Abstract
Objective:
To investigate patient factors predictive of gamma glutamyltransferase (GGT) normalization following Ursodeoxycholic acid (UDCA) therapy in children with primary sclerosing cholangitis (PSC).
Study design:
We retrospectively reviewed patient records at 46 centers. We included patients with a baseline serum GGT level ≥50 IU/L at PSC diagnosis, who initiated UDCA therapy within one month and continued therapy for at least 1 year. We defined ‘normalization’ as a GGT level < 50 IU/L without experiencing portal hypertensive or dominant stricture events, liver transplantation or death during the first year.
Results:
We identified 263 patients, median age 12.1 years at diagnosis, treated with UDCA at a median dose of 15 mg/kg/day. Normalization occurred in 46%. Patients with normalization had a lower prevalence of Crohn’s disease, lower total bilirubin level, lower AST to Platelet Ratio Index, higher platelet count, and higher serum albumin level at diagnosis. The 5-year survival with native liver was 99% in those patients who achieved normalization vs 77% in those who did not
Conclusions:
Less than half of the patients treated with UDCA have a complete GGT normalization in the first year after diagnosis, but this subset of patients has a favorable five-year outcome. Normalization is less likely in patients with a Crohn’s disease phenotype or a laboratory profile suggestive of more advanced hepatobiliary fibrosis. Patients who do not achieve normalization could reasonably stop UDCA, as they are likely not receiving clinical benefit. Alternative treatments with improved efficacy are needed, particularly for patients with already-advanced disease.
Keywords: Juvenile, Cholestasis, Autoimmune, Surrogate Endpoint, Treatment
Primary sclerosing cholangitis (PSC) is a rare cholestatic liver disease characterized by progressive destruction of the bile ducts.1 Within 10 years of diagnosis, 30% of children with PSC will require liver transplantation and 50% of children will develop complications, including biliary stricturing and portal hypertension2. To date there is no medical intervention proven to prolong patient survival3. PSC is recognized as one the largest unmet needs in hepatology4.
Ursodeoxycholic acid (UDCA) is a hydrophilic bile acid with hepatobiliary cytoprotective and immune-modulating effects5–7. The role of UDCA in modifying disease behavior in PSC and prolonging patient survival is controversial. UDCA has not been shown to improve patient survival in adult clinical trials8–12. Given the unclear benefit, as well as a possibility that higher doses of UDCA may cause harm12, the current practice guideline of the American Association of the Study of Liver Diseases (AASLD) recommends against the use of UDCA in adults with PSC13. There are no formal guidelines for the use of UDCA in children with PSC.
A subset of adults normalize serum alkaline phosphatase levels during UDCA therapy and have improved survival compared with those with persistently elevated levels14–16. We showed an analogous effect in children with PSC using gamma glutamyl transferase (GGT) as a biomarker17. Children whose GGT declined to < 50 IU/L at one year after PSC diagnosis had favorable long-term outcomes. Untreated patients who spontaneously normalized serum GGT levels also had good outcomes. The proportion of patients who achieved GGT normalization was higher in those treated with UDCA however, with an approximate number needed to treat of four patients to achieve one normalization that would not have occurred on its own. Thus, there appears to be a subgroup of children treated with UDCA who have a favorable response. UDCA is the most common medication used in children with PSC, with over 80% currently receiving long-term therapy2,18. More data is needed to better define which patients may be receiving a clinical benefit from UDCA therapy. There are presently no predictors of which patients will respond. The aim of this study was to identify baseline differences between children with complete vs. incomplete biochemical normalization on UDCA.
Methods:
The Pediatric PSC Consortium is an active research collaboration that includes 49 centers in Europe, North America, the Middle East, and Asia. We retrospectively reviewed medical records on all known PSC patients with disease onset prior to 18 years of age at each institution, as previously described2. For each patient we collected data on basic demographics, and diagnostic cholangiography and histopathology studies. We collected laboratory data at liver disease diagnosis and approximately one year later (within 46–58 weeks after diagnosis). To account for the wide range of normal alkaline phosphatase (ALP) values in children at various ages due to bone turnover and growth, we normalized all values for age using Mayo Medical Laboratories reference values19.
