Abstract
Objectives:
To evaluate the diagnostic accuracy of ultrasound elastography with acoustic radiation force impulse (ARFI) to detect congenital hepatic fibrosis and portal hypertension in children with autosomal recessive polycystic kidney disease (ARPKD).
Study design:
Cross sectional study of 25 children with ARPKD and 24 healthy controls. US ARFI elastography (Acuson S3000, Siemens Medical Solutions USA) was performed to measure shear wave speed (SWS) in the right and left liver lobes and the spleen. Liver and spleen SWS were compared in controls vs ARPKD, and ARPKD without vs with portal hypertension. Linear correlations between liver and spleen SWS, spleen length, and platelet counts were analyzed. Receiver operating characteristic (ROC) analysis was used to evaluate diagnostic accuracy of US ARFI elastography.
Results:
ARPKD participants had significantly higher median liver and spleen SWS than controls. At a proposed SWS cut-off value of 1.56 m/s, the left liver lobe had the highest sensitivity (92%) and specificity (96%) for distinguishing ARPKD participants from controls [ROC area 0.92 (95% CI: 0.82–1.00)]. ARPKD participants with portal hypertension (splenomegaly and low platelet counts) had significantly higher median liver and spleen stiffness than those without portal hypertension. The left liver lobe also had the highest sensitivity and specificity for distinguishing ARPKD subjects with portal hypertension.
Conclusions:
US ARFI elastography of the liver and spleen, particularly of the left liver lobe, is a useful non-invasive biomarker to detect and quantify liver fibrosis and portal hypertension in children with ARPKD.
Keywords: congenital hepatic fibrosis, portal hypertension, shear wave elastography, pediatrics
Autosomal recessive polycystic kidney disease (ARPKD) is an inherited hepatorenal fibrocystic disorder characterized by progressive chronic kidney disease (CKD) and liver disease consisting of dilated biliary ducts, congenital hepatic fibrosis (CHF), and portal hypertension (Caroli syndrome).1 Liver-related complications in ARPKD can include ascending cholangitis and symptoms of portal hypertension, such as hypersplenism and esophageal varices. Severe bleeding complications and/or the need for portosystemic shunting occur in 10–40% of patients with ARPKD, and about 7% of patients eventually require liver transplantation.2–7
Current clinical methods to monitor the severity and progression of liver disease in ARPKD have significant limitations. Unlike typical cirrhosis, the liver fibrosis in CHF consists of dense, slowly progressive peri-portal fibrosis that is usually not accompanied by inflammation. Patients typically have well-preserved liver synthetic function despite progressive portal hypertension, making markers of liver synthetic function testing uninformative for quantifying severity of CHF.3 Performing a liver biopsy is generally not recommended due to the risk of complications and lack of prognostic value.8 Splenomegaly and thrombocytopenia resulting from hypersplenism correlate with the severity of portal hypertension,3 but are not universally present or may be under-recognized. Indeed, in one cohort study, 2 of 4 patients with ARPKD without splenomegaly had esophageal varices on endoscopy,3 raising concern that serious bleeding complications could occur in patients without clinical examination findings concerning for portal hypertension. The presence of esophageal varices on endoscopy can confirm portal hypertension, but current expert recommendations do not advise prospective surveillance endoscopy and primary prophylaxis for children with portal hypertension, due to invasiveness and lack of data on risk of variceal bleeding.10,11 Standard greyscale ultrasound provides only qualitative assessment of liver echotexture and biliary tract dilatation, and Doppler assessments of portal blood flow lack sensitivity for detection of portal hypertension.9
Novel non-invasive imaging biomarkers are therefore needed to quantify the severity of CHF and portal hypertension in ARPKD and could inform anticipatory guidance and plans for expediting medical care in case of variceal bleeding.10,11 The need to develop new imaging biomarkers has been highlighted by an expert panel, which recommended further study of “noninvasive tests…as a means to help triage children for endoscopy to screen for esophageal varices.”10
US elastography with acoustic radiation force impulse (ARFI) imaging has emerged as a valuable method to quantify liver fibrosis. ARFI is integrated into a conventional US machine and assesses tissue stiffness by measuring shear wave speed (SWS) of transverse waves generated in the tissue following an acoustic pulse, with higher SWS indicating higher stiffness. In adults and children with chronic liver diseases, US ARFI elastography has been shown to have good diagnostic accuracy for non-invasive staging of liver fibrosis.12–14
The objective of this study is to evaluate the use of US ARFI elastography to quantify the severity of liver fibrosis and portal hypertension in children and young adults with ARPKD. Our specific aims are to determine if liver and spleen stiffness measured by ARFI can distinguish ARPKD participants from healthy controls; ARPKD participants without clinical signs of portal hypertension from healthy controls; and ARPKD participants with vs. without clinical signs of portal hypertension.
Methods
In this cross-sectional study, children and young adults ≤21 years old with a clinical diagnosis of ARPKD were recruited from the nephrology practice at the Children’s Hospital of Philadelphia (CHOP). Individuals who had received a liver transplant or a portosystemic shunt were excluded from this analysis. A reference population of 24 healthy children with no personal history of hypertension, obesity, hematologic or rheumatologic disease, and no family history of kidney or liver disease, was recruited from CHOP primary care practices. The reference population consisted of an equal number of males and females in each of the following age groups<5 years old (n=6); 5 to <10 years old (n=6); 10 to <15 years old (n=6); and ≥ 15 years old (n=6). This reference population approach was chosen to allow comparison of our ARFI measurements with the largest published study of ARFI normative values in children.15 The CHOP Institutional Review Board approved this study (IRB 14–10785), and informed consent was obtained from all participants/guardians.
Measurements
Data collected during the single study visit included demographic information, current medications, and medical and family history. Physical examination included measurements of height, weight, and manual blood pressure. Laboratory measurements were performed only in participants with ARPKD, and included complete blood counts and tests of kidney and liver function. Clinical signs of portal hypertension were defined as presence of splenomegaly or thrombocytopenia. “Definitive” portal hypertension was defined as presence of both splenomegaly and thrombocytopenia, and absence of portal hypertension was defined as the presence of neither splenomegaly nor thrombocytopenia. Splenomegaly was defined as spleen length >90th percentile for height,16 and spleen length index was calculated as actual/90th percentile spleen length. Thrombocytopenia was defined as platelet count <150×103/μL. Known varices were defined as esophageal or gastric varices diagnosed on upper endoscopy. Estimated glomerular filtration rate (eGFR) was calculated based on the bedside CKD in Children (CKiD) Study equation.17
US was performed with the Siemens Acuson S3000 with Virtual Touch tissue quantification system (Siemens Medical Solutions USA, Inc., Malvern, PA), using age- and size-appropriate linear or convex transducers (4–9 MHz). Participants were asked to fast prior to US for age-appropriate durations according to hospital protocols, unless medically contraindicated. Greyscale US of the liver and spleen were obtained in supine position according to standard clinical protocols, including measurements of liver and spleen sagittal (craniocaudal) length. ARFI elastography was performed in Virtual Touch quantification (VTq) mode to obtain point SWS measurements (in meters per second, m/s) in the right and left liver lobes and spleen mid-pole, using an inter- or sub-costal approach. Regions of interest (ROIs) for SWS measurements were placed perpendicular to the organ capsule at a depth of 2–7 cm, based on participants’ organ size and body habitus, avoiding areas of visible bile ducts, cysts, or vessels. The minimum pressure needed to obtain an adequate greyscale image was applied to the transducer. Measurements were performed during a brief breath-hold (without deep inspiration) in participants who were able to comply, and at end-expiration for non-cooperative participants. Ten valid SWS measurements were obtained to calculate the mean SWS for each site.
