Abstract
Objective
To assess the performance of a standardized age-based metric for scoring clinical actionability to evaluate conditions for inclusion in newborn screening (NBS), and compare it with the results from other contemporary methods.
Study design
The North Carolina Newborn Exome Sequencing for Universal Screening (NC NEXUS) study developed an age-based, semi-quantitative metric (ASQM) to assess the clinical actionability of gene-disease pairs and classify them with respect to age of onset or timing of interventions. This categorization was compared with the gold standard Recommended Uniform Screening Panel (RUSP) and other methods to evaluate gene-disease pairs for newborn genomic sequencing.
Results
We assessed 822 gene-disease pairs, enriched for pediatric onset of disease and suspected actionability. Of these, 466 were classified as having childhood onset and high actionability, analogous to conditions selected for the RUSP core panel. Another 245 were classified as having childhood onset and low to no actionability, 25 were classified as having adult onset and high actionability, 19 were classified as having adult onset and low to no actionability, and 67 were excluded due to controversial evidence and/or prenatal onset.
Conclusions
This study describes a novel method to facilitate decisions about the potential use of genomic sequencing for newborn screening. These categories may assist parents and physicians in making informed decisions about the disclosure of results from voluntary genomic sequencing in children.
Keywords: Genetics, actionability, disclosure, decision-making, NSIGHT, NC NEXUS, NGS-NBS, ASQM
Newborn screening (NBS), the largest screening program in the United States, aims to identify children with medically actionable conditions and intervene prior to the onset of symptoms. Early diagnosis has prevented morbidity and mortality in thousands of children.(1) To encourage consistency between states, the Health Resources and Services Administration commissioned a task force to establish a Recommended Uniform Screening Panel (RUSP) of core conditions and additional “secondary” conditions to identify through screening.(2,3) A key criterion is the ability of state-run laboratories to implement testing based on the required technology.(4) Currently, no conditions are screened primarily through genetic sequencing (although it is used as a second-tier test, eg, cystic fibrosis).(5) However, similar to the introduction of tandem mass spectrometry that triggered an expansion in the number of conditions screened for,(6) advances in next-generation sequencing (NGS) have likewise driven an exploration of its use in NBS.(7–9)
Genomic sequencing is a powerful diagnostic tool for individuals suspected to have monogenic conditions (9–17) and some have proposed using it to screen healthy populations of adults.(18–22) The application of NGS to NBS would dramatically increase the number of conditions that could be identified presymptomatically.(23) Anticipating the need for data about the technical, clinical, social, and ethical issues associated with sequencing in newborns, the NIH has funded a consortium to investigate the possible applications.(24,25) The ability to detect virtually any genetic condition with a known molecular basis requires selection of conditions appropriate for analysis in a public health setting and that merit disclosure to the parents of asymptomatic newborns.(26–28) Despite the possible health benefits of genomic sequencing, there is also a potential for psychological and physical harm (e.g. risks associated with interventions, parental anxiety regarding positive or uncertain genomic findings, social stigma or loss of confidentiality).(1,7,18,24,26–36) Therefore, the careful selection of disorders for inclusion in next-generation sequencing newborn screening (NGS-NBS) is a public health issue (37) requiring consideration of factors such as the condition’s natural history, the availability of confirmatory testing, and an assessment of the clinical actionability of the condition.
Our research group has previously described a semi-quantitative metric (SQM) for evaluating clinical actionability assessing five key criteria: the severity and likelihood of manifesting a particular condition, the efficacy and acceptability of intervention, and the overall knowledge base of the gene-disease association.(38) To address the challenges of implementing genomic sequencing in newborn screening, the North Carolina Newborn Exome Sequencing for Universal Screening (NC NEXUS) study optimized the SQM to classify gene-disease pairs into categories by including age-based factors. This age-based semi-quantitative metric (ASQM) allows a priori categorization of the large amount of information potentially generated by genomic sequencing to facilitate decision-making about incorporating genomic sequencing into the care of newborns.
METHODS
A multidisciplinary committee, comprised of members with demonstrated expertise based on credentials, publication records, and professional experience in pediatrics, newborn screening, genetic/genomic analysis, health behavior and communication, biomedical ethics, and medical genetics, was established to review and classify gene-disease pairs. Ten committee members had participated in the development and application of the previously described semi-quantitative metric.(38) Additional individuals with domain-specific clinical practice expertise were included for group discussions of specific disorders. The gene list included categories of conditions related to NBS (e.g. metabolic, hearing loss, immunodeficiency), as well other genes previously curated by our group. Trained staff biocurators reviewed the primary literature and online genetic resources (e.g. GeneReviews, ClinVar, OMIM) to curate the natural history, treatments, interventions, and evidence linking the gene to the disease into a REDCap database. A primary reviewer (committee member or biocurator) presented each gene and disease and suggested preliminary ASQM scores for each of the criteria, which the committee then discussed for consensus scoring and classification. Cases for which consensus was not immediately reached were further researched and discussed until a consensus decision was identified.
Scoring criteria for medical actionability
The previously published method for assessing clinical actionability(38) was optimized for newborn screening by adjusting the scoring rubric and incorporating age of onset and age at which interventions would begin.
Defining the Intervention
Healthy infants suspected of being at risk for a genetic condition may undergo pre-symptomatic evaluation and/or a cascade of interventions for a spectrum of possible health and developmental concerns. To address this variability in symptoms and interventions, the committee considered genetic conditions and interventions broadly rather than pairing specific health outcomes with direct interventions as described in the SQM.
