for IMS). We selected a cut-off value attempting to match the TPR of guidelines and described the corresponding FPR and 95%CI using non-parametric bootstrapping (6000-replicates)[25], [27]. This approach avoids erroneously narrow CIs for FPR that can result from traditional methods. Analyses were conducted using R (R Core Team, 2017).
RESULTS
Our study included 123 patients (Figure 1). Table 1 shows the demographic and clinical characteristics of the cohort. Eighty-six percent underwent IMS, 78% underwent resection, and 90% were ultimately diagnosed with NSCLC. A median of 1 [range 0–3] hilar node and 4 [range 1–8] mediastinal nodes were sampled by IMS and/or intraoperatively (e-Appendix B-C). The prevalence of nodal disease was 25% (95%CI 17–33%). Eleven percent (95%CI 5.7–17%) had multi-station N2 and/or N3 disease. Table 2 shows the prevalence of radiographic risk-factors and nodal disease.
Figure 1.
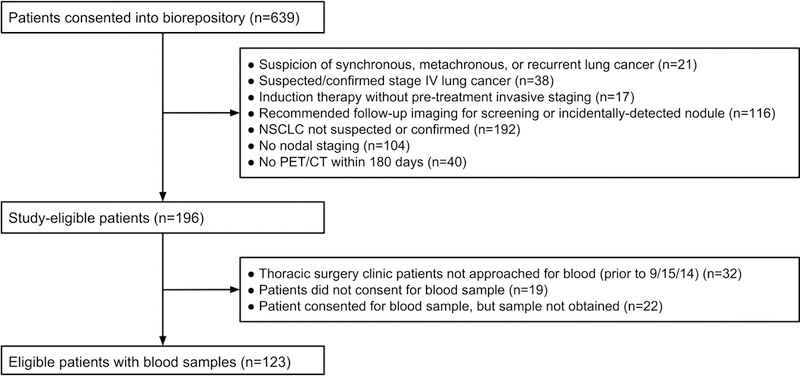
Patient accrual and cohort selection. Vascular endothelial growth factor C (VEGFC), Positron emission tomography (PET), Computed tomography (CT), Non-small Cell Lung Cancer (NSCLC)
Table 1.
Cohort Characteristics
| n=123 | |
|---|---|
| Median age, years (IQR) | 68 (12) |
| Men (%) | 68 (55%) |
| Race (%) | |
| White | 112 (91%) |
| Black | 1 (0.81%) |
| Asian | 5 (4.1%) |
| Native Hawaiian/Pacific Islander | 1 (0.81%) |
| American Indian/Alaska Native | 4 (3.3%) |
| Comorbid conditions (%) | |
| Hypertension | 79 (64%) |
| Coronary artery disease | 31 (25%) |
| Congestive heart failure | 4 (3.3%) |
| Cerebrovascular disease | 6 (4.9%) |
| Peripheral vascular disease | 9 (7.3%) |
| Chronic obstructive pulmonary disease | 43 (35%) |
| Pulmonary hypertension | 4 (3.3%) |
| Diabetes mellitus | 24 (20%) |
| Smoking status (%) | |
| Former | 78 (63%) |
| Current | 26 (21%) |
| Never | 19 (16%) |
| Median pack-yearsa (IQR) | 40 (34) |
| Median FEV1, % predicted (IQR) | 81 (32) |
| Missing (%) | 8 (6.5%) |
| Median Diffusing capacity of the lung for carbon monoxide, % predicted (IQR) | 67 (25) |
| Missing (%) | 15 (12%) |
| Clinical Stage (%) | |
| Stage IA | 57 (46%) |
| Stage IB | 16 (13%) |
| Stage IIA | 18 (15%) |
| Stage IIB | 5 (4.1%) |
| Stage IIIA | 21 (17%) |
| Stage IIIB | 6 (4.9%) |
| Invasive mediastinal staging (%) | |
| None | 17 (14%) |
| Mediastinoscopy | 86 (70%) |
| Endobronchial ultrasound | 8 (6.5%) |
| Endobronchial ultrasound/mediastinoscopy | 12 (9.8%) |
| Final diagnosis (%) | |
| NSCLC | 112 (90%) |
| Small cell lung cancer | 1 (0.81%) |
| Metastasis (non-lung primary) | 4 (3.3%) |
| Infection | 2 (1.6%) |
| Benign lesion | 4 (3.3%) |
| Final Pathologic Stage b (%) | |
| Stage 0 | 1 (0.89%) |
| Stage IA | 39 (35%) |
| Stage IB | 31 (28%) |
| Stage IIA | 9 (8.0%) |
| Stage IIB | 7 (6.3%) |
| Stage IIIA | 23 (21%) |
| Stage IIIB | 2 (1.8%) |
| Prevalence of nodal disease b (%) | |
| N0 | 81 (72%) |
| N1 | 11 (9.8%) |
| N2 | 18 (16%) |
| N3 | 2 (1.8%) |
Interquartile range (IQR), Non-small cell lung cancer (NSCLC), Maximum standard uptake value (SUVMax), Vascular endothelial growth factor-C (VEGF-C), Forced expiratory volume in the first second (FEV1),, Fluorodeoxyglucose (FDG).
