Abstract
Objective:
To prospectively assess whether the infant psychosocial environment was associated with cardiometabolic risk as early as adolescence.
Study design:
Participants were recruited in Santiago, Chile, and have been followed from infancy. Inclusion criteria included healthy infants with birth weight ≥3kg and a stable caregiver. The psychosocial environment, including depressive symptoms, stressful life events, poor support for child development, father absence, and socioeconomic status, was reported by mothers at 6–12 months. BMI z-score was assessed at 5 and 10 years. BMI z-score, waist-to-hip ratio, systolic and diastolic blood pressure, fat mass and body fat percentage, fasting glucose, total and HDL cholesterol, and homeostatic model of insulin resistance were tested in adolescence.
Results:
Adolescents ranged from 16–18y (N = 588; 48.1% female). A poorer infant psychosocial environment was associated with BMI z-score at 10 years (β = 0.10, 95% CI = 0.00–0.19) and in adolescence (β = 0.15, 95% CI = 0.06–0.24) but not at 5 years. A poorer infant psychosocial environment was associated with higher blood pressure (β = 0.15, 95% CI = 0.05–0.24), greater anthropometric risk (β = 0.13, 95% CI = 0.03–0.22), greater biomarker (triglycerides, HOMA-IR, total cholesterol) risk (β = 0.12, 95% CI = 0.02–0.22), and a higher likelihood of metabolic syndrome in adolescence (aOR = 1.50; 95% CI = 1.06–2.12).
Conclusions:
These findings demonstrate that a poorer infant psychosocial environment was associated with greater adolescent cardiometabolic risk. The results support screening for infants’ psychosocial environments and further research into causality, mechanisms, prevention, and intervention.
Keywords: Infancy, cardiometabolic risk, adolescence, BMI, blood pressure, metabolic syndrome, stress
Psychosocial stress and poor psychosocial environments in childhood predict greater likelihood of developing cardiovascular disease (CVD) and metabolic syndrome (MetS) in adulthood (1–5). However, most studies examining these associations are cross-sectional, using retrospective reports of childhood stress and environment obtained in adulthood. Of the few prospective studies (6–10), even fewer have examined whether differences in CVD and MetS risk emerge as early as adolescence – or even earlier in childhood. There is debate about how early these differences emerge, with some evidence that changes in cardiovascular health following a poor childhood psychosocial environment may be reliably detected during adolescence, and other studies suggest that changes can be detected starting in adulthood (4). Discrepancies may result because differences in the prevalence of obesity, MetS, or CVD between individuals with and without histories of a poor psychosocial environment may not emerge until adulthood, but underlying risk, including increased body fat, blood pressure, and biomarkers (eg, triglycerides, glucose, insulin) may emerge earlier. The early psychosocial environment could also be more associated with some biomarkers of risk than others before disease onset in adulthood. Early detection of risk profiles for CVD and MetS following a poor early psychosocial environment is important for prevention of later disease.
The aim of this study was to examine whether a poor psychosocial environment in infancy was associated with a higher likelihood of MetS in adolescence, controlling for the concurrent environment, and to test whether the associations were specific to three clusters of risk: blood pressure, anthropometric risk, and biomarkers of cardiometabolic risk. The association between infant psychosocial environment and body mass index (BMI) z-scores was also tested throughout childhood to investigate how early cardiometabolic risk emerges following a poor infant psychosocial environment.
Methods
The current study is based on a longitudinal cohort that began as an infancy iron deficiency anemia preventive trial with follow-up at 5 years, 10 years and several time points in adolescence. From 1991–1996, 6-month-old infants were enrolled at clinics in working-class communities in Santiago, Chile. Inclusion criteria for the infancy study included birth weight ≥3.0 kg, singleton term birth, stable caregiver, vaginal delivery, and residence in the selected communities. Exclusion criteria included birth complications, major congenital anomaly, phototherapy, illness, hospitalization longer than 5 days, iron therapy, another infant less than 12 months old in the household, infant in day care, or a caregiver who was illiterate or psychotic (11). The appropriate Institutional Review Boards approved the initial and follow-up studies (supplementary Methods, Appendix [available at www.jpeds.com]). Informed consent was obtained from the parent at each time point.
Questionnaires about the infant psychosocial environment were administered to mothers by clinical psychologist interviews when the infants were 6–12 months old to assess family-level psychosocial environment. The psychosocial environment was assessed as a composite variable using the top quartile of risk in 7 categories: maternal depressive symptoms (12, 13), home support for child development (14), maternal stress (15, 16), father absence, socioeconomic status (17), and maternal and paternal education (Appendix). Descriptive statistics were calculated, and participants in the risk quartile for each category were assigned a score of 1, and those in the other 3 quartiles were assigned a score of 0. These values were added to create a risk score from 0–7. If items were missing, the score was prorated using the non-missing items (using the mean across the available items). If a participant was missing more than 3 items, they were given a missing value for the composite. The same mother-reported measures were used to create psychosocial environment composites at 5 years, 10 years, and adolescence.
Height and weight were assessed at 5y and 10y by a research nurse at the Instituto de Nutrición y Tecnología de los Alimentos at the Universidad de Chile, and BMI was converted to z-scores using World Health Organization (WHO) standards.