Scoring Criteria
Each gene-disease pair was scored (0–3 points) on five criteria: severity of the condition’s outcome of interest, likelihood of manifesting the outcome of interest (penetrance), efficacy of the intervention(s) for that outcome, acceptability or burden of the intervention(s), and knowledge base of the gene-disease association (Table I). Scorers assumed that a newborn screened positive for the condition and considered the range of healthcare interventions thereafter (see the Appendix [available at www.jpeds.com] for more details).
Table 1.
Criteria for Age-based Semi-Quantitative Metric for Scoring Actionability
| Category | Description | Score | Examples / details |
|---|---|---|---|
|
SEVERITY “What is the effect on morbidity or mortality to an individual carrying a pathogenic variant in this gene?” |
Sudden Death or Unavoidable Death in Childhood (<10yo) | 3 | Sudden death as a result of cardiac arrhythmia or aortic dissection; death before 10 years of age; other fatal infantile neurodegenerative conditions |
| Possible Death due to disease or severe intellectual impairment | 2 | Cancer or multiple organ failure with potential for mortality; moderate to severe intellectual disability; loss of multiple senses | |
| Serious Morbidity or moderate intellectual impairment | 1 | Intellectual disability, growth disorders, non-fatal organ dysfunction | |
| Modest or No Morbidity | 0 | Benign biochemical phenotypes, later-onset neurosensory deficits | |
|
LIKELIHOOD “What is the chance that a threat will materialize?” (this is akin to penetrance, but may be scored for a single outcome of a condition) |
≥ 50% | 3 | Likelihood is related to the chance that the outcome of interest will manifest. If likelihood information is not readily available, scores may be estimated through pedigree analysis and available segregation data. A score of 0 is assigned if there is little data available and likelihood cannot be reasonably estimated. Recessive conditions are typically assumed to have high penetrance unless evidence exists to the contrary. |
| 5–49% | 2 | ||
| 1–5% | 1 | ||
| <1 % | 0 | ||
|
EFFICACY “How effective are interventions for preventing the harm?” |
Highly Effective | 3 | Completely prevents or substantially reduces morbidity in most patients |
| Modestly Effective | 2 | Works relatively well, but still has some residual symptoms or potential for death | |
| Minimally Effective | 1 | Alleviates some symptoms, but not enough to effectively reduce or prevent morbidity. Also, may be able to treat some parts of the condition but not others. | |
| No Effective Intervention | 0 | Nothing can be done to prevent the morbidity in presymptomatic patients, typically assigned if interventions only relate to symptomatic management. | |
|
ACCEPTABILITY “How acceptable are the interventions in terms of the burdens or risks placed on the individual?” |
Highly Acceptable | 3 | Little or no impact or burden. Yearly blood tests, noninvasive imaging screening tests, oral medications with low side effect profile |
| Modestly Acceptable | 2 | Modest impact or burden. Invasive screening tests, daily lifestyle/diet modification, medications with substantial side effect profile | |
| Minimally Acceptable | 1 | Significant impact or burden. Removal of a non-vital organ or transplantation with frequent complications | |
| No Effective Intervention | 0 | Extreme impact or burden. Removal of a vital organ. Also assigned when no effective intervention is available | |
|
KNOWLEDGE “What is the evidence base for decisions about the natural history of the disease, and interventions used for preventing serious outcomes?” |
Substantial Evidence and/or Practice Guidelines | 3 | Sufficient evidence to confidently score all categories for the specific gene-disease pair, based on high quality information from peer-reviewed journals, consensus statements, professional society practice guidelines. |
| Modest Evidence | 2 | Sufficient evidence to score all categories, potentially based on inferences from a closely related condition or the molecular/biochemical mechanism of the disease, based on good quality information with minor limitations. | |
| Minimal Evidence | 1 | Unable to score one or more categories, due to limited information about the specific condition or closely related genes or phenotypes, with sparse primary literature. | |
