Abstract
Background:
We systematically analyzed the synergistic effect of: i) cytokine-mediated inflammatory activation of endothelial cells (ECs), and ii) shear-mediated platelet activation (SMPA) as a potential contributory mechanism to intraventricular thrombus formation in the setting of Left Ventricular Assist Device (LVAD) support.
Methods:
Intact and shear-activated human platelets were exposed to nonactivated and cytokine-activated ECs. To modulate the level of LVAD-related shear activation, platelets were exposed to shear stress patterns of varying magnitude (30, 50, 70 dynes/cm2, 10min) via a Hemodynamic Shearing Device. ECs were activated via exposure to inflammatory Tumor Necrosis Factor-α (TNF-α, 10 and 100 ng/mL, 24h), consistent with inflammatory activation recorded in patients on LVAD circulatory support.
Results:
Adhesivity of shear-activated platelets to ECs was significantly higher than that of intact/unactivated platelets, regardless of the initial activation level (70 dynes/cm2 shear-activated platelets vs. intact platelets: +80%, p<0.001). Importantly, inflammatory activation of ECs amplified platelet prothrombinase activity progressively with increasing shear stress magnitude and TNF-α concentration: thrombin generation of 70 dynes/cm2 shear-activated platelets was 2.6-fold higher following exposure and adhesion to 100 ng/mL TNF-α-activated ECs (p<0.0001).
Conclusions:
We demonstrated synergistic effect of SMPA and cytokine-mediated EC inflammatory activation to enhance EC-platelet adhesion and platelet prothrombotic function. These mechanisms might potentially contribute to intraventricular thrombosis in the setting of mechanical circulatory support.
INTRODUCTION
Mechanical circulatory support devices, i.e., Left Ventricular Assist Devices (LVADs), are increasingly utilized for hemodynamic restoration in patients with advanced and end-stage heart failure.1 The latest generation of miniaturized, continuous-flow LVADs has afforded enhanced patient survival and quality of life compared to earlier pulsatile pneumatic systems, and is now approved for both bridge-to-transplant as well as for definitive destination therapy.1 Despite clinical efficacy of these devices, LVAD thrombosis, either in the form of pump thrombosis or as thromboembolic events, remains a common and vexing complication, severely affecting long-term outcomes.2
Clinical studies to date have identified a range of contributing factors that increase the likelihood of LVAD thrombosis.3–12 Nevertheless, the mechanisms underlying and driving thrombosis have only been partially elucidated, limiting the development of effective therapeutic strategies to prevent adverse clinical events.
Shear-mediated platelet activation (SMPA) is a major driver of LVAD thrombosis.13 SMPA is defined as the prothrombotic activation of platelets due to accumulation of supraphysiologic shear stress-mediated injury over time, as a result of platelet recirculation through the device.14
We have previously reported that SMPA may occur directly in free-flowing blood regions of the LVAD characterized by high levels of intermittent or sustained shear exposure and that platelets “register” and accumulate shear damage via repetitive exposure to shear stress.14–19 We have also reported that LVAD hemodynamic shear stress has a sensitizing effect, in that platelets exposed to very high shear stress – i.e. during passage through an LVAD - continue to activate despite subsequent exposure to low shear stress - as is encountered downstream of the LVAD - with a residual incremental response compounding that of the initial high shear pulses.20 Moreover, we identified specific hemodynamic components of the shear stress, namely high shear temporal oscillations, that is the “dynamicity” of the shear stress, as major contributors to SMPA.21
Complementing these preclinical studies, we have recently reported a clear relationship between progressive accumulation of LVAD-mediated platelet injury in vivo and post-implant thromboembolic complications.22–24 Notably, our analysis of shear-mediated platelet prothrombinase activity correlates well with other studies that described altered platelet function and enhanced thrombotic propensity in response to non-physiological shear stress of blood contacting medical devices, including LVADs.25–29
An important issue in LVAD thrombosis is the site of thrombus initiation and propagation. Thrombus formation has been clinically observed to initiate often within the LVAD-implanted ventricle, at the LV apex, with ingestion of endoventricular thrombi into the pump.2,4 Ingested thrombi may drive and worsen pump thrombosis, leading to severe pump dysfunction, low pump output and cardiogenic shock, seriously jeopardizing patient survival. Further, ingested thrombi propelled downstream through the LVAD may result in thromboembolic events, ischemic stroke and severe neurologic dysfunction.4
A range of mechanisms have been suggested to promote specifically endoventricular thrombus formation, including: i) the reduced contractility of the dysfunctional left ventricle, where activated and/or sensitized platelets may accumulate in the apical region, being exposed to additional prothrombotic factors, i.e., low dynamic shear stress for elongated durations, areas of blood stasis, and stagnant/recirculation flow;30,31 ii) the surgical LVAD implantation technique, e.g., particular LVAD inflow cannula placement and angulation;32–34 and iii) biomaterial/device factors, i.e., surface contact activation of the coagulation cascade associated with the LVAD inflow cannula material.31 On the other hand, the role of the ventricular endothelium in LVAD thrombosis, specifically the role of inflammatory activation of endocardial endothelial cells (ECs), has not been examined.
Previous studies have reported high plasma levels of inflammatory cytokines such as Tumor Necrosis Factor-α (TNF-α) in patients with end-stage heart failure, and even higher values have been recorded in patients on LVAD circulatory support.36 TNF-α is known to enhance the procoagulant activity state of ECs: TNF-α binds the cognate EC receptor TNF-R1, which in turn leads to rapid translocation of the transcription factor NF-kB from the cytoplasm to the cell, promoting the expression of proadhesive and procoagulant molecules, including integrin αvβ337, intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), and endothelial selectin (E-Selectin).38–41 As such, TNF-α-mediated EC activation induces a switch from a quiescent bystander phenotype to a more proinflammatory, proadhesive and procoagulant behavior.
In the present study, we hypothesized that exposure to high TNF-α levels might result in EC inflammatory activation of the LVAD-implanted ventricular apex and that it may synergize with SMPA in enhancing EC-platelet adhesion and further drive platelet activation and thrombin generation, thus contributing to enhanced overall prothrombosis. As such, being these mechanisms concomitantly operative in the LVAD-implanted ventricle, this synergistic effect may mechanistically contribute to progressive intraventricular thrombosis. Accordingly, in the present study we sought to dissect and systematically analyze the synergistic effect of SMPA and TNF-α-mediated EC inflammatory activation as for the development of progressive prothrombotic conditions. We first examined the adhesivity of intact and shear-activated platelets to nonactivated and TNF-α treated ECs. We then examined potential synergies of shear- and cytokine-mediated prothrombosis as for platelet thrombin generation and associated prothrombotic activity.