Between 16–18y, we examined the following cardiometabolic risk factors: BMI z-score (WHO), waist circumference, fat mass index (fat mass/height2), percent fat mass (fat mass/total body mass), systolic and diastolic blood pressure (BP), fasting plasma glucose and insulin for homeostatic model assessment of insulin resistance (HOMA-IR), triglycerides, total and HDL cholesterol, and MetS. Anthropometry and blood pressure were measured by research physicians. A serum sample was obtained after a 12-hour overnight fast. We used the International Diabetes Federation (IDF) criteria for MetS (18): waist circumference ≥ 94cm for males and ≥ 80cm for females, plus any two of the following four factors: 1) triglycerides ≥ 150 mg/dL, 2) HDL-cholesterol <40 mg/dL for males and <50 mg/dL for females, 3) systolic blood pressure ≥130mm Hg or diastolic blood pressure ≥85mm Hg, 4) fasting plasma glucose ≥100 mg/dL.
SPSS version 25 was used for analyses. Covariates are detailed in the Appendix. First, timing of cardiometabolic alterations was assessed using 3 linear multivariable regression analyses using BMI z-score at 5y, 10y, and adolescence as the dependent variables in the separate analyses.
Second, MetS risk was examined as 3 separate composites informed by factor analysis (Appendix) to examine whether the psychosocial environment in infancy was more strongly associated with separate components of cardiometabolic risk. Factor analysis has been used to create composites of cardiometabolic risk in both adolescent and adult samples (19, 20). Principal component analysis indicated that 3 factors had favorable psychometric properties to allow for creation of continuous variables: 1) blood pressure, 2) anthropometric risk, and 3) biomarker risk. To account for sex differences in individual cardiometabolic risk variables, each of the variables in the composites were standardized within sex such that the final composites were sex-adjusted. Blood pressure was the mean of the z-scores for systolic and diastolic blood pressure measures (factor loadings ≥ .90; α = .77). Anthropometric risk was the mean of the z-scores for waist circumference, fat mass index (fat mass/height2), percent fat mass (fat mass/total body mass), and BMI (z-score from WHO, age-and sex-adjusted; factor loadings ≥ .88; α = .94). Biomarker risk was the mean of the z-scores for HOMA-IR, total cholesterol, and triglycerides measures (factor loadings ≥ .70; α = .60). Three multivariable linear regressions were conducted with each of these variables as the dependent variable.
Third, to test whether a poorer psychosocial environment in infancy was associated with MetS in adolescence, multivariable logistic regression was conducted with MetS (0 = no MetS, 1 = MetS) as the dependent variable. Control variables were entered into the first step of the regression, and psychosocial environment in infancy was entered into the second step. Additional analyses of individual cardiometabolic risk variables by infant psychosocial environment quartiles of risk (lowest quartile to highest quartile) controlling for covariates were conducted to demonstrate effect sizes by quartile of risk (Figure and Table 4 [available at www.jpeds.com]). Alpha levels of .05 were used to determine significance. Sex differences were tested by adding an interaction term between the mean-centered infant psychosocial environment and sex variables for each outcome. Finally, if infant psychosocial environment was associated with either age 5 or 10 BMI z-score, these variables will be added to the final models with adolescent outcomes to test for mediation through increases in BMI z-score at earlier time points.
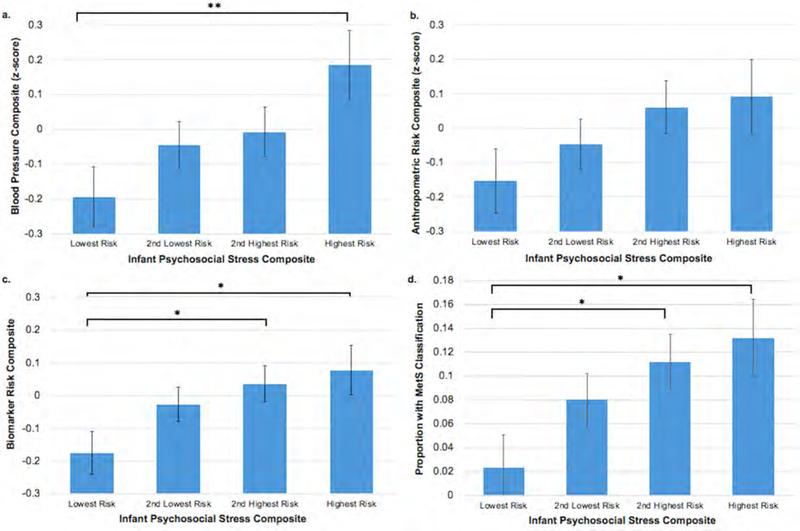
Figure.
Infant psychosocial environment composite risk quartiles by a) the blood pressure composite, b) the anthropometric risk composite, c) the biomarker risk composite, and d) metabolic syndrome (MetS) classification, all controlling for covariates including sex. Lowest quartile of risk = 10 on the composite (N = 121), 2nd lowest is > 0 but ≤ 1.17 (N = 189), 2nd highest is ≥ 1.18 but ≤ 2.34 (N = 173), highest risk ≥ 2.35 (N = 105). *p < .05, **p < .01.