The diagnosis of PSC required elevated hepatobiliary inflammatory markers (GGT or aspartate aminotransferase [AST] or alanine aminotransferase [ALT]) and/or bilirubin. When characteristic changes on cholangiography were seen, large duct PSC was diagnosed. When cholangiography was normal but liver histopathology showed typical changes, small duct PSC was diagnosed13. Cases of sclerosing cholangitis secondary to any other cause were excluded.
Autoimmune hepatitis (AIH) was diagnosed in patients who met the simplified AIH criteria, which have been validated in children20, based on histopathology, positive autoantibodies, elevated serum globulins and exclusion of viral hepatitis. We documented the presence and type of associated inflammatory bowel disease (IBD).
We created a retrospective cohort of all patients followed from date of PSC diagnosis to the date of several clinical endpoints: 1. the development of portal hypertensive complications (ascites, hepatic encephalopathy, or esophageal varices with or without bleeding); 2. biliary complications (a clinical picture of biliary obstruction as evidenced by a biliary stricture requiring an intervention in the form of endoscopic or percutaneous stenting, balloon dilation, or drainage); 3. Cholangiocarcinoma; 4. liver transplantation; or 5. death from liver disease. Survival free of any of these endpoints was termed event-free survival. Patients were censored at the date of last known follow-up. We excluded patients who presented with portal hypertensive or biliary complications within 3 months of PSC diagnosis from this analysis, because their baseline laboratory studies were likely reflective of the outcomes of interest already being present. Data was abstracted and de-identified at local study sites and reviewed and stored centrally. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Utah21.
In this analysis, we included patients with baseline serum GGT level > 50 IU/L, who started UDCA within one month of PSC diagnosis and continued taking it for at least one year. We defined ‘normalization’ as a decline in GGT to ≤ 50 IU/L without experiencing clinical endpoints during the first year. The threshold of 50 IU/L for GGT represents 1–2 times the upper limit of normal for most non-infant pediatric age groups at most laboratories and was validated as an endpoint in our prior study17. We compared baseline demographic, phenotypic and laboratory data between these 2 groups. We assessed differences in the change in laboratory studies between diagnosis and at one year. We compared transplant-free survival after 1 year between groups.
The Wilcoxon rank sum and chi squared tests were used to assess differences between groups. We used the Kaplan-Meier method to calculate annual outcome probabilities. The Logrank test assessed survival differences between groups. Logistic regression was used to assess the association between baseline predictor variables and treatment success or failure. Variables with P value < .1 in univariate analyses were included in a multivariate model. The model was optimized using variance inflation factor diagnostics, removing any collinear variables. Calculations were done using Stata version 15 (StataCorp, College Station, TX). All research work was approved by the institutional review board of each participating center.
Results:
We identified 344 patients with a serum GGT level > 50 IU/L at diagnosis who had laboratory data one year later. We excluded 63 patients who did not receive UDCA treatment and 18 patients with clinical complications at or within 3 months after diagnosis. The remaining 263 patients (60% male, median age 12.1 years old, median GGT 290 (IQR 163–431) were followed for a median of 5.4 years with a total of 1736 person-years of observation. The median UDCA dose administered was 15 mg/kg/day [IQR: 15–19].
Serum GGT normalization occurred in 46% (122/263) of patients. In the remainder, non-normalization occurred in 16% (23/141) due to occurrence of clinical events and in 84% due to persistently elevated GGT at one year. Patients with normalization vs. non-normalization had similar PSC phenotypes. At diagnosis however, the response group had a lower prevalence of Crohn’s disease, lower total bilirubin, lower AST to Platelet Ratio Index (APRI), higher platelet count, and higher serum albumin (Table 1). In addition to the primary endpoint of GGT reduction, the normalization group experienced larger improvements in ALT, AST, ALP and APRI compared with those with non-normalization in the year after PSC diagnosis (Table 2).