| Controversial or Poor Evidence | 0 | Unable to reasonably score most or all categories due to controversial or minimal information about the specific condition, lack of primary literature, or conflicting information. | |
| TOTAL | 0–15 |
Categorical Framework
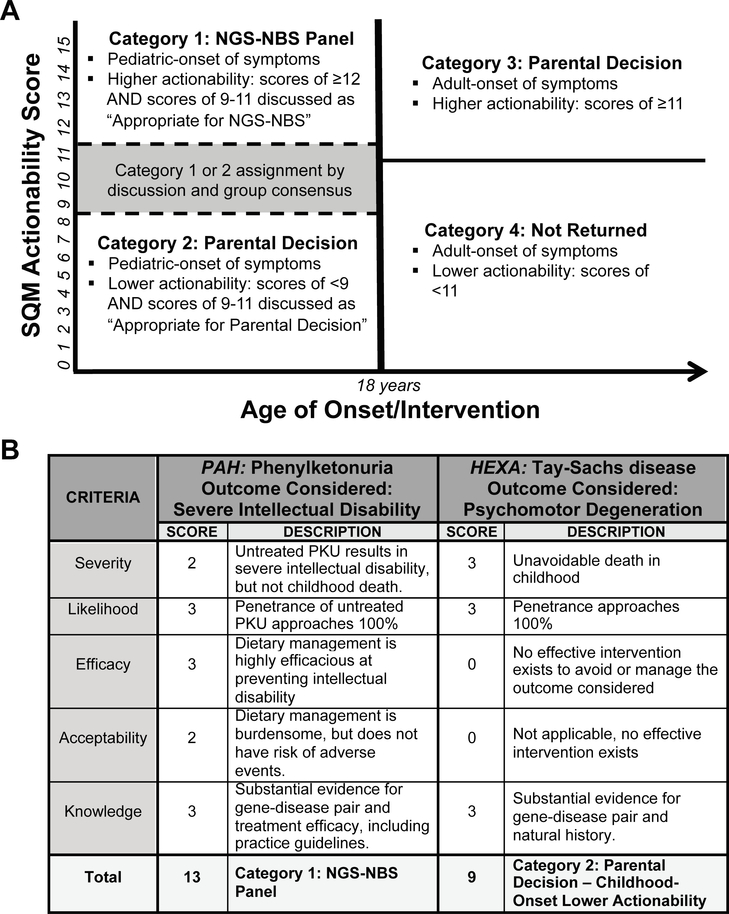
The typical age at onset (when symptoms would most likely begin) and the typical age at intervention (when medical intervention, including screening evaluation, if any, would begin) were assessed for each gene-disease pair. Gene-disease pairs were considered as having pediatric onset and/or pediatric actionability, if either would likely occur prior to age 18. Based on the final ASQM score, the age of onset/actionability, and consensus review by the committee, gene-disease pairs were placed into one of four categories: Category 1: pediatric conditions with high actionability; Category 2: pediatric conditions with low or no actionability; Category 3: adult conditions with high actionability; and Category 4: adult conditions with low or no actionability (Figure 1, A). In the NC NEXUS study, genes in category 1 are analyzed and pathogenic and likely pathogenic variants are disclosed to all participants. To investigate parental decision-making about additional genomic information beyond category 1, parents are randomized to either a control group or a group that decides whether to initiate analysis of genes in category 2, category 3, or carrier status for recessive disorders. Gene-disease pairs in category 4 are neither analyzed nor disclosed to any participants.
Figure 1. Implementation of the Age-based Semi-Quantitative Metric (ASQM).
A.) The four NC NEXUS categories, separated on dimensions of actionability and age of onset/intervention. ASQM scores were assigned by a multi-disciplinary review committee. The gray box represents the gene-disease pairs with scores of 9–11, for which the ASQM score alone was not sufficient to make a classification of Category 1 or Category 2. Separation of categories 1 and 2 from categories 3 and 4 was made at age of onset/intervention of 18 because of the ethical principle of preserving future autonomy for children undergoing genetic testing,(32) but we also collected more granular data regarding ages of onset and intervention in anticipation that these might be useful for defining age-targeted screening panels (Table 2). B.) Examples of scores for conditions in Category 1 and Category 2. Scores for each criterion and the underlying rationale are provided. Left, PKU is a classic example of a high-scoring condition that is found on the RUSP and was placed in Category 1, pediatric conditions with high actionability. Right, Tay-Sachs is a severe childhood condition with no efficacious intervention and therefore a low-scoring condition placed in Category 2, pediatric conditions with low or no actionability.
Validation of the ASQM
In order to evaluate the ability of the NC NEXUS categories to reliably represent the pediatric actionability of monogenic conditions, we compared ASQM scores for primary and secondary RUSP conditions(39) and for published lists of conditions curated the BabySeq project.(40) We obtained supplementary files for BabySeq lists and manually matched gene-disease association pairs to ensure compatibility between studies during analysis. Mann-Whitney and Kruskal-Wallis tests were performed to compare ASQM scores between RUSP lists and BabySeq categories respectively.
RESULTS
Categorization of gene-disease pairs using the ASQM
A total of 822 gene-disease pairs were curated and 755 gene-disease pairs were categorized (Figure 1, A and Table 2 [available at www.jpeds.com]). Our previous analysis showed that the top quintile of randomly selected genes scored 11 or higher.(38) In the current analysis, gene-disease pairs with more conservative ASQM scores ≥12 (N=269) were automatically placed in Category 1, and those with ASQM scores <9 (N=135) were automatically placed in Category 2. Representative examples of scoring for phenylketonuria (MIM #261600, PAH) and Tay-Sachs disease (MIM #272800, HEXA) are shown in Figure 1, B.(41–43) The most common reasons that Category 2 genes did not meet our criteria for disclosure to parents of healthy newborns included the lack of an effective intervention and/or insufficient knowledge about the gene-disease relationship. As more evidence accumulates and/or interventions become available, these genes and their categorizations can be reassessed and modified.