Statistical Analyses
Clinical and demographic variables were reported as median and interquartile range (IQR) for continuous variables, and as frequency and percentage for binary variables. Group differences were compared using Wilcoxon rank sum test for continuous variables and the Fisher exact test for binary variables. Liver and spleen stiffness (measured as SWS) were compared between control and ARPKD groups, between controls and ARPKD participants without portal hypertension (neither splenomegaly nor low platelets), and between ARPKD participants without vs. with definitive portal hypertension (both splenomegaly and low platelets), using Wilcoxon rank sum tests. To perform age-matched analysis, each ARPKD participant was matched manually 1:1 to the healthy control participant closest in age, dropping any ARPKD or control participants without a match. Following matching, unpaired group analyses were performed. Linear fit plots and Spearman correlation were performed to examine relationships between liver and spleen SWS, age, spleen length index, and platelet counts. Non-parametric bootstrapping with 1000 replications was performed to calculate 95% confidence intervals (CI) for the Spearman rho. Receiver operating characteristic (ROC) analysis was performed to evaluate the diagnostic performance of ARFI elastography to distinguish between children with ARPKD and controls, and between ARPKD participants with vs. without clinical signs of portal hypertension. Diagnostic value of SWS cut-offs was evaluated using the sensitivity, specificity, and percent of subjects correctly classified. SWS cut-offs were chosen to maximize the percent of subjects correctly classified. Statistical analyses were performed using Stata 13.1 (StataCorp, College Station, TX).
Results
US measurements were obtained on 26 participants with ARPKD and 24 healthy controls. One participant with ARPKD was excluded from this analysis due to history of a portosystemic shunt, leaving 25 ARPKD participants in the final analysis. Twenty three of 24 (96%) healthy controls and 22 of 25 (88%) of ARPKD participants were fasting prior to US. All participants who did not fast were infants ≤16 months old. Five ARPKD participants had previously received a kidney transplant.
Clinical and demographic characteristics of the ARPKD and control groups are shown in Table 1. The median age of the ARPKD participants was younger than healthy controls (4.6 vs. 10.5 years), but this difference was not statistically significant. Participants with ARPKD had median white blood cell (WBC) and platelet counts within the normal range, but 9 (36%) of the ARPKD participants had low platelets (<150×103/μL). Splenomegaly was significantly more common in the ARPKD group compared with controls (56% vs. 4%, P < .001), with median spleen length index of 1.10 in the ARPKD group compared with 0.83 in controls (p<0.001) (Table I).
Table 1.
Clinical and demographic characteristics of healthy controls and ARPKD participants.
| Characteristic | Healthy controls (n = 24) | ARPKD (n = 25) | p |
|---|---|---|---|
| Age, years | 10.5 [5.2, 15.0] | 4.6 [1.9, 13.2] | 0.1 |
| Male sex | 12 (50%) | 15 (60%) | 0.6 |
| eGFR* (mL/min/1.73m2) | - | 65.2 [42.5, 90.4] | - |
| WBC count (×103/μL) | - | 6.3 [4.4, 10.6] | - |
| <150×103/μL | 9 (36%) | ||
| >90th percentile | 1 (4%) | 14 (56%) | <0.001 |
Continuous variables given as median [IQR]; binary variables as count (%).
includes 5 participants with kidney transplant
ARPKD, autosomal recessive polycystic kidney disease; eGFR, estimated glomerular filtration rate; WBC, white blood cell count
Of the 25 ARPKD participants, 11 had no clinical signs of portal hypertension (i.e. neither splenomegaly nor low platelets); 14ARPKD participants had splenomegaly, and 9 of these individuals also had low platelets. Clinical and demographic characteristics of ARPKD participants without and with clinical signs of portal hypertension are shown in Table 2 (available at www.jpeds.com). ARPKD participants with either splenomegaly or low platelets had a higher median age than those without signs of portal hypertension. As expected, ARPKD participants with splenomegaly (n=14) had lower median WBC and platelet counts than those without splenomegaly (n=11) (WBC 6.1 vs. 11.0 ×103/μL, p=0.03; platelets 142 vs. 322 ×103/μL, p=0.0002). All participants with low platelets had splenomegaly, and were defined as having “definitive” portal hypertension (n=9). However, 5 participants with splenomegaly had normal platelets, suggesting that low platelets may be a later sign of portal hypertension or that platelet counts may be affected by other clinical factors. Participants with definitive portal hypertension (i.e. both splenomegaly and low platelets, n=9) had a higher median age and lower WBC count than those without portal hypertension (n=11) (median age 7.7 vs. 1.9 years, p=0.03; WBC 4.4 vs. 11.0 ×103/μL, p=0.01). Three participants had a history of endoscopically-confirmed esophageal or gastric varices, all of whom had both splenomegaly and low platelets. One participant had a history of ascending cholangitis; this individual had both splenomegaly and low platelets, but did not have a known history of varices. Of the 5 ARPKD participants who had received kidney transplants, all had splenomegaly and 2 also had low platelets (i.e. definitive portal hypertension). Kidney transplant recipients (n=5) had slightly higher median WBC and slightly lower median platelet counts than non-transplant ARPKD participants (n=20), but these differences were not statistically significant (WBC 7.5 vs. 6.1 ×103/μL, p=0.9; platelets 199 vs. 271 ×103/μL, p=0.5, Table 3; available at www.jpeds.com). Liver and spleen stiffness were not significantly different between kidney transplant recipients and non-transplant ARPKD participants (Table 3).
Table 2.