MATERIALS AND METHODS
To evaluate EC-platelet prothrombotic interaction mechanisms, three experimental protocols were performed. Protocol 1: the effect of differing extents of SMPA induced by platelet exposure to 30, 50 and 70 dynes/cm2 time-constant shear stress patterns for 10 min in a Hemodynamic Shearing Device42 was examined as for platelet adhesion to untreated ECs. Platelet prothrombinase activity was also evaluated via the PAS assay.16,20 Protocol 2: the effect of cytokine-mediated EC activation (TNF-α concentrations: 10 and 100 ng/mL; incubation time: 24 h) was evaluated as to its impact on platelet adhesivity and prothrombinase activity (both intact and shear-activated platelets). Protocol 3: the combined effect of SMPA and TNF-α-mediated EC activation was examined to demonstrate synergistic platelet-EC adhesivity and prothrombotic function.
Full experimental details related to i) platelet isolation, shear activation, and characterization of platelet prothrombinase activity, ii) EC culture and inflammatory activation via TNF-α, iii) characterization of EC-platelet adhesion, and iv) characterization of EC soluble markers of activation, and EC and platelet surface markers of activation are reported as Supplementary Material.
RESULTS
Shear-mediated platelet activation
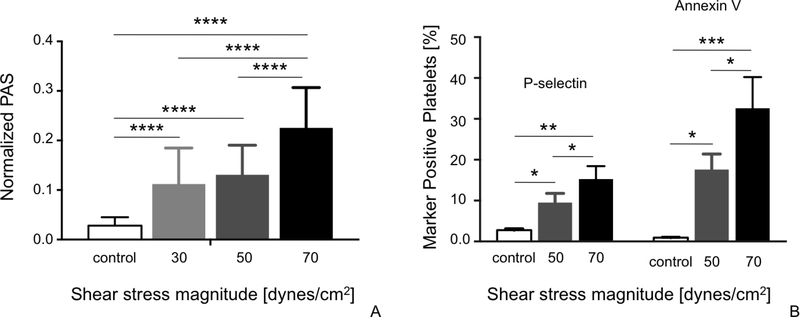
The extent of SMPA increased progressively with increasing shear stress magnitude. In detail, PAS values of platelets stimulated in the HSD with different levels of shear stress (30, 50 and 70-dynes/cm2) were significantly higher than basal values measured in control/non-stimulated platelet samples (Fig. 1A; p<0.0001). PAS values of platelets stimulated with 30 and 50 dynes/cm2 were comparable (Fig. 1A; p>0.05), while thrombin generation of platelets stimulated with 70 dynes/cm2 was significantly higher (Fig. 1A; p<0.0001).
Figure 1:
Prothrombinase activity (A), P-selectin expression and phosphatidylserine externalization (annexin V binding) (B) of platelets activated with increasing shear stress magnitude as compared to basal values of intact platelets (control). Data are reported as mean ± SD. *p<0.05; **p<0.001; ***p<0.0005; ****p<0.0001.
Consistent with PAS data, expression of P-selectin and phosphatidylserine externalization (annexin V binding) of 50 and 70 dynes/cm2-stimulated platelets were significantly higher than basal values measured in control samples (Fig. 1B; P-selectin: p=0.0015; Annexin V: p=0.0007). Furthermore, these values increased with increasing shear stress magnitude, with both P-selectin expression and phosphatidylserine externalization measured in 70 dynes/cm2-stimulated platelets being significantly higher than those measured following stimulation with 50 dynes/cm2 (Fig. 1B; p<0.05). In contrast, P-Selectin expression increased at a lower rate when compared with annexin V externalization: specifically, a 5.5-fold increase of P-Selectin expression was observed in 70-dynes/cm2 sheared platelets vs. controls, while annexin-V externalization was 34-fold higher in 70-dynes/cm2 sheared platelets vs. controls (Fig. 1B).
TNF-α inflammatory activation of endothelial cells
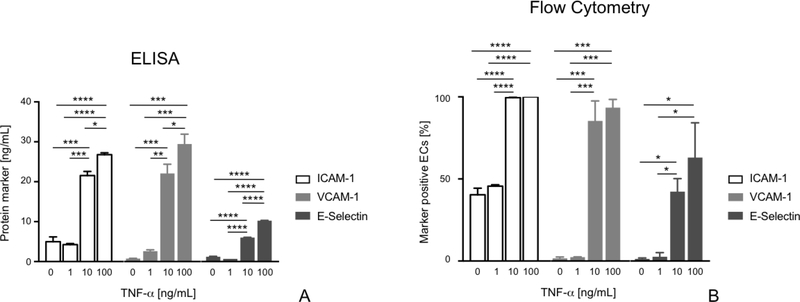
We observed significant TNF-α dose-dependent EC inflammatory activation, as indicated by the increased expression of activation markers with increasing TNF-α concentration. In detail: ICAM-1, VCAM-1 and E-Selectin soluble expression of ECs incubated with 1 ng/mL TNF-α was not significantly different with respect to untreated control cells (Fig. 2A; p>0.05); conversely, we observed a significantly greater expression of these markers in ECs incubated with 10 ng/mL TNF-α in comparison to untreated control cells (Fig. 2A; ICAM: p<0.001, V-CAM: p<0.01, E-Selectin: p<0.0001). Furthermore, significantly higher expression of I-CAM1, V-CAM1 and E-Selectin was found in ECS incubated with 100 ng/mL TNF-α vs. those stimulated with 10 ng/mL TNF-α (Fig. 2A; I-CAM: p<0.05, V-CAM: p<0.05, E-Selectin: p<0.0001). Expression of surface activation markers assessed via flow cytometry showed analogous TNF-α dose dependent expression of EC activation markers: incubation with 1 ng/mL TNF-α did not induce significantly higher expression of ICAM-1, VCAM-1, and E-selectin with respect to control (i.e., nonactivated) ECs (Fig. 2B; p>0.05); expression of activation markers was significantly higher in ECs incubated with 10 ng/mL TNF-α vs. control cells (Fig. 2B; I-CAM: p<0.0001; VCAM-1: p<0.001; E-Selectin: p<0.01); ECs incubated with 100 ng/mL TNF-α exhibited comparable expression of surface markers of activation with respect to samples incubated with 10 ng/mL (Fig. 2B; p>0.05).
