Abstract
In vitro studies showed that high-frequency pulsed electromagnetic fields (HF-PEMFs) increase the activity/expression of early and late osteogenic markers and enhance bone mineralization. The main aim of this study was to investigate the in vivo effects of HF-PEMFs on fracture healing using a rat model. A femur fracture was established by surgery in 20 male Wistar rats. Titanium nails were implanted to reduce and stabilize the fracture. After surgery, 20 rats were equally divided into untreated control and treated group (from the first postoperative day HF-PEMFs at 400 pulses/sec [pps] were applied for 10 minutes/day, for two weeks). Quantitative and qualitative assessment of bone formation was made at two and eight weeks following surgery and included morphological and histological analysis, serological analysis by ELISA, micro-computed tomography (micro-CT), and three-point bending test. At two weeks in HF-PEMF group, soft callus was at a more advanced fibrocartilaginous stage and the bone volume/total tissue volume (BV/TV) ratio in the callus area was significantly higher compared to control group (p = 0.047). Serum concentration of alkaline phosphatase (ALP) and osteocalcin (OC) was significantly higher in HF-PEMF group (ALP p = 0.026, OC p = 0.006) as well as the mechanical strength of femurs (p = 0.03). At eight weeks, femurs from HF-PEMF group had a completely formed woven bone with dense trabeculae, active bone marrow, and had a significantly higher BV/TV ratio compared to control (p = 0.01). HF-PEMFs applied from the first postoperative day, 10 minutes/day for two weeks, enhance bone consolidation in rats, especially in the early phase of fracture healing.
Keywords: Fracture healing, electromagnetic fields, high-frequency pulsed electromagnetic fields, PEMFs, titanium 6-aluminum-4-vanadium, osteogenic markers, bone remodeling, bone consolidation, in vivo rat model
INTRODUCTION
Impaired bone healing affects 5% to 10% of the 6.2 million fractures that occur annually in the United States and can lead to delayed union or nonunion. These conditions are associated with increased use of healthcare resources and high economic costs, placing a financial burden on patients and healthcare systems. For instance, a cost analysis of treatment of long bone fracture nonunions showed that the costs for humeral, femoral and tibia nonunions range from £15 566 to £17 200 [1,2]. In addition, surgical treatment of long bone nonunions is complex, often aggressive and with a failure rate of 5% to 40%, representing an important challenge in orthopedic surgery [2].
Biophysical methods for the treatment of bone fractures, particularly pulsed electromagnetic field (PEMF) devices, have several advantages over the conventional pharmacological and surgical treatments. These advantages include noninvasive design, limited systemic effects, high patient compliance, and reduced costs [3]. In the 1950s, Fukuda and Yasuda carried out several studies on piezoelectric properties of bone and the role of piezoelectric fields in osseous healing; later, they showed the effect of electric current passing through bone on callus formation [3,4]. Since then, numerous preclinical and clinical studies investigated the use of electromagnetic fields (mostly low-frequency PEMFs, 5–30 Hz) to enhance bone healing in delayed unions, nonunions (i.e., pseudarthrosis), or osteoporosis [4,5]. In patients with primary and secondary osteoporosis, low-frequency PEMFs relieve chronic bony pain with no side effects [6], and in ovariectomized (OVX) rats, treatment with low-frequency PEMFs prevents bone loss and deterioration of bone microarchitecture and strength, possibly via the Wnt/β-catenin signaling pathway [7]. In vitro studies showed that PEMFs at different low frequencies increase alkaline phosphatase level/activity, osteoblast marker gene expression, extracellular matrix production and osteoblast proliferation, as well as inhibit bone resorption [7,8]. In acute diaphyseal fractures, PEMF stimulation accelerates the time to radiological and clinical union [9]. Teven et al. [10] first demonstrated that high-frequency (HF) PEMFs (≥1 MHz), delivered by a Food and Drug Administration (FDA)-approved device for the treatment of soft tissue discomfort and edema, are capable of inducing osteogenic differentiation in murine osteoprogenitor cells, without the addition of costimulants [10]. However, the precise molecular mechanisms underlying the beneficial effects of PEMFs are yet to be determined.
In their systematic review on the effects of PEMFs on knee osteoarthritis in 930 patients, Ryang We et al. did not show any local or systemic adverse reactions of PEMFs stimulation [11]. Nevertheless, a considerable variability exists between studies concerning the effects of PEMF stimulation on bone formation/growth and healing, mostly due to differences in methodology. For example, there is a lack of agreement regarding the optimal timing of PEMF treatment, duration of daily exposure, and the overall duration of treatment [12]. Also, there is no consensus on the spectral characteristics of PEMF waveforms and energy output of PEMF devices. Depending on these parameters, different signaling mechanisms may be activated in target cells [13], which can facilitate or suppress differentiation in osteoclast-like cells [14]. In addition, most previous studies on the biological effects of PEMFs are limited by the fact that they based their conclusions on only one quantitative method (e.g., micro-computed tomography [micro-CT] or mechanical strength testing) with the other methods being qualitative, such as observer-based clinical and radiological scoring, evaluation of histological images, and pain questionnaires [15].
In this study, we aimed to investigate the effects of HF-PEMF stimulation on fracture healing using a rat model. After a femur fracture model was established by surgery, 20 rats were equally divided into control and HF-PEMF group. Starting from the first postoperative day, rats in HF-PEMF group were exposed to PEMFs at 400 pulses/sec (pps) for 10 minutes/day, for two weeks. Quantitative and qualitative assessment of bone formation was made at two and eight weeks following surgery and included morphological analysis, serological analysis of bone formation markers by enzyme-linked immunosorbent assay (ELISA), micro-CT, three-point bending test, and histological analysis.