Table 1:
Baseline phenotypic and laboratory data
| Normalization n=122 |
Non-normalization n=141 |
p | |
|---|---|---|---|
| Age (years) | 12.0 [7.8–15] |
12.4 [8.6–15] |
0.416 |
| Gender (% male) | 60% | 60% | 0.941 |
| Ulcerative colitis (% with) | 74% | 59% | 0.012 |
| Crohn disease (% with) | 10% | 18% | 0.017 |
| No IBD (% with) | 16% | 23% | 0.203 |
| Autoimmune hepatitis (%) | 39% | 39% | 0.955 |
| Large duct phenotype | 73% | 76% | 0.586 |
| Hemoglobin (g/dL) | 12.4 [11.5–13.2] |
12.1 [11–13.4] |
0.137 |
| Platelet count (k/uL) |
325 [260–438] |
298 [196–375] |
<0.001 |
| INR | 1.1 [M.2] |
1.1 [1–1.2] |
0.331 |
| ALT(U/L) | 167 [87–297] |
146 [87–229] |
0.278 |
| AST (U/L) | 125 [71–226] |
140 [78–192] |
0.697 |
| ALP (U/L) | 406 [281–720] |
448 [275–717] |
0.918 |
| ALP (× ULN) | 1.0 [0.7–1.6] |
1.0 [0.6–1.5] |
0.461 |
| GGT (U/L) | 290 [148–427] |
290 [192–465] |
0.158 |
| Total Bilirubin (mg/dL) |
0.7 [0.4–1] |
0.9 [0.5–1.6] |
0.002 |
| Albumin (g/L) |
4.1 [3.7–4.5] |
3.9 [3.6–4.3] |
0.008 |
| APRI |
0.9 [0.5–1.9] |
1.3 [0.7–2.3] |
0.023 |
Table 2:
Change in biochemistry over one year of ursodeoxycholic acid treatment
| Normalization n=122 |
Non-normalization n=141 |
p | |
|---|---|---|---|
| Hemoglobin (g/dL) | +0.8 [−0.4 to +1.6] |
+0.6 [−0.4 to +1.5] |
0.382 |
| Platelet count (k/uL) | −34 [−114 to +5] |
−32 [−87 to +22] |
0.132 |
| INR | 0 [−0.1 to +0.1] |
0 [−0.1 to +0.2] |
0.600 |
| ALT (U/L) |
−141 [−266 to −59] |
−70 [−165 to +3] |
<0.001 |
| AST (U/L) |
−92 [−197 to −40] |
−62 [−151 to −7] |
<0.001 |
| ALP (U/L) |
−278 [−411 to +2] |
−129 [−618 to +80] |
<0.001 |
| ALP(× ULN) |
−0.6 [−1.3 to −0.2] |
−0.3 [−0.9 to 0] |
<0.001 |
| GGT (U/L) |
−258 [−406 to −127] |
−142 [−271 to −42] |
<0.001 |
| Total Bilirubin (mg/dL) | −0 [−0.4 to +0.2] |
+0.1 [−0.7 to +0.2] |
0.567 |
| Albumin (g/L) | +0.2 [−0.1 to +0.6] |
+0.1 [−0.3 to +0.4] |
0.038 |
| APRI |
−0.7 [−1.6 to −0.2] |
−0.4 [−1.3 to 0] |
0.023 |
The presence or absence of a Crohn’s disease IBD phenotype, as well as baseline platelet count, total bilirubin and albumin at diagnosis showed significant univariate correlation with treatment response (Table 3; available at www.jpeds.com). After regression diagnostics including all variables with p<0.10 in univariate analysis, hemoglobin was removed for excessive covariance. In the final multivariate model, a Crohn phenotype decreased the odds of normalization. Increased platelet count and serum albumin significantly increased the odds of a normalization (Table 4; available at www.jpeds.com).