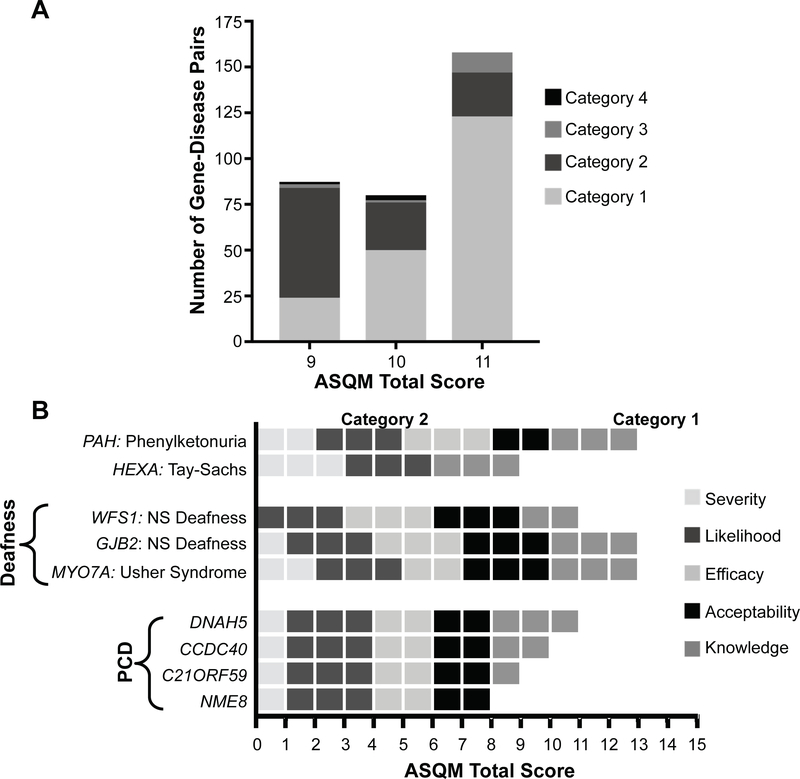
Gene-disease pairs with ASQM scores within the range of 9, 10, or 11 (N=331) posed a challenge because some were considered to have potential actionability. Therefore, the committee held consensus discussions about these conditions, resulting in 197 being assigned to Category 1 for a total of 466, and 110 being assigned to Category 2 for a total of 245. (Figure 2, A). For example, patients with mucopolysaccharidosis type IVa (MPS4A/Morquio type A, MIM #253000, GALNS), characterized by skeletal dysplasia and respiratory failure, have improved with enzyme replacement therapy.(44) Although no substantial evidence supports presymptomatic administration, based on published evaluation the committee decided that its early use was potentially beneficial and that a reasonable parent would value disclosure. Therefore, although the total score for MPS4A was only a 9, by consensus it was placed it into Category 1.
Figure 2. Nuances of categorical designation and scoring rubric.
A.) Consensus categorization of gene-disease pairs scoring 9–11. After scoring conditions, the review committee identified that there was no single threshold that was adequate to delineate actionability among those gene-conditions having scores of 9, 10, or 11. For this reason, each gene-condition with childhood age of onset/intervention and score in this range was further discussed and a consensus decision among the committee was made regarding placement in category 1 or 2. As would be predicted, the majority of those with a score of 11 were assigned to Category 1 and the majority of those with a score of 9 were assigned to Category 2, illustrating that scores in this range reflect a grey zone that may not capture the entirety of the decision-making process. B.) Comparison of ASQM scores based on condition-specific rubrics. Each horizontal bar represents the total score for a gene-disease pair broken down into its scores for each ASQM criterion. This process can also be illustrated by the example of Primary Ciliary Dyskinesia (PCD) for which 35 genes have been published as causative. Specialty-area experts for clinical and molecular knowledge of PCD were consulted to develop our approach for scoring and categorization, and determined that presymptomatic intervention would be somewhat beneficial despite the paucity of evidence in the scientific literature. Although there is substantial locus heterogeneity for PCD, we considered the condition as a whole to have generally equivalent efficacy and acceptability of medical intervention regardless of the genetic cause, based on the highly similar clinical presentations. Therefore, scores varied most significantly in terms of the knowledge base establishing a particular gene as causative (Table 2). Genes associated with PCD for which the knowledge score was at least 1 received a total ASQM score ≥9. The decision was made for all of these gene-disease pairs to be placed in Category 1, acknowledging the potential for benefit through presymptomatic intervention.
A total of 25 gene-disease pairs were placed in Category 3; adult-onset conditions with high actionability (recommended interventions starting in adulthood), such as Lynch syndrome (MIM #609310, MLH1). There were 19 gene-disease pairs assigned to Category 4; adult-onset conditions with low or no actionability. An additional 67 gene-disease pairs, including 24 with scores of 9 or 10, were not categorized due to controversial evidence and/or the association with congenital manifestations making the condition inappropriate for newborn screening.
For conditions with substantial locus heterogeneity, such as deafness and primary ciliary dyskinesia (PCD), a general rubric was established to maintain consistency between scores (Figure 2, B). For hearing loss, the scoring for the efficacy and acceptability of early audiology surveillance was held constant, whereas the severity score varied between 2, 1, and 0 depending on age of onset (congenital, pre- or post-lingual respectively), or the presence of syndromic features. For PCD, scores for the other four criteria were generally held constant, but the knowledge score varied between the well-known sub-types and those with limited evidence.