MATERIALS AND METHODS
Experimental design
We used a rat model of femur fracture to investigate the effects of HF-PEMFs on fracture healing. Twenty male Wistar albino rats were provided by the Centre of Experimental Medicine of University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca. The Ethics Committee of “Iuliu Hatieganu” University of Medicine and Pharmacy and Veterinary Sanitary Committee of Cluj County approved the study protocol and experimental procedures (Approval No. 85/19.07.2017). Previous studies have shown that 20 rats is the optimal sample size to obtain significant results at two and eight weeks following surgical procedure. Orthopedic surgeons performed a femur surgery in 20 rats at the Centre of Experimental Medicine of University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca. The rats were divided into two equal groups (n = 10): untreated control group (CG) and group treated with HF-PEMFs.
Surgery technique
The rats were two months old and weighted 223.7 ± 17.1 g. General anesthesia was induced with 0.04 mL of xylazine (Bioveta, Romania, Xylazine Bio 2%) and 0.08 mL of ketamine hydrochloride (Biotur, Romania, Ketamine 10%) per 100 g body weight, administered intramuscularly (i.m). The left femur was used in each case. The animal was placed in the supine position, and the surgical site was shaved, cleaned with iodine solution and draped for aseptic surgery. A longitudinal incision of 1.5 to 2 cm was made on the lateral aspect of the femur, through the skin, subcutaneous tissue and iliotibial band, and blunt dissection of the intermuscular septum between biceps femoris and vastus lateralis was performed. After the exposure and inspection of the bone, a transverse fracture was produced with a blade in the middle of the femoral shaft. The femoral canal was opened through the intercondylar fossa of femur, using a needle. After the fracture reduction, 20 × 1 mm medical grade titanium nails (Ti90Al6V4) were implanted retrograde into the medullary canal down to the trochanteric region to stabilize the fracture. At the end of surgery, the muscle and subcutaneous layers were closed using resorbable sutures and the tegument was closed using nonresorbable sutures. After the surgery, the animals were housed in four cages (n = 5 rats/cage) under controlled conditions (12/12 hours light/dark cycle, room temperature of 22–23 °C) and fed ad libitum.
We performed clinical evaluation, gait inspection, and incision examination in rats on a daily basis for two or eight weeks. Any pathological findings were noted.
Rats were euthanized by anesthetic overdose at two (10/20 rats, n = 5 rats/group) or eight weeks (10/20, n = 5/group) after the surgery. The left femurs were harvested and the soft tissue around the bone was cleaned taking care not to damage or disrupt the bone callus. The samples were placed separately in 10% formaldehyde.
Pulsed electromagnetic field therapy
Starting from the first postoperative day, rats in HF-PEMF group were exposed to PEMFs (400 pps, a mean power output of 25.35 W [peak power output of 975 W], average field strength of 50 mW/cm2), 10 minutes/day, seven days/week, for two weeks (14 sessions). The total energy was 15.21 kJ. HF-PEMFs were generated by a Diapulse device (Diapulse Corporation of America, USA), which delivers pulsed, non-thermal electromagnetic waves at a (high) frequency of 27.12 MHz and a wavelength of 11.06 m. The pulses have a duration of 65 microseconds and a frequency of 400 pps. The mean power output and total energy were calculated using the formulas: mean power [W] = peak power [W] × pulse duration [s] × pulse frequency [Hz], and total energy [kJ] = mean power [W] × application time [s].
The electromagnetic filed was delivered through a drum-shaped head of 9 inches in diameter, placed above the area that was treated. Two rats at a time were placed in a 3-inch-high plastic box and covered with the diaphragm emitter. The drum-shaped head was positioned at a distance of 3 to 5 cm from rat bodies, to maximize the effect of PEMFs (Figure 1).
FIGURE 1.

Starting from the first postoperative day, rats in HF-PEMF group were exposed to PEMFs (400 pps, a mean power output of 25.35 W), 10 minutes/day for two weeks (14 sessions). HF-PEMFs were generated by a Diapulse device. The electromagnetic filed was delivered through a drum-shaped head of 9 inches in diameter, placed above the treated area. Two rats at a time were placed in a 3-inch-high plastic box and covered with the diaphragm emitter. The drum-shaped head was positioned at a distance of 3 to 5 cm from rat bodies, to maximize the effect of PEMFs. HF-PEMF: High-frequency pulsed electromagnetic field.
Alkaline phosphatase and osteocalcin analysis
Blood was collected from the retro-orbital sinus (0.6 ml/rat/examination) at the following time points: before the surgery (day 0, n = 20 rats), after the completion of PEMF therapy (day 14, n = 20), and at the end of the experiment (day 56, n = 10). Markers of bone formation, osteocalcin (OC) and alkaline phosphatase (ALP), were evaluated with commercially available, tissue non-specific, Rat OC/BGP (Osteocalcin) ELISA kit ER1205 (Wuhan Fine Biological Technology Co, Ltd, China) and ALP reagent OSR6504 used with the AU680 system (Beckman Coulter, USA).
Micro-CT and histological analysis
The femurs were cleansed with saline solution (0.9%) to remove excessive formaldehyde and left for 2 hours at room temperature to dry. The samples were scanned using the Bruker micro-CT SkyScan 1172 (Bruker-microCT, Belgium). The scanned data were transformed into images using CTVol v.2.2.1 (Bruker-microCT, Belgium) and CTAn v.1.12 (Bruker-microCT, Belgium). The region of interest (ROI) was established at the upper and inferior part of bone callus. The mean height of bone callus was 4.7 ± 0.72 mm. From the ROI, the total tissue volume (TV), total bone volume (BV), and bone volume relative to total tissue volume (BV/TV) were calculated. The trabecular bone architecture was assessed by calculating the trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N). The nomenclature and units were according to the American Society of Bone and Mineral Research (ASBMR) Histomorphometry Nomenclature Committee recommendations.
