Abstract
Objectives:
To characterize the day-night activity patterns of children after major surgery and describe differences in children’s activity patterns between the PICU and inpatient floor setting.
Study design:
In this prospective observational study, we characterized the daytime activity ratio estimate (DARE; ratio between mean daytime activity [08:00-20:00] and mean 24-h activity [00:00-24:00]) for children admitted to the hospital after major surgery. The study sample included 221 infants and children ages 1 day to 17 years admitted to the pediatric intensive care unit (PICU) at a tertiary, academic children's hospital. Subjects were monitored with continuous accelerometry from postoperative day 1 until hospital discharge. NHANES accelerometry data were utilized for normative data to compare DARE in a community sample of U.S. children to hospitalized children.
Results:
The mean DARE over 2,271 hospital days was 57.8%, with a significant difference between the average DARE during PICU days and inpatient floor days (56% vs. 61%, P <.0001). The average subject DARE ranged from 43% to 73%. In a covariate-adjusted mixed effects model, PICU location, lower age, orthopedic or urologic surgery, and intubation time were associated with decreased DARE. Hospitalized children had significantly lower DARE than NHANES subjects in all age groups studied, with the largest difference in the youngest PICU group analyzed (6-9 years; 59% vs 75%, p<0.0001). A subset analysis of children older than 2 years (n=144) showed that DARE was <50% on 15% of hospital days.
Conclusions:
Children hospitalized after major surgery experience disruptions in day-night activity patterns during their hospital stay that may reflect disturbances in circadian rhythm.
Keywords: children, critical illness, surgery, activity, actigraphy, accelerometry, acute rehabilitation, delirium, sleep, intensive care units, hospital, circadian rhythms
Sleep plays an integral role in brain development, which is reflected by the constantly evolving sleep needs of infants and children as they grow.(1-3) Hospitalized children are exposed to several factors that can disrupt sleep continuity during a critical time in brain development and recovery, including pain, noise, light, and interruptions for nursing care. After major surgery, children admitted to the pediatric intensive care unit (PICU) are often exposed to sedative and analgesic medications that also have negative effects on circadian rhythms.(4-6) Normal sleep-wake homeostasis is critical for thermoregulation, minimizing inflammation, and maintaining normal immunity.(5, 7-9) Additionally, circadian rhythm impairments may increase the risk of delirium in the PICU, which is associated with increased cost, hospital length of stay, and mortality.(10-14) Moreover, these alterations in sleep continuity that begin after surgery may persist beyond the PICU on the inpatient floor and even after discharge to home.(15, 16)
Although polysomnography is considered the gold standard for objective assessment of sleep architecture, its application is limited for continuous, longitudinal measurement in the hospital setting. Actigraphy is a noninvasive, well-tolerated, reliable, and objective method to assess 24-h patterns of day-night activity over several days to weeks.(17-21) Validated for sleep assessment in infants and children,(22, 23) the wristwatch-like device produces minute-to-minute activity counts to monitor activity patterns over the course of hospitalization.
To date, no studies have objectively described the day-night activity patterns of hospitalized children. Adult studies have shown that circadian rhythm disturbances persist during the course of short- and long-term recovery from surgery.(24, 25) Such findings may have even more extensive implications in a population undergoing active neurocognitive development. Therefore, it is critical to characterize rest-activity patterns in hospitalized children to inform potential targets for intervention. The primary objective of this study was to characterize the day-night activity patterns of infants and children after major surgery and describe the differences in children’s activity patterns as they transition from the PICU to an inpatient floor setting. Additionally, we aimed to demonstrate the feasibility of continuous actigraphy monitoring in a heterogeneous group of pediatric patients in the hospital setting and compare their activity patterns to those of a community sample of U.S. children using a novel analytic technique, the daytime activity ratio estimate (DARE).
METHODS
The reporting of this study conforms to the STROBE statement for reporting of observational studies.(26) Children were eligible for inclusion if they were admitted to the Johns Hopkins PICU after major cardiac, orthopedic, or urologic surgery. We chose to include these groups of surgical patients because they comprise a large proportion of surgical PICU admits; capture a wide range of ages while minimizing the heterogeneity in comorbid conditions that could confound the DARE (i.e. neurosurgery, general pediatric surgery) and are generally admitted for greater than 1 night after surgery. The Johns Hopkins PICU is a 40-bed, academic, tertiary-care PICU, which serves all medical and surgical patients who require critical care in the pediatric setting. Informed consent was obtained on the day after surgery from the child’s parent or legally authorized representative, and assent was obtained from the child when age or developmentally appropriate, unless the child was mechanically ventilated and receiving sedatives. Consent was delayed until postoperative day 1 to avoid increased parental stress on the day of surgery in the setting of a high-risk procedure. Patients were excluded if they had evidence of seizure activity at the time of screening, a history of traumatic brain injury, severe obstructive sleep apnea, ischemic or hemorrhagic stroke, or neuromuscular disease. The study was approved by the Johns Hopkins Institutional Review Board.
Instruments and Measures
Clinical variables
Demographic and clinical data collected by daily chart review through the duration of actigraphy recording included age, sex, type of surgery (cardiac/orthopedic/urologic), length of PICU stay, floor and hospital length of stay, pediatric risk of mortality score (PRISM-3), intubation status, and duration of mechanical ventilation.