Comparison to other published efforts
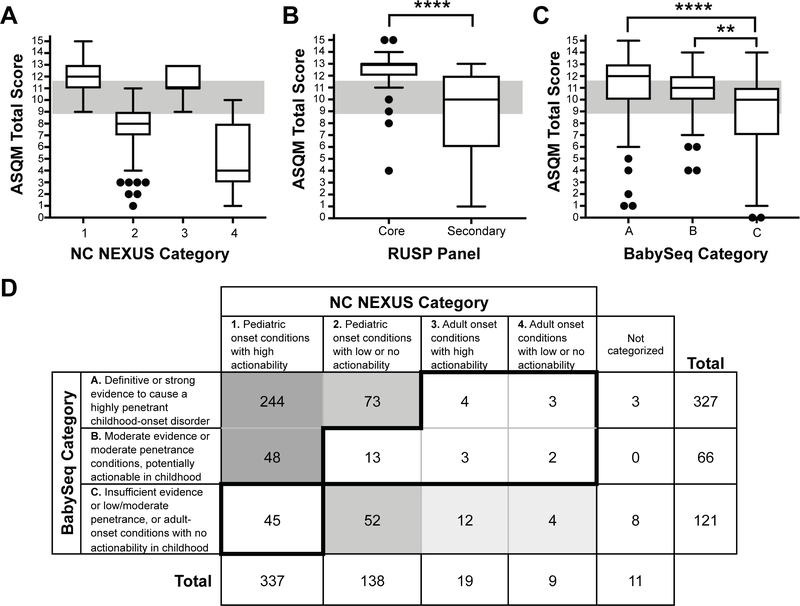
We analyzed the overall distribution of ASQM scores for genes included in each of the four NC NEXUS categories (Figure 3, A) and for genes responsible for the RUSP primary and secondary conditions (Figure 3, B). Of the 34 genes representing RUSP primary conditions, 29 (85%) had scores ≥ 12, demonstrating that ASQM scores generally correlated well with conditions already established as having high actionability. Four gene-disease pairs had scores in the lower range (4–10); adrenoleukodystrophy (MIM #300100, ABCD1), primary systemic carnitine deficiency (MIM #212140, SLC22A5) and 3-methylcrotonylglycinuria (MIM #210200, MCCC1; and MIM #210210, MCCC2) suggesting that actionability of conditions currently included in the RUSP varies. Secondary conditions identified by tandem mass spectrometry are generally considered less actionable than primary conditions. The ASQM scores of the primary conditions reflected this difference, as they were significantly higher than the scores for secondary conditions (p < 0.0001). Of the 35 gene-disease pairs associated with secondary RUSP conditions, 23 (66%) scored an 11 or lower (Figure 3, B).
Figure 3. Comparison of ASQM scores across different lists of conditions.
Box and whisker plots summarizing ASQM scores for A.) genes curated into NC NEXUS Categories 1–4, B.) 34 genes related to 25 primary RUSP conditions (32 were placed in Category 1 and 2 were assigned to Category 2) and 35 genes related to 22 secondary RUSP conditions (18 pairs were assigned to Category 1, 15 pairs were assigned to Category 2, and 2 pairs (ACAD8: isobutyryl-CoA dehydrogenase and AUH: 3-methylglutaconic aciduria, type I) were not assigned to any category and are only on the diagnostic list, and C.) gene-disease associations curated by the BabySeq Project into Categories A, B, and C. D.) Comparing gene-disease pairs categorized by both NC NEXUS and BabySeq, 244 gene-disease pairs were placed in both NC NEXUS category 1 and BabySeq category A, suggesting that this group may consist of conditions with definitive or strong clinical validity, high penetrance, and high actionability. Similarly, 48 gene-disease pairs were placed in NC NEXUS category 1 and BabySeq category B, indicating that they may have slightly lower knowledge base and/or reduced penetrance, but have strong consensus to be actionable in childhood. Together, these 292 conditions (indicated by dark shading) might represent a consensus list of gene-disease pairs for inclusion in NGS-NBS. In contrast, 73 gene-disease pairs were placed in NC NEXUS category 2 and BabySeq category A, suggesting that these disorders may be strongly associated with highly penetrant childhood diseases without substantial actionability. Similarly, 52 gene-disease pairs were placed in NC NEXUS category 2 and BabySeq category C, indicating that they are likely associated with lower strength of evidence, lower penetrance, and/or lower overall actionability. Together, these 125 conditions (indicated by medium shading) might comprise a group of gene-disease pairs that would not generally meet consensus criteria for newborn screening. The 16 gene-disease pairs that were placed in BabySeq category C and either NC NEXUS category 3 or category 4 (indicated by light shading) are consensus adult-onset conditions, with or without actionability. In contrast, 45 gene-disease pairs were categorized in NC NEXUS category 1 for return to all participants, but placed in BabySeq category C and not returned to any participants; 13 gene-disease pairs were placed in BabySeq category B (potentially actionable), but in NC NEXUS category 2 (low or no actionability); and 12 gene-disease pairs were placed in BabySeq categories A and B, but were assigned to NC NEXUS categories 3 and 4 due to adult onset, with or without actionability. Additionally, 11 gene-disease pairs were evaluated but were not assigned a NC NEXUS category whereas 3 of these gene-disease pairs were placed in BabySeq Category A and 8 were placed in Category C (Table 2).
We applied the ASQM to a selection of genes-disease pairs evaluated by the BabySeq project.(40) Their framework to categorize gene-disease pairs relied on three criteria: validity of the gene-disease association, earliest reported age of onset, and penetrance. Conditions were classified into Category A (“genes … with definitive or strong evidence to cause a highly penetrant childhood-onset disorder”), Category B (“genes included … based on actionability during childhood”), or Category C (“genes that did not meet criteria to be returned”). These categories are described in more detail in the Appendix. To assess the concordance between the two methods, 514 of the 1514 gene-disease pairs evaluated for the BabySeq project were assessed using the ASQM (Figure 3, C). In pairwise comparisons, there was no significant difference between the ASQM scores in Category A as compared with those in Category B (p=0.66). However, there were significant differences between scores for those in Category A and those in Category C (P < .0001) and between those in Category B and those in Category C (p<0.0016). The BabySeq criteria did not map directly to the ASQM method described here, so we examined the distribution of gene-disease pairs between the two different curation systems (Figure 3, D). We identified 292 conditions that could represent a consensus list for inclusion in NGS-NBS and an additional 125 conditions that could be considered for optional disclosure. The main outliers were 45 conditions placed in NC NEXUS Category 1 for disclosure to all participants but placed in Category C in BabySeq and thus ineligible for disclosure (see the Appendix for details).