Table 4.
Individual adolescent cardiometabolic risk variables by quartiles of infant psychosocial environment risk.
| Variable | Infant Psychosocial Stress Score | |||||||
|---|---|---|---|---|---|---|---|---|
| Lowest risk (score = 0) N = 121 | 2nd lowest risk (0 < score ≤ 1.17) N = 189 | 2nd highest risk (1.18 ≤ score ≤ 2.34) N = 173 | Highest risk (score ≥ 2.35) N = 105 | |||||
| M | 95% CI | M | 95% CI | M | 95% CI | M | 95% CI | |
| SBP (mmHg) | 109.4 | 107.4–111.3 | 111.0 | 109.4–112.5 | 112.1* | 110.5–113.7 | 113.3* | 111.1–115.5 |
| DBP (mmHg) | 67.9 | 66.6–69.2 | 68.9 | 67.9–69.9 | 68.6 | 67.5–69.6 | 70.4* | 68.9–71.9 |
| BMI z-score | 0.45 | 0.23–0.68 | 0.64 | 0.46–0.81 | 0.77* | 0.59–0.96 | 0.77 | 0.51–1.03 |
| Fat mass index | 6.83 | 6.17–7.49 | 7.11 | 6.59–7.63 | 7.56 | 7.02–8.11 | 7.56 | 6.79–8.32 |
| Percent total fat mass | 28.5 | 26.9–30.1 | 28.6 | 27.4–29.9 | 29.7 | 28.3–31.0 | 29.5 | 27.7–31.4 |
| Waist circumference (cm) | 79.0 | 76.9–81.2 | 80.9 | 79.2–82.6 | 81.7 | 79.9–83.5 | 83.1* | 80.6–85.6 |
| HOMA-IR | 1.48 | 1.29–1.66 | 1.71 | 1.56–1.85 | 1.81* | 1.66–1.96 | 1.89* | 1.68–2.10 |
| Total cholesterol (mg/dL) | 150.1 | 145.2–154.9 | 153.4 | 149.6–157.2 | 152.6 | 148.6–156.6 | 154.3 | 148.7–160.0 |
| Triglycerides (mg/dL) | 79.5 | 71.1–88.0 | 83.8 | 77.1–90.5 | 91.1* | 84.2–98.1 | 88.4 | 78.6–98.2 |
| Fasting glucose (mg/dL) | 89.6 | 87.9–91.4 | 88.2 | 86.8–89.6 | 88.3 | 86.9–89.8 | 87.3 | 85.2–89.3 |
| HDL (mg/dL) | 41.3 | 39.3–43.3 | 39.8 | 38.2–41.4 | 39.7 | 38.0–41.3 | 39.2 | 36.9–41.5 |
Note. Mean and 95% confidence intervals are presented for adolescents grouped by infant psychosocial environment scores (range: 0–7) controlling for all covariates including sex. SBP = systolic blood pressure; DBP = diastolic blood pressure; BMI = body mass index; HOMA-IR = homeostatic model assessment- insulin resistance; HDL = high density lipoprotein.
Significantly different than lowest risk group, p < .05.
Results
A total of 588 adolescents were included in analyses (283 female; 48.1%). At the 5-year assessment, children ranged from 5.5–6.0y (M = 5.5 y, SD = .04), and 9.9–10.4y at the 10-year assessment (M = 10.0y, SD = .05). At the first adolescent assessment, adolescents ranged from 11.9–17.8y (M = 14.1y, SD = 1.5). Adolescents ranged from 16.4–18.1y at the cardiometabolic risk assessment (M = 16.8y, SD = 0.3). All participants were of Hispanic/Latino origin. Among males, 22.0% had overweight (>1 SD above WHO median) and an additional 14.1% had obesity (>2 SD above WHO median) in adolescence. Among females, 27.2% had overweight and an additional 15.5% had obesity. Descriptive characteristics by sex are included in Table I. Little’s Missing Completely at Random Test indicated that the cardiometabolic risk, psychosocial environment, and background variables were missing at random.
Table 1.
Participant Characteristics (N = 588).