Actigraphy
The Daytime Activity Ratio Estimate (DARE)
Though the actigraph can estimate the duration of sleep and wakefulness using algorithms that quantify the reduced movement associated with sleep, these algorithms may overestimate the amount of time in sleep owing to a state of bedrest or immobility in the hospital setting, particularly in the ICU.(27-29) In healthy children, activity is consolidated during the daytime hours, and the rest-activity cycle can be utilized as an indirect measure of the sleep-wake cycle. For this reason, we introduce a simple, reliable, and normalized measure of differences between day and night activity patterns that uses an explicit, easy-to-reproduce algorithm based directly on the raw motor activity data.(30) The daytime activity ratio estimate (DARE) is defined as the ratio between the mean daytime activity (08:00-20:00) and the mean 24-h activity (00:00-24:00). When minute-level activity data are available, computing DARE is straightforward and reproducible across studies. Major advantages of DARE are that it is intuitive and simple, has the same interpretation across subjects and days, and is reproducible across platforms and software.(31, 32) The DARE can be compared easily across subjects regardless of age, disease process, or neuromuscular strength.
Actigraphy in Hospitalized Children
An actigraphy watch (MicroMotionlogger, Ambulatory Monitoring, Inc.) was placed on the child’s wrist or ankle on postoperative day 1 in the PICU, and remained in place until the day of hospital discharge. The MicroMotionlogger has been used widely to document rest-activity patterns in infants and children.(33) Motion sensors inside the accelerometry device capture data on patient movement using the Zero Crossing Mode, which is measured as the number of accelerations per minute. The devices used in this study are capable of collecting data for up to 12 weeks. If the child was intubated at the time of actigraphy initiation, data analysis began at the time of extubation due to the confounding effects of sedation and immobilization (ie, restraints) on activity data. Act Millennium software was used for downloading the actigraph data, and initial data pre-processing was conducted using ActionW 2.7 software.
A member of the research team monitored each subject's device daily to document that it was in place, comfortable, functioning, and not interfering with the child’s medical care. A log placed at the bedside of each subject was used to record times that the actigraphy device was removed for patient care activities (e.g., IV placement, baths); travel to surgical, diagnostic, or interventional areas (e.g., MRI); or parent/child request. The research team reviewed this log daily during visits and queried family and nursing staff to ensure completeness.
Actigraphy in a Community Sample
Accelerometry data used to obtain estimates of normative DARE in a representative sample of children in the United States was collected during the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and 2005-2006 waves (34) and is available in processed format in the rnhanesdata package.(35) This NHANES sample examined individuals 6 years of age and older. The device employed was a uniaxial accelerometer manufactured by Actigraph (Pensacola, FL), placed at the hip. The study protocol instructed participants to remove their accelerometers during sleep. However, using established algorithms for estimating non-wear time, it was determined that a subset of participants was non-compliant and wore the device during sleep on one or more days. (36, 37) In estimating normative DARE we included days with more than 22 hours of estimated wear-time, resulting in a total of 1122 days across 565 participants in 3 age groups (6-9 years, 10-13 years, and 14-17 years). The number of participants in each age group was 161, 175, and 229, respectively. The NHANES sample included more males (324) than females (241), and was 15% white, 23% Mexican-American, 54% black, and 8% other race. Age trends in DARE were consistent across race in this sample.
Actigraphy Data Analysis
For subjects with complete actigraphy data, activity profiles represented by 1440 min-level (24 h) activity counts were analyzed. A log(count+1) transformation was applied to reduce the strong skewness of the activity count distributions. Transformed activity counts were averaged within two specific time periods: daytime (08:00-20:00) and night-time (20:00-08:00). Day-night activity patterns were estimated by using DARE, defined as the ratio of the average daytime activity to total 24-h activity. More precisely, , where and are the average log activity counts (per minute) for an individual during the daytime and night-time hours, respectively. A DARE of 100% indicates that all activity is occurring during the daytime period with consolidation of rest at night, whereas a DARE of 50% suggests that the level of activity is equally distributed between day and night periods. Assuming an average DARE of 75% in healthy children, we aimed to enroll 200 subjects to demonstrate a 15% decrease in average DARE in hospitalized children with α=0.05 and 90% power.
Thus, DARE was calculated for every child and every day. To account for the multilevel structure of the hospital data (multiple days measured within the same child), a mixed effects model with child-specific random intercepts was used. We examined the fixed effects for the PICU, sex, age, race, PRISM, cohort, and time intubated in minutes. Because the distribution of time intubated was highly skewed, the transformation log(time intubated + 1) was applied. All analyses were performed using the lme() function in the nlme (v. 3.1-131) package in R 3.4.2.(38)
RESULTS
During the study period, 250 children were enrolled in the PICU, 221 of whom met the criteria for completion of the study with >24 h of analyzable actigraphy data (n=2271 hospital days). Eighty-four percent of eligible admissions (n=333) were approached for informed consent during the study period and 90% of these patients were enrolled. Only 6 children dropped out of the study before hospital discharge owing to some discomfort in wearing the actigraphy watch. However, the watch was associated with no adverse events (e.g., irritation, constriction). One watch was lost or misplaced before study completion. Demographic and clinical characteristics of the patient sample are shown in Table I.
Table 1.
Baseline Patient Characteristics by Surgical Cohort
| Parameter | Cardiac | Orthopedic | Urologic | All patients |
|---|---|---|---|---|
| N (%) | 143 (64) | 64 (29) | 14 (6) | 221 |
| Age, mean (SD), months | 61 (68) | 149 (55) | 58 (74) | 86 (76) |
| Weight, mean (SD), kg | 21 (20) | 36 (18) | 19 (18) | 25 (21) |
| Male sex, n (%) | 92 (64) | 23 (36) | 9 (64) | 124 (56) |
| PRISM score, mean (SD) | 8 (5) | 6 (5) | 3 (5) | 7 (5) |
| PICU LOS, mean (SD), days | 9 (17) | 7 (9) | 11 (13) | 9 (15) |
| Intubated at admission, n (%) | 70 (49) | 18 (28) | 0 | 88 (40) |
| Race, n (%) | ||||
| White | 73 (51) | 37 (58) | 12 (86) | 122 (55) |
| Black | 39 (27) | 9 (14) | 0 | 48 (22) |
| Other | 31 (22) | 18 (28) | 2 (14) | 51 (23) |
LOS = length of stay; PICU = pediatric intensive care unit; PRISM = pediatric risk of mortality score.