Figure 2:
Expression of EC soluble (A) and surface (B) activation markers following 24h incubation with different concentrations of TNF-α (0, 1, 10 and 100 ng/mL). Data are reported as mean ± SD. * p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Prothrombotic interaction of unactivated endothelial cells and nonstimulated platelets
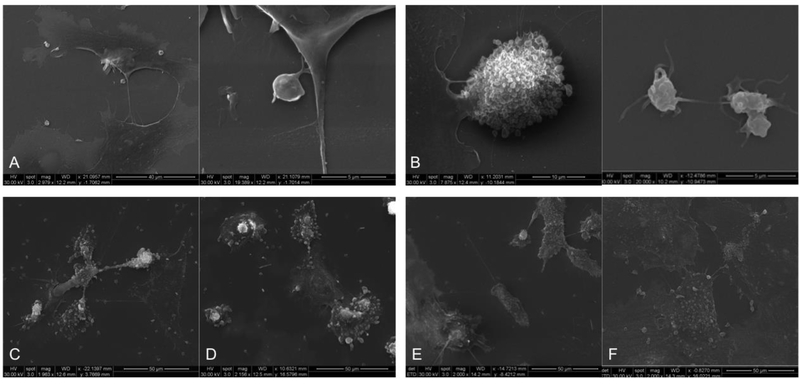
Unstimulated platelets bind minimally to nonactivated ECs (Fig. 3A, left) and preserve their basal physiologic, discoid shape (Fig. 3A, right). Flow cytometry confirmed low adhesivity, consistent with comparable αvβ3 integrin expression of ECs incubated with unstimulated platelets vs. ECs alone (positive cells % = 11.40±1.20 vs. 15.60±0.70, respectively; p>0.05); in addition, PAS values of unstimulated platelets incubated with nonactivated ECs were not statistically different with respect to those of unstimulated platelets alone (PAS = 0.03±0.02 vs. 0.01±0.004, respectively; p>0.05).
Figure 3:
Scanning electron microscopy images acquired following 60 min of EC-platelet incubation. (A) Intact platelets exposed to nonactivated ECs; (B) Shear-activated platelets (70 dynes/cm2) exposed to nonactivated ECs; (C) Intact platelets exposed to 10 ng/mL TNF-α activated ECs; (D) Intact platelets exposed to 100ng/mL TNF-α activated ECs; (E) Shear-activated platelets (70 dynes/cm2) exposed to 10 ng/mL TNF-a activated ECs; (F) Shear-activated platelets (70 dynes/cm2) exposed to 100ng/mL TNF-a activated ECs.
Prothrombotic interaction of unactivated endothelial cells and shear-activated platelets
Shear-activation of platelets significantly enhanced adhesivity to ECs. Extensive EC-platelet adhesion was observed for 70 dynes/cm2 shear-activated platelets (Fig. 3B, left), which was accompanied by morphological changes of adherent platelets that switched from nonactivated, smooth disc-shape morphology to an activated morphology displaying blebs and pseudopodia (Fig. 3B, right). Consistent with SEM images, flow cytometry revealed that platelet adhesivity to ECs increased with shear-activation: in detail, EC αvβ3 integrin expression of ECs incubated with shear-activated platelet was significantly lower than that of ECs incubated with intact platelets (Table 1; p<0.0001), with this reduction of surface expression being the result of platelet occupancy of EC adhesion sites; αvβ3 integrin expression of ECs incubated with platelets stimulated with 50 and 70-dynes/cm2 shear stress was comparable (Table 1). On the other hand, the PAS assay revealed that exposure of shear-activated platelets to nonactivated ECs did not enhance their prothrombinase activity (PAS = 0.22±0.08 vs. 0.26±0.09 in 70 dynes/cm2-activated platelets and 70 dynes/cm2-activated platelets incubated with untreated ECs, respectively; p=0.39). These results suggest that ECs provide a proadhesive substrate for sear-activated platelets.
Table 1:
EC expression of αvβ3 integrin (percentage of αvβ3-positive cells) as modulated by shear-mediated platelet activation (50 and 70 dynes/cm2) and TNF-α EC inflammatory activation (10 and 100 ng/mL TNF-α)
| ECs + nP | ECs + 50P | ECs + 70P | p-value |
| 22.73 ± 2.44 | 8.70 ± 0.84 | 5.70 ± 0.28 | <0.0001 |
| 10ECs + nP | 10ECs + 50P | 10ECs + 70P | p-value |
| 18.08 ± 2.01 | 9.05 ± 1.20 | 9.77 ± 1.21 | 0.0006 |
| 100ECs + nP | 100ECs + 50P | 100ECs + 70P | p-value |
| 26.55 ± 5.54 | 5.73 ± 1.05 | 4.10 ± 2.26 | 0.0004 |
ECs: nonactivated ECs; nP: nonactivated platelets; 50P: 50 dynes/cm2-activated platelets; 70P: 70dynes/cm2-activated platelets; 10ECs: ECs activated with 10ng/mL TNF-α; 100ECs: ECs activated with 100ng/mL TNF-α
Prothrombotic interaction of activated endothelial cells and nonstimulated platelets
We observed diffuse adhesion of intact platelets to activated ECs (Fig. 3C,D). Analysis of αvβ3 integrin expression revealed low platelet occupancy of EC adhesion sites, which was comparable to that of intact platelets incubated with nonactivated ECs (Table 1; p>0.05). Analysis of PAS values showed a significant, 3.5-fold increase in platelet prothrombinase activity following exposure to activated ECs (10 ng/mL and 100ng/mL TNF-α) vs. that of intact platelets alone (p<0.0001). PAS values of platelets exposed to 10 ng/mL and 100ng/mL TNF-α activated ECs were comparable (0.035±0.022 and 0.034±0.019, respectively). These results suggest that EC inflammatory activation ampifies platelet prothrombotic activity.