Table 3:
Univariate regression analysis of baseline predictors of normalization on ursodeoxycholic acid
| Univariate Odds Ratio | p | |
|---|---|---|
| Age | 0.97 | 0.328 |
| Gender | 0.98 | 0.941 |
| UC | 1.35 | 0.235 |
| Crohn | 0.41 | 0.026 |
| No IBD | 0.57 | 0.094 |
| AIH | 1.01 | 0.955 |
| Large duct phenotype | 0.84 | 0.586 |
| Hemoglobin | 1.15 | 0.071 |
| Platelet count | 1.43 | <0.001 |
| INR | 0.82 | 0.795 |
| ALT | 1.00 | 0.660 |
| AST | 1.00 | 0.710 |
| ALP | 0.99 | 0.943 |
| ALP | 1.01 | 0.906 |
| GGT | 0.99 | 0.092 |
| Total Bilirubin | 0.87 | 0.005 |
| Albumin | 1.84 | 0.008 |
| APRI | 0.95 | 0.155 |
Table 4:
Multivariate model of baseline predictors of normalization on ursodeoxycholic acid
| Multivariate Odds Ratio | p | |
|---|---|---|
| Crohn (vs. UC or no IBD) | 0.33 | 0.012 |
| Platelet count (per 100,000 k/uL) | 1.55 | <0.001 |
| GGT (per U/L) | 0.99 | 0.119 |
| Total Bilirubin (per mg/dL) | 0.88 | 0.167 |
| Albumin (per g/dL) | 1.98 | 0.009 |
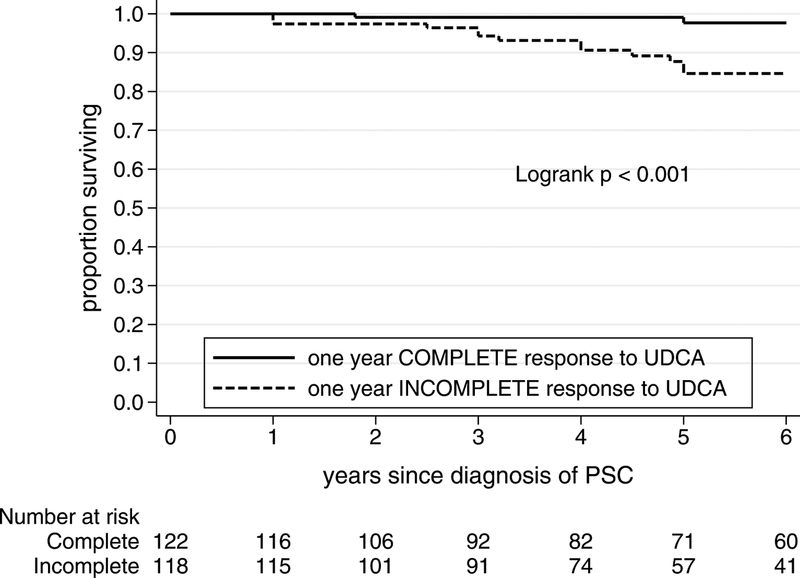
Long-term survival with native liver after one year was better in the normalization group than the incomplete response group (Figure 1). Following the first year of UDCA treatment, the annual event rate (deaths or liver transplants per group per year) was 0.7%/year in the normalization group vs. 4.3%/year in the non-normalization group, Logrank p<0.001. The 5-year survival with native liver was 99% [95%CI 94–100] in those with normalization, compared with 77% [95%CI 68–84] in those who did not normalize, p<0.001.
Figure 1.
Long-term survival with native liver in ursodeoxycholic acid treated patients
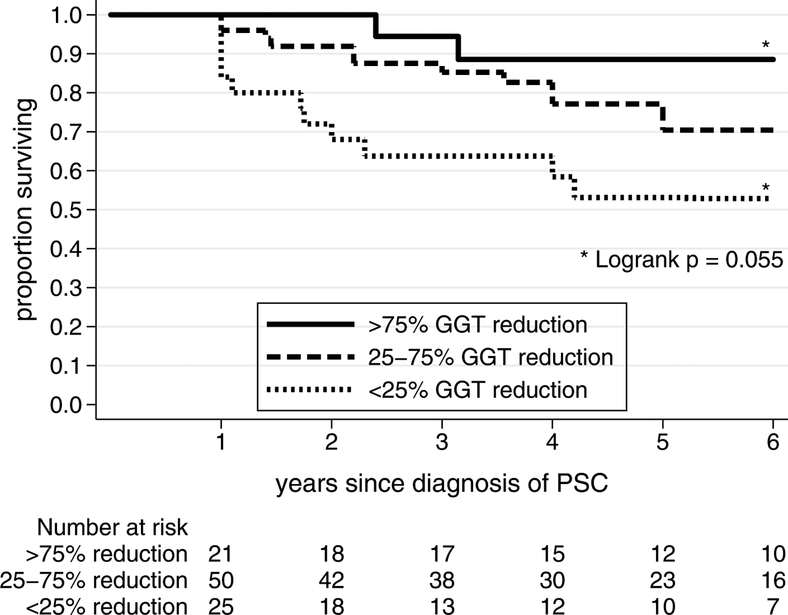
Amongst those who did not normalize GGT on UDCA who did not experience an adverse clinical event within the first year, event-free survival from years 1–6 was different based on the percentage GGT reduction achieved by one year (Figure 2). There was a trend towards the best event-free survival for patients with the largest relative GGT reductions between diagnosis and year one. Those with a > 75% reduction in GGT, compared with a < 25% reduction in GGT, had an 88% vs. 52% event-free survival over the next five years, p=0.055.