At two or eight weeks postoperatively, the titanium nails were removed and tissues samples from each group were stained with hematoxylin and eosin (H&E). Qualitative analysis of chondrocytes, collagen fibers, and bone matrix was performed in the callus area using an optical microscope.
Three-point bending test
After the imaging analysis was completed, six femurs per experimental or control group (i.e., n = 3 femurs/group at two and eight weeks postoperatively) were selected for the three-point bending test. The test was carried out using the ProLine Material Testing Device (Zwick Roell, Germany) at the Mechanical Faculty, Technical University of Cluj-Napoca. The results were processed using a software associated with the test device and included mechanical strength in the fracture focal point (N) and the degree of elastic deformation (mm) until the fracture was produced. A custom-made device was used, with a 6 mm diameter steel cylinder as the loading pin and two supporting pins placed 20 mm apart from each other.
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 21.0. (IBM Corp., Armonk, NY) and GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California USA). Results were expressed as mean ± standard deviation and minimum and maximum values. Differences between the groups were tested using the two-tailed Student’s t-test. Results were considered statistically significant if p < 0.05.
RESULTS
No deaths were observed during the experiment. One rat from the control group acquired a thigh infection and was excluded from further analysis. Starting from the first postoperative day, all rats could walk without putting weight on the operated limb. At the end of the experiment, the rats weighted 276.2 ± 12.6 g and had no changes in the overall aspect of the lower limb or gait.
Figure 2A-F shows complications observed in two groups at two and eight weeks following surgery. At two weeks, bone callus in control group was composed of fibrous tissue (Figure 2C), while in HF-PEMF group it had calcified islands (Figure 2D). At eight weeks, there was a case of delay in consolidation in control group (Figure 2A), and a case of a valgus deformation in HF-PEMF group (Figure 2B). In control group, the femur diameter at the fracture site was larger than the diameter of the adjacent proximal and distal regions (Figure 2E). In HF-PEMF group, the diameter of the femur was almost normal at eight weeks (Figure 2F).
FIGURE 2.
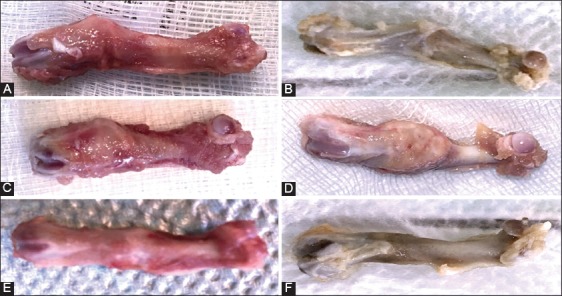
Macroscopic findings in control and HF-PEMF group at two and eight weeks after surgery. A) There was a delay in consolidation in control group, but B) valgus consolidation was observed in HF-PEMF group. C) At two weeks, bone callus in control group was composed of fibrous tissue, while D) in HF-PEMF group it had calcified islands. E) At eight weeks in control group, the femur diameter at the fracture site was larger than the diameter of the adjacent proximal and distal regions. F) In HF-PEMF group, the diameter of the femur was almost normal at eight weeks. HF-PEMF: High-frequency pulsed electromagnetic field.
ALP and OC analysis
During the first two weeks postoperatively, the serum concentration of ALP and OC increased in both groups (Figure 3), with a significant difference between the first and 14th postoperative day (p < 0.005 for ALP and p < 0.002 for OC). At two weeks, the concentration of ALP and OC was significantly higher in HF-PEMF compared to control group (for ALP 777.1 ± 51.48 U/L HF-PEMF vs. 638.6 ± 25.30 U/L control, p = 0.026; for OC 39.21 ± 1.81 pg/ml HF-PEMF vs. 32.27 ± 1.31 pg/ml control, p = 0.006). From the second to eighth week, the concentration of ALP and OC decreased in both groups (for ALP p < 0.001 in both groups; for OC p = 0.1 in control and p < 0.001 in HF-PEMF). At eight weeks, there were no significant differences between two groups in the concentration of ALP (367.5 ± 28.53 U/L HF-PEMF vs. 441.2 ± 13.22 U/L control, p = 0.056) and OC (21.47 ± 1.79 pg/ml HF-PEMF vs. 26.83 ± 3.42 pg/ml control, p = 0.19).
FIGURE 3.
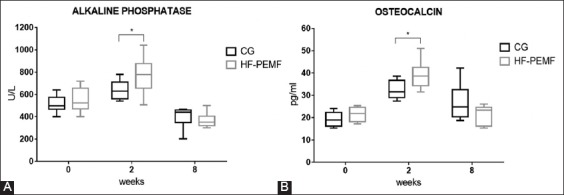
Serum concentration of ALP (A) and OC (B) in control and HF-PEMF group, at the beginning of the experiment (before surgery), and at two and eight weeks after surgery. During the first two weeks postoperatively, the serum concentration of ALP and OC increased in both groups. At two weeks, the concentration of ALP and OC was significantly higher in HF-PEMF compared to control group (for ALP p = 0.026; for OC p = 0.006). From the second to eighth week, the concentration of ALP and OC decreased in both groups. At eight weeks, there were no significant differences between two groups in the concentration of ALP (p = 0.056) and OC (p = 0.19). *indicates statistically significant difference. CG: Control group; HF-PEMF: High-frequency pulsed electromagnetic field; ALP: Alkaline phosphatase; OC: Osteocalcin.