| Variable | Males (N = 305) | Females (N = 283) | ||||
|---|---|---|---|---|---|---|
| No. (%) | M (SD) | % Missing | No. (%) | M (SD) | % Missing | |
| Birth weight (g) | 3598.4 (369.4) | 0 | 3517.5 (362.0) | 0 | ||
| Weight increase from birth-6 months (g) | 4728.1 (868.0) | 0 | 4213.0 (707.8) | 0 | ||
| Formula/milk intake (average mL/day) | 381.0 (211.6) | 0.3 | 379.0 (199.5) | 0.4 | ||
| Supplementation group | 0 | 0 | ||||
| Iron supplemented | 154 (50.5%) | 171 (60.4%) | ||||
| No added iron | 151 (49.5%) | 112 (39.6%) | ||||
| Maternal age at birth (y) | 26.56 (6.23) | 0 | 26.15 (5.90) | 0.4 | ||
| Mother’s education in infancy (y) | 9.46 (2.46) | 0 | 9.74 (2.55) | 0 | ||
| Father’s education in infancy (y) | 10.02 (2.44) | 0 | 9.60 (2.79) | 0 | ||
| Father absence | 42 (13.8%) | 0 | 44 (15.5%) | 0 | ||
| Graffar (SES) in infancy | 21.46 (5.88) | 0 | 21.35 (5.55) | 0 | ||
| HOME score in infancy | 30.51 (4.76) | 0 | 29.94 (4.73) | 0 | ||
| Maternal stress in infancy | 4.67 (2.62) | 0.3 | 4.51 (2.54) | 0 | ||
| Maternal depressive symptoms in infancy | 17.02 (9.47) | 0 | 16.26 (10.09) | 0 | ||
| Psychosocial Environment Score | ||||||
| Infancy | 1.57 (1.18) | 0 | 1.62 (1.18) | 0 | ||
| 5y | 1.85 (1.40) | 0 | 1.93 (1.51) | 0 | ||
| 10y | 1.97 (1.52) | 9.5 | 1.92 (1.48) | 12.4 | ||
| Adolescence | 2.09 (1.56) | 4.9 | 2.11 (1.54) | 4.9 | ||
| BMI z-score | ||||||
| 5y | 1.07 (1.23) | 0 | 0.97 (1.13) | 1.1 | ||
| 10y | 1.20 (1.21) | 9.2 | 0.99 (1.10) | 12.4 | ||
| Adolescence | 0.60 (1.20) | 0 | 0.76 (1.16) | 0 | ||
| Adolescent Assessment | ||||||
| Participant age (y) | 16.80 (0.24) | 0 | 16.83 (0.27) | 0 | ||
| Parent: diabetes at adolescent assessment | 41 (13.4%) | 8.2 | 29 (11.2%) | 8.5 | ||
| Parent: hypertension at adolescent assessment | 115 (37.7%) | 4.6 | 108 (39.3%) | 2.8 | ||
| MetS present (IDF) | 24 (7.9%) | 0.3 | 25 (8.8%) | 0 | ||
| Waist Circumference (cm) | 81.25 (11.02) | 0 | 81.35 (11.61) | 0 | ||
| Fat Mass Index (fat mass/height2) | 5.58 (3.27) | 0.7 | 9.16 (3.51) | 1.4 | ||
| Percent Fat Mass (%) | 22.27 (8.88) | 0.7 | 36.61 (7.35) | 1.4 | ||
| Systolic Blood Pressure (mmHg) | 114.91 (10.33) | 0 | 108.07 (9.46) | 0 | ||
| Diastolic Blood Pressure (mmHg) | 70.48 (6.84) | 0 | 67.39 (6.83) | 0 | ||
| HOMA-IR | 1.70 (1.01) | 0.3 | 1.75 (0.95) | 0 | ||
| Triglycerides (mg/dL) | 85.67 (44.87) | 0.3 | 86.45 (41.25) | 0 | ||
| Total cholesterol (mg/dL) | 147.34 (24.54) | 0.3 | 156.94 (23.92) | 0 | ||
| HDL (mg/dL) | 37.89 (10.04) | 0.3 | 42.58 (10.65) | 0 | ||
| Glucose (mg/dL) | 90.52 (8.97) | 0.3 | 86.36 (8.92) | 0 | ||
Note. Values are n (%) for categorical variables and mean (SD) for continuous variables. The scale of the psychosocial stress score is 0–7. Percentages calculated for those with non-missing data on each variable. MetS = metabolic syndrome; SBP = systolic blood pressure; DBP = diastolic blood pressure; BMI = body mass index; HOMA-IR = homeostatic model assessment- insulin resistance; HDL = high density lipoprotein
A poorer psychosocial environment in infancy was not associated with BMI z-score at age 5 (β = 0.07, SE = .05, 95% CI = −0.03–0.16; p = .16) controlling for age 5 psychosocial environment. A poorer psychosocial environment in infancy was associated with higher BMI z-score at age 10 (β = 0.10, SE = 0.05, 95% CI = 0.00–0.19; P = .05), controlling for age 10 psychosocial environment, and with higher BMI z-scores at the adolescent assessment (β = 0.15, SE = .05, 95% CI = 0.06–0.24, p = .002), controlling for adolescent environment (Table 2). The interaction between sex and infant psychosocial environment did not significantly predict BMI z-score at ages 5, 10, or adolescence, ps > .50.
Table 2.