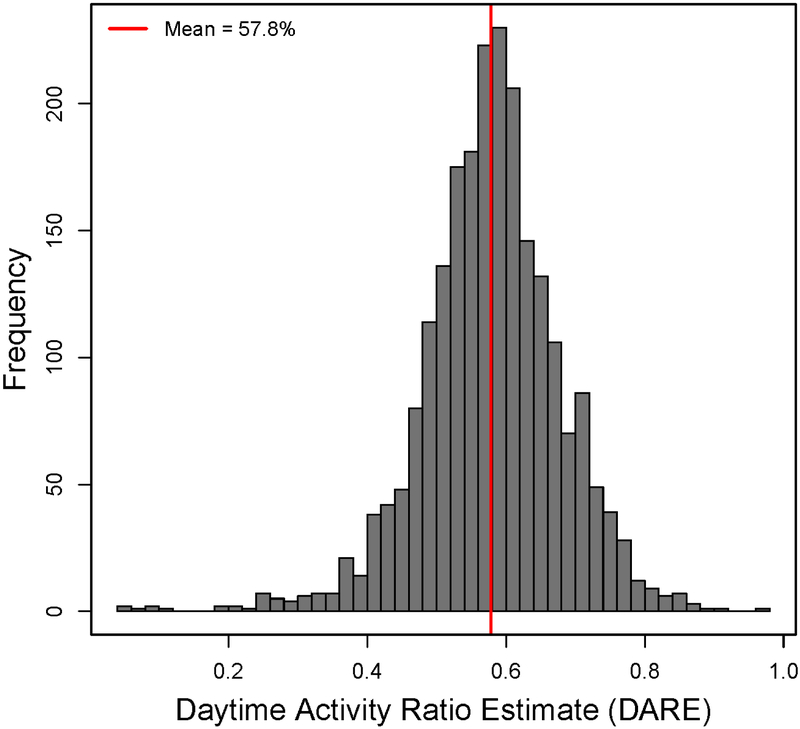
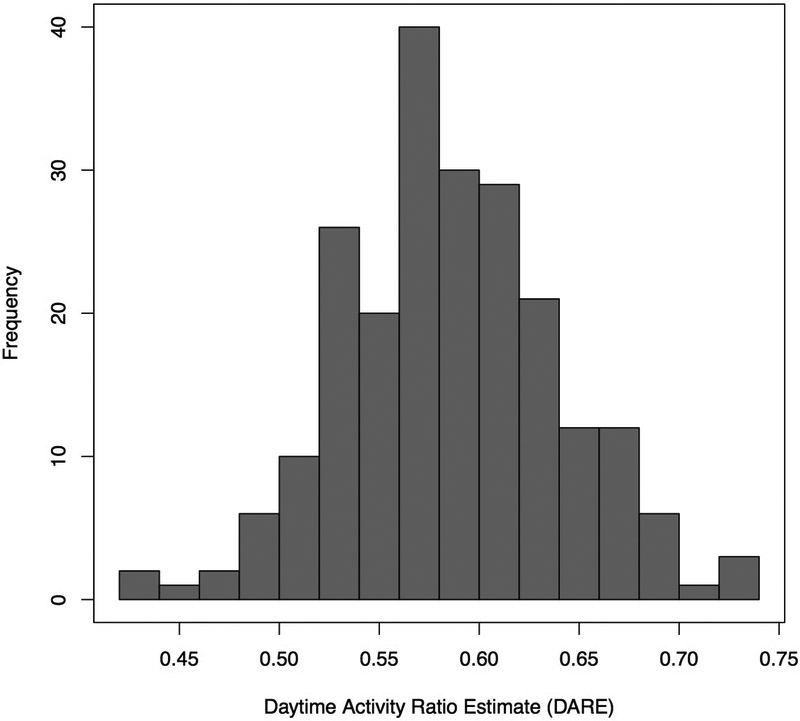
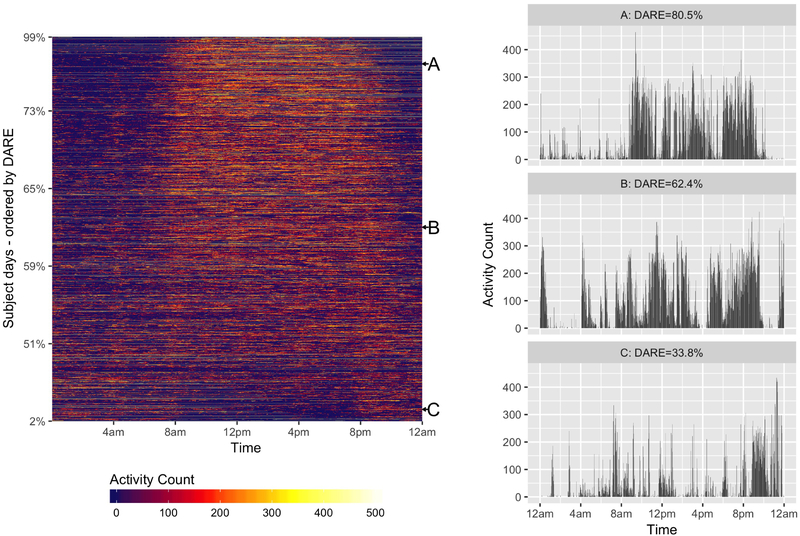
When all patients (n=221) and hospital days (n=2271) were included, the mean daytime activity represented 57.8% (95% CI, 57.4%-58.2%) of total activity while in the hospital and ranged from 4.6% to 98.6% (Figure 1). The mean DARE was 55.9% (95% CI, 55.4-56.4) during 1346 PICU days and 60.8% (95% CI, 60.1%-61.5%) during the 905 days on the inpatient floor (P<0.0001). Children in the cardiology cohort had the highest mean DARE (58.2%; 95% CI, 57.7-58.7) when compared with the orthopedic (57.5%; 95% CI, 56.6-58.4) and urology (57.0%; 95% CI, 56.1-57.9) cohorts. The average subject DARE ranged from 43.1% to 73.2%, and 11 subjects (5%) had <50% of their average daily activity occur during daytime hours (Figure 2; available at www.jpeds.com). Eighteen percent of all hospital days had a DARE less than 50%. Figure 3 illustrates actigraphy plots for a spectrum of DAREs, with all patient days organized from lowest to highest DARE.