Imaging analysis
At two weeks postoperatively, the total tissue and bone volumes were significantly larger in HF-PEMF compared to control group (total tissue volume: 254.4 ± 10.7 mm3 vs. 221.1 ± 22.2 mm3, p = 0.038; total bone volume: 87.16 ± 8.75 mm3 vs. 66.17 ± 8.5 mm3, p = 0.041). The BV/TV ratio, indicating bone density in the total tissue volume, was also higher in HF-PEMF vs. control group [34.22% ± 2.55 vs. 29.87% ± 1.11, p = 0.047] (Figure 4A). On the other hand, there were no statistical differences between two groups in the trabecular thickness (0.58 ± 0.11 mm HF-PEMF vs. 0.49 ± 0.05 mm control, p = 0.21), trabecular separation (0.86 ± 0.12 mm vs. 1.03 ± 0.14 mm, p = 0.18) and trabecular number [0.72 ± 0.07/mm2 vs. 0.72 ± 0.03/mm2, p = 0.13] (Figure 4B).
FIGURE 4.
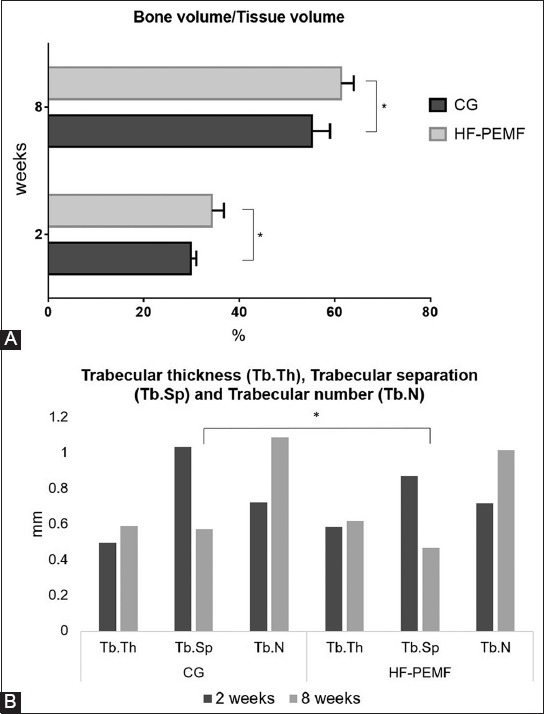
Micro-CT analysis of TV, BV, BV/TV ratio, and trabecular bone architecture in the ROI within callus, in control and HF-PEMF group at two and eight weeks after surgery. A) At two weeks postoperatively, the TV and BV were significantly larger (p = 0.038 and p = 0.041, respectively) and the BV/TV ratio was significantly higher (p = 0.047) in HF-PEMF compared to control group. At eight weeks postoperatively, the TV was significantly larger in control compared to HF-PEMF group (p = 0.006), but no significant difference in the BV was observed between two groups (p = 0.1). The BV/TV ratio was significantly higher in HF-PEMF vs. control group (p = 0.01). B) At two weeks postoperatively, there were no statistical differences between two groups in the Tb.Th (p = 0.21), Tb.Sp (p = 0.18) and Tb.N (p = 0.13). At eight weeks postoperatively, there was a significant difference between two groups in the Tb.Sp (p = 0.018) but not in the Tb.Th (p = 0.41) and Tb.N (p = 0.1). *indicates statistically significant difference. Micro-CT: Micro-computed tomography; ROI: Region of interest; TV: Total tissue volume; BV: Total bone volume; BV/TV: Bone volume relative to total tissue volume; Tb.Th: Trabecular thickness; Tb.Sp: Trabecular separation; Tb.N: Trabecular number; HF-PEMF: High-frequency pulsed electromagnetic field; CG: Control group.
At eight weeks postoperatively, the total tissue volume was significantly larger in control compared to HF-PEMF group (151.70 ± 6.70 mm3 vs. 127.29 ± 15.25 mm3, p = 0.006), but no significant difference in the total bone volume was observed between two groups (77.85 ± 7.42 mm3 HF-PEMF vs. 83.63 ± 4.01 mm3 control, p = 0.1). Thus, the BV/TV ratio was significantly higher in HF-PEMF vs. control group [61.34% ± 2.61 vs. 55.23% ± 3.8, p = 0.01] (Figure 4A). There was a significant difference between two groups in the trabecular separation (0.46 ± 0.01 mm HF-PEMF vs. 0.57 ± 0.08 mm control, p = 0.018) but not in the trabecular thickness (0.61 ± 0.04 mm HF-PEMF vs. 0.59 ± 0.05 mm control, p = 0.41) and trabecular number (1.02 ± 0.12/mm2 HF-PEMF vs. 1.08 ± 0.05/mm2 control, p = 0.1).
Three-point bending test
At two weeks following surgery, the mechanical strength of femurs was higher in HF-PEMF compared to control group (35.1 N ± 5.6 vs. 21.83 N ± 4.2, p = 0.03), and the elastic deformation until fracture was smaller [0.7 ± 0.06 mm HF-PEMF vs. 1.03 ± 0.12 mm control, p < 0.01] (Figure 5A).
FIGURE 5.