Clinical and demographic characteristics of ARPKD participants without vs. with signs of portal hypertension (splenomegaly and low platelets). All participants without splenomegaly had normal platelet counts and were categorized as “Definitive No pHTN.” All participants with low platelets also had splenomegaly and were categorized as “Definitive Yes pHTN.”
| Characteristic | Without splenomegaly (Definitive No pHTN) (n = 11) |
With splenomegaly (n = 14) |
P | Without low Platelets (n = 16) |
With low platelets (Definitive Yes pHTN) (n = 9) |
P | P (Definitive pHTN No vs. Yes) |
|---|---|---|---|---|---|---|---|
| Age, years | 1.9 [0.5, 8.4] | 6.9 [4.4, 16.8] | 0.02 | 3.3 [0.7, 9.6] | 7.7 [4.5, 16.8] | 0.06 | 0.03 |
| Male sex | 8 (73%) | 7 (50%) | 0.4 | 9 (56%) | 6 (67%) | 0.7 | 0.9 |
| eGFR* (mL/min/1.73 m2) |
69.8 [30.2, 95.2] | 62.3 [48.6, 89.8] | 0.8 | 68.0 [42.6, 92.8] | 59.3 [42.5, 82.4] | 0.9 | 0.9 |
| WBC count (×103/μL) |
11.0 [5.0, 13.5] | 6.1 [4.1, 7.5] | 0.03 | 8.8 [6.2, 12.8] | 4.4 [3.1, 5.9] | 0.002 | 0.01 |
| <150×103/μL | 0 (0%) | 9 (65%) | 0.001 | 0 (0%) | 9 (100%) | n/a | n/a |
| >90th percentile | 0 (0%) | 14 (100%) | n/a | 5 (31%) | 9 (100%) | 0.001 | n/a |
| With bleeding | n/a | 2 (14%) | - | n/a | 2 (22%) | - | |
| History of ascending cholangitis | 0 (0%) | 1 (7%) | - | 0 (0%) | 1 (11%) | - | n/a |
Continuous variables given as median [IQR]; binary variables as count (%).
includes 5 participants with kidney transplant
ARPKD, autosomal recessive polycystic kidney disease; eGFR, estimated glomerular filtration rate; pHTN, portal hypertension; WBC, white blood cell count
Table 3.
Comparison of ARPKD kidney transplant recipients vs. those without kidney transplant: clinical and demographic characteristics, and liver and spleen shear wave speeds measured by ARFI US elastography.
| Characteristic | ARPKD kidney transplant recipients (n = 5) |
ARPKD without kidney transplant (n = 20) |
P |
|---|---|---|---|
| Age, years | 6.1 [4.5, 18.7] | 4.3 [0.9, 12.0] | 0.2 |
| eGFR* (mL/min/1.73m2) |
65.2 [53.7, 82.4] | 62.7 [34.7, 102.7] | 0.9 |
| WBC count (×103/μL) |
7.5 [6.3, 7.6] | 6.1 [4.3, 11.6] | 0.9 |
| <150×103/μL | 2 (40%) | 7 (35%) | 0.9 |
| >90th percentile | 5 (100%) | 9 (45%) | 0.05 |
| Definite pHTN | 2 (40%) | 7 (35%) | 0.2 |
| Spleen | 3.27 [2.97, 3.37] | 3.13 [2.86, 3.55] | 0.9 |
Continuous variables given as median [IQR]; binary variables as count (%).
ARPKD, autosomal recessive polycystic kidney disease; eGFR, estimated glomerular filtration rate; pHTN, portal hypertension (Definite pHTN = splenomegaly + low platelets); SWS, shear wave speed; WBC, white blood cell count
Liver and spleen stiffness in control vs ARPKD groups
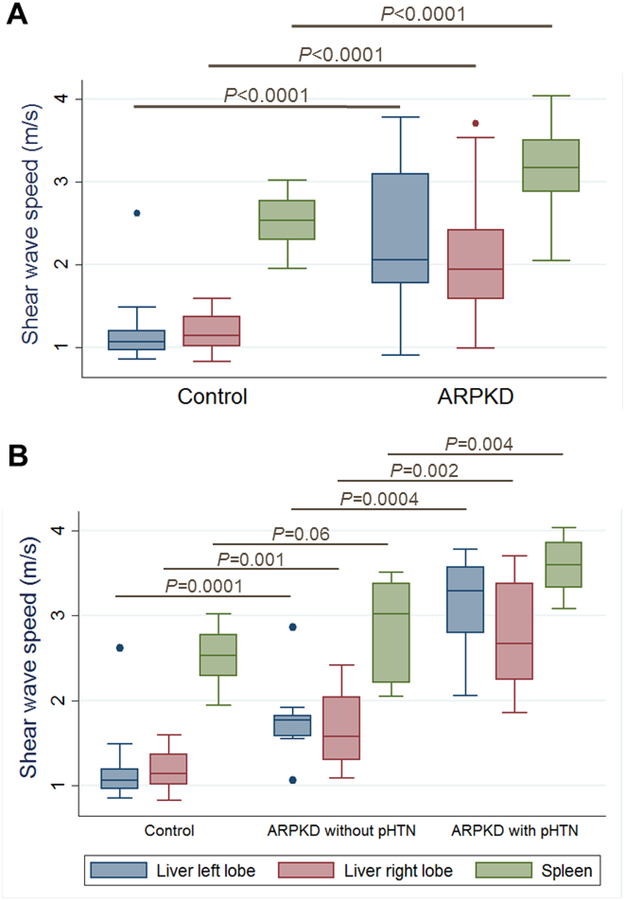
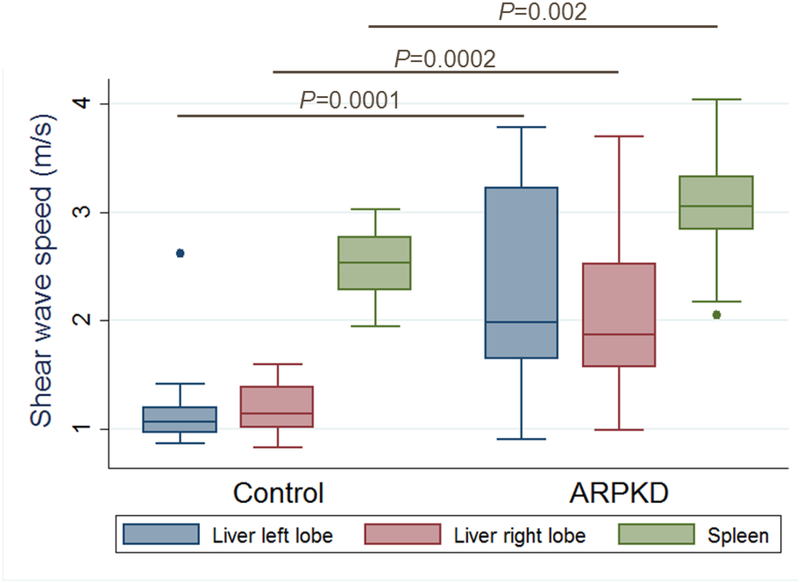
Overall, ARPKD participants had significantly higher median liver and spleen stiffness than healthy controls. Median SWS for controls (n=24) vs. all ARPKD (n=25) participants was 1.07 vs. 2.06 m/s in the left liver lobe (p<0.0001), 1.15 vs. 1.95 m/s in the right liver lobe (p<0.0001), and 2.53 vs. 3.17 m/s in the spleen (p<0.0001) (Figure 1, A). To explore whether US ARFI elastography can detect milder forms of ARPKD liver disease, we compared liver and spleen stiffness between healthy controls and ARPKD participants without portal hypertension. Median SWS for controls (n=24) vs. ARPKD participants without portal hypertension (n=11) was 1.07 vs. 1.77 m/s in the left liver lobe (p=0.0001), 1.15 vs. 1.58 m/s in the right liver lobe (p=0.001), and 2.53 vs. 3.02 m/s in the spleen (p=0.06) (Figure 1, B).