DISCUSSION
Commentators have suggested that current newborn screening should be augmented or even replaced with genomic sequencing.(45–47) However, the successful incorporation of NGS technology into routine newborn care will require that the scientific and medical communities address significant challenges, including the development of a robust, reproducible and transparent method to adjudicate and classify the different types of genomic findings for potential disclosure in a pediatric setting. Here, we propose an evidence-based method to guide informed decision-making by policy-makers, physicians, and parents.
We validated our framework against the gold-standard RUSP, and the high ASQM scores assigned to most RUSP conditions indicate that this metric recapitulates the components of actionability that led to their inclusion for standard NBS. Interestingly, the RUSP does include a small number of conditions, such as 3-MCC deficiency, that received lower scores largely due to their variable expression, lack of efficacious interventions, and/or limited knowledge base due to their rare occurrence. Furthermore, the metric effectively differentiates between the highly actionable RUSP core conditions and less actionable secondary conditions, most of which were placed into NC NEXUS Category 2 with the exception of a small number, including 3-methylglutaconic aciduria type II (Barth syndrome; MIM #302060, TAZ) and DOPA-responsive dystonia (MIM #128230, GCH1), that scored higher and were included in Category 1. Overall, we suggest that the ASQM could serve as a reproducible method for scaling the assessment of conditions for possible inclusion in screening, and that conditions with scores in the same range as the RUSP could be considered as strong candidates for an NGS-NBS panel.
The relatively few adult-onset highly actionable conditions included in Category 3 may be surprising, given that the American College of Medical Genetics and Genomics recommended 59 gene/disease pairs be disclosed as secondary findings from clinical genomic sequencing(48). However, we found that 50 of the 59 have a pediatric onset of symptoms and/or interventions, thereby meriting their placement into Category 1.
Comparison of between the NC NEXUS framework and the BabySeq method of categorization for sequencing healthy newborns revealed differences due to the criteria used to define those categories. The ASQM integrates several components together to achieve a consistent score representing actionability, whereas the BabySeq criteria differ between each of the three categories. BabySeq category A focuses on pediatric onset conditions with genes of high penetrance and strong evidence for the gene-disease relationship but did not include consideration of actionability; therefore, the widely dispersed ASQM scores reflect that many conditions, although well-understood, have little to no actionability. BabySeq category B contains a mixture of gene-disease pairs with moderate strength of evidence and/or moderate penetrance, and potential actionability that scored primarily within the boundary between NC NEXUS category 1 and category 2 (9–11 points) based on the lower ASQM scores for both likelihood and knowledge base. BabySeq category C conditions are much harder to parse because they include insufficient evidence, low/moderate penetrance, or adult-onset conditions without noninvasive interventions in childhood. ASQM scores for this heterogeneous category ranged widely and the gene-disease pairs were broadly distributed into all four of the NC NEXUS categories.
Though NC NEXUS Category 1 (466 gene-disease pairs) is relatively large, one explanation for its size is that the intervention scored for the efficacy and acceptability criteria was often based upon a presumed NGS-NBS program of early referral to specialists, surveillance and early intervention, for which there is plausible, but not yet proven, evidence of net benefit. Another potential explanation is that the extensive locus heterogeneity of some phenotypic subgroups (e.g. hearing loss and PCD) resulted in the inclusion of gene-disease pairs that were scored by analogy to other more well-known examples within the subgroup. In order to fully substantiate this list for targeted NGS-NBS, we recommend restricting this category to include only gene-disease pairs that meet the criteria of “Definitive/Strong” clinical validity evidence based on the ClinGen framework(49) (approximated by the intersection between BabySeq Category A and NC NEXUS Category 1).
Large prospective studies of healthy individuals are needed to amass sufficient outcomes data to support the inclusion of these conditions on a NGS-NBS panel and to establish thresholds of variant pathogenicity for disclosure in a screening setting that will appropriately balance case finding and false positive rates. Although the criteria for RUSP conditions have traditionally been limited to neonatal or infantile onset, incorporating a broader range of conditions with symptoms that manifest during childhood would enable higher rates of early diagnosis and the elucidation of subclinical states that may represent key intervention points. It is important to acknowledge, however, that broadening the age range would also expose newborns and children (and their families) to potentially numerous screening evaluations and possible interventions that convey risks and may contribute to a state of health anxiety. One potential solution would be to implement targeted age-based genomic screening occurring over the lifespan, to deliver actionable genomic information at age-appropriate time points (e.g. infant, early childhood, adolescent) while reducing potential ethical and psychosocial concerns associated with other types of non-actionable genomic information.(50)
Implementation of universal NGS-NBS will require both a standardized method for determining which conditions should be screened for and the development of guidelines for incorporating additional genes over time. The ASQM method of categorization provides a robust framework to identify conditions that may be appropriate and a starting point for policy decisions, additional criteria such as disease prevalence and economic considerations would still need to be weighed. Pediatricians will need to engage in this decision-making process by first understanding the potential actionability of different genetic conditions and then considering the impact of screening for these conditions on routine care of infants and children. Further research into the clinical outcomes and psychosocial impacts experienced by families, elucidation of disease penetrance and efficacy of interventions, and cost effectiveness will be required before widespread population screening can be responsibly initiated.