Figure 1 –
Histogram of DARE over all hospital days.
Figure 2 online –
Histogram of average DARE by subject.
Figure 3 –
Left: Heat map of 24-h activity arranged by DARE. Note the clear transition to consolidated rest-activity patterns above 60%. Right: Twenty-four-hour actigrams for DAREs at points A, B, and C in the left panel.
To determine predictors of DARE, we fit a mixed model with random intercept for each subject while controlling for age, sex, patient location, surgical cohort, PRISM score, log of time intubated, and race (Table 2; available at www.jpeds.com). PICU days were associated with a significantly lower DARE (−5.3%, P<0.0001) than were days when the subject was on the floor. After adjustment, the urology cohort exhibited the lowest DARE when compared with the reference cardiology cohort (−5%, P=0.0001). The log intubation time was associated with lower DARE. As an example, for each 20% increase in intubation time (e.g., from 10 days to 12 days), the DARE decreased on average by 0.12%. Older children had a higher DARE, with a 0.18% increase for each year of age. Severity of illness at PICU admission was not associated with the DARE after adjusting for the other variables.
Table 2.
Mixed Effects Regression Model for DARE
| Variable | Coefficient | Standard error | T-statistic | P value |
|---|---|---|---|---|
| PICU | −0.0530 | 0.0047 | −11.341 | <0.0001 |
| Sex (F) | 0.0001 | 0.0067 | 0.086 | 0.9931 |
| PRISM | 0.0000 | 0.0007 | −0.047 | 0.9628 |
| Race | ||||
| Black | −0.0081 | 0.0086 | −0.941 | 0.3479 |
| Other | 0.0063 | 0.0081 | 0.775 | 0.4390 |
| Age | 0.0018 | 0.0006 | 2.732 | 0.0068 |
| Cohort | ||||
| Cardiology (ref) | ||||
| Orthopedic surgery | −0.0331 | 0.0091 | −3.627 | 0.0004 |
| Urology | −0.0495 | 0.0124 | −3.977 | 0.0001 |
| Time intubated (log - scale) | −0.0025 | 0.0010 | −2.569 | 0.0109 |
PICU = pediatric intensive care unit; PRISM = pediatric risk of mortality score.
Comparing Normative DARE to Hospital DARE
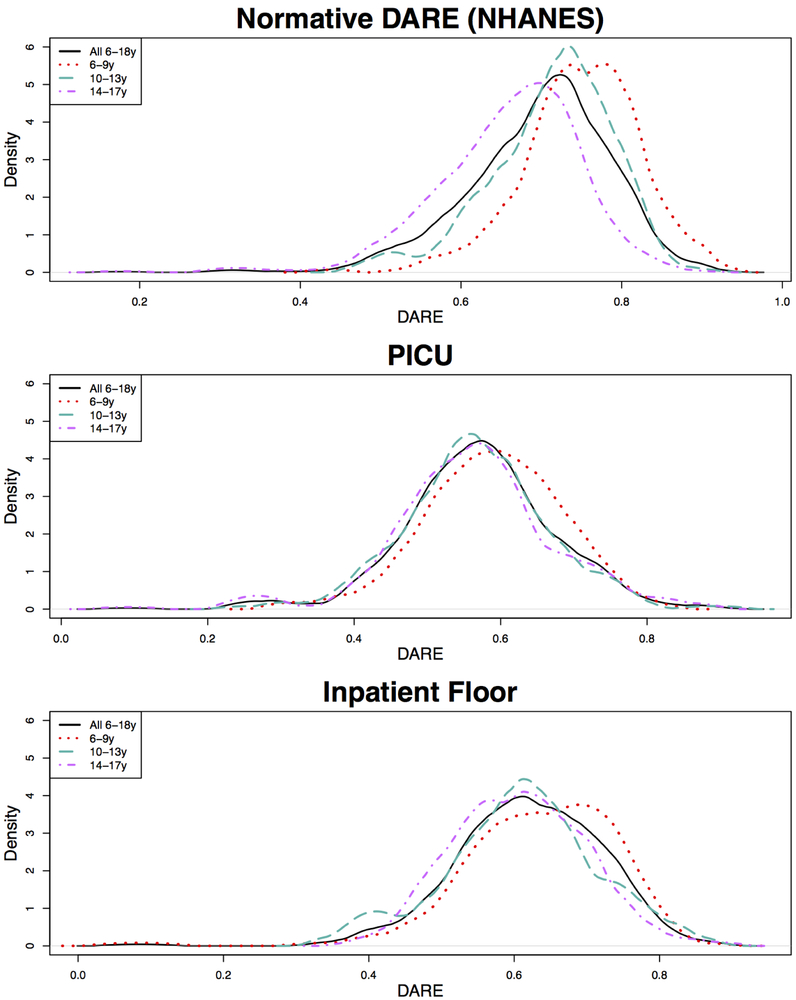
The average DARE across days for the normative NHANES sample, PICU patients, and inpatient floor patients in three age categories (6-9 years, 10-13 years, and 14-17 years) is shown in Table 3 (available at www.jpeds.com). Figure 4 displays the DARE distributions colored by age category. The trend of decreasing DARE with increasing age was observed in all groups. The average DARE in each age category and overall was significantly higher in the normative sample than both PICU and inpatient floor patients (p<0.0001) with the greatest difference in the 6-9 year old PICU patients (75% vs. 59%).