Linear regressions predicting BMI z-score at 5y, 10y, and 16–18y.
| Variable | β | 95% CI | P-value |
|---|---|---|---|
| 5y BMI z-score | |||
| Poor infant psychosocial environment | 0.07 | −0.03, 0.16 | .16 |
| Age | 0.01 | −0.07, 0.08 | .90 |
| Female | 0.05 | −0.04, 0.13 | .26 |
| Birth weight | 0.10* | 0.02, 0.18 | .015 |
| Weight change from 0–6 months | 0.28*** | 0.20, 0.37 | <.001 |
| Formula intake (mL/day) | 0.01 | −0.07, 0.09 | .82 |
| Received medicinal iron | −0.07 | −0.15, 0.02 | .12 |
| Randomized to iron supplementation | −0.04 | −0.13, 0.05 | .42 |
| Poor 5y psychosocial environment | −0.03 | −0.13, 0.06 | .51 |
| 10y BMI z-score | |||
| Poor infant psychosocial environment | 0.10* | 0.00, 0.19 | .050 |
| Age | 0.01 | −0.07, 0.10 | .79 |
| Female | −0.03 | −0.12, 0.07 | .57 |
| Birth weight | 0.11** | 0.03, 0.20 | .009 |
| Weight change from 0–6 months | 0.20*** | 0.11, 0.29 | < .001 |
| Formula intake (mL/day) | −0.07 | −0.16, 0.02 | .12 |
| Received medicinal iron | −0.04 | −0.13, 0.05 | .27 |
| Randomized to iron supplementation | 0.01 | −0.09, 0.10 | .86 |
| Poor 10y psychosocial environment | −0.08 | −0.18, 0.02 | .10 |
| Adolescent BMI z-score (16–18y) | |||
| Poor infant psychosocial environment | 0.15** | 0.06, 0.24 | .002 |
| Age | 0.04 | −0.04, 0.12 | .35 |
| Female | 0.12** | 0.04, 0.21 | .005 |
| Birth weight | 0.13*** | 0.05, 0.22 | .001 |
| Weight change from 0–6 months | 0.18*** | 0.09, 0.26 | < .001 |
| Formula intake (mL/day) | −0.08 | −0.16, 0.01 | .074 |
| Received medicinal iron | −0.02 | −0.11, 0.07 | .68 |
| Randomized to iron supplementation | −0.02 | −0.11, 0.08 | .73 |
| Poor adolescent psychosocial environment | −0.03 | −0.12, 0.07 | .57 |
| Adolescent Blood Pressure Risk Composite | |||
| Poor infant psychosocial environment | 0.15** | 0.05, 0.24 | .003 |
| Age | 0.10* | 0.01, 0.18 | .031 |
| Female | 0.00 | −0.09, 0.09 | .98 |
| Birth weight | 0.10* | 0.01, 0.18 | .033 |
| Weight change from 0–6 months | 0.04 | −0.05, 0.14 | .36 |
| Formula intake (mL/day) | −0.03 | −0.23, 0.06 | .53 |
| Received medicinal iron | −0.07 | −0.16, 0.02 | .15 |
| Randomized to iron supplementation | −0.03 | −0.12, 0.07 | .60 |
| Parent diabetes | 0.09* | 0.00, 0.18 | .049 |
| Parent hypertension | 0.09* | 0.00, 0.18 | .044 |
| Poor adolescent psychosocial environment | −0.07 | −0.16, 0.03 | .19 |
| Adolescent Anthropometric Risk Composite | |||
| Poor infant psychosocial environment | 0.13* | 0.03, 0.22 | .011 |
| Age | 0.09* | 0.00, 0.18 | .050 |
| Female | 0.06 | −0.03, 0.15 | .20 |
| Birth weight | 0.14** | 0.05, 0.23 | .003 |
| Weight change from 0–6 months | 0.15*** | 0.06, 0.25 | .001 |
| Formula intake (mL/day) | −0.10* | −0.19, −0.01 | .037 |
| Received medicinal iron | 0.00 | −0.09, 0.09 | .96 |
| Randomized to iron supplementation | −0.03 | −0.13, 0.06 | .49 |
| Parent diabetes | 0.04 | −0.05, 0.13 | .43 |
| Parent hypertension | 0.04 | −0.05, 0.13 | .40 |
| Poor adolescent psychosocial environment | 0.00 | −0.10, 0.10 | .99 |
| Adolescent Biomarker Composite | |||
| Poor infant psychosocial environment | 0.12* | 0.02, 0.22 | .016 |
| Age | 0.16*** | 0.07, 0.24 | < .001 |
| Female | −0.01 | −0.10, 0.09 | .87 |
| Birth weight | 0.00 | −0.09, 0.09 | .93 |
| Weight change from 0–6 months | 0.05 | −0.04, 0.15 | .27 |
| Formula intake (mL/day) | −0.02 | −0.11, 0.07 | .70 |
| Received medicinal iron | −0.03 | −0.12, 0.07 | .58 |
| Randomized to iron supplementation | 0.01 | −0.09, 0.11 | .83 |
| Parent diabetes | 0.07 | −0.02, 0.16 | .14 |
| Parent hypertension | 0.05 | −0.04, 0.14 | .25 |
| Poor adolescent psychosocial environment | −0.11* | −0.21, 0.00 | .041 |
p ≤ .05
p ≤ .01
p ≤ .001.