Figure 1: Liver and spleen stiffness in healthy controls and ARPKD participants.
Liver and spleen shear wave speeds measured by ARFI US elastography in: A. Healthy controls (n=24) and participants with ARPKD (n=25), and B. Healthy controls (n=24), ARPKD participants without portal hypertension (n=11), and ARPKD participants with definitive portal hypertension (n=9).
Given the older median age of healthy controls compared with the ARPKD group, we performed comparisons of age-matched ARPKD and control groups, and also explored whether there were any age-related changes in liver and spleen stiffness in the ARPKD and control groups. In age-matched analysis, ARPKD participants again showed significantly higher median liver and spleen stiffness than healthy controls. Median SWS for age-matched groups of controls (n=18, median age 8.4 [3.8, 14.9] years) vs. ARPKD (n=18, median age 8.1 [4.0, 14.7] years) was 1.07 vs. 1.99 m/s in the left liver lobe (p=0.0001), 1.15 vs. 1.87 m/s in the right liver lobe (p=0.0002), and 2.53 vs. 3.05 m/s in the spleen (p=0.002) (Figure 2; available at www.jpeds.com).
Figure 2: Liver and spleen stiffness in age-matched healthy controls and ARPKD participants.
Liver and spleen shear wave speeds measured by ARFI US elastography in age-matched groups of healthy controls (n=18) and participants with ARPKD (n=18).
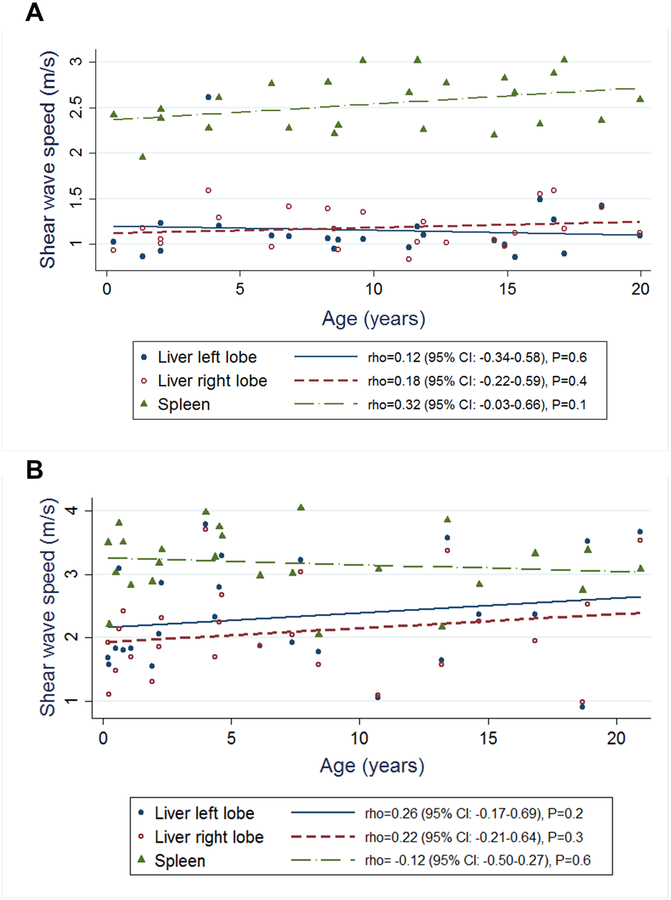
In healthy controls (n=24), liver stiffness did not correlate with age (left liver lobe: rho=0.12 (95% CI: −0.34–0.58), P=0.6; right liver lobe: rho=0.18 (95% CI: −0.22–0.59, P=0.4). Spleen stiffness showed a slight positive correlation with age in healthy controls, but this was not statistically significant (rho=0.32 (95% CI: −0.03–0.66), P=0.1). In the ARPKD group (n=25), liver stiffness showed a slight positive but statistically non-significant trend with age, and spleen stiffness did not correlate with age (left liver lobe: rho=0.26 (95% CI: −0.17–0.69), P=0.2; right liver lobe: rho=0.22 (95% CI: −0.21–0.64), P=0.3; spleen: rho=−0.12 (95% CI: −0.50–0.27), P=0.6) (Figure 3; available at www.jpeds.com).
Figure 3: Relationship of liver and spleen stiffness with age.
Relationship between liver and spleen shear wave speed measured by ARFI US elastography with age in A. healthy controls (n=24) and B. ARPKD participants (n=25)
Liver and spleen stiffness in ARPKD participants without vs. with clinical portal hypertension
Liver and spleen stiffness in ARPKD participants without vs. with splenomegaly
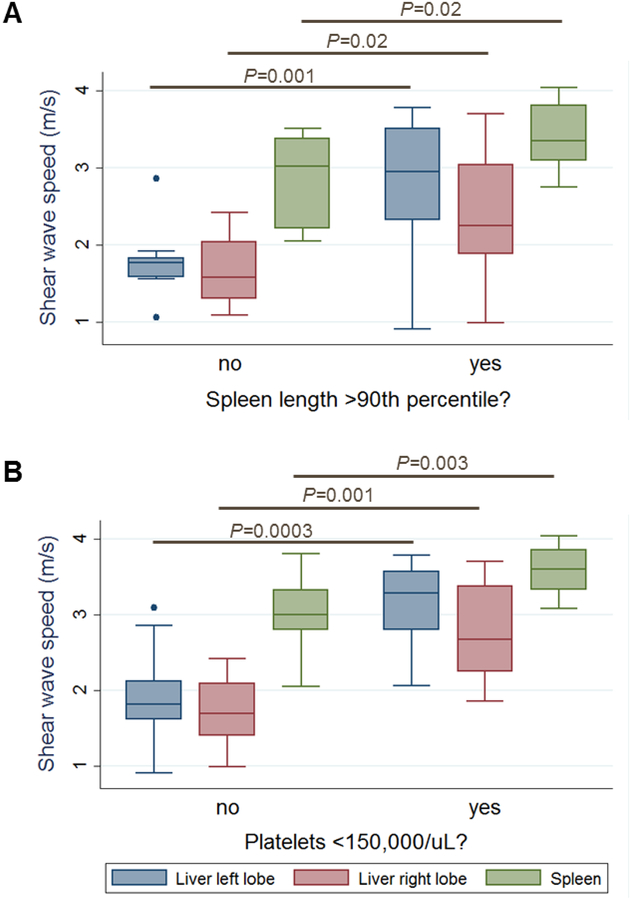
ARPKD participants with splenomegaly had significantly higher median liver and spleen stiffness than those without splenomegaly. Median SWS for ARPKD participants without vs. with splenomegaly was 1.77 vs. 2.94 m/s in the left liver lobe (p=0.001), 1.58 vs. 2.25 m/s in the right liver lobe (p=0.02), and 3.02 vs. 3.35 m/s in the spleen (p=0.02) (Figure 4, A; available at www.jpeds.com).