Prothrombotic interaction of activated endothelial cells and shear-activated platelets
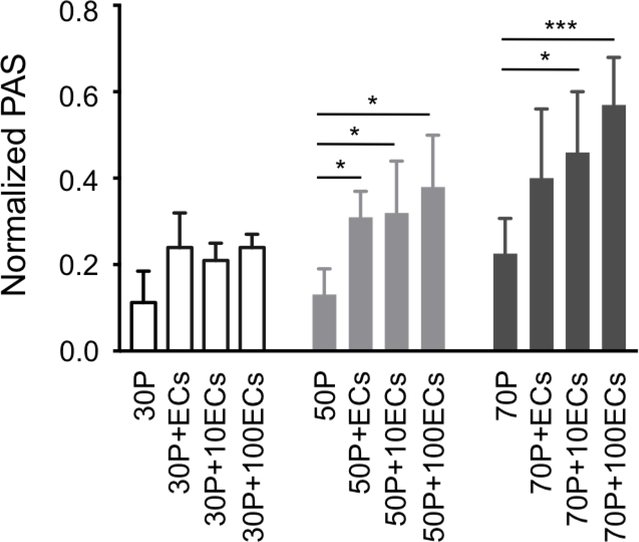
Analysis of the synergistic effect of shear-activation of platelets and TNF-α activation of ECs showed extensive EC-platelet adhesivity (Fig. 3E,F), which increased with increasing shear-stress and EC inflammatory activation: in detail, a significant decrease in αvβ3 levels was measured in 10ng/mL TNF-α activated ECs incubated with 50 and 70 dynes/cm2-activated platelets vs. that of activated ECs incubated with intact platelets (Table 1: p=0.0006); likely, a significant decrease in αvβ3 levels was measured in 100ng/mL TNF-α activated ECs incubated with 50 and 70 dynes/cm2-activated platelets vs. that of activated ECs incubated with intact platelets (Table 1: p=0.0004). Moreover, SMPA and EC TNF-α-activation synergistically contributed to increased platelet prothrombinase activity: comparison of PAS values before and after exposure of platelets to TNF-α-activated ECs showed a progressive increase of platelet thrombin generation with increasing shear stress magnitude and TNF-α concentration (Fig. 4); specifically, PAS values measured in 70 dynes/cm2-activated platelets exposed to 10 and 100 ng/mL TNF-α-activated ECs were higher than those measured in platelets stimulated with lower shear stresses (30 and 50 dynes/cm2) but exposed to analogous EC prothrombotic environments (i.e., 10 and 100 ng/mL TNF-α; Fig. 4). In addition, PAS values of 70 dynes/cm2 activated platelets exposed to 100 ng/mL TNF-α-activated ECs were 2.6-fold, 1.4-fold and 1.24-fold higher than those of 70 dynes/cm2-shear-activated platelets alone, 70 dynes/cm2-shearactivated platelets incubated with nonactivated ECs, and 70 dynes/cm2-shear-activated platelets incubated with 10 ng/mL TNF-α activated ECs, respectively (Fig. 4). These results suggest a combined role of the activated endothelium and of (high) mechanical - shear-mediated - platelet activation to further enhance prothrombosis.
Figure 4:
Platelet prothrombinase activity mediated by the combined synergistic effect of shear stress activation and EC inflammatory cytokine activation. 30P, 50P and 70P indicate 30 dynes/cm2, 50 dynes/cm2 and 70 dynes/cm2 shear-activated platelets, respectively; ECs, 10ECs and 100ECs indicate nonactivated, 10 ng/mL and 100 ng/mL TNF-α-activated ECs, respectively. Data are reported as mean ± SD. * p<0.05; *** p<0.001.
DISCUSSION
We examined the potential synergistic effects of mechanical - shear-mediated - platelet activation (SMPA) and inflammatory - cytokine-mediated - endothelial cell (EC) activation as to their functional interaction to enhance EC-platelet surface adhesion and prothrombosis.
We found that shear-activated platelets bind to ECs, regardless of the level of shear-activation as well as of EC activation, i.e., they bind to non-activated ECs as well. As such, ECs are a pro-adhesive substrate for shear-activated platelets, with significant EC αvβ3 integrin occupancy and ligand-binding activity mediating firm adhesion (Table 1). However, importantly, we demonstrated that EC activation amplifies platelet activation resulting in enhanced platelet thrombin generation. Indeed, analysis of prothrombinase activity of shear-activated platelets as measured via the PAS assay demonstrated that PAS values of platelets exposed to high shear stress magnitude (i.e., 70 dynes/cm2) were further modulated by the prothrombotic milieu of TNF-α activated ECs, and increased progressively concomitant with the increase of EC inflammatory activation (Fig. 4). In particular, the highest PAS values were measured in platelets activated with high shear stress stimulation (70 dynes/cm2) and exposed to highly-inflamed (100 ng/mL TNF-α) ECs (Fig. 4). These data suggest, a possible synergistic contribution of EC inflammatory activation compounding that of SMPA alone in promoting platelet thrombin generation. While it has been reported that EC activation will bind and activate previously quiescent platelets, the demonstration that binding of shear-activated platelets to activated ECs leads to enhanced platelet thrombin generation has not been reported. Here we demonstrated that the extent of prothrombinase activity is dependent upon the extent of both EC activation and SMPA.
We hypothesized that our findings might have functional implications as an additional contributory mechanism operative in vivo in the setting of mechanical circulatory support, specifically, a potential role in endoventricular thrombus formation and thromboembolic complications in patients with implanted LVADs. Indeed, i) SMPA has been shown to correlate with LVAD-related thrombosis,14,22,29,31,32 and ii) high pro-inflammatory TNF-α plasma concentrations have been measured in LVAD patients,36 and might contribute to triggering an activated prothrombotic phenotype for the endocardium, which might serve as the prothrombotic substrate allowing shear-activated platelets to adhere and promoting progressive thrombosis.
Accordingly, our results suggest that anatomically, the ventricular apex, that is the site of direct LVAD-left ventricle interfacing, where shear-activated circulating platelets come in direct contact with activated endocardial ECs, may have a mechanistic role in LVAD endoventricular thrombosis (Fig. 5). Clinical imaging and surgical studies have previously shown this interface site to be a nidus for thrombus localization and accumulation.2,4,43–45 Our findings are consistent with, and provide a mechanistic rationale for, these clinical observations suggesting that shear activated platelets adhere to activated ECs within the LVAD-implanted ventricular apex, with subsequent apposition on the LVAD inflow cannula (Fig. 5).