Table 3.
Comparison of Normative Dare vs. Hospitalized Patients ages 6-17
| Age Group | No. of Days |
No. of Subjects |
DARE (95% CI) |
|---|---|---|---|
| 6-9 yrs | |||
| Normative | 264 | 157 | 75% (74.1 to 75.9) |
| PICU | 117 | 25 | 59% (57.4 to 60.7) |
| Inpatient floor | 117 | 21 | 63.5%(61.6 to 65.4) |
| 10-13 yrs | |||
| Normative | 354 | 176 | 71.3% (70.5 to 72.1) |
| PICU | 148 | 31 | 56.7% (55.1 to 58.3) |
| Inpatient Floor | 71 | 22 | 61% (58.5 to 63.4)) |
| 14-17 yrs | |||
| Normative | 504 | 232 | 66% (65.2 to 66.8) |
| PICU | 230 | 40 | 56.3% (54.8 to 57.7) |
| Inpatient floor | 90 | 29 | 60.7% (58.9 to 62.5) |
| All 6-17 yrs | |||
| Normative | 1122 | 565 | 69.8% (69.3 to 70.3) |
| PICU | 495 | 96 | 57% (56.1 to 57.9) |
| Inpatient floor | 278 | 72 | 58.8% (58.1 to 59.5) |
Figure 4 –
Comparison of normative, PICU and inpatient floor DARE in children ages 6-17 by age category.
Comparing last day of PICU to first day on the floor
When the unadjusted difference in DARE between the last day in the PICU and the first day on the floor were compared within subjects, we found an estimated mean increase of 2.3% DARE on the floor (95% CI, 0.7%-4%; P=0.006). After adjustment for age, sex, surgical cohort, PRISM score, log of time intubated, and race, the difference was similar (2.4%; P=0.02).
Subset analysis of children >2 years
Because infants have less consolidation of activity during daytime hours than do older children, we performed a subset analysis to evaluate the DARE in children over 2 years of age (n=144). The average DARE by subject was 59.7% (95% CI, 58.8-60.6%) in children older than 2 years compared with 56.5% (95% CI, 55.4%-57.5%) in those 2 years and under (P<0.0001). Fifteen percent of hospital days in older children exhibited a DARE less than 50%. After adjusting for demographic variables, only PICU, cohort (orthopedic and urology), and intubation at admission were predictors of decreased DARE in children over 2 years. The highest subject DARE was 73.2%.
DISCUSSION
The results of this study confirm that children recovering from major surgery experience disturbances in day-night activity patterns both in the PICU and after transfer to the inpatient floor setting. As the first study to characterize day-night activity patterns in a large cohort of infants and children admitted to the hospital, we also demonstrate the feasibility of actigraphy for continuous monitoring in this setting. Additionally, we describe the novel application of the DARE to characterize day-night activity patterns in healthy and hospitalized children. These results provide important insights into how children’s rest-activity cycles are distributed throughout the 24-h period, suggesting that hospital setting and interventions, medications, and patient-level factors such as pain may disrupt the normal circadian rhythm after major surgery.
Our finding that, on average, only 56% of activity occurs between 8 a.m. and 8 p.m. in the PICU confirms that critically ill children have the greatest increase in the ratio of nighttime activity with absence of rest-activity cycle consolidation. These findings are consistent with those of previous studies in which actigraphy was used to measure sleep-wake patterns in hospitalized adults after major surgery. Such studies showed that total sleep time and sleep efficiency were reduced with increased awakenings.(19) As shown by the normative DARE data in our analysis, even the oldest children experience disruptions in day-night activity patterns in the hospital, but this difference was most prominent in children ages 6-9 years, a critical period of neurodevelopment. In a study of adult patients hospitalized after traumatic brain injury, rest-activity cycles were consolidated on only 46.6% of all hospital days, with a linear trend of improvement observed over time.(30) In the current study cohort of children without acute neurologic disease, the average DARE on the floor setting was slightly higher than that in the PICU setting, suggesting that day-night patterns do improve over time. However it is not known whether this is a consequence of the environment, improvement in pain, or both. Although the increase in DARE (2%) between the last day in the PICU and the first day on the floor was statistically significant, a change this small is unlikely to be clinically meaningful, suggesting that the hospital setting itself, not just the PICU, is disruptive to sleep-wake homeostasis.