Figure 4: Liver and spleen stiffness in ARPKD participants without vs. with clinical signs of portal hypertension.
Liver and spleen shear wave speeds measured by ARFI US elastography in ARPKD participants A. without (n=11) vs. with (n=14) splenomegaly, B. without (n=16) vs. with (n=9) low platelets.
Liver and spleen stiffness in ARPKD participants without vs. with low platelets
ARPKD participants with low platelets had significantly higher median liver and spleen stiffness than those without low platelets. Median SWS for ARPKD participants without vs. with low platelets was 1.82 vs. 3.29 m/s in the left liver lobe (p=0.0003), 1.70 vs. 2.67 m/s in the right liver lobe (p=0.001), and 2.99 vs. 3.60 m/s in the spleen (p=0.003) (Figure 4, B; available at www.jpeds.com).
Liver and spleen stiffness in ARPKD participants without vs. with definitive portal hypertension
ARPKD participants with definitive portal hypertension (both splenomegaly and low platelets) had significantly higher median liver and spleen stiffness than those without portal hypertension. Median SWS for ARPKD participants without (n=11) vs. with (n=9) definitive portal hypertension was 1.77 vs. 3.29 m/s in the left liver lobe (p=0.0004), 1.58 vs. 2.67 m/s in the right liver lobe (p=0.002), and 3.02 vs. 3.60 m/s in the spleen (p=0.004) (Figure 1, B).
Relationship of liver and spleen stiffness with spleen length and platelet count in ARPKD participants
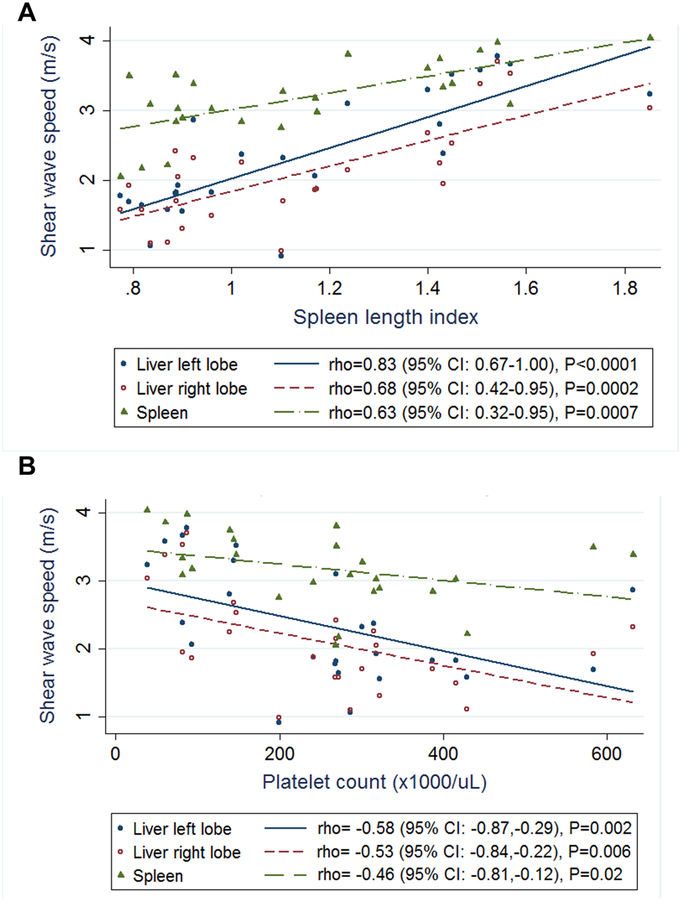
To further explore the relationship of liver and spleen stiffness with clinical signs of portal hypertension in children with ARPKD, we examined the linear correlations of liver and spleen SWS with spleen length and platelet count. Liver and spleen stiffness were strongly positively correlated with spleen length index [left liver lobe: rho=0.83 (95% CI: 0.67–1.00), p<0.0001; right liver lobe: rho=0.68 (95% CI: 0.42–0.95), p=0.0002; spleen: rho=0.63 (95% CI: 0.32–0.95), p=0.0007] (Figure 5, A). Liver and spleen stiffness were negatively correlated with platelet count [left liver lobe: rho=−0.58 (95% CI: −0.87,−0.29), p=0.002; right liver lobe: rho=−0.53 (95% CI: −0.84,−0.22), p=0.006; spleen: rho=−0.46 (95% CI: −0.81,−0.12), p=0.02] (Figure 5, B).
Figure 5: Linear correlations of liver and spleen stiffness with spleen length index and platelet count in ARPKD participants.
Relationship of liver and spleen shear wave speeds measured by ARFI US elastography with: A. spleen length index and B. platelet count.