Figure 5:
Proposed schema of prothrombotic interaction between inflamed ECs and shear-activated platelets in the LVAD-implanted ventricle. (A) the LVAD-implanted ventricle; (B) cross-sectional view of the LVAD-implanted ventricular apex: here, shear-activated circulating platelets that follow secondary/peripheral blood flow not entering the LVAD inflow cannula accumulate and get trapped, facing with (C) inflammatory activated endocardial endothelial cells (ECs), with associated integrin-mediated platelet adherence and apposition on the LVAD inflow cannula.
John et al. have previously reported activation of vascular ECs in LVAD-supported patients.46 Moreover, Diehl and colleagues reported an increased extent of platelet-, leukocyte- and EC-activation in LVAD patients.47 However, a paucity of data exists as to the direct contributory interaction of shear-activated platelets with activated ECs to LVAD thrombosis. Here we demonstrate that inflamed endothelium increases platelet prothrombinase activity additively with shear mediated activation, potentially contributing to endoventricular thrombus formation, with the LVAD-implanted activated endocardium serving as the substrate for prothrombotic activity of shear-activated platelets.
In addition, our results further corroborate our previous findings, which analyzed the mechanistic effect of LVAD-related shear stress upon platelet function and thrombosis.22–24 Our present report provides insight into a potential contributory mechanism that may be involved in the pathogenesis of LVAD thrombosis, linking the mechanobiological responsiveness of circulating shear-activated platelets to the synergistic effect provided by the prothrombotic milieu of inflammatory ECs.
These results also suggest that prothrombotic EC-platelet functional interactions may be a possible therapeutic target to prevent intraventricular thrombosis. In this context, recent studies have questioned the actual efficacy of current antiplatelet therapeutic strategies in the setting of LVAD therapy as a means of preventing LVAD-related thrombosis,48–51 Thus, anatomic device targets, i.e., LV apex-LVAD interactions, as suggested by our findings, offer the potential for modulation of thrombosis in the setting of mechanical circulatory support.
The experimental design utilized in this study allowed us to examine in vitro the selective and mutual contribution to thrombus formation of SMPA and EC cytokine-mediated activation. First, the Hemodynamic Shearing Device-based protocol for platelet activation allowed effective modulation of the level of SMPA (Fig. 1). Our findings agree with previous studies showing that shear-activated platelets increase shedding of GPIIb/IIIa,25,26 increase the release of specific proteins contained within α-granules (beta-thromboglobulin and platelet factor 4),27, as well as δ granules28 are characterized by abnormal ristocetin-induced aggregation,29 upregulate expression of pro-adhesive proteins, such as P-selectin,51 and significantly expose negatively charged phospholipids phosphatidylserine and phosphatidylethanolamine, thus providing a catalytic surface for thrombin generation contributing to prothrombotic state formation and propagation.52 This study also shows that platelet annexin V externalization is dominant vs. P-Selectin expression in response to shear stress exposure (Fig. 1). Moreover, although time-constant shear stress patterns where employed in this study, which differ from those generating within the LVAD system,54 PAS values measured in platelets stimulated with shear stress patterns of greater stress magnitude were comparable to those reported when dynamic shear stress waveforms pertinent to commercial LVADs were used to stimulate platelets.17,18,54 Accordingly, the stimulation protocol utilized in this study was relevant to replicate and analyze prothrombinase activity of LVAD-stimulated platelets. Secondly, despite use of non-physiologic TNF-α concentrations to activate ECs, our results about EC activation are consistent with those reported by Bruggink et al. in vivo in LVAD patients: indeed, in that study the authors reported high levels of circulating TNF-α in LVAD patients,36 which we hypothesized to induce endocardial inflammatory activation: accordingly, we replicated in vitro this condition and incubated ECs with 10 and 100ng/mL TNF-α. TNF–α concentrations used in this study were selected based on literature studies that have previously focused on in vitro expression of EC activation markers (ICAM-1, VCAM-1, E-Selectin) in response to TNF-α activation.55–59 Moreover, modulation of EC activation with differing concentrations of TNF-α utilized in this study facilitated examination of the platelet response to differing proadhesive and prothrombotic EC inflammatory milieu (no EC activation, mild EC activation, strong EC activation). Consistent with previous works, we observed TNF-α dose-dependent activation of ECs, that is, higher expression of activation markers following EC exposure to progressively higher TNF-α concentrations (Fig. 2).55–59 Jain and colleagues have previously reported that the altered expression of ICAM-1, VCAM-1 and E-selectin by TNF-α-activated ECs have a role in hemostatic disorders and support thrombus formation.60 Similarly Girdhar et al. previously demonstrated that EC activation with other inflammatory mediators increases E-selectin and coordinate platelet adhesion and activation.61 Here, we suggest relevance of this mechanism in enhancing platelet prothrombosis in the LVAD-implanted ventricle.
Study limitations
In this study, Human Umbilical Vein Endothelial Cells (HUVECs) were utilized as a model for endocardial ECs to examine the effect of cytokine activation. Despite differences between the two cell types, HUVECs are a general but reliable model to the study of TNF-α-mediated activation of endocardial ECs. Indeed, HUVECs have been shown to be responsive to physiological and/or pathological stimuli and to express those endothelial markers and signaling molecules we sought to analyze here, i.e., ICAM-1, VCAM-1 and E-selectin.62 Accordingly, under proper conditions, HUVECs may be effectively employed in advanced models to better understand the behavior of ECs in vivo. On the other hand, further confirmation employing primary endocardial ECs obtained from ventricular biopsy in patients undergoing LVAD implantation, as well as validation studies using platelets from LVAD patients are warranted.