We found that pediatric orthopedic and urologic surgery patients have lower DARE than cardiac surgery patients, which is surprising given that cardiac surgery patients often undergo longer surgeries including use of cardiopulmonary bypass. This observation may be a reflection of neuromuscular disease and other complicating comorbidities associated with sleep disturbances in orthopedic surgery patients. Similarly, urology patients (e.g., those with bladder extrophy or hypospadia) are usually admitted to the PICU with epidurals and complex pain management including opioids and benzodiazepines to facilitate surgical healing. Opioids and benzodiazepines can be detrimental to sleep continuity and quality.(5)
Not surprisingly, duration of intubation and mechanical ventilation was associated with decreased DARE. As such, this finding implies that the effect of mechanical ventilation and/or sedation practices may have a lasting impact on sleep-wake patterns after extubation and underlines the importance of sleep promotion both during and after mechanical ventilation. Numerous studies in adults have demonstrated severe disruption in sleep architecture during invasive mechanical ventilation, including decreased rapid-eye movement (REM) sleep and slow wave sleep and loss of circadian rhythm.(5, 39, 40) A cross-sectional study investigating the temporal characteristics of the sleep electroencephalogram in mechanically ventilated children found that PICU patients had more slow wave activity during daytime hours than nighttime hours, and had none of the expected ultradian variability in the EEG power spectra. (41)
The current study also provides important information to support the feasibility of longterm, continuous actigraphy use in a heterogeneous population of infants and children in the inpatient setting. Actigraphy monitoring was successfully completed in 241 children from PICU admission to hospital discharge over 2,333 hospital days (including intubation days). As a lower-cost and practical alternative to continuous polysomnography, actigraphy can characterize the evolution of activity patterns over time.(42) Clinical and research applications of actigraphy to evaluate children’s sleep quality in the home environment have been well-described in the literature.(18, 43-45) However, studies to evaluate activity and sleep with actigraphy in the inpatient setting, including the ICU, have focused largely on adults.(28-30, 46-51) In a recent narrative review that covered actigraphic assessment of perioperative sleep and activity in pediatric patients, the authors included studies primarily in outpatient surgery patients.(51, 52) Our DARE data in the NHANES sample demonstrated consistency with normal sleep patterns in children, including a delayed sleep phase (later bedtime) with the onset of adolescence.(53) Therefore, the DARE is a useful method for objective assessment of day-night activity patterns.
The results of our study confirming disruption in day-night activity patterns highlight an opportunity for staff caring for children in the both the PICU and inpatient floor setting. Hospitals are noisy and brightly lit environments where a patient’s ability to sleep is challenged by numerous disruptive factors.(54) Hospital routines are not typically coordinated to optimize sleep hygiene, as x-rays and baths are often performed during night-time or early morning hours for staff convenience in a shift-based work environment. The PICU environment can be particularly stressful for both children and their families, with monitors, alarms, and staff conversation contributing to sleep disruption in critically ill children.(55) In a recent survey of pediatric intensivists, only 16% of respondents were aware of any protocols in place for sleep promotion, such as lighting or noise reduction efforts.(56)
Circadian rhythm disturbance is a risk factor for delirium, with a prevalence of approximately 25% in PICU patients.(13, 57) Pediatric delirium increases hospital length of stay and mortality.(12, 14) Additionally, although pain can negatively impact sleep, sleep disruption alone can exacerbate pain and analgesic requirements.(58, 59) Sleep promotion and optimizing circadian rhythm is a non-invasive, inexpensive and feasible approach to prevent delirium and decrease pain and analgesic needs. These interventions can be singular or multicomponent, and have been shown to improve outcomes in ICU patients.(57) In fact, the most recent update of the Society for Critical Care Medicine Pain, Agitation and Delirium (PAD) guidelines has now incorporated management of sleep disruption and immobility as key components of optimizing adult ICU care (PADIS).(60)
This study has several limitations. First, as a single-center cohort study our findings may not be generalizable to other pediatric hospital settings. Second, we did not account for comorbidities, such as neuromuscular weakness, in assessment of the DARE. However, when used over multiple days, DARE allows the child to be his/her own reference. Third, our analysis did not consider pain, sedatives, or anesthetic medications before or during the period of actigraphic analysis, factors that may play a significant role in activity patterns and sleep quality. Fourth, age-based normative data are not available for DARE in children under 6 years of age. Therefore, we could not draw comparisons with younger patients in our inpatient cohort. However, it is likely that similar differences would be observed in younger patients. Finally, although the difference in DARE between the hospitalized and normative sample of children is significant, it is important to note that the 2 populations, although similar in age, could differ vastly due to other factors. NHANES aimed to gather a representative sample of the noninstitutionalized population in the United States using a complex, multistage probability design.(36) Thus, selection bias due to possible differences in overall health status, height, weight, obese status, and other characteristics not accounted for may affect the significance of the difference in DARE between the two populations. We did not have baseline data.
Children admitted to the PICU after major surgery experience disruptions in day-night activity patterns during their hospital stay that could reflect disturbances in circadian rhythm. Given the potential impact of circadian rhythm disturbances on the developing brain and importance of sleep during recovery from surgery, additional research is needed to identify methods of optimizing sleep hygiene and circadian rhythms in the perioperative period.
Acknowledgments
We thank Claire Levine, MS, in the Department of Anesthesiology and Critical Care Medicine at Johns Hopkins University School of Medicine for her editorial assistance with this manuscript.
S.K. was funded by the National Center for Advancing Translational Sciences (5KL2RR025006), the American Thoracic Society Outstanding Early Stage Investigator Award, and the Foundation for Anesthesia Education and Research (FAER) Research in Fellowship Award. These funding sources provided support for conduct of the research but did not play a role in the study design, data collection, analysis, or decision to submit the paper for publication. The authors declare no conflicts of interest.