ROC analysis
Healthy controls vs. ARPKD participants
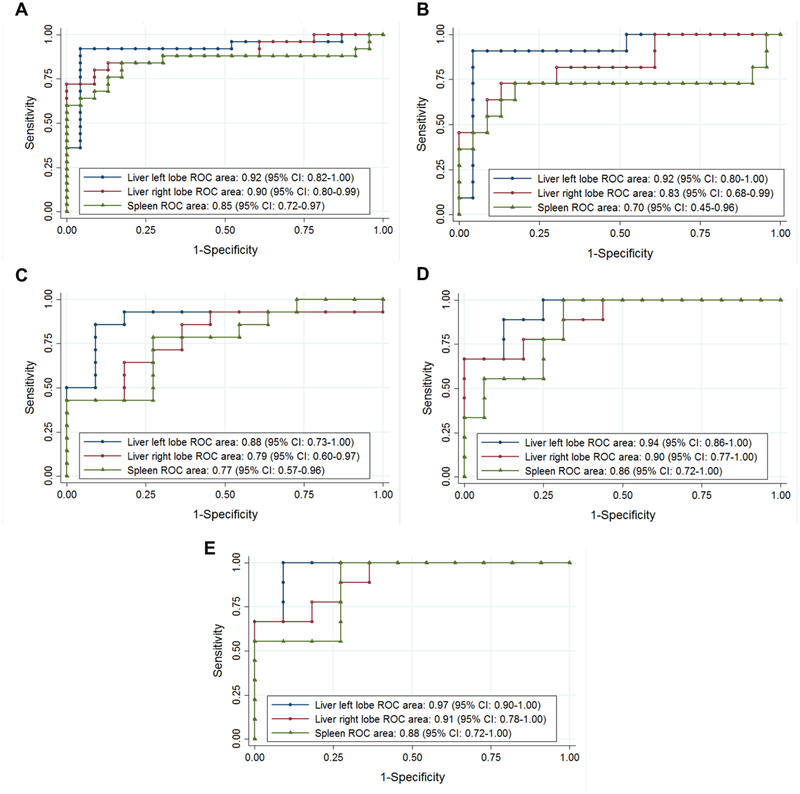
ROC analysis showed that ARFI US elastography of the liver and spleen had high accuracy in distinguishing ARPKD participants from healthy controls, with areas under the ROC curve (AUROC) of 0.92 (95% CI: 0.82–1.00) for the left liver lobe, 0.90 (95% CI: 0.80–0.88) for the right liver lobe, and 0.85 (95% CI 0.72–0.97) for the spleen (Figure 6, A). At a proposed SWS cut-off value of 1.56 m/s, the left liver lobe had the highest sensitivity (92%) and specificity (96%) for distinguishing ARPKD participants from healthy controls (Table 4; available at www.jpeds.com).
Figure 6: Receiver operating characteristic (ROC) curves to evaluate diagnostic performance of liver and spleen stiffness.
ROC curves to evaluate diagnostic performance of liver and spleen shear wave speeds measured by ARFI US elastography to distinguish between: A. Healthy controls vs. ARPKD participants; B. Healthy controls vs. ARPKD participants without portal hypertension; C. ARPKD participants without vs. with splenomegaly; D. ARPKD participants without vs. with low platelets; and E. ARPKD participants without vs. with definitive portal hypertension (both splenomegaly and low platelets). Areas under the ROC curves with 95% confidence intervals (CI) are as shown.
Table 4.
Diagnostic accuracy of cut-off values for liver and spleen shear wave speeds (SWS) measured by ARFI US elastography to distinguish between: A. Healthy controls vs. ARPKD participants; B. Healthy controls vs. ARPKD participants without portal hypertension; C. ARPKD participants without vs. with splenomegaly; D. ARPKD participants without vs. with low platelet counts; and E. ARPKD participants without vs. with definitive portal hypertension (i.e. both splenomegaly and low platelet counts)
| Site | Proposed SWS cut-off (m/s) | Sensitivity (%) | Specificity (%) | Correctly classified (%) |
|---|---|---|---|---|
| A. Healthy controls vs. ARPKD participants | ||||
| Liver left lobe | 1.56 | 92 | 96 | 94 |
| Liver right lobe | 1.49 | 84 | 88 | 86 |
| Spleen | 2.83 | 84 | 83 | 84 |
| B. Healthy controls vs. ARPKD participants without portal hypertension | ||||
| Liver left lobe | 1.56 | 91 | 96 | 94 |
| Liver right lobe | 1.49 | 73 | 83 | 83 |
| Spleen | 2.83 | 73 | 83 | 80 |
| C. ARPKD participants without vs. with splenomegaly | ||||
| Liver left lobe | 2.06 | 86 | 91 | 88 |
| Liver right lobe | 1.86 | 86 | 64 | 76 |
| Spleen | 3.08 | 79 | 73 | 76 |
| D. ARPKD participants without vs. with low platelet counts | ||||
| Liver left lobe | 2.37 | 89 | 88 | 88 |
| Liver right lobe | 2.52 | 67 | 100 | 88 |
| Spleen | 3.60 | 56 | 94 | 80 |
| E. ARPKD participants without vs. with definitive portal hypertension | ||||
| Liver left lobe | 2.06 | 100 | 91 | 95 |
| Liver right lobe | 2.52 | 67 | 100 | 85 |
| Spleen | 3.08 | 100 | 73 | 85 |
Healthy controls vs. ARPKD participants without portal hypertension
To examine the accuracy of ARFI US elastography to detect milder forms of ARPKD liver disease, we performed ROC analysis to distinguish ARPKD participants without portal hypertension from healthy controls. AUROCs were 0.92 (95% CI: 0.80–1.00) for the left liver lobe, 0.83 (95% CI: 0.68–0.99) for the right liver lobe, and 0.70 (95% CI 0.45–0.96) for the spleen (Figure 6, B). At a proposed SWS cut-off value of 1.56 m/s, the left liver lobe had the highest sensitivity (91%) and specificity (96%) for distinguishing ARPKD participants without portal hypertension from healthy controls (Table 4).
ARPKD participants without vs. with portal hypertension
Within the ARPKD group, we examined the accuracy of ARFI US elastography to distinguish between participants without vs. with clinical signs of portal hypertension, namely splenomegaly or low platelets.
For distinguishing ARPKD participants without vs. with splenomegaly, AUROCs were 0.88 (95% CI: 0.73–1.00) for the left liver lobe, 0.79 (95% CI: 0.60–0.97) for the right liver lobe, and 0.77 (95% CI 0.57–0.96) for the spleen (Figure 6, C). At a proposed SWS cut-off value of 2.03 m/s, the left liver lobe had the highest sensitivity (86%) and specificity (91%) for distinguishing ARPKD participants with vs. without splenomegaly (Table 4C).
For distinguishing ARPKD participants without vs. with low platelets, AUROCs were 0.94 (95% CI: 0.86–1.00) for the left liver lobe, 0.90 (95% CI: 0.77–1.00) for the right liver lobe, and 0.86 (95% CI 0.72–1.00) for the spleen (Figure 6, D). At a proposed SWS cut-off value of 2.37 m/s, the left liver lobe had the highest sensitivity (89%) and specificity (88%) for distinguishing ARPKD participants with vs. without low platelets (Table 4).
For distinguishing ARPKD participants without vs. with definitive portal hypertension (both splenomegaly and low platelets), AUROCs were 0.97 (95% CI: 0.90–1.00) for the left liver lobe, 0.91 (95% CI: 0.78–1.00) for the right liver lobe, and 0.88 (95% CI 0.72–1.00) for the spleen (Figure 6, E). At a proposed SWS cut-off value of 2.06 m/s, the left liver lobe had the highest sensitivity (100%) and specificity (91%) for distinguishing ARPKD participants with vs. without definitive portal hypertension (Table 4).