CONCLUSIONS
We provide new mechanistic insights into the prothrombotic interaction of mechanical - shear-mediated - stimulation of platelets with inflammatory activation of ECs. Our findings demonstrate a synergy of this interaction in enhancing overall prothrombosis. Specifically, our results provide evidence that EC activation, here cytokine-mediated, combine with shear-mediated platelet activation to drive and sustain platelet adhesion to the inflamed endothelium and further prothrombotic activity. Indeed, we demonstrate that, besides being completely adhesive for activated platelets, the inflammatory activated EC environment contributes to a further increase in thrombin production by shear-activated platelets. The conditions studied - both anatomically and functionally - might reflect in vivo conditions in the cardiac ventricular apex of patients implanted with LVADs. As such, our observations might offer new insight as to a possible additional contributory mechanism operative in the pathogenesis of intraventricular LVAD thrombosis. Further, our findings underscore the importance of looking at LVAD and - in general mechanical circulatory support - thrombosis as s “system” issue composed of many local, regional contributors, beyond the “pump” alone. Our findings also open the potential for development of new therapeutic strategies specifically targeting ventricular prothrombotic EC-platelet interactions as a means of reducing the burden of life-threatening thrombotic complications in LVAD patients. Future studies performing experiments with platelets from LVAD patients, and examination of explanted hearts to localize thrombus formation at the LVAD inflow cannula-left ventricle interface are important next steps to validate in vitro results.
Supplementary Material
Highlights.
We demonstrate prothrombotic synergistic functional interaction of i) cytokine-mediated inflammatory activation of endothelial cells (ECs) and ii) shear-mediated platelet activation
We suggest that these mechanisms are operative in vivo and contribute to endoventricular thrombosis and thromboembolic complications in LVAD patients
Our findings open the potential for the development of new therapeutic strategies specifically targeting prothrombotic EC-platelet interactions to modulate platelet prothrombosis and prevent intraventricular thrombosis in the setting of mechanical circulatory support
Acknowledgments:
This study was supported by Fondazione CARIPLO grant n. 2015-1044 (awarded to FC and MJS), Fondazione Cariplo and Regione Lombardia Grant 2016-0901 (awarded to AR), NIH Cardiovascular Biomedical Engineering Training Grant T32 HL007955 (awarded to KRA), National Institute of Biomedical Imaging and Bioengineering (Quantum Grant Award No. 5U01EB012487-00 (awarded to DB and MJS) and Arizona Center for Accelerated Biomedical Innovation (ACABI)/Tech Launch Arizona Grant UA 15-035 (awarded to MJS).
Footnotes
Disclosures: The Authors have no conflicts of interest relevant to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–504. [DOI] [PubMed] [Google Scholar]
- 2.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, Thuita L, Mountis MM, Soltesz EG, Lytle BW, Smedira NG. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370(1):33–40. [DOI] [PubMed] [Google Scholar]
- 3.Tarzia V, Buratto E, Bortolussi G, Gallo M, Bejko J, Bianco R, Bottio T, Gerosa G. Hemorrhage and thrombosis with different LVAD technologies: a matter of flow? Ann Cardiothorac Surg. 2014;3(6):582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, Tatooles AJ, Moazami N, Kormos RL, Hathaway DR, Najarian KB, Bhat G, Aaronson KD, Boyce SW; HVAD Bridge to Transplant ADVANCE Trial Investigators. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33(1):23–34. [DOI] [PubMed] [Google Scholar]
- 5.Uriel N, Han J, Morrison KA, Nahumi N, Yuzefpolskaya M, Garan AR, Duong J, Colombo PC, Takayama H, Thomas S, Naka Y, Jorde UP. Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant. 2014;33:51–59. [DOI] [PubMed] [Google Scholar]
- 6.Lopilato AC, Doligalski CT, Caldeira C. Incidence and Risk Factor Analysis for Gastrointestinal Bleeding and Pump Thrombosis in Left Ventricular Assist Device Recipients. Artif Organs. 2015;39(11):939–44. [DOI] [PubMed] [Google Scholar]
- 7.Nassif ME, LaRue SJ, Raymer DS, Novak E, Vader JM, Ewald GA, Gage BF. Relationship Between Anticoagulation Intensity and Thrombotic or Bleeding Outcomes Among Outpatients With Continuous-Flow Left Ventricular Assist Devices. Circ Heart Fail. 2016;9(5): e002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah P, Tantry US, Bliden KP, Gurbel PA. Bleeding and thrombosis associated with ventricular assist device therapy. J Heart Lung Transplant. 2017;36(11):1164–1173. [DOI] [PubMed] [Google Scholar]
- 9.Yarboro LT, Mehaffey JH, Hawkins RB, Kron IL, Ailawadi G, Kern JA, Ghanta RK. Pre-implant left ventricular apex position predicts risk of HeartMate II pump thrombosis. J Card Surg. 2017;32(12):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han JJ, Sooppan R, Johnson AP, Chen CW, Gaffey AC, Phillips EC, Howard J, Rame JE, Acker MA, Atluri P. Higher Body Mass Index Increases Risk of HeartMate II Pump Thrombosis But Does Not Adversely Affect Long-Term Survival. Circ J. 2017;81(2):213–219. [DOI] [PubMed] [Google Scholar]
- 11.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012;125:3038–3047. [DOI] [PubMed] [Google Scholar]
- 12.de Biasi AR, Manning KB, Salemi A. Science for surgeons: understanding pump thrombogenesis in continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2015;149(3):667–73. [DOI] [PubMed] [Google Scholar]
- 13.Selmi M, Chiu WC, Chivukula VK, Melisurgo G, Beckman JA, Mahr C, Aliseda A, Votta E, Redaelli A, Slepian MJ, Bluestein D, Pappalardo F, Consolo F. Blood Damage in Left Ventricular Assist Devices (LVADs): Pump Thrombosis or LVAD System Thrombosis? Int J Artif Organs. 2018. DOI: 10.1177/0391398818806162. [DOI] [PubMed] [Google Scholar]