Abbreviations:
- DARE
daytime activity ratio estimate
- PICU
pediatric intensive care unit
- PRISM
pediatric risk of mortality score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dumoulin Bridi MC, Aton SJ, Seibt J, Renouard L, Coleman T, Frank MG. Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci Adv. 2015;1:e1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahl RE. Sleep and the developing brain. Sleep. 2007;30:1079–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72:56–65. [DOI] [PubMed] [Google Scholar]
- 4.Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the Intensive Care Unit. Am J Respir Crit Care Med. 2015;191:731–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudchadkar S, Sterni L, Yaster M, Easley RB. Sleep in the Intensive Care Unit. Contemporary Critical Care. 2009;7:1–12. [Google Scholar]
- 6.Best KM, Boullata JI, Curley MA. Risk factors associated with iatrogenic opioid and benzodiazepine withdrawal in critically ill pediatric patients: a systematic review and conceptual model. Pediatr Crit Care Med. 2015;16:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabor JY, Cooper AB, Hanly PJ. Sleep disruption in the intensive care unit. Current Opinion in Critical Care. 2001;7:21–7. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol (1985). 1991;71:1112–8. [DOI] [PubMed] [Google Scholar]
- 9.Landis CA, Savage MV, Lentz MJ, Brengelmann GL. Sleep deprivation alters body temperature dynamics to mild cooling and heating not sweating threshold in women. Sleep. 1998;21:101–8. [DOI] [PubMed] [Google Scholar]
- 10.Creten C, Van Der Zwaan S, Blankespoor RJ, Leroy PL, Schieveld JN. Pediatric delirium in the pediatric intensive care unit: a systematic review and an update on key issues and research questions. Minerva Anestesiol. 2011;77:1099–107. [PubMed] [Google Scholar]
- 11.Schieveld JN, van der Valk JA, Smeets I, Berghmans E, Wassenberg R, Leroy PL, et al. Diagnostic considerations regarding pediatric delirium: a review and a proposal for an algorithm for pediatric intensive care units. Intensive Care Med. 2009;35:1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traube C, Mauer EA, Gerber LM, Kaur S, Joyce C, Kerson A, et al. Cost Associated With Pediatric Delirium in the ICU. Critical Care Medicine. 2016;44:e1175–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traube C, Silver G, Reeder RW, Doyle H, Hegel E, Wolfe HA, et al. Delirium in Critically Ill Children: An International Point Prevalence Study. Crit Care Med. 2017;45:584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traube C, Silver G, Gerber L, Kaur S, Mauer EA, Kerson AG, et al. Delirium and Mortality in Critically Ill Children: Epidemiology and Outcomes of Pediatric Delirium. Crit Care Med. 2017;45:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrup EA, Wieczorek B, Kudchadkar SR. Characteristics of postintensive care syndrome in survivors of pediatric critical illness: A systematic review. World J Crit Care Med 2017;6:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabbitts JA, Zhou C, Narayanan A, Palermo TM. Longitudinal and Temporal Associations Between Daily Pain and Sleep Patterns After Major Pediatric Surgery. J Pain. 2017;18:656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoey LM, Fulbrook P, Douglas JA. Sleep assessment of hospitalised patients: a literature review. Int J Nurs Stud. 2014;51:1281–8. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16:463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen MT, Rosenberg J, Gogenur I. Actigraphy for measurement of sleep and sleep-wake rhythms in relation to surgery. J Clin Sleep Med. 2013;9:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos AR, Weng J, Wallace DM, Petrov MR, Wohlgemuth WK, Sotres-Alvarez D, et al. Sleep Patterns and Hypertension Using Actigraphy in the Hispanic Community Health Study/Study of Latinos. Chest. 2018;153:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.So K, Buckley P, Adamson TM, Horne RS. Actigraphy correctly predicts sleep behavior in infants who are younger than six months, when compared with polysomnography. Pediatr Res. 2005;58:761–5. [DOI] [PubMed] [Google Scholar]
- 23.Belanger ME, Bernier A, Paquet J, Simard V, Carrier J. Validating actigraphy as a measure of sleep for preschool children. J Clin Sleep Med. 2013;9:701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao WC, Huang CY, Huang TY, Hwang SL. A systematic review of sleep patterns and factors that disturb sleep after heart surgery. J Nurs Res. 2011;19:275–88. [DOI] [PubMed] [Google Scholar]
- 25.Gogenur I, Middleton B, Burgdorf S, Rasmussen LS, Skene DJ, Rosenberg J. Impact of sleep and circadian disturbances in urinary 6-sulphatoxymelatonin levels, on cognitive function after major surgery. J Pineal Res. 2007;43:179–84. [DOI] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. [DOI] [PubMed] [Google Scholar]
- 27.Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ. Sleep monitoring in the intensive care unit: Comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Medicine. 2008;34:2076–83. [DOI] [PubMed] [Google Scholar]
- 28.van der Kooi AW, Tulen JH, van Eijk MM, de Weerd AW, van Uitert MJ, van Munster BC, et al. Sleep monitoring by actigraphy in short-stay ICU patients. Crit Care Nurs Q. 2013;36:169–73. [DOI] [PubMed] [Google Scholar]
- 29.Schwab KE, Ronish B, Needham DM, To AQ, Martin JL, Kamdar BB. Actigraphy to Evaluate Sleep in the Intensive Care Unit: A Systematic Review. Ann Am Thorac Soc. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duclos C, Dumont M, Blais H, Paquet J, Laflamme E, de Beaumont L, et al. Rest-Activity Cycle Disturbances in the Acute Phase of Moderate to Severe Traumatic Brain Injury. Neurorehabil Neural Repair. 2014;28:472–82. [DOI] [PubMed] [Google Scholar]