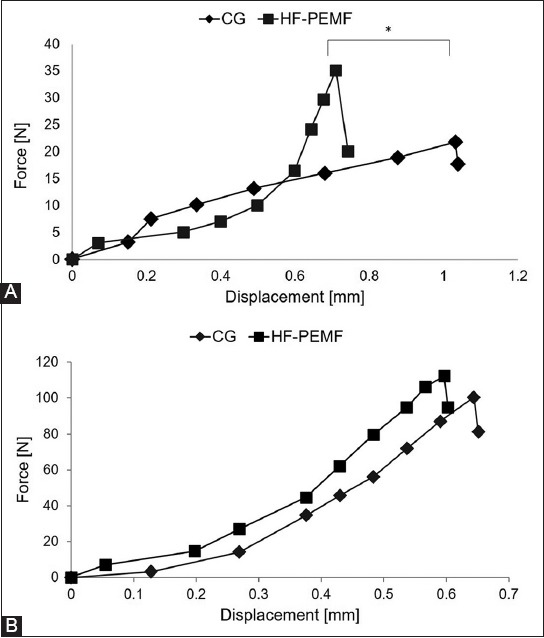
Results of three-point bending test in control and HF-PEMF group at A) two and B) eight weeks after surgery. At two weeks postoperatively, the mechanical strength of femurs was higher in HF-PEMF compared to control group (35.1 N ± 5.6 vs. 21.83 N ± 4.2, p = 0.03), and the elastic deformation until fracture was smaller (0.7 ± 0.06 mm HF-PEMF vs. 1.03 ± 0.12 mm control, p < 0.01). At eight weeks postoperatively, the bending resistance of femurs increased in both groups, with no significant difference in mechanical strength (112.3 N ± 6.7 HF-PEMF vs. 100.5 N ± 7.2 control, p = 0.09) and elastic deformation (0.59 ± 0.06 mm HF-PEMF vs. 0.64 ± 0.08 mm control, p = 0.23) between two groups. *indicates statistically significant difference. HF-PEMF: High-frequency pulsed electromagnetic field; CG: Control group.
At eight weeks following surgery, the bending resistance of femurs increased in both groups, with no significant difference in mechanical strength (112.3 N ± 6.7 HF-PEMF vs. 100.5 N ± 7.2 control, p = 0.09) and elastic deformation [0.59 ± 0.06 mm HF-PEMF vs. 0.64 ± 0.08 mm control, p = 0.23] between two groups (Figure 5B).
Histology
At two weeks after surgery, femurs from control group showed a persistent infiltration of inflammatory cells (yellow arrow) and numerous chondrocytes (green arrow) at the fracture site (Figure 6A). In femurs from HF-PEMF group, soft callus was at a more advanced, fibrocartilaginous stage and there was synthesis of new collagen fibers (blue arrow). These samples had no inflammatory infiltrates and had less chondrocytes [green arrow] (Figure 6C). In addition, fibrous tissue was more abundant in HF-PEMF group and was more pronounced towards the periphery of the callus.
FIGURE 6.
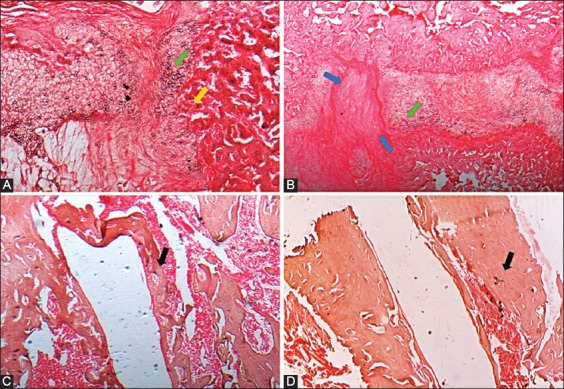
Histological analysis of femurs in control and HF-PEMF group at two and eight weeks after surgery (4×, hematoxylin and eosin [H&E]). A) At two weeks postoperatively, femur samples from control group showed a persistent infiltration of inflammatory cells (yellow arrow) and numerous chondrocytes (green arrow) at the fracture site. B) At eight weeks, there was a lower amount of bone marrow in the medullary cavity and less defined woven bone trabeculae (black arrow) in femurs from control group. C) In HF-PEMF group at two weeks, soft callus was at a more advanced, fibrocartilaginous stage and there was synthesis of new collagen fibers (blue arrow). These samples had no inflammatory infiltrates and had less chondrocytes (green arrow). D) At eight weeks, femurs from HF-PEMF group had a completely formed woven bone (black arrow) with dense trabeculae and had active bone marrow. HF-PEMF: High-frequency pulsed electromagnetic field.
At eight weeks after surgery, there was a lower amount of bone marrow in the medullary cavity and less defined woven bone trabeculae (black arrow) in femurs from control group (Figure 6B). In contrast, the samples from HF-PEMF group had a completely formed woven bone (black arrow) with dense trabeculae and had active bone marrow (Figure 6D).
DISCUSSION
Bone healing is a complex process involving inflammation, bone formation and remodeling. In this study, we used a rat model of femur fracture to investigate the effects of HF-PEMFs (at 400 pps, applied for 10 minutes/day for two weeks) on the acute phase of fracture healing. The acute phase of bone healing includes the formation of hematoma, infiltration of inflammatory cells, and formation of soft callus. The transition from the inflammatory to the soft callus stage occurs within 10 to 14 days of injury [16].
Previous research has shown that PEMFs can accelerate healing of soft tissue injuries, e.g. contusion, sprain, luxation and hematomas, by 30% to 50% [17]. In clinical setting, four to eight PEMF sessions are required to treat acute and subacute conditions and 10 to 15 sessions are needed for the treatment of chronic conditions [18]. Based on the previous studies, we selected 14 sessions as the optimal number of PEMF treatments.
Bombonica Dogaru et al. showed that HF-PEMFs stimulation of 35 rats, at 400 pps, applied for 10 minutes/day affects the synthesis and secretion of adrenal hormones (cortisol and aldosterone) [19]. HF-PEMFs also promote the healing of damaged bone tissue in patients with algoneurodystrophy [20]. In our study, HF-PEMFs accelerated fracture healing in rats by increasing the rate of callus mineralization. Specifically, the BV/TV ratio at two and eight weeks postoperatively and the concentration of early (ALP) and late (OC) osteogenic markers at two weeks postoperatively were significantly higher in HF-PEMF compared to control group. Moreover, the mechanical strength test showed a higher bending strength and smaller elastic deformation of femurs in HF-PEMF group. Overall, our results are indicative of a more advanced stage of bone healing in HF-PEMF vs. control group at two and eight weeks postoperatively.