Supplementary Material
Acknowledgments
We thank a number of colleagues who have contributed to the development or implementation of the ASQM over the course of the NC NEXUS project including Don Bailey, Jessica Booker, Ali S. Calikoglu, Natario Couser, Brian Jensen, Michael R. Knowles, Joseph Muenzer, Arti Pandya, Christie M. Turcott, andMaimoona Zariwala.
Funded by NICHD U19 HD077632. J.B. is a recipient of the UNC Yang Family Biomedical Scholars Award. The authors declare no conflicts of interest.
List of Abbreviations
- (NBS)
Newborn Screening
- (NC NEXUS)
The North Carolina Newborn Exome Sequencing for Universal Screening
- (ASQM)
Age-based, Semi-Quantitative Metric
- (RUSP)
Recommended Uniform Screening Panel
- (NGS)
Next-Generation Sequencing
- (NGS-NBS)
Next-Generation Sequencing for Newborn Screening
- (PCD)
primary ciliary dyskinesia
- (MPS4A)
Mucopolysaccharidosis type IVa
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Newborn screening: A blueprint for the future executive summary: newborn screening task force report. Pediatrics. 2000;106:386–388. [PubMed] [Google Scholar]
- 2.Newborn screening: toward a uniform screening panel and system. Genet Med. 2006;8:1S–252S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Medical Genetics Newborn Screening Expert Group. Newborn screening: toward a uniform screening panel and system-executive summary. Pediatrics. 2006;117:S296–307. [DOI] [PubMed] [Google Scholar]
- 4.Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003;348:2304–2312. [DOI] [PubMed] [Google Scholar]
- 5.Gregg RG, Wilfond BS, Farrell PM, Laxova A, Hassemer D, Mischler EH. Application of DNA analysis in a population-screening program for neonatal diagnosis of cystic fibrosis (CF): comparison of screening protocols. Am J Hum Genet. 1993;52:616–626. [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaldo P, Tortorelli S, Matern D. Recent developments and new applications of tandem mass spectrometry in newborn screening. Curr Opin Pediatr. 2004;16:427–433. [DOI] [PubMed] [Google Scholar]
- 7.Bodian DL, Klein E, Iyer RK, Wong WSW, Kothiyal P, Stauffer D, et al. Utility of whole-genome sequencing for detection of newborn screening disorders in a population cohort of 1,696 neonates. Genet Med. 2016;18:221–230. [DOI] [PubMed] [Google Scholar]
- 8.Landau YE, Lichter-Konecki U, Levy HL. Genomics in newborn screening. J Pediatr. 2014;164:14–19. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharjee A, Sokolsky T, Wyman SK, Reese MG, Puffenberger E, Strauss K, et al. Development of DNA confirmatory and high-risk diagnostic testing for newborns using targeted next-generation DNA sequencing. Genet Med. 2015;17:337–347. [DOI] [PubMed] [Google Scholar]
- 10.Biesecker LG, Green RC. Diagnostic clinical genome and exome sequencing. N Engl J Med. 2014;370:2418–2425. [DOI] [PubMed] [Google Scholar]
- 11.Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015;3:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18:1090–1096. [DOI] [PubMed] [Google Scholar]
- 13.Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4:154ra135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller NA, Farrow EG, Gibson M, Willig LK, Twist G, Yoo B, et al. A 26-hour system of highly sensitive whole genome sequencing for emergency management of genetic diseases. Genome Med. 2015;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Z, Greenwood R, Felix ACG, Shiloh-Malawsky Y, Tennison M, Roche M, et al. GCH1 heterozygous mutation identified by whole-exome sequencing as a treatable condition in a patient presenting with progressive spastic paraplegia. J Neurol. 2014;261:622–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strande NT, Berg JS. Defining the Clinical Value of a Genomic Diagnosis in the Era of Next-Generation Sequencing. Annu Rev Genomics Hum Genet. 2016;17:303–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong JX, Buckingham KJ, Jhangiani SN, Boehm C, Sobreira N, Smith JD, et al. The genetic basis of mendelian phenotypes: discoveries, challenges, and opportunities. Am J Hum Genet. 2015;97:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans JP, Berg JS, Olshan AF, Magnuson T, Rimer BK. We screen newborns, don’t we?: realizing the promise of public health genomics. Genet Med. 2013;15:332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prince AER, Cadigan RJ, Henderson GE, Evans JP, Adams M, Coker-Schwimmer E, et al. Is there evidence that we should screen the general population for Lynch syndrome with genetic testing? A systematic review. Pharmgenomics Pers Med. 2017;10:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams MC, Evans JP, Henderson GE, Berg JS. The promise and peril of genomic screening in the general population. Genet Med. 2016;18:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury MJ, McCabe LL, McCabe ERB. Population screening in the age of genomic medicine. N Engl J Med. 2003;348:50–58. [DOI] [PubMed] [Google Scholar]