Among smokers.
Among 112 patients with confirmed NSCLC
Table 2.
Prevalence of Risk Factors and Nodal Disease
|
All (n=123) |
No Nodal Disease (n=92) |
Nodal Disease (n=31) |
Prevalence of Nodal Disease (%) |
||
|---|---|---|---|---|---|
| Tumor size by CT (%) | |||||
| ≤3cm | 72 (59%) | 64 (70%) | 8 (26%) | 11% | |
| >3–7cm | 45 (38%) | 25 (27%) | 20 (65%) | 44% | |
| >7cm | 6 (4.9%) | 3 (3.0%) | 3 (10%) | 50% | |
| Central tumor location | |||||
| Primary study definition (%) | 63 (51%) | 40 (43%) | 23 (74%) | 37% | |
| ACCP definition (%) | 51 (42%) | 29 (32%) | 22 (71%) | 43% | |
| NCCN definition (%) | 62 (50%) | 39 (42%) | 23 (74%) | 37% | |
| SUVMax primary tumor | |||||
| Quartile 1 (0.80–3.50) | 32 (26%) | 31 (34%) | 1 (3.2%) | 3.1% | |
| Quartile 2 (3.60–7.10) | 31 (25%) | 27 (29%) | 4 (13%) | 13% | |
| Quartile 3 (7.20–13.5) | 30 (24%) | 18 (20%) | 12 (39%) | 40% | |
| Quartile 4 (13.6–44.9) | 30 (24%) | 16 (17%) | 14 (45%) | 47% | |
| Lymphadenopathy (%) | |||||
| N0 | 80 (65%) | 69 (75%) | 11 (36%) | 14% | |
| N1 | 25 (20%) | 9 (9.8%) | 16 (52%) | 64% | |
| N2 | 31 (25%) | 18 (20%) | 13 (42%) | 42% | |
| N3 | 3 (2.4%) | 2 (2.2%) | 1 (3.2%) | 33% | |
| N1–3 | 43 (35%) | 23 (25%) | 20 (65%) | 47% | |
|
FDG-uptake by hilar/ mediastinal nodes (%) |
|||||
| N0 | 69 (56%) | 60 (65%) | 9 (29%) | 13% | |
| N1 | 47 (38%) | 27 (29%) | 20 (65%) | 43% | |
| N2 | 32 (26%) | 16 (17%) | 16 (52%) | 50% | |
| N3 | 10 (8.1%) | 7 (8%) | 3 (10%) | 30% | |
| N1–3 | 54 (44%) | 32 (35%) | 22 (71%) | 42% | |
| Plasma levels VEGF-C (pg/mL) | |||||
| Quartile 1 (106–329) | 31 (25%) | 22 (24%) | 9 (29%) | 30% | |
| Quartile 2 (340–530) | 31 (25%) | 24 (26%) | 7 (23%) | 23% | |
| Quartile 3 (543–865) | 31 (25%) | 25 (27%) | 6 (19%) | 19% | |
| Quartile 4 (868–3,796) | 30 (25%) | 21 (23%) | 9 (29%) | 30% | |
Non-small cell lung cancer (NSCLC), ACCP (American College of Chest Physicians), NCCN (Nacional Comprehensive Cancer Network), Maximum standard uptake value (SUVMax), fluorodeoxygluose (FDG), Vascular endothelial growth factor-C (VEGF-C).