PEMF treatments vary in EMF frequency, pulse duration/shape, duration of exposure, and type of stimulator [21]. Depending on the configuration and dose of electric or electromagnetic input, different transmembrane signaling mechanisms may be activated in target cells. Previous studies have shown that the maximum effective frequency range of PEMFs for humans is between 1 and 50 Hz, while frequencies >100 Hz are not effective and result in osteoclastogenesis [21]. In PEMFs, longer intervals between pulses eliminate heat, producing a nonthermal biological effect [22]. The frequency of impulses has thus been calculated so that the effect of each impulse continues on the biological effect of a previous impulse. The first study on the osteoinductive effects of HF-PEMFs (≥1 MHz) showed that HF-PEMF stimulation increases ALP activity (marker of early osteogenic differentiation), as well as OC expression and matrix mineralization (both are indicators of late stage osteogenic differentiation) in murine osteoprogenitor cells [10]. Similarly, our in vivo study in rats showed higher levels of ALP and OC in HF-PEMF vs. control group at two weeks of HF-PEMF stimulation.
Currently, there is a lack of agreement regarding the onset time for PEMF treatment. In animal studies, some authors suggest that PEMF treatment should be started as early as possible after the fracture is induced [15]. Others indicate that the bone trauma should progress to an inactive state before PEMF treatment is applied [15], modeling thus a delayed union or nonunion phenotype [23]. In the current study, we started HF-PEMF treatment in rats shortly after the fracture was surgically induced, i.e., from the first day postoperatively.
Clinical studies in the fields of orthopedics and traumatology showed that PEMF therapy decreases edema, reduces pain and wound healing time [5], as well as accelerates hematoma resolution [24] and bone consolidation [21]. It is well-known that callus formation is stimulated by controlled micromovements applied to the fracture focus. The patient is usually immobilized and unable to move in the first days following the trauma. A direct application of electric current and associated electromagnetic field at the fracture focal point mimics the effect of mechanical stress to which the bone is subjected, promoting the formation of new bone and mineralization [25].
Duration of exposure to PEMFs is also an important factor, especially in terms of patient compliance. In clinical setting, the transmitter is placed at a very low distance from the human body (at a maximum 5 cm distance), as the air layer between the PEMF-emitting region and body surface causes electromagnetic waves to disperse [26]. In our study, the rats were smaller in size than the emitting area of Diapulse device and the exposure to PEMFs was systemic.
PEMF devices with a mean power output of less than 38 W do not produce thermal effects nor do they cause burns or other skin lesions in the treated area [27]. To limit the side effects, we used a PEMF device with the mean power output of 25.35 W and we exposed the rats to HF-PEMFs for 10 minutes/day. Pulsed short-wave diathermy activates enzymatic reactions, increases cell metabolism and tissue metabolic rate, as well as alters cell membrane permeability due to increased arterial blood flow velocity [28]. The exposure of the gastrocnemius muscle to an average root mean square output of 48 W increases its temperature by 1.36 ± 0.90 °C at 5 minutes of exposure, 2.87 ± 1.44 °C at 10 minutes, 3.78 ± 1.19 °C at 15 minutes, and 3.49 ± 1.13 °C at 20 minutes [29]. Long-term exposure (28 days) to extremely low-frequency (ELF)-PEMFs induces oxidative stress in cerebral cortex and pathological lesions in immune organs (thymus, spleen) of mice [30]. On the other hand, repeated exposure of human osteoblasts (hOBs) to ELF-PEMFs for one week stimulates their differentiation, by producing nontoxic amounts of reactive oxygen species (ROS) and consequently inducing antioxidative defense mechanisms in osteoblasts. Therefore, ELF-PEMFs may be a useful supplement to conventional therapy in fracture healing [31]. Similarly, repeated exposure of primary human osteoblasts to specific ELF-PEMFs for 21 days significantly increases the total protein content, mitochondrial activity, ALP activity, and enhances the formation of mineralized matrix during their differentiation [32].
In the current study, we used 20 × 1 mm medical grade titanium nails (Ti90Al6V4) for fracture stabilization and reduction, because of their enhanced mechanical properties and biocompatibility [33]. While some studies indicated that, in the medullary canal of rabbit long bones, PEMFs promote bone formation around implants by modulating the activity of primary activators in bone cells [34], others showed no difference in bone formation around titanium surface implanted in tibiae of rabbits between PEMF and control group [35]. However, in the latter study, the authors suggested that duration of stimulation and intensity of electromagnetic power may have affected their results [35].
Most previous studies on the biological effects of PEMFs are limited by the fact that they relied primarily on the qualitative assessment of newly formed bone volume during fracture healing and included very few quantitative measures. In the current study, we used several types of quantitative parameters of bone formation, including serum markers (ALP and OC), imaging measures (TV, BV, BV/TV) and three-point bending test values (mechanical strength and elastic deformation), supplemented by the histological analysis.
Overall, our results indicate that HF-PEMFs generated by a Diapulse device facilitate bone healing by shortening the time to consolidation.
A limitation to our study is that the exposure to HF-PEMFs was systemic. In clinical setting, local PEMF stimulation is more plausible and also increases patient compliance to treatment. We also did not use a histomorphometric system to quantify parameters such as bone volume, tissue volume, osteoid volume and interlabel width based on the histological images.