- 14.Slepian MJ, Sheriff J, Hutchinson M, Tran P, Bajaj N, Garcia JG, Scott Saavedra S, Bluestein D. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J Biomech. 2017;50:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheriff J, Girdhar G, Chiu WC, Jesty J, Slepian MJ, Bluestein D. Comparative efficacy of in vitro and in vivo metabolized aspirin in the DeBakey ventricular assist device. J Thromb Thrombolysis. 2014. May;37(4):499–506. doi: 10.1007/s11239013-0997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consolo F, Valerio L, Brizzola S, Rota P, Marazzato G, Vincoli V, Reggiani S, Redaelli A, Fiore G. On the Use of the Platelet Activity State Assay for the In Vitro Quantification of Platelet Activation in Blood Recirculating Devices for Extracorporeal Circulation. Artif Organs. 2016;40(10):971–980. [DOI] [PubMed] [Google Scholar]
- 17.Consolo F, Dimasi A, Rasponi M, Valerio L, Pappalardo F, Bluestein D, Slepian MJ, Fiore GB, Redaelli A. Microfluidic approaches for the assessment of blood cell trauma: a focus on thrombotic risk in mechanical circulatory support devices. Int J Artif Organs. 2016;39(4):184–93. [DOI] [PubMed] [Google Scholar]
- 18.Dimasi A, Rasponi M, Consolo F, Fiore GB, Bluestein D, Slepian MJ, Redaelli A. Microfludic platforms for the evaluation of anti-platelet agent efficacy under hyper-shear conditions associated with ventricular assist devices. Med Eng Phys. 2017;48:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nobili M, Sheriff J, Morbiducci U, Redaelli A, Bluestein D. Platelet activation due to hemodynesamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J. 2008;54(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheriff J, Tran PL, Hutchinson M, DeCook T, Slepian MJ, Bluestein D, Jesty J. Repetitive Hypershear Activates and Sensitizes Platelets in a Dose-Dependent Manner. Artif Organs. 2016;40(6):586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Consolo F, Sheriff J, Gorla S, Magri N, Bluestein D, Pappalardo F, Slepian MJ, Fiore GB, Redaelli A. High Frequency Components of Hemodynesamic Shear Stress Profiles are a Major Determinant of Shear-Mediated Platelet Activation in Therapeutic Blood Recirculating Devices. Sci Rep. 2017;7(1):4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valerio L, Consolo F, Bluestein D, Tran P, Slepian M, Redaelli A, Pappalardo F. Shear-mediated platelet activation in patients implanted with continuous flow LVADs: A preliminary study utilizing the platelet activity state (PAS) assay. Conf Proc IEEE Eng Med Biol Soc. 2015. August;2015:1255–8. [DOI] [PubMed] [Google Scholar]
- 23.Consolo F, Sferrazza G, Motolone G, Contri R, Valerio L, Lembo R, Pozzi L, Della Valle P, De Bonis M, Zangrillo A, Fiore GB, Redaelli A, Slepian MJ, Pappalardo F. Platelet Activation is a pre-operative risk factor to the development of thromboembolic complications in patients with continuousflow Left Ventricular Assist Device. Eur J Heart Fail. 2018;20(4):792–800. [DOI] [PubMed] [Google Scholar]
- 24.Consolo F, Sferrazza G, Motolone G, Pieri M, De Bonis M, Zangrillo A, Rdaelli A, Slepian MJ, Pappalardo F. Shear-Mediated Platelet Activation Enhances Thrombotic Complications in Patients With LVADs and Is Reversed After Heart Transplantation ASAIO J 2018. doi: 10.1097/MAT.0000000000000842. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Griffith BP, Wu ZJ. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Mol Cell Biochem. 2015. November;409(1–2):93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Mondal NK, Zheng S, Koenig SC, Slaughter MS, Griffith BP, Wu ZJ. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets. 2017. November 28:1–8. doi: 10.1080/09537104.2017.138542. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisen U, Brehm K, Trummer G, Berchtold-Herz M, Heilmann C, Beyersdorf F, Schelling J, Schlagenhauf A, Zieger B. Platelet Secretion Defects and Acquired von Willebrand Syndrome in Patients With Ventricular Assist Devices. J Am Heart Assoc. 2018. January 13;7(2). pii: e006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawahito K, Mohara J, Misawa Y, Fuse K., Platelet damage caused by the centrifugal pump: in vitro evaluation by measuring the release of alpha-granule packing proteins. Artif Organs. 1997;21:1105–1109. [DOI] [PubMed] [Google Scholar]
- 29.Steinlechner B, Dworschak M, Birkenberg B, Duris M, Zeidler P, Fischer H, Milosevic L, Wieselthaler G, Wolner E, Quehenberger P, Jilma B. Platelet dysfunction in outpatients with left ventricular assist devices. Ann Thorac Surg. 2009;87:131–137. [DOI] [PubMed] [Google Scholar]
- 30.Rossini L, Martinez-Legazpi P, Vu V, Fernández-Friera L, et al. A clinical method for mapping and quantifying blood stasis in the left ventricle. J Biomech. 2016;49(11):2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehra MR, Stewart CG, Uber PA. The vexing problem of thrombosis in long-term mechanical circulatory support. J Heart Lung Transplant. 2014;33(1):1–11. [DOI] [PubMed] [Google Scholar]
- 32.Taghavi S, Ward C, Jayarajan SN, Gaughan J, Wilson LM, Mangi AA. Surgical technique influences HeartMate II left ventricular assist device thrombosis. Ann Thorac Surg. 2013;96:1259–1265. [DOI] [PubMed] [Google Scholar]
- 33.Uriel N, Han J, Morrison KA, Nahumi N, Yuzefpolskaya M, Garan AR, Duong J, Colombo PC, Takayama H, Thomas S, Naka Y, Jorde UP. Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant. 2014;33:51–59. [DOI] [PubMed] [Google Scholar]
- 34.Chiu WC, Alemu Y, McLarty AJ, Einav S, Slepian MJ, Bluestein D. Ventricular Assist Device Implantation Configurations Impact Overall Mechanical Circulatory Support System Thrombogenic Potential. ASAIO J. 2017;63(3):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chivukula VK, Beckman JA, Prisco AR, Dardas T, Lin S, Smith JW, Mokadam NA, Aliseda A, Mahr C. Left Ventricular Assist Device Inflow Cannula Angle and Thrombosis Risk. Circ Heart Fail. 2018;11(4):e004325. [DOI] [PubMed] [Google Scholar]
- 36.Bruggink AH, van Oosterhout MF, De Jonge N, Gmelig-Meyling FH, De Weger RA. TNFalpha in patients with end-stage heart failure on medical therapy or supported by a left ventricular assist device. Transpl Immunol. 2008;19(1):64–8. [DOI] [PubMed] [Google Scholar]