Figure 2:
Event-free survival after one year in patients with incomplete biochemical response to ursodeoxycholic acid, stratified by percentage reduction in GGT from baseline.
Discussion:
Although only a minority of children experience normalization of their serum GGT level in response to UDCA therapy, those who do show a decreased likelihood of liver transplant or death during follow-up. We identified clinically-relevant patient factors at baseline associated with non-normalization on UDCA treatment including a Crohn’s disease IBD phenotype, relative thrombocytopenia, hypoalbuminemia, or hyperbilirubinemia, and increased APRI, a surrogate marker of fibrosis.
The 46% normalization rate observed in this study is similar to a 41% biochemical response seen in an adult prospective trial of UDCA15. In our pediatric cohort, outcomes were best in those with GGT < 50 IU/L at one year after diagnosis. Even amongst those with GGT ≥ 50 IU/L at one year, outcomes were better with progressively larger reductions in GGT between diagnosis and one year. Those who had a reduction in GGT of .> 75% had nearly the same long-term survival as those with GGT < 50 IU/L at one year. This data further supports the utility of GGT reduction as a candidate surrogate endpoint for future clinical trials. Clinically, children with PSC on long-term UDCA who have not had a >75% reduction in GGT or complete normalization of GGT can stop the medicine as they are unlikely to be receiving a clinical benefit and could minimize unnecessary drug exposure.
Of note, we do not know how many of the UDCA-treated patients would have had spontaneous GGT normalization and no adverse liver events without UDCA therapy. We previously showed that nearly one third of children who are UDCA-naïve have spontaneous GGT normalization by one year. These untreated patients’ long-term survival outcomes are indistinguishable from UDCA-treated patients with GGT normalization17. A larger proportion of UDCA-treated patients achieved GGT normalization however. The number of PSC patients who would need to be treated with UDCA to achieve one case of GGT normalization that would not have occurred spontaneously without treatment was approximately four (number needed to treat = 4). A recent clinical trial evaluated the impact of UDCA withdrawal from children with PSC who had been on chronic therapy with normal liver biochemistries. Upon complete withdrawal of the medication for 12 weeks, one third of patients maintained serum GGT level < 29 IU/L22. Whether the third of patients who may have spontaneously normalized GGT and the third of patients who maintained a normal GGT upon complete withdrawal of UDCA represent the same group is speculative, but it is likely that a large proportion of children on chronic UDCA are receiving no clinical benefit. More data on the natural history of GGT in UDCA-exposed and unexposed patients within the first 3 to 6 months after diagnosis are needed to determine the optimal length of a trial of UDCA therapy.
Liver disease phenotype was not associated with normalization: patients with large and small duct disease had equivalent normalization rates, and the presence of autoimmune hepatitis overlap features did not affect outcome. Similarly, no phenotypic differences were observed in UDCA complete vs. incomplete biochemical changes in an adult randomized-controlled trial10. Biochemical differences between groups at baseline suggest that patients with more extensive hepatobiliary fibrosis were less likely to achieve normalization on UDCA. There was a consistent trend across several markers: decreased serum albumin and platelet count, and increased APRI and bilirubin in non-normalizers. Length of time with PSC before diagnosis is likely an important, but ultimately unmeasurable, factor. Patient age at diagnosis was similar in response and incomplete response groups. We speculate that there may be additional subphenotypes of PSC that are more slowly-progressive and more responsive to therapy, perhaps based on microbiome or metabolome profiles.
Crohn’s disease had a negative impact on the probability of normalization. This was in contrast to the association between Crohn’s disease and a favorable PSC prognosis in general. The presence of IBD overall (either ulcerative colitis or a Crohn’s disease phenotype) is a favorable predictor of outcome in pediatric PSC2, and a Crohn’s disease phenotype was a favorable predictor in adult PSC23. patients with Crohn’s disease may be more likely to receive immunomodulatory or biologic medicines than patients with ulcerative colitis (UC) or those with no IBD, though such medicines have not shown a survival detriment or benefit in PSC24,25. Differences in the microbiome of patients with Crohn’s disease may not favor useful, disease-modifying metabolism of UDCA in some patients. Indeed, PSC-Crohn, PSC-UC, and PSC patients with no IBD each have distinct microbiota signatures26, with underrepresentation of Butyricoccus species in PSC-Crohn compared with the others. the microbiome may impact for PSC progression itself and, metabolism of and potential responsiveness to UDCA. Additional research is needed to more fully characterize the relative effectiveness of medical therapies for PSC in patients with different microbiome profiles and across the spectrum of objectively-staged hepatobiliary fibrosis and duct stricturing.