CONCLUSION
In conclusion, HF-PEMFs applied from the first postoperative day, 10 minutes/day for two weeks, enhance bone consolidation in rats, especially in the early phase of fracture healing. Future studies should elucidate the molecular mechanisms underlying stimulatory effects of HF-PEMFs on bone formation as well as the impact of HF-PEMFs on fracture healing in humans.
ACKNOWLEDGMENTS
The results of this study were presented at the Romanian Congress of Rehabilitation, Physical Medicine and Balneology 2018 and published in the Abstracts Volume of Balneo Research Journal 2018;9(2):155 DOI 10.12680/balneo.2018.182. Daniel Oltean-Dan received an internal grant (contract nr. 1680/14/10.01.2018) from the “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca.
DECLARATION OF INTERESTS
The authors declare no conflict of interests
REFERENCES
- 1.Kanakaris NK, Giannoudis PV. The health economics of the treatment of long-bone non-unions. Injury. 2007;38(Suppl 2):S77–S84. doi: 10.1016/s0020-1383(07)80012-x. https://doi.org/10.1016/S0020-1383(07)80012-X. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Bances I, Perez-Basterrechea M, Perez-Lopez S, Nunez Batalla D, Fernandez Rodriguez MA, Alvarez-Vlejo M, et al. Repair of long-bone pseudoarthrosis with autologous bone marrow mononuclear cells combined with allogenic bone graft. Cytotherapy. 2013;15(5):571–7. doi: 10.1016/j.jcyt.2013.01.004. https://.org/10.1016/j.jcyt.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Cook JJ, Summers NJ, Cook EA. Healing in the new millennium:Bone Stimulators:An overview of where we've been and where we may be heading. Clin Podiatr Med Surg. 2015;32(1):45–59. doi: 10.1016/j.cpm.2014.09.003. https://doi.org/10.1016/j.cpm.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Griffin XL, Warner F, Costa M. The role of electromagnetic stimulation in the management of established non-union of long bone fractures:What is the evidence? Injury. 2008;39(4):419–29. doi: 10.1016/j.injury.2007.12.014. https://doi.org/10.1016/j.injury.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Guo L, Kubat NJ, Nelson TR, Isenberg RA. Meta-analysis of clinical efficacy of pulsed radio frequency energy treatment. Ann Surg. 2012;255(3):457–67. doi: 10.1097/SLA.0b013e3182447b5d. https://doi.org/10.1097/SLA.0b013e3182447b5d. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, He H, Yang L, Chen S, Guo H, Xia L, et al. Effects of pulsed electromagnetic fields on bone mass and Wnt/β-catenin signaling pathway in ovariectomized rats. Arch Med Res. 2012;43(4):274–82. doi: 10.1016/j.arcmed.2012.06.002. https://doi.org/10.1016/j.arcmed.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Huang LQ, He HC, He CQ, Chen J, Yang L. Clinical update of pulsed electromagnetic fields on osteoporosis. Chin Med J (Engl) 2008;121(20):2095–9. [PubMed] [Google Scholar]
- 8.Tsai MT, Li WJ, Tuan RS, Chang WH. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthop Res. 2009;27(9):1169–74. doi: 10.1002/jor.20862. https://doi.org/10.1002/jor.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannemann PFW, Mommers EH, Schots JPM, Brink PR, Poeze M. The effects of low-intensity pulsed ultrasound and pulsed electromagnetic fields bone growth stimulation in acute fractures:A systematic review and meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2014;134(8):1093–106. doi: 10.1007/s00402-014-2014-8. https://doi.org/10.1007/s00402-014-2014-8. [DOI] [PubMed] [Google Scholar]
- 10.Teven CM, Greives M, Natale RB, Su Y, Luo Q, He BC, et al. Differentiation of osteoprogenitor cells is induced by high-frequency pulsed electromagnetic fields. J Craniofac Surg. 2012;23(2):586–93. doi: 10.1097/SCS.0b013e31824cd6de. https://doi.org/10.1097/SCS.0b013e31824cd6de. [DOI] [PubMed] [Google Scholar]
- 11.Ryang We S, Koog YH, Jeong KI, Wi H. Effects of pulsed electromagnetic field on knee osteoarthritis:A systematic review. Rheumatology (Oxford) 2013;52(5):815–24. doi: 10.1093/rheumatology/kes063. https://doi.org/10.1093/rheumatology/kes063. [DOI] [PubMed] [Google Scholar]
- 12.Dimitriou R, Babis GC. Biomaterial osseointegration enhancement with biophysical stimulation. J Musculoskelet Neuronal Interact. 2007;7(3):253–65. [PubMed] [Google Scholar]
- 13.Ciombor DM, Aaron RK. The role of electrical stimulation in bone repair. Foot Ankle Clin. 2005;10(4):579–93. doi: 10.1016/j.fcl.2005.06.006. https://doi.org/10.1016/j.fcl.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Chang K, Chang WH, Huang S, Huang S, Shih C. Pulsed electromagnetic fields stimulation affects osteoclast formation by modulation of osteoprotegerin, RANK ligand and macrophage colony-stimulating factor. J Orthop Res. 2005;23(6):1308–14. doi: 10.1016/j.orthres.2005.03.012.1100230611. https://doi.org/10.1016/j.orthres.2005.03.012.1100230611. [DOI] [PubMed] [Google Scholar]