- 37.Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J Exp Med. 1998. February 2;187(3):329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Modur V, Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996;271(22):13094–102. [DOI] [PubMed] [Google Scholar]
- 39.Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. Exp Med 1993;177(5):1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med 1986;163(3):740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehme MW, Raeth U, Scherbaum WA, Galle PR, Stremmel W. Interaction of endothelial cells and neutrophils in vitro: kinetics of thrombomodulin, intercellular adhesion molecule-1 (ICAM-1), E-selectin, and vascular cell adhesion molecule-1 (VCAM-1): implications for the relevance as serological disease activity markers in vasculitides. Clin Exp Immunol. 2000;119(1):250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bluestein D, Girdhar G, Einav S, Slepian MJ. Device thrombogenicity emulation: a novel methodology for optimizing the thromboresistance of cardiovascular devices. J Biomech. 2013;46(2):338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garbade J, Bittner HB, Mohr FW, Barten MJ. Fluoroscopy-guided resolution of ingested thrombus leading to functional disturbance of a continuous-flow left ventricular assist device. Case Rep Surg. 2012;2012:791056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eulert-Grehn JJ, Krabatsch T, Potapov E. A case of an obstructive inflow thrombus in a HeartMate 3 from the left ventricle into the pump. J Heart Lung Transplant. 2018;37(1):172–173. [DOI] [PubMed] [Google Scholar]
- 45.Stulak JM, Dunlay SM, Sharma S, Haglund NA, Davis MB, Cowger J, Shah P, Masood F, Aaronson KD, Pagani FD, Maltais S. Treatment of device thrombus in the HeartWare HVAD: Success and outcomes depend significantly on the initial treatment strategy. J Heart Lung Transplant. 2015;34(12):1535–41. [DOI] [PubMed] [Google Scholar]
- 46.John R, Panch S, Hrabe J, Wei P, Solovey A, Joyce L, Hebbel R. Activation of endothelial and coagulation systems in left ventricular assist device recipients. Ann Thorac Surg. 2009;88(4):1171–9. [DOI] [PubMed] [Google Scholar]
- 47.Diehl P, Aleker M, Helbing T, Sossong V, Beyersdorf F, Olschewski M, Bode C, Moser M. Enhanced microparticles in ventricular assist device patients predict platelet, leukocyte and endothelial cell activation. Interact Cardiovasc Thorac Surg. 2010;11(2):133–7. [DOI] [PubMed] [Google Scholar]
- 48.Netuka I, Litzler PY, Berchtold-Herz M, Flecher E, Zimpfer D, Damme L, Sundareswaran KS, Farrar DJ, Schmitto JD; EU TRACE Investigators. Outcomes in HeartMate II Patients With No Antiplatelet Therapy: 2-Year Results From the European TRACE Study. Ann Thorac Surg. 2017;103(4):1262–1268. [DOI] [PubMed] [Google Scholar]
- 49.Litzler PY, Smail H, Barbay V, Nafeh-Bizet C, Bouchart F, Baste JM, Abriou C, Bessou JP. Is anti-platelet therapy needed in continuous flow left ventricular assist device patients? A single-centre experience. Eur J Cardiothorac Surg. 2014;45(1):55–9. [DOI] [PubMed] [Google Scholar]
- 50.Valerio L, Tran PL, Sheriff J, Brengle W, Ghosh R, Chiu WC, Redaelli A, Fiore GB, Pappalardo F, Bluestein D, Slepian MJ. Aspirin has limited ability to modulate shear-mediated platelet activation associated with elevated shear stress of ventricular assist devices. Thromb Res. 2016. April;140:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valerio L, Sheriff J, Tran PL, Brengle W, Redaelli A, Fiore GB, Pappalardo F, Bluestein D, Slepian MJ. Routine clinical anti-platelet agents have limited efficacy in modulating hypershear-mediated platelet activation associated with mechanical circulatory support. Thromb Res. 2018;163:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merten M, Thiagarajan P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation. 2000;102(16):1931–6. [DOI] [PubMed] [Google Scholar]
- 53.Tzima E, Trotter PJ, Orchard MA, Walker JH. Annexin V relocates to the platelet cytoskeleton upon activation and binds to a specific isoform of actin. Eur J Biochem 2000;267(15):4720–30. [DOI] [PubMed] [Google Scholar]
- 54.Chiu WC, Girdhar G, Xenos M, Alemu Y, Soares JS, Einav S, Slepian M, Bluestein D. Thromboresistance comparison of the HeartMate II ventricular assist device with the device thrombogenicity emulation-optimized HeartAssist 5 VAD. J Biomech Eng. 2014;136(2):021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Modur V, Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996;271(22):13094–102. [DOI] [PubMed] [Google Scholar]
- 56.Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. Exp Med 1993;177(5):1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, Ware RE, Fletcher DA, Lam WA. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122(1):408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163(3):740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boehme MW, Raeth U, Scherbaum WA, Galle PR, Stremmel W. Interaction of endothelial cells and neutrophils in vitro: kinetics of thrombomodulin, intercellular adhesion molecule-1 (ICAM-1), E-selectin, and vascular cell adhesion molecule-1 (VCAM-1): implications for the relevance as serological disease activity markers in vasculitides. Clin Exp Immunol. 2000;119(1):250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain A, van der Meer AD, Papa AL, Barrile R, Lai A, Schlechter BL, Otieno MA, Louden CS, Hamilton GA, Michelson AD, Frelinger AL 3rd, Ingber DE. Assessment of whole blood thrombosis in a microfluidic device lined by fixed human endothelium. Biomed Microdevices. 2016;18(4):73. [DOI] [PMC free article] [PubMed] [Google Scholar]; Malek AM, Alper SL, Izumo S. Hemodynesamic shear stress and its role in atherosclerosis. JAMA. 1999;282(21):2035–42. [DOI] [PubMed] [Google Scholar]
- 61.Girdhar G, Xu S, Jesty J, Bluestein D. In vitro model of platelet-endothelial activation due to cigarette smoke under cardiovascular circulation conditions. Ann Biomed Eng. 2008;36(7):1142–51. [DOI] [PubMed] [Google Scholar]
- 62.Cao Y, Gong 1, Liu L, Zhou Y, Fang X, Zhang C, Li Y, Li J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: a review. J Appl Toxicol. 2017;37(12):1359–1369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.