Discussion
In this study, we found that individuals with ARPKD have significantly higher liver and spleen stiffness than healthy controls. ARFI US elastography had a high predictive value for differentiating individuals with ARPKD from healthy controls, with a left liver lobe cut-off of 1.56 m/s showing the highest sensitivity (92%) and specificity (96%) (AUROC=0.92). This high sensitivity and specificity for left liver lobe stiffness in detecting ARPKD liver disease persisted even when comparing healthy controls to ARPKD participants without evidence of portal hypertension. In clinical settings, ARFI US elastography, particularly of the left liver lobe, could therefore be useful to detect liver involvement in children in whom the diagnosis of ARPKD is unclear, or to detect early signs of portal hypertension in children with known ARPKD.
Within the ARPKD group, participants with either splenomegaly or low platelets had significantly higher liver and spleen stiffness than those without these clinical signs of portal hypertension. Overall, median liver and spleen stiffness measurements were higher in ARPKD participants with low platelets compared with those with splenomegaly, suggesting that low platelets are sign of more advanced portal hypertension. This is consistent with our observation that only a subset (64%) of participants with splenomegaly also had low platelets. Liver and spleen stiffness both showed strong linear correlations with spleen size and platelet count, suggesting that the ARFI measures track reliably with increasing severity of portal hypertension. Again, the left liver lobe showed the highest accuracy for distinguishing clinical signs of portal hypertension with high sensitivity and specificity.
Overall, our results indicate that ARFI US elastography of the liver and spleen appears to be a promising biomarker of the severity of congenital hepatic fibrosis and portal hypertension in ARPKD. In particular, elastography of the left liver lobe appears particularly sensitive and specific in detecting ARPKD-related liver disease. Disproportionate involvement of the left liver lobe is consistent with our own clinical observations of left liver lobe enlargement palpable under the xiphoid in our patients with ARPKD. It has also been shown on magnetic resonance imaging in an NIH cohort study, where the left liver lobe was disproportionately enlarged in 35 of 51 patients (69%).3 Our finding of higher sensitivity and specificity of left liver lobe elastography measurements is in contrast to previous studies of US ARFI elastography in other disease processes in adults. For example, one study compared US ARFI elastography of the left and right liver lobes to histologic grading of fibrosis in adults undergoing hepatectomy for hepatocellular carcinoma, chronic viral hepatitis, and/or alcoholic hepatitis, and found that the left liver lobe had lower AUROC than the right for diagnosing histologic fibrosis.18 Similarly, some studies in a meta-analysis in which US ARFI elastography measurements were performed in both liver lobes showed lower AUROC in the left lobe13 Our findings of higher diagnostic accuracy of the left liver lobe may represent a unique aspect of ARPKD-related liver disease, or perhaps a finding specific to pediatric liver disease. Further studies in larger ARPKD populations and in children with other liver diseases are therefore needed.
A previous small study evaluating a different type of US elastography, transient elastography (TE, Fibroscan), found children with ARPKD had higher liver stiffness than controls.19 TE is widely used in adults to quantify liver fibrosis in various chronic liver diseases and has the advantage of device portability. However, inability to obtain a reliable reading is about three times as common with TE compared with ARFI, with even higher failure rates in obese patients20. In addition, TE does not provide any anatomic images and thus does not allow precise ROI placement. TE also cannot be used in patients with ascites.20 Therefore, ARFI is likely to be a more useful modality in children with ARPKD across a wide range of body sizes, and provides the advantage of a “one-stop” evaluation of both anatomic US and stiffness measurements in the same examination. In the current study, we were able to successfully perform ARFI US elastography along with anatomic US imaging in children ranging in age from 2 months to 20 years. The elastography measurements add only 5–7 minutes to the anatomic US study, making them easy to integrate into routine clinical imaging examinations.
Current expert guidelines do not recommend routine primary endoscopic surveillance for varices in children with portal hypertension, due to lack of published evidence on the risk of bleeding and efficacy of primary prophylaxis such as non-selective beta blockers or endoscopic ligation.10,21 However, ARFI US elastography measures could provide additional data to stratify patients’ risk of varices, and could help to more appropriately select patients who may benefit from primary endoscopic surveillance and prophylaxis.10 Because the number of participants with varices in this study was small, we could not perform ROC analysis to explore an SWS cutoff to differentiate children at risk for varices.
In research settings, US ARFI elastography of the liver could potentially serve as a surrogate endpoint for clinical trials of disease-modifying therapies in ARPKD. The somatostatin analogs octreotide and pasireotide22 and the multi-kinase inhibitor tesevatinib23 have been shown to ameliorate ARPKD-related liver disease in rodent models, and a Phase 1 clinical trial is now underway for tesevatinib.24 If any of these agents progresses to efficacy trials, reliable biomarkers of ARPKD liver disease severity will be needed to monitor response to therapy. Because US ARFI elastography can detect increased liver stiffness even in ARPKD children without clinical signs of portal hypertension, it appears to be more useful than clinical measures such as spleen size and platelet count to monitor ARPKD liver disease severity.
We acknowledge that larger multicenter studies will be needed to further validate the SWS cutoffs identified in the current study, and to identify SWS cut-offs that can predict more severe complications such as varices. Another strength of our study was the recruitment of a reference population of healthy control children. The SWS values for liver and spleen obtained in our healthy controls were similar to those obtained in the largest published study of ARFI normative values in children,15 which supports the external validity of our findings.
A major limitation in any study attempting to investigate a new non-invasive biomarker is the lack of a clinical “gold standard” by which to judge the accuracy of the imaging measure. We therefore had to rely on clinical measures of portal hypertension, namely splenomegaly and low platelets, to gauge the reliability of the ARFI elastography measures. However, spleen size and platelet counts are themselves imperfect measures of portal hypertension. As noted previously, esophageal varices have been reported even in patients with normal spleen size.3 In addition, platelet counts can vary in the context of viral or other illnesses. “Hard” clinical endpoints for portal hypertension, such as esophageal variceal bleeding, are potentially more reliable; however, as observed in the current study, such severe complications are thankfully relatively infrequent in a pediatric population. Another limitation of our study is its cross-sectional nature. Although we found that liver and spleen SWS correlated with clinical signs of portal hypertension across ARPKD participants, we cannot yet determine whether an individual’s liver and spleen SWS will increase over time with progression of liver fibrosis and portal hypertension. Our ongoing longitudinal study of ARPKD participants will allow us to address this question in the future.
In summary, this study supports the use of US ARFI elastography of the liver and spleen, an in particular elastography of the left liver lobe, to measure the severity of liver fibrosis and portal hypertension in children with ARPKD.