A strength of this study was its large size and inclusion of patients from a diverse mix of secondary and tertiary referral centers. There are weaknesses to this study. The retrospective design prevented a standardized diagnostic and therapeutic algorithm, and misclassification bias may be present. We did not have data on compliance with prescribed UDCA, which may have been poor especially in teenage patients. GGT and general biochemistry was only available at the time of diagnosis and one year later. Data at earlier time points to report on rapidity of GGT improvement was not available. Similarly, longer-term data are needed to define the durability of GGT normalization on and off chronic UDCA therapy. Future studies should control for the severity of intestinal disease and concomitant immunosuppression for IBD and autoimmune hepatitis, which we were unable to do here. Finally, objective markers of disease severity and response are needed, including performing liver biopsy and cholangiography before and during therapy. These data are part of a larger, more extensive data collection that is currently underway within the Pediatric PSC Consortium to address these shortfalls.
Supplementary Material
Acknowledgments
Supported by PSC Partners Seeking A Cure, the Primary Children’s Hospital Foundation, the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR001065 and 8UL1TR000105 [formerly UL1RR025764]). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. M.D. served as a consultant for HighTide Biopharmaceuticals LLC. B.K. is a consultant for Retrophin. T.M. is a consultant, serves on the advisory board, and serves on the speaker board for Alexion. P.M. has received grants from Gilead.
List of abbreviations (in order of appearance):
- PSC
primary sclerosing cholangitis
- UDCA
ursodeoxycholic acid
- GGT
gamma glutamyl transferase
- ALP
alkaline phosphatase
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- AIH
autoimmune hepatitis
- IBD
inflammatory bowel disease
- APRI
aspartate aminotransferase to platelet ratio index
- UC
ulcerative colitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
Contributor Information
Mark Deneau, University of Utah, Salt Lake City, UT, USA.
Emily Perito, University of California San Francisco, San Francisco, CA, USA.
Amanda Ricciuto, The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada.
Nitika Gupta, Emory University School of Medicine, Atlanta, GA, USA.
Binita M. Kamath, The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada
Sirish Palle, Oklahoma University, Oklahoma City, OK, USA.
Bernadette Vitola, Medical College of Wisconsin, Milwaukee, WI,USA.
Vratislav Smolka, Palacky University, Olomouc, Czech Republic.
Federica Ferrari, Sapienza University of Rome, Rome, Italy.
Achiya Z. Amir, The Dana-Dwek Children’s Hospital, Tel-Aviv University, Tel Aviv, Israel
Tamir Miloh, Texas Children’s Hospital, Houston, TX, USA.
Alexandra Papadopoulou, University of Athens, Athens, Greece.
Parvathi Mohan, Children’s National Medical Center, Washington, DC, USA.
Cara Mack, University of Colorado School of Medicine, Aurora, CO, USA.
Kaija-Leena Kolho, University of Helsinki, Helsinki, Finland.
Raffaele Iorio, University of Naples Federico II, Naples, Italy.
Wael El-Matary, University of Manitoba, Winnipeg, Manitoba, Canada.
Veena Venkat, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Albert Chan, University of Rochester Medical Center, Rochester, NY, USA.
Lawrence Saubermann, University of Rochester Medical Center, Rochester, NY, USA.
Pamela L. Valentino, Yale University School of Medicine, New Haven, CT, USA
Uzma Shah, Harvard University, Boston, MA, USA.
Alexander Miethke, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
Henry Lin, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
MK Jensen, University of Utah, Salt Lake City, UT, USA.