A poorer psychosocial environment in infancy was associated with higher adolescent blood pressure (β = 0.15, SE = 0.05, 95% CI = 0.05–0.24; p = .003; Table 2), higher adolescent anthropometric risk (β = 0.13, SE = 0.05, 95% CI = 0.03–0.22; p = .011), and higher biomarker (HOMA-IR, total cholesterol, and triglycerides) risk (β = 0.12, SE = .05, 95% CI = 0.02–0.22; p = .016). The interaction between sex and infant psychosocial environment was not significant for any of the adolescent risk composites, ps > .22. After controlling for the significant effect of BMI z-score at 10 years (β = 0.31, SE = 0.04, 95% CI = 0.22–0.40; p < .001), the infant psychosocial environment remained associated with blood pressure risk (β = 0.11, SE = 0.05, 95% CI = 0.02–0.21; p = .022). After controlling for BMI z-score at 10 years (β = 0.80, SE = 0.03, 95% CI = 0.74–0.86; p < .001), the infant psychosocial environment was no longer associated with anthropometric risk (β = 0.05, SE = 0.03, 95% CI = −0.02–0.11; p = .15), suggesting at least partial mediation. After controlling for BMI z-score at 10 years (β = 0.37, SE = 0.05, 95% CI = 0.27–0.46; p < .001), the infant psychosocial environment was no longer associated with biomarker risk (β = 0.08, SE = .05, 95% CI = −0.02–0.18; p = .13), suggesting partial mediation.
A poorer psychosocial environment in infancy was associated with greater likelihood of MetS in adolescence (aOR = 1.50; 95% CI = 1.06–2.12; p = .024), even after controlling for the adolescent psychosocial environment (Figure and Table 3). The interaction between sex and infant psychosocial environment was not significant, p = .88. After controlling for BMI z-score at 10 years (aOR = 3.82, 95% CI = 2.41–6.08; p < .001), the infant psychosocial environment was no longer associated with adolescent metabolic syndrome (aOR = 1.18; 95% CI = 0.80–1.76; p = .41), suggesting at least partial mediation. Adolescent data by quartiles of infant psychosocial environment (lowest to highest quartiles of risk) are presented in the Figure and Table 4 to demonstrate effect sizes. Follow-up analyses indicated that the total psychosocial environment variable predicted cardiometabolic risk better than the socioeconomic/parental education and psychosocial risk (depressive symptoms, life stress, home support for child development, father absence) variables separately for all outcomes except anthropometric and blood pressure risk. The psychosocial risk variable (excluding socioeconomic/education) predicted blood pressure risk (β = 0.11, SE = 0.04, 95% CI = 0.02–0.20; p = .013), anthropometric risk (β = 0.13, SE = 0.05, 95% CI = 0.04–0.22; p = .003), and biomarker risk (β = 0.12, SE = 0.05, 95% CI = 0.03–0.21; p = .012) as well as the total infant environment variable.
Table 3.
Logistic regression predicting MetS at 16–18y
| Variable | β | SE | P-value | aOR | 95% CI |
|---|---|---|---|---|---|
| Poor infant psychosocial environment | 0.40* | 0.18 | .024 | 1.50 | 1.06, 2.12 |
| Age | 0.23 | 0.16 | .15 | 1.26 | 0.92, 1.71 |
| Female | 0.12 | 0.17 | .49 | 1.13 | 0.80, 1.59 |
| Birth weight | 0.18 | 0.16 | .28 | 1.19 | 0.87, 1.64 |
| Weight change from 0–6 months | 0.34* | 0.17 | .045 | 1.41 | 1.01, 1.97 |
| Formula intake (mL/day) | 0.04 | 0.17 | .82 | 1.04 | 0.74, 1.46 |
| Received medicinal iron | −0.20 | 0.18 | .25 | 0.82 | 0.57, 1.16 |
| Randomized to iron supplementation | −0.20 | 0.18 | .28 | 0.82 | 0.57, 1.17 |
| Parent diabetes | −0.09 | 0.17 | .61 | 0.92 | 0.66, 1.27 |
| Parent hypertension | 0.29 | 0.16 | .077 | 1.33 | 0.97, 1.84 |
| Poor adolescent psychosocial environment | 0.00 | 0.19 | .98 | 1.00 | 0.69, 1.45 |
SE = standard error; aOR = adjusted odds ratio; CI = confidence interval.
p < .05.
Discussion
The scientific statement from the American Heart Association on the associations between childhood/adolescent stress and cardiometabolic health (4) calls for more prospective studies of the types of stressors and environments associated with cardiometabolic risk, and studies that investigate prevention and intervention targets. The current study informs the prospective literature and adds information about the type and timing of environmental risk that could confer greater cardiometabolic risk. We found that a poorer psychosocial environment, prospectively measured in infancy, was associated with increased odds of MetS, and greater blood pressure, anthropometric, and biomarker risk in adolescence. These associations were found within biologically low-risk infants (normal birth weight, no perinatal complications) and remained significant after controlling for the current environment.
In contrast to some studies (7, 21), the current analyses suggest that variations in the infant psychosocial environment were associated with increased BMI z-score at 10 years, but not as early as 5 years, controlling for the concurrent environment. These differences may be due to our broader measurement of the infant psychosocial environment rather than severe stressors such as maltreatment, which could capture more chronic negative experiences in infancy. Prior reports of these associations have frequently focused on children exposed to more extreme forms of stress and poor environments such as abuse, neglect, and adverse childhood experiences (ACEs; (1, 2, 7, 21). Our findings suggest that a poorer psychosocial environment in infancy has a dose-response association with adolescent cardiometabolic risk rather than a threshold effect (Figure) and that these associations can be seen even if the environmental exposures are less severe than some of the stressors included in the ACEs.