- 15.Midura RJ, Ibiwoye MO, Powell KA, Sakai Y, Doehring T, Grabiner MD, et al. Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J Orthop Res. 2005;23(5):1035–46. doi: 10.1016/j.orthres.2005.03.015. https://doi.org/10.1016/j.orthres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Morgan EF, De Giacomo A, Gerstenfeld LC. Overview of skeletal repair (fracture healing and its assessment) Methods Mol Biol. 2014;1130:13–31. doi: 10.1007/978-1-62703-989-5_2. https://doi.org/10.1007/978-1-62703-989-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinney B. Pulsed electromagnetic field therapy in plastic surgery. Aesthet Surg J. 2005;25(1):87–91. doi: 10.1016/j.asj.2004.12.001. https://doi.org/10.1016/j.asj.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Prentice WE, Quillen WS, Underwood F. Therapeutic modalities in rehabilitation. 4th ed. New York, United States: McGraw-Hill Education/Medical; 2005. [Google Scholar]
- 19.Bombonica Dogaru G, Crăciun C, Rusu M, Bodizs G, Calinici T, Pop L. Histoenzimological and biomechanical cortisol and aldosterone changes in rats exposed to pulsed short waves (Diapulse) Ann RSCB. 2010;15(2):79–86. [Google Scholar]
- 20.Comorosan S, Pana I, Pop L, Craciun C, Cirlea AM, Paslaru L. The influence of pulsed high peak power electromagnetic energy (Diapulse) treatment on posttraumatic algoneurodystrophies. Rev Roum Physiol (1990) 1991;28(3-4):77–81. [PubMed] [Google Scholar]
- 21.Biggane P, Jackson X, Nazarian A. Bone composition and healing:Open electromagnetic and biomechanical problems. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:6026–9. doi: 10.1109/EMBC.2016.7592102. https://doi.org/10.1109/EMBC.2016.7592102. [DOI] [PubMed] [Google Scholar]
- 22.Dogaru GB, Bódizs G, Calinici T, Onac I, Pop L. Biochemical changes in rats submitted to the action of pulsed short waves (Diapulse) Ann RSCB. 2012;17(1):133–5. [Google Scholar]
- 23.Assiotis A, Sachinis NP, Chalidis BE. Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature. J Orthop Surg Res. 2012;7:24. doi: 10.1186/1749-799X-7-24. https://doi.org/10.1186/1749-799X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee EW, Maffulli N, Li CK, Chan KM. Pulsed magnetic and electromagnetic fields in experimental achilles tendonitis in the rat:A prospective randomized study. Arch Phys Med Rehabil. 1997;78(4):399–404. doi: 10.1016/s0003-9993(97)90232-x. https://doi.org/10.1016/S0003-9993(97)90232-X. [DOI] [PubMed] [Google Scholar]
- 25.Fredericks DC, Nepola JV, Baker JT, Abbott J, Simon B. Effects of pulsed electromagnetic fields on bone healing in a rabbit tibial osteotomy model. J Orthop Trauma. 2000;14(2):93–100. doi: 10.1097/00005131-200002000-00004. https://doi.org/10.1097/00005131-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Nunes FD, Vasconcelos TC, Bezerra M, Weiner J. Electromagnetic energy density in dispersive and dissipative media. J Opt Soc Am B. 2011;28(6):1544–52. https://doi.org/10.1364/JOSAB.28.001544. [Google Scholar]
- 27.Sussman C, Bates-Jensen B. Wound Care:A collaborative practice manual for health professionals. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 28.De Sousa NTA, Guirro ECDO, Calió JG, De Quelz MC, Guirro RRDJ. Application of shortwave diathermy to lower limb increases arterial blood flow velocity and skin temperature in women:A randomized controlled trial. Braz J Phys Ther. 2017;21(2):127–37. doi: 10.1016/j.bjpt.2017.03.008. https://doi.org/10.1016/j.bjpt.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draper DO, Knight K, Fujiwara T, Castel JC. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sport Phys Ther. 1999;29(1):13–22. doi: 10.2519/jospt.1999.29.1.13. https://doi.org/10.2519/jospt.1999.29.1.13. [DOI] [PubMed] [Google Scholar]
- 30.Luo X, Chen M, Duan Y, Duan W, Zhang H, He Y, et al. Chemoprotective action of lotus seedpod procyanidins on oxidative stress in mice induced by extremely low-frequency electromagnetic field exposure. Biomed Pharmacother. 2016;82:640–8. doi: 10.1016/j.biopha.2016.06.005. https://doi.org/10.1016/j.biopha.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Ehnert S, Fentz AK, Schreiner A, Birk J, Wilbrand B, Ziegler P, et al. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of •O2− and H2O2. Sci Rep. 2017;7:14544. doi: 10.1038/s41598-017-14983-9. https://doi.org/10.1038/s41598-017-14983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehnert S, Falldorf K, Fentz AK, Ziegler P, Schroler S, Freude T, et al. Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure - Clinical implication possible. Bone Rep. 2015;3:48–56. doi: 10.1016/j.bonr.2015.08.002. https://doi.org/10.1016/j.bonr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rau JV, Antoniac I, Cama G, Komlev VS, Ravaglioli A. Bioactive materials for bone tissue engineering. Biomed Res Int. 2016;2016:3741428. doi: 10.1155/2016/3741428. https://doi.org/10.1155/2016/3741428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spadaro JA. Mechanical and electrical interactions in bone remodeling. Bioelectromagnetics. 1997;18(3):193–202. https://doi.org/ 10.1002/(SICI) 1521-186X (1997) 18:3 <193:AID-BEM1>3.0. CO;2-Y. [PubMed] [Google Scholar]
- 35.Buzzá EP, Shibli JA, Barbeiro RH, Barbosa JR. Effects of electromagnetic field on bone healing around commercially pure titanium surface:Histologic and mechanical study in rabbits. Implant Dent. 2003;12(2):182–7. doi: 10.1097/01.id.0000058385.23346.4d. https://doi.org/10.1097/01.ID.0000058385.23346.4D. [DOI] [PubMed] [Google Scholar]