References:
- 1.Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587–1599. [DOI] [PubMed] [Google Scholar]
- 2.Deneau MR, El-Matary W, Valentino PL, et al. The natural history of primary sclerosing cholangitis in 781 children: A multicenter, international collaboration. Hepatology. 2017;66(2):518–527. [DOI] [PubMed] [Google Scholar]
- 3.Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. Journal of hepatology. 2017. [DOI] [PubMed] [Google Scholar]
- 4.Dyson JK, Webb G, Hirschfield GM, et al. Unmet clinical need in autoimmune liver diseases. J Hepatol. 2015;62(1):208–218. [DOI] [PubMed] [Google Scholar]
- 5.Galle PR, Theilmann L, Raedsch R, Otto G, Stiehl A. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990;12(3 Pt 1):486–491. [DOI] [PubMed] [Google Scholar]
- 6.Stiehl A, Rudolph G, Raedsch R, et al. Ursodeoxycholic acid-induced changes of plasma and urinary bile acids in patients with primary biliary cirrhosis. Hepatology. 1990;12(3 Pt 1):492–497. [DOI] [PubMed] [Google Scholar]
- 7.Calmus Y, Gane P, Rouger P, Poupon R. Hepatic expression of class I and class II major histocompatibility complex molecules in primary biliary cirrhosis: effect of ursodeoxycholic acid. Hepatology. 1990;11(1):12–15. [DOI] [PubMed] [Google Scholar]
- 8.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691–695. [DOI] [PubMed] [Google Scholar]
- 9.Cullen SN, Rust C, Fleming K, Edwards C, Beuers U, Chapman RW. High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol. 2008;48(5):792–800. [DOI] [PubMed] [Google Scholar]
- 10.Olsson R, Boberg KM, de Muckadell OS, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129(5):1464–1472. [DOI] [PubMed] [Google Scholar]
- 11.Harnois DM, Angulo P, Jorgensen RA, Larusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. The American journal of gastroenterology. 2001;96(5):1558–1562. [DOI] [PubMed] [Google Scholar]
- 12.Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50(3):808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660–678. [DOI] [PubMed] [Google Scholar]
- 14.Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. Journal of hepatology. 2013;58(2):329–334. [DOI] [PubMed] [Google Scholar]
- 15.Lindstrom L, Hultcrantz R, Boberg KM, Friis-Liby I, Bergquist A. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;11(7):841–846. [DOI] [PubMed] [Google Scholar]
- 16.Stanich PP, Bjornsson E, Gossard AA, Enders F, Jorgensen R, Lindor KD. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2011;43(4):309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deneau MR, Mack C, Abdou R, et al. Gamma glutamyltransferase reduction is associated with favorable outcomes in pediatric primary sclerosing cholangitis. Hepatology Communications (In Press - accepted 13-Jul-2018). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valentino PL, Wiggins S, Harney S, Raza R, Lee CK, Jonas MM. The Natural History of Primary Sclerosing Cholangitis in Children: A Large Single-Center Longitudinal Cohort Study. Journal of pediatric gastroenterology and nutrition. 2016;63(6):603–609. [DOI] [PubMed] [Google Scholar]
- 19.Laboratories MM. Alkaline Phosphatase, Total and Isozymes, Serum: Clinical and Interpretive. http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/89503. Accessed August 18, 2016.
- 20.Mileti E, Rosenthal P, Peters MG. Validation and modification of simplified diagnostic criteria for autoimmune hepatitis in children. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(4):417–421 e411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black DMC, Kerkar N, Miloh T, Sundaram S, Anand R, Gupta A, Alonso E, Arnon R, Bulut P, Karpen S, Lin C, Rosenthal P, Ryan M, Squires R, Valentino P, Schneider B. Initial Results of the WUPPSC Study - Prospective Multicenter Withdrawal of Ursodeoxycholic Acid in Pediatric Primary Sclerosing Cholangitis [Abstract}. Gastroenterology. 2018;154(6 S1):S1209. [Google Scholar]
- 23.Weismuller TJ, Trivedi PJ, Bergquist A, et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology. 2017;152(8):1975–1984 e1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tse CS, Loftus EV Jr., Raffals LE, Gossard AA, Lightner AL. Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2018;48(2):190–195. [DOI] [PubMed] [Google Scholar]
- 25.Peng X, Luo X, Hou JY, et al. Immunosuppressive Agents for the Treatment of Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis. Dig Dis. 2017;35(5):478–485. [DOI] [PubMed] [Google Scholar]
- 26.Sabino J, Vieira-Silva S, Machiels K, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65(10):1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.