Abstract
Hematopoietic stem cell transplantation is commonly used in patients with certain hematological or bone marrow tumors. Total body irradiation combined with chemotherapy is part of the preconditioning protocol that was the most commonly used before hematopoietic stem cell transplantation. However, total body irradiation preconditioning damages other normal cells in bone marrow. Therefore