Ninety-one (74%) patients had at least one guideline-recommended indication for IMS resulting in a TPR of 100% (97.5%CI 89–100%) and FPR of 65% (95%CI 55–75%) (Table 3).
Table 3.
Performance Characteristics of Guideline-Recommended and Prediction Model-Based Risk Stratification Strategies
| Guideline-Recommended Invasive Mediastinal Staging Strategya |
Prediction Model-Based Risk Stratification Strategy |
|
|---|---|---|
| True-Positive Rate (Sensitivity) | 100% (97.5%CI 89–100%) | 97%b |
| False-Positive Rate (1-Specificity) | 65% (95%CI 55–75%) | 44% (95%CI 34–77%) |
| Rate of Selection for Invasive Mediastinal Staging | 74% (95%CI 66–82%) | 57% (95%CI 49–67%) |
Confidence interval (CI)
A guideline-recommended strategy assumes adherence to minimum indications for invasive mediastinal staging (tumor>3cm, central location, hilar/mediastinal lymphadenopathy, or FDG-uptake by hilar/mediastinal nodes).
Because the threshold for binary risk classification was chosen to best match the true-positive rate of guidelines (i.e. “fixing”sensitivity), it is not appropriate to estimate a measure of uncertainty for the true-positive rate of the prediction model.
The final prediction model included five variables (Table 4). Predicted probabilities of nodal disease ranged from 4.3%−82%. The uncorrected c-statistic was 0.82 (95%CI 0.74–0.90). Internal validation revealed an optimism-corrected c-statistic of 0.78 (95%CI 0.71–0.89) (Figure 2). Despite no evidence of a poor-fitting model on goodness-of-fit test (p=0.55), visual inspection of the calibration plot (Figure 3) suggests the model underestimates risk of nodal disease for predicted probabilities <30% and overestimates risk for predicted probabilities >30%. Likelihood ratio testing indicated that adding VEGF-C to the model did not significantly improve prediction (p=0.89). Pre-specified sensitivity analyses showed no meaningful differences in our results across varying definitions of central location, using mediastinal nodal disease as our dependent variable, nor across subgroups with confirmed NSCLC or ≥3 sampled mediastinal nodal stations (e-Appendix C).
Table 4.
Prediction Model Parameters, Coefficients, and Standard Errors
| Final Model with Radiographic Predictors |
||
|---|---|---|
| Coefficient (log-odds) |
Standard error |
|
| Parameter | ||
| Size ≥3cm | 0.803 | 0.622 |
| Central tumor | 0.511 | 0.569 |
| SUVMax primary tumor | 0.060 | 0.038 |
| Hilar/mediastinal lymphadenopathy | 0.831 | 0.554 |
| FDG-uptake in hilar/mediastinal lymph nodes | 0.643 | 0.558 |
| Constant | −3.150 | 0.583 |
Maximum standard uptake value (SUVMax), Fluorodeoxyglucose (FDG)
Figure 2.