- 22.Linderman MD, Nielsen DE, Green RC. Personal genome sequencing in ostensibly healthy individuals and the peopleseq consortium. J Pers Med. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg JS, Powell CM. Potential Uses and Inherent Challenges of Using Genome-Scale Sequencing to Augment Current Newborn Screening. Cold Spring Harb Perspect Med. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg JS, Agrawal PB, Bailey DB, Beggs AH, Brenner SE, Brower AM, et al. Newborn sequencing in genomic medicine and public health. Pediatrics. 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milko LV, Rini C, Lewis MA, Butterfield RA, Lin F-C, Paquin RS, et al. Evaluating parents’ decisions about next generation sequencing for their child in the NC NEXUS (North Carolina Newborn Exome Sequencing for Universal Screening) study: a randomized controlled trial protocol. Trials. 2018;19:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andermann A, Blancquaert I, Beauchamp S, Dery V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ. 2008;86:317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borry P, Evers-Kiebooms G, Cornel MC, Clarke A, Dierickx K, Public and Professional Policy Committee (PPPC) of the European Society of Human Genetics (ESHG). Genetic testing in asymptomatic minors: background considerations towards ESHG Recommendations. Eur J Hum Genet. 2009;17:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullough LB, Brothers KB, Chung WK, Joffe S, Koenig BA, Wilfond B, et al. Professionally responsible disclosure of genomic sequencing results in pediatric practice. Pediatrics. 2015;136:e974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borry P, Sénécal K, Knoppers BM. Do It Yourself Newborn Screening. JAMA Pediatr. 2016;170:523–524. [DOI] [PubMed] [Google Scholar]
- 30.Roche MI, Berg JS. Incidental Findings with Genomic Testing: Implications for Genetic Counseling Practice. Curr Genet Med Rep. 2015;3:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Botkin JR, Belmont JW, Berg JS, Berkman BE, Bombard Y, Holm IA, et al. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet. 2015;97:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross LF, Saal HM, David KL, Anderson RR, American Academy of Pediatrics, American College of Medical Genetics and Genomics. Technical report: Ethical and policy issues in genetic testing and screening of children. Genet Med. 2013;15:234–245. [DOI] [PubMed] [Google Scholar]
- 33.Jansen ME, Metternick-Jones SC, Lister KJ. International differences in the evaluation of conditions for newborn bloodspot screening: a review of scientific literature and policy documents. Eur J Hum Genet. 2016;25:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman JM, Cornel MC, Goldenberg AJ, Lister KJ, Senecal K, Vears DF, et al. Genomic newborn screening: public health policy considerations and recommendations. BMC Med Genomics. 2017;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clayton EW, McCullough LB, Biesecker LG, Joffe S, Ross LF, Wolf SM, et al. Addressing the ethical challenges in genetic testing and sequencing of children. Am J Bioeth. 2014;14:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis MA, Paquin RS, Roche MI, Furberg RD, Rini C, Berg JS, et al. Supporting parental decisions about genomic sequencing for newborn screening: the NC NEXUS decision aid. Pediatrics. 2016;137:S16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansen ME, Lister KJ, van Kranen HJ, Cornel MC. Policy making in newborn screening needs a structured and transparent approach. Front Public Health. 2017;5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg JS, Foreman AKM, O’Daniel JM, Booker JK, Boshe L, Carey T, et al. A semiquantitative metric for evaluating clinical actionability of incidental or secondary findings from genome-scale sequencing. Genet Med. 2016;18:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kemper AR, Green NS, Calonge N, Lam WKK, Comeau AM, Goldenberg AJ, et al. Decision-making process for conditions nominated to the recommended uniform screening panel: statement of the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Genet Med. 2014;16:183–187. [DOI] [PubMed] [Google Scholar]
- 40.Ceyhan-Birsoy O, Machini K, Lebo MS, Yu TW, Agrawal PB, Parad RB, et al. A curated gene list for reporting results of newborn genomic sequencing. Genet Med. 2017;19:809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jancar J Increased life expectancy in people with untreated phenylketonuria. J Intellect Disabil Res. 1998;42:97–99. [DOI] [PubMed] [Google Scholar]
- 42.Eijgelshoven I, Demirdas S, Smith TA, van Loon JMT, Latour S, Bosch AM. The time consuming nature of phenylketonuria: a cross-sectional study investigating time burden and costs of phenylketonuria in the Netherlands. Mol Genet Metab. 2013;109:237–242. [DOI] [PubMed] [Google Scholar]
- 43.Kaback MM, Desnick RJ. Hexosaminidase A Deficiency In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, et al. , editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 1993. [Google Scholar]
- 44.Elosulfase alfa for treating mucopolysaccharidosis type IVa | Guidance and guidelines | NICE [Internet]. [cited 2017 Oct 26]. Available from: https://www.nice.org.uk/guidance/hst2
- 45.Boemer F, Fasquelle C, d Otreppe S, Josse C, Dideberg V, Segers K, et al. A next-generation newborn screening pilot study: NGS on dried blood spots detects causal mutations in patients with inherited metabolic diseases. Sci Rep. 2017;7:17641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almannai M, Marom R, Sutton VR. Newborn screening: a review of history, recent advancements, and future perspectives in the era of next generation sequencing. Curr Opin Pediatr. 2016;28:694–699. [DOI] [PubMed] [Google Scholar]
- 47.Francescatto L, Katsanis N. Newborn screening and the era of medical genomics. Semin Perinatal. 2015;39:617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med. 2017;19:249–255. [DOI] [PubMed] [Google Scholar]
- 49.Strande NT, Riggs ER, Buchanan AH, Ceyhan-Birsoy O, DiStefano M, Dwight SS, et al. Evaluating the Clinical Validity of Gene-Disease Associations: An Evidence-Based Framework Developed by the Clinical Genome Resource. Am J Hum Genet. 2017;100:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mollison L, Berg JS. Genetic screening: birthright or earned with age? Expert Rev Mol Diagn. 2017;17:735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.