The association between infant psychosocial environment and BMI z-score was stronger in adolescence than at age 10, which is consistent with other studies (10, 22, 23), demonstrating that cardiometabolic risk following psychosocial stress may be more clearly detected in adolescence rather than childhood, as markers of cardiometabolic risk emerge. Many of these studies include the psychosocial environment measured more broadly than solely maltreatment (10, 23), which may also contribute to this consistency. These findings are also in agreement with a large study showing associations between ACEs and both BMI and waist circumference in 11-to 14-year-olds but did not find an association between ACEs and blood pressure at this age (24). We hypothesize that the timing of the blood pressure measurement may be a reason for this difference, as the current study examined blood pressure in 16-to 18-year-olds. It is likely that differences in BMI z-score may precede alterations in blood pressure, which may be better detected in adolescence. However, blood pressure was not measured at ages 5 or 10 years in our sample, and we cannot directly test this hypothesis. Our analyses suggest that after controlling for age 10 BMI z-score, the infant psychosocial environment was no longer associated with adolescent metabolic syndrome, anthropometric risk, or biomarker risk, which could indicate that age 10 BMI z-score at least partially mediates these associations. However, the association between infant psychosocial environment and adolescent blood pressure risk remained significant even after controlling for age 10 BMI z-score—though the β was reduced from 0.15 to 0.11—suggesting that at least part of the association between infant psychosocial environment and adolescent blood pressure risk was independent of age 10 BMI z-score.
The infant, but not adolescent, psychosocial environment was related to MetS classification and all three of the cardiometabolic risk composites, suggesting that the psychosocial environment in infancy may be more closely related to cardiometabolic health in adolescence than the adolescent environment. In addition, a poorer adolescent environment was associated with lower biomarker risk, suggesting timing of environmental risk may play a role in cardiometabolic risk profiles. These findings support the developmental origins of disease hypothesis, which predicts programming effects of the early environment on later health (25). Potential mechanisms include, but are not limited to, alterations in DNA methylation (26), deleterious changes in the hypothalamic-pituitary-adrenal axis and immune system (27, 28), continued stress (29), living in an obesogenic environment or being exposed to obesogenic agents (e.g., pollution and secondhand smoke; (30), altered food intake (31), less sensitive and responsive parenting (32), and poorer parental health behaviors.
The historical context of the current findings is important to consider. Chile experienced a major increase in obesity concurrent with a rapid economic transition in which the food and physical activity environment changed dramatically (33). In Chile, the obesity rate for 6-year-olds increased from 8.9% to 17.0% between 1990 and 2000 for boys and from 10.1% to 18.6% for girls (34). Thus, our sample grew up during a rapid economic and nutritional transition which created risk, especially for those experiencing psychosocial risk, leading to excess weight gain in childhood and adolescence and associated cardiometabolic risk (35, 36).
There were limitations that must be considered. The study recruited from low-to middle-income communities in Santiago, Chile, which may constrain generalizability. Missing data for some participants also limits generalizability. The psychosocial environment composite included mostly objective measures of the environment, including socioeconomic status, father absence, and occurrence of stressors. There may be stronger or weaker associations of cardiometabolic risk with subjective measures of the environment by the mother or adolescent, such as perceived level of stress or perceptions of income inequality, as objective and subjectively measured stress and the environment can have different associations with outcomes (37). However, the strengths of the longitudinal study include prospective measurements of the environment and health, and objective measures of cardiometabolic risk.
Our findings suggest that resources given to reduce psychosocial risk in families with infants could promote long-term cardiometabolic health. It is unknown whether these associations may be reversible later in life, but the current results suggest that the infant environment is more strongly associated with adolescent cardiometabolic risk than the adolescent environment. More research is needed to understand the potential biological, behavioral, and environmental mechanisms that could transmit risk to infants, including research investigating how early cardiometabolic risk following infant psychosocial risk emerges and examining whether these associations strengthen over time.
Supplementary Material
Acknowledgements:
We thank the families who have participated and continue to participate in this research.
Funded by the National Institutes for Health (F32HD088029 [to J.D.], R01HD14122 [to B.L.], R01HD33487 [to B.L. and S.G.], and R01HL088530 [to S.G.]). The funder had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the manuscript for publication.