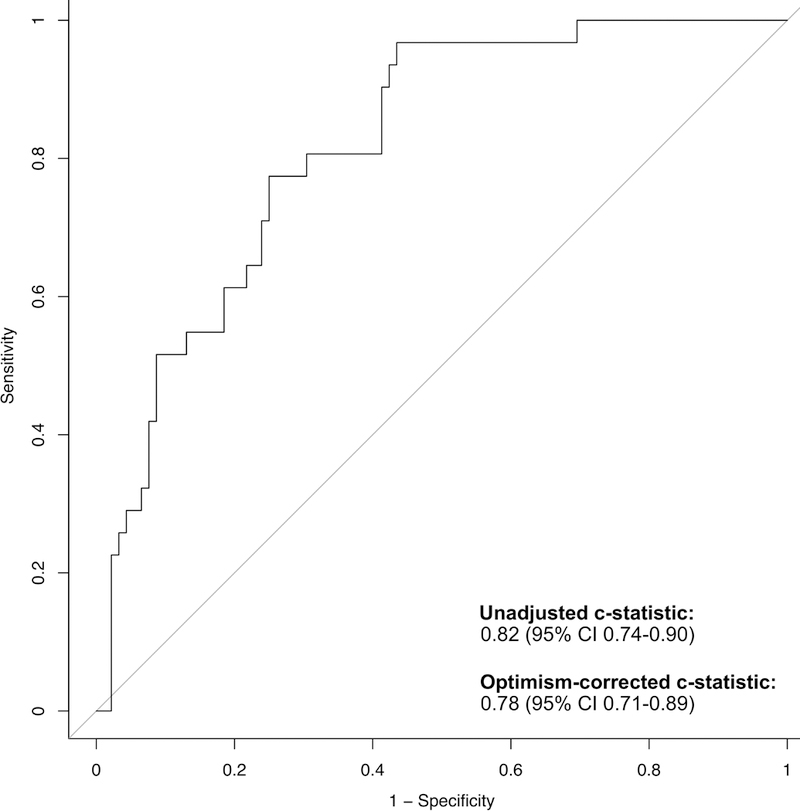
Receiver Operating Characteristics, Optimism-correction is a statistical method used to mitigate bias arising from validation of a prediction model in the cohort in which it was derived[25], [26].
Figure 3.
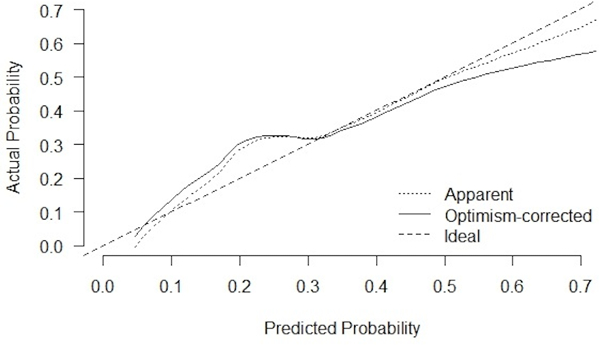
Calibration Pot, Optimism-correction is a statistical method used to mitigate bias arising from validation of a prediction model in the cohort in which it was derived[25], [26].
To explore the potential clinical impact of our model, we selected a cut-off probability (>11%) to attempt to best match the 100% TPR of guidelines while allowing for slight improvement in FPR. Doing so resulted in a 97%TPR and a 44% FPR (95%CI 34–77%) (Table 3). Both strategies correctly selected all thirteen patients with multi-station N2 and/or N3 disease for IMS.
COMMENT
A guideline-recommended strategy for IMS identified 100% of patients with true nodal disease for IMS; however, it also selected 65% of patients without true nodal disease to undergo IMS. This finding is novel and motivates an alternative approach to IMS. We developed and cross-validated a prediction model for nodal disease—based on five radiographic factors—with excellent discriminatory ability. The addition of VEGF-C—a growth factor for lymphangiogenesis—to the model was motivated by a body of literature showing that lymphangiogenesis facilitates nodal metastases in patients with epithelial tumors[15]; however, VEGF-C did not improve prediction in this cohort.
Our research team has characterized the performance characteristics of guideline-recommended IMS. A small study motivating our current work showed identical TPR and FPR for guidelines[22]. Another study—evaluating a slightly different population (PET-negative mediastinum)—also demonstrated a 100% TPR for guidelines but a higher 76% FPR[13]. The significance of these findings is two-fold: guideline-recommendations reproducibly achieve their goal of selecting node-positive patients for IMS, but they do so inefficiently—selecting a majority of node-negative patients for IMS. This inefficiency is amplified by the relatively low prevalence of nodal disease (25%). These performance characteristics reveal an opportunity to increase the value of lung cancer staging.