- 31.Bai J, Di C, Xiao L, Evenson KR, LaCroix AZ, Crainiceanu CM, et al. An Activity Index for Raw Accelerometry Data and Its Comparison with Other Activity Metrics. PLoS One. 2016;11:e0160644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai J, He B, Shou H, Zipunnikov V, Glass TA, Crainiceanu CM. Normalization and extraction of interpretable metrics from raw accelerometry data. Biostatistics. 2014;15:102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rupp TL, Balkin TJ. Comparison of Motionlogger Watch and Actiwatch actigraphs to polysomnography for sleep/wake estimation in healthy young adults. Behav Res Methods. 2011;43:1152–60. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control. National Health and Nutrition Examination Survey [Available from: http://www.cdc.gov/nchs/nhanes.htm]
- 35.Leroux A rnhanesdata: NHANES Accelerometry Data Pipeline. R package version 1.0. [Google Scholar]
- 36.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. [DOI] [PubMed] [Google Scholar]
- 37.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindstrom MJ, Bates DM. Newton-Raphson and EM Algorithms for Linear Mixed-Effects Models for Repeated-Measures Data. Journal of the American Statistical Association. 1988;83:1014–22. [Google Scholar]
- 39.Ozsancak A, D'Ambrosio C, Garpestad E, Schumaker G, Hill NS. Sleep and Mechanical Ventilation. Critical Care Clinics. 2008;24:517–31. [DOI] [PubMed] [Google Scholar]
- 40.Gehlbach BK, Chapotot F, Leproult R, Whitmore H, Poston J, Pohlman M, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35:1105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kudchadkar SR, Yaster M, Punjabi AN, Quan SF, Goodwin JL, Easley RB, et al. Temporal Characteristics of the Sleep EEG Power Spectrum in Critically Ill Children. J Clin Sleep Med. 2015;11(12): 1449–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kudchadkar SR, Aljohani OA, Punjabi NM. Sleep of critically ill children in the pediatric intensive care unit: A systematic review. Sleep Med Rev. 2014;18:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotagal S, Pianosi P. Sleep disorders in children and adolescents. BMJ. 2006;332:828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fawkes DB, Malow BA, Weiss SK, Reynolds AM, Loh A, Adkins KW, et al. Conducting actigraphy research in children with neurodevelopmental disorders--a practical approach. Behav Sleep Med. 2015;13:181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadeh A The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67 [DOI] [PubMed] [Google Scholar]
- 46.Beveridge C, Knutson K, Spampinato L, Flores A, Meltzer DO, Van Cauter E, et al. Daytime Physical Activity and Sleep in Hospitalized Older Adults: Association with Demographic Characteristics and Disease Severity. J Am Geriatr Soc. 2015;63:1391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaiswal SJ, McCarthy TJ, Wineinger NE, Kang DY, Song J, Garcia S, et al. Melatonin and sleep in preventing hospitalized delirium: A randomized clinical trial. Am J Med. 2018;131(9): 1110–1117.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krane-Gartiser K, Henriksen TE, Vaaler AE, Fasmer OB, Morken G. Actigraphically assessed activity in unipolar depression: a comparison of inpatients with and without motor retardation. The J Clin Psychiatry. 2015;76:1181–7. [DOI] [PubMed] [Google Scholar]
- 49.Kamdar BB, Kadden DJ, Vangala S, Elashoff DA, Ong MK, Martin JL, et al. Feasibility of Continuous Actigraphy in Patients in a Medical Intensive Care Unit. Am J Crit Care. 2017;26:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raj R, Ussavarungsi K, Nugent K. Accelerometer-based devices can be used to monitor sedation/agitation in the intensive care unit. J Crit Care. 2014;29:748–52. [DOI] [PubMed] [Google Scholar]
- 51.Tanev KS, Winokur A, Pitman RK. Sleep Patterns and Neuropsychiatric Symptoms in Hospitalized Patients With Dementia. J Neuropsychiatry Clin Neurosci. 2017;29:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conrad N, Karlik J, Lewandowski Holley A, Wilson AC, Koh J. A Narrative Review: Actigraphy as an Objective Assessment of Perioperative Sleep and Activity in Pediatric Patients. Children (Basel). 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–84.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart NH, Arora VM. Sleep in Hospitalized Older Adults. Sleep Med Clin. 2018;13:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kudchadkar SR, Beers MC, Ascenzi JA, Jastaniah E, Punjabi NM. Nurses' Perceptions of Pediatric Intensive Care Unit Environment and Work Experience After Transition to Single-Patient Rooms. Am J Crit Care. 2016;25:e98–e107. [DOI] [PubMed] [Google Scholar]
- 56.Kudchadkar SR, Yaster M, Punjabi NM. Sedation, Sleep Promotion, and Delirium Screening Practices in the Care of Mechanically Ventilated Children: A Wake-Up Call for the Pediatric Critical Care Community. Crit Care Med. 2014;42:1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamdar BB, Needham DM. Bundling sleep promotion with delirium prevention: ready for prime time? Anaesthesia. 2014;69:527–31. [DOI] [PubMed] [Google Scholar]
- 58.Karmann AJ, Kundermann B, Lautenbacher S. [Sleep deprivation and pain: a review of the newest literature]. Schmerz. 2014;28:141–6. [DOI] [PubMed] [Google Scholar]
- 59.Fisher K, Laikin AM, Sharp KMH, Criddle CA, Palermo TM, Karlson CW. Temporal relationship between daily pain and actigraphy sleep patterns in pediatric sickle cell disease. J Behav Med. 2018;41:416–22. [DOI] [PubMed] [Google Scholar]
- 60.Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46:e825–e73. [DOI] [PubMed] [Google Scholar]