Abbreviations:
- MetS
metabolic syndrome
- CVD
cardiovascular disease
- BMI
body mass index
- HOMA-IR
homeostatic model assessment of insulin resistance
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- HDL
high density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American journal of preventive medicine 1998;14:245–58. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert LK, Breiding MJ, Merrick MT, Thompson WW, Ford DC, Dhingra SS, et al. Childhood adversity and adult chronic disease. American journal of preventive medicine 2015;48:345–9. [DOI] [PubMed] [Google Scholar]
- 3.Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012;129:e232–e46. [DOI] [PubMed] [Google Scholar]
- 4.Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Clark CJ, Danese A, et al. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation 2018;137:e15–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas C, Hyppönen E, Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics 2008;121:e1240–e9. [DOI] [PubMed] [Google Scholar]
- 6.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of pediatrics & adolescent medicine 2009;163:1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Molecular psychiatry 2014;19:544. [DOI] [PubMed] [Google Scholar]
- 8.Hakulinen C, Pulkki-Råback L, Elovainio M, Kubzansky LD, Jokela M, Hintsanen M, et al. Childhood psychosocial cumulative risks and carotid intima-media thickness in adulthood: the cardiovascular risk in young Finns study. Psychosomatic medicine 2016;78:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klassen SA, Chirico D, O’Leary DD, Cairney J, Wade TJ. Linking systemic arterial stiffness among adolescents to adverse childhood experiences. Child abuse & neglect 2016;56:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Wells NM, Evans GW, Beavis A, Ong AD. Early childhood poverty, cumulative risk exposure, and body mass index trajectories through young adulthood. American journal of public health 2010;100:2507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 2003;112:846–54. [PubMed] [Google Scholar]
- 12.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement 1977;1:385–401. [Google Scholar]
- 13.Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of youth and adolescence 1991;20:149–66. [DOI] [PubMed] [Google Scholar]
- 14.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment (Revised Edition) Little Rock: University of Arkansas; 1984. [Google Scholar]
- 15.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Med 1967;11:213–8. [DOI] [PubMed] [Google Scholar]
- 16.Bradley RH, Corwyn RF, Whiteside‐Mansell L. Life at home: same time, different places—an examination of the HOME inventory in different cultures. Infant and Child Development 1996;5:251–69. [Google Scholar]
- 17.Alvarez M, Muzzo S, Ivanovic D. Escala para la medicion del nivel socioeconomico en el area de la salud. Revista Medica de Chile 1985;113:243–9. [PubMed] [Google Scholar]
- 18.Alberti G, Zimmet P, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The IDF consensus definition of the metabolic syndrome in children and adolescents. Pediatric Diabetes 2007;8:299–306. [DOI] [PubMed] [Google Scholar]
- 19.Goodman E, Dolan LM, Morrison JA, Daniels SR. Factor analysis of clustered cardiovascular risks in adolescence: obesity is the predominant correlate of risk among youth. Circulation 2005;111:1970–7. [DOI] [PubMed] [Google Scholar]
- 20.Tsai CH, Li TC, Lin CC, Tsay HS. Factor analysis of modifiable cardiovascular risk factors and prevalence of metabolic syndrome in adult Taiwanese. Endocrine 2011;40:256–64. [DOI] [PubMed] [Google Scholar]
- 21.Power C, Pereira SMP, Li L. Childhood maltreatment and BMI trajectories to mid-adult life: follow-up to age 50y in a British birth cohort. PLoS One 2015;10:e0119985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin SH, Miller DP. A longitudinal examination of childhood maltreatment and adolescent obesity: results from the National Longitudinal Study of Adolescent Health (AddHealth) Study. Child abuse & neglect 2012;36:84–94. [DOI] [PubMed] [Google Scholar]
- 23.Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain, behavior, and immunity 2012;26:239–50. [DOI] [PubMed] [Google Scholar]
- 24.Pretty C, D O’Leary D, Cairney J, Wade TJ. Adverse childhood experiences and the cardiovascular health of children: a cross-sectional study. BMC pediatrics 2013;13(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gluckman PD, Hanson MA. Developmental plasticity and the developmental origins of health and disease. Early Life Origins Of Human Health and Disease: Karger Publishers; 2009. p. 1–10. [Google Scholar]
- 26.Hao G, Youssef NA, Davis CL, Su S. The role of DNA methylation in the association between childhood adversity and cardiometabolic disease. International journal of cardiology 2018;255:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biological psychiatry 2016;80:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 29.Raposa EB, Hammen CL, Brennan PA, O’callaghan F, Najman JM. Early adversity and health outcomes in young adulthood: The role of ongoing stress. Health Psychology 2014;33:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang C-C, et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environmental health perspectives 2015;123:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition 2007;23(11):887–94. [DOI] [PubMed] [Google Scholar]
- 32.Evans GW, Kim P. Childhood Poverty, Chronic Stress, Self‐Regulation, and Coping. Child Development Perspectives 2013;7:43–8. [Google Scholar]
- 33.Albala C, Vio F, Kain J, Uauy R. Nutrition transition in Chile: determinants and consequences. Public health nutrition 2002;5:123–8. [DOI] [PubMed] [Google Scholar]
- 34.Kain J, Uauy R, Vio F, Albala C. Trends in overweight and obesity prevalence in Chilean children: comparison of three definitions. European journal of clinical nutrition 2002;56:200. [DOI] [PubMed] [Google Scholar]
- 35.Khuc K, Blanco E, Burrows R, Reyes M, Castillo M, Lozoff B, et al. Adolescent metabolic syndrome risk is increased with higher infancy weight gain and decreased with longer breast feeding. International journal of pediatrics 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacheco LS, Blanco E, Burrows R, Reyes M, Lozoff B, Gahagan S. Peer Reviewed: Early Onset Obesity and Risk of Metabolic Syndrome Among Chilean Adolescents. Preventing chronic disease 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior 1983:385–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.