We reasoned that a model-based alternative would make better use of radiographic information to predict nodal disease, possibly resulting in better selection of patients for IMS. Specifically, the model does not assume that all predictors confer equal risk, but assumes that multiple risk factors have an additive effect on risk. Furthermore, our model also incorporates risk information about the primary tumor SUVMax. One consequence of developing a model in a population with suspected or confirmed NSCLC is that some patients will not be diagnosed with lung cancer, potentially reducing model performance. However, sensitivity analyses revealed no performance decrements, presumably because a non-NSCLC diagnosis occurred infrequently (10%). In settings where providers more frequently suspect NSCLC incorrectly, model performance may suffer. For this reason, validation across other practice settings is critical. Other reasons for pursuing further validation are: 1) our inconsistent statistical and graphical results regarding model calibration; 2) the fact that we validated performance in the same sample used to develop the model, which can lead to optimistic performance characteristics[26]; but also 3) enthusiasm over our model’s excellent ability to discriminate between patients with and without nodal disease.
Despite evidence supporting VEGF-C as a biologically-plausible predictor of nodal disease in lung cancer patients[15], VEGF-C did not improve prediction. Epidemiologic studies demonstrated an association between nodal disease and VEGF-C expression[16]–[20]. Previously, our team demonstrated that the use of VEGF-C levels and PET results better predicts nodal disease than PET findings alone[22]. Inexplicably, this study found no evidence of an association between plasma VEGF-C and nodal disease. Our protocol for blood draws, specimen processing, and storage did not change over the study period. Measurement error is an unlikely explanation as we used a commercially-available assay and our coefficient-of-variation—a measure of within-subject intra-assay variability[29]—was low (3.8%) and matched that of the manufacturer (3.4–6.9%). VEGF-C expression among node-negative patients was similar to prior reports[16], [17], [22]. It is possible that the lack of association between VEGF-C and nodal disease—and failure to improve prediction—is simply due to chance.
Our study has several limitations. Importantly, the sample size was too small for independent validation. We attempted to mitigate bias arising from validation in the same sample as development using an optimism-corrected c-statistic[26] and graphical evaluation of calibration[26], [30]. Our single-institution study may have limited generalizability; however, patient demographics, risk-factor prevalence, and prevalence of nodal disease were similar to other studies. We may have misclassified radiographic risk-factors for nodal disease because we ascertained these risk-factors from radiology reports rather than independent, expert radiology review. Misclassification could decrease model performance. Yet, our approach represents model performance in a “real-world” setting where providers do not always have access to independent, expert radiology review. Patients did not receive a uniform evaluation of lymph nodes, as evidenced by the variable number of nodal stations sampled. We anticipated that non-uniform lymph node evaluation would diminish the performance characteristics of the model (conservative bias). However, evaluation of model performance in a subpopulation with ≥3 mediastinal nodal stations sampled revealed similar results as our primary analysis. Finally, our model may have limited value in patients with radiographically bulky nodal disease.
Post-hoc exploratory analyses reveal both a potential benefit and unintended consequence of using our model in practice. The TPR of the model matches (within a margin of error) that of guideline recommendations, thus maintaining the high accuracy of selecting node-positive patients for IMS. By reducing IMS among node-negative patients, the model may reduce exposure to procedure-related adverse events, patient burden, and associated costs. Similar or better outcomes at lower cost yield higher value care[1]. The model failed to correctly classify one node-positive patient in our sample, raising concerns over harm arising from omission of IMS. This patient had a centrally-located 2cm biopsy-proven right lower lobe NSCLC without radiographic evidence of nodal disease and underwent endobronchial ultrasound-guided aspiration of level-7 and a hilar node (both negative) followed by mediastinoscopy sampling five mediastinal stations including level-7 (all negative). A level-7node was found to be positive at the time of resection, and the patient received adjuvant therapy. There is no evidence that induction therapy followed by resection leads to superior long-term outcomes compared to resection and adjuvant therapy[31]. Therefore, we cannot conclude that this patient would have been harmed due to misclassification. Misclassification could harm a patient if the model fails to identify multi-station N2 or N3 disease because these patients should not undergo resection. Fortunately, such misclassification did not occur. A substantially larger cohort is needed to better characterize the risks (e.g. unnecessary surgery in patients with multi-station N2 or N3 disease) and benefits (e.g. equivalent patient outcomes at lower costs) of our model. We recognize that others may have a different view of how this model might be implemented in practice. To that end, we have included a mock-up of a risk calculator (e-Appendix D). We currently recommend against the use of this tool in clinical practice.