Acknowledgements
We thank Norma Latham, Marci Hutchinson, Trudi Morgan, Nina Laney, Daniel Elchediak, and Mohini Dutt for their invaluable assistance with data collection and management. We thank Kathleen Loomes, MD for her advice and critical review of the manuscript, and Rachel Rogers, MS, for biostatistical advice. We also thank the network of primary care clinicians, their patients, and families for their contribution to this project and clinical research facilitated through the Pediatric Research Consortium (PeRC) at CHOP. Study data were collected and managed using REDCap (Research Electronic Data Capture)25 hosted at CHOP, a secure web-based application designed to support data capture for research studies, providing a validated data entry interface; audit trails for tracking data manipulation and export procedures; automated export procedures to common statistical packages; and procedures for importing data from external sources.
Funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH) (5-K23-DK109203 to E.H.]) and by the National Center for Advancing Translational Sciences (NCATS), NIH, (5-KL2-TR-000139 [PI: Fitzgerald]). The Clinical and Translational Research Center at the CHOP is supported by the National Center for Research Resources and NCATS, NIH (UL1RR024134 and UL1TR000003). The funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication. E.H. served as a consultant for and receives research funding from Kadmon Corporation, LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
Portions of this study were presented as an oral presentation at the Pediatric Radiology / International Pediatric Radiology Conjoint Meeting, May 15–20, 2016 Chicago, Illinois, and as a poster at the American Society of Nephrology Kidney Week, November 15–20, 2016, Chicago, Illinois.
References
- 1.Hartung EA, Guay-Woodford LM. Autosomal Recessive Polycystic Kidney Disease: A Hepatorenal Fibrocystic Disorder With Pleiotropic Effects. Pediatrics. 2014. August 11;134:e833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy S, Dillon MJ, Trompeter RS, Barratt TM. Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatr Nephrol. 1997. June;11:302–6. [DOI] [PubMed] [Google Scholar]
- 3.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman Gerstein M, Piwnica-Worms K, Choyke P, et al. Characteristics of congenital hepatic fibrosis in a large cohort of patients with autosomal recessive polycystic kidney disease. Gastroenterology. 2013;144:112–121.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adeva M, El-Youssef M, Rossetti S, Kamath PS, Kubly V, Consugar MB, et al. Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine (Baltimore). 2006;85:1–21. [DOI] [PubMed] [Google Scholar]
- 5.Capisonda R, Phan V, Traubuci J, Daneman A, Balfe JW, Guay-Woodford LM. Autosomal recessive polycystic kidney disease: outcomes from a single-center experience. Pediatr Nephrol. 2003;18:119–26. [DOI] [PubMed] [Google Scholar]
- 6.Gagnadoux MF, Habib R, Levy M, Brunelle F, Broyer M. Cystic renal diseases in children. Adv Nephrol Necker Hosp. 1989;18:33–57. [PubMed] [Google Scholar]
- 7.Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics. 2003. May;111:1072–80. [DOI] [PubMed] [Google Scholar]
- 8.Guay-Woodford LM, Bissler JJ, Braun MC, Bockenhauer D, Cadnapaphornchai MA, Dell KM, et al. Consensus expert recommendations for the diagnosis and management of autosomal recessive polycystic kidney disease: report of an international conference. J Pediatr. 2014. September 8;165:611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berzigotti A, Piscaglia F, EFSUMB Education and Professional Standards Committee. Ultrasound in portal hypertension--part 2--and EFSUMB recommendations for the performance and reporting of ultrasound examinations in portal hypertension. Ultraschall Med. 2012. February 9;33:8–32; quiz 30–1. [DOI] [PubMed] [Google Scholar]
- 10.Shneider BL, Bosch J, de Franchis R, Emre SH, Groszmann RJ, Ling SC, et al. Portal Hypertension in Children: Expert Pediatric Opinion on the Report of the Baveno V Consensus Workshop on Methodology of Diagnosis and Therapy in Portal Hypertension. Pediatr Transplant. 2012. August;16:426–37. [DOI] [PubMed] [Google Scholar]
- 11.Shneider BL, de Ville de Goyet J, Leung DH, Srivastava A, Ling SC, Duché M, et al. Primary prophylaxis of variceal bleeding in children and the role of MesoRex Bypass: Summary of the Baveno VI Pediatric Satellite Symposium. Hepatology. 2016. April;63:1368–80. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012. February;19:e212–9. [DOI] [PubMed] [Google Scholar]
- 13.Nierhoff J, Chávez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2013. June 26;23:3040–53. [DOI] [PubMed] [Google Scholar]
- 14.Noruegas MJ, Matos H, Gonçalves I, Cipriano MA, Sanches C. Acoustic radiation force impulse-imaging in the assessment of liver fibrosis in children. Pediatr Radiol. 2012. February;42:201–4. [DOI] [PubMed] [Google Scholar]
- 15.Lee M-J, Kim M-J, Han KH, Yoon CS. Age-related changes in liver, kidney, and spleen stiffness in healthy children measured with acoustic radiation force impulse imaging. Eur J Radiol. 2013. June;82:e290–4. [DOI] [PubMed] [Google Scholar]
- 16.Megremis SD, Vlachonikolis IG, Tsilimigaki AM. Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology. 2004. April;231:129–34. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009. March;20:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toshima T, Shirabe K, Takeishi K, Motomura T, Mano Y, Uchiyama H, et al. New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver. J Gastroenterol. 2011. May 26;46:705–11. [DOI] [PubMed] [Google Scholar]
- 19.Kummer S, Sagir A, Pandey S, Feldkötter M, Habbig S, Körber F, et al. Liver fibrosis in recessive multicystic kidney diseases: transient elastography for early detection. Pediatr Nephrol. 2011. May 1;26:725–31. [DOI] [PubMed] [Google Scholar]
- 20.Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013. September;33:1138–47. [DOI] [PubMed] [Google Scholar]
- 21.Wehrman A, Kriegermeier A, Wen J. Diagnosis and Management of Hepatobiliary Complications in Autosomal Recessive Polycystic Kidney Disease. Front Pediatr. 2017. May 29;5:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masyuk TV, Radtke BN, Stroope AJ, Banales JM, Gradilone SA, Huang B, et al. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2013. July;58:409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweeney WE, Frost P, Avner ED. Tesevatinib ameliorates progression of polycystic kidney disease in rodent models of autosomal recessive polycystic kidney disease. World J Nephrol. 2017. July 6;6:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A Safety, Pharmacokinetic, Single Ascending Dose Study of Tesevatinib in Pediatric Subjects With Autosomal Recessive Polycystic Kidney Disease (ARPKD) [Internet]. ClinicalTrials.gov. [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. April;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]