In conclusion, a guideline-recommended IMS strategy selects all patients with true nodal disease to undergo IMS, but exposes a majority of node-negative patients to invasive procedures. Further studies are needed to determine whether our prediction model for nodal disease can serve as a safe alternative to guideline-recommendations and lead to higher value care.
Supplementary Material
ACKNOWLEDGEMENTS
This project was funded by a grant from The CHEST Foundation (principal investigator: Farjah). Dr. Verdial was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (Award Number T32DK070555). The content is solely the authors’ responsibility and does not necessarily represent the views of the National Institutes of Health.
ABREVIATIONS
- ACCP
American College of Chest Physicians
- CI
Confidence interval
- CT
Computed tomography
- FEV1
Forced expiratory volume in the first second
- FDG
Fluorodeoxygluose
- FPR
False-positive rate
- IMS
Invasive mediastinal staging
- IQR
Interquartile range
- NCCN
National Comprehensive Cancer Network
- NSCLS
Non-small cell lung cancer
- PET
Positron emission tomography
- SUVMax
Maximum standardized uptake
- TPR
True-positive rate
- VEGF-C
Vascular endothelial growth factor-C
Footnotes
Presented at the CHEST® annual meeting in Toronto, Canada November 1, 2017.
CLASSIFICATIONS: Lung cancer, diagnosis; Mediastinal lymph nodes; EBUS; Mediastinoscopy
CONFLICT-OF-INTEREST STATEMENT: Dr. Mulligan reports consultant fees from Covidien. Dr. Wood reports consultant fees from Olympus Respiratory America and GRAIL.
REFERENCES
- 1.Porter ME. What is value in health care. N Engl J Med. 2010;363(26):2477–2481. [DOI] [PubMed] [Google Scholar]
- 2.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for Staging Non-small Cell Lung Cancer. Chest. 2013;143(5):e211S–e250S. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Clinical practice guidelines in oncology v.4.2016: Non-small cell lung cancer. NCCN Guidel. 2016. [DOI] [PubMed] [Google Scholar]
- 4.Lemaire A, Nikolic I, Petersen T, et al. Nine-Year Single Center Experience With Cervical Mediastinoscopy: Complications and False Negative Rate. Ann Thorac Surg. 2006;82(4):1185–1190. [DOI] [PubMed] [Google Scholar]
- 5.Eapen GA, Shah AM, Lei X, et al. Complications, Consequences, and Practice Patterns of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Chest. 2013;143(4):1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardio-Thoracic Surg. 2008;33(1):104–109. [DOI] [PubMed] [Google Scholar]
- 7.Kanzaki R, Higashiyama M, Fujiwara A, et al. Occult mediastinal lymph node metastasis in NSCLC patients diagnosed as clinical N0–1 by preoperative integrated FDG-PET/CT and CT: Risk factors, pattern, and histopathological study. Lung Cancer. 2011;71(3):333–337. [DOI] [PubMed] [Google Scholar]
- 8.Lee PC, Port JL, Korst RJ, Liss Y, Meherally DN, Altorki NK. Risk Factors for Occult Mediastinal Metastases in Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg. 2007;84(1):177–181. [DOI] [PubMed] [Google Scholar]
- 9.Trister AD, Pryma DA, Xanthopoulos E, Kucharczuk J, Sterman D, Rengan R. Prognostic Value of Primary Tumor FDG Uptake for Occult Mediastinal Lymph Node Involvement in Clinically N2/N3 Node-negative Non–Small Cell Lung Cancer. Am J Clin Oncol. 2014;37(2):135–139. [DOI] [PubMed] [Google Scholar]
- 10.Koike T, Koike T, Yamato Y, Yoshiya K, Toyabe S. Predictive Risk Factors for Mediastinal Lymph Node Metastasis in Clinical Stage IA Non–Small-Cell Lung Cancer Patients. J Thorac Oncol. 2012;7(8):1246–1251. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Sun Y, Xiang J, Zhang Y, Hu H, Chen H. A prediction model for N2 disease in T1 non–small cell lung cancer. J Thorac Cardiovasc Surg. 2012;144(6):1360–1364. [DOI] [PubMed] [Google Scholar]
- 12.Chen K, Yang F, Jiang G, Li J, Wang J. Development and validation of a clinical prediction model for N2 lymph node metastasis in non-small cell lung cancer. Ann Thorac Surg. 2013;96(5):1761–1768. [DOI] [PubMed] [Google Scholar]
- 13.Farjah F, Backhus LM, Varghese TK, et al. External validation of a prediction model for pathologic N2 among patients with a negative mediastinum by positron emission tomography. J Thorac Dis. 2015;7(4):576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J, Yang X, Zhong W, et al. Association of maximum standardized uptake value with occult mediastinal lymph node metastases in cN0 non-small cell lung cancer. Eur J cardio-thoracic Surg. 2016;50(5):1–6. [DOI] [PubMed] [Google Scholar]
- 15.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438(7070):946–953. [DOI] [PubMed] [Google Scholar]
- 16.Tamura M, Oda M, Tsunezuka Y, et al. Chest CT and serum vascular endothelial growth factor-C level to diagnose lymph node metastasis in patients with primary non-small cell lung cancer. Chest. 2004;126(2):342–346. [DOI] [PubMed] [Google Scholar]
- 17.Tamura M, Ohta Y. Serum vascular endothelial growth factor-C level in patients with primary nonsmall cell lung carcinoma. Cancer. 2003;98(6):1217–1222. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Meng X, Zeng H, et al. Serum vascular endothelial growth factor-C levels: A possible diagnostic marker for lymph node metastasis in patients with primary non-small cell lung cancer. Oncol Lett. 2013;6(2):545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nwogu CE, Yendamuri S, Tan W, et al. Lung cancer lymph node micrometastasis detection using real-time polymerase chain reaction: correlation with vascular endothelial growth factor expression. J Thorac Cardiovasc Surg. 2013;145(3):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maekawa S, Iwasaki A, Shirakusa T, et al. Correlation between lymph node metastasis and the expression of VEGF-C, VEGF-D and VEGFR-3 in T1 lung adenocarcinoma. Anticancer Res. 2007;27(6 A):3735–3741. [PubMed] [Google Scholar]
- 21.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farjah F, Madtes DK, Wood DE, et al. Vascular endothelial growth factor C complements the ability of positron emission tomography to predict nodal disease in lung cancer. J Thorac Cardiovasc Surg. 2015;150(4):796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–718. [DOI] [PubMed] [Google Scholar]
- 24.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28(5):964–974. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19(9):1141–1164. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE, Lee KL, Mark DB. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med. 1996;15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 27.Fawcett T An introduction to ROC analysis. 2005. doi: 10.1016/j.patrec.2005.10.010. [DOI] [Google Scholar]
- 28.Pepe MS, Janes H, Li CI. Net Risk Reclassification P Values: Valid or Misleading? JNCI J Natl Cancer Inst. 2014;106(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed GF, Lynn F, Meade BD. Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol. 2002;9(6):1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steyerberg E Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. 1st ed New York: Springer-Verlag; 2009. [Google Scholar]
- 31.Martins RG, D’Amico TA, Loo BWJ, et al. The management of patients with stage IIIA non-small cell lung cancer with N2 mediastinal node involvement. J Natl Compr Canc Netw. 2012;10(5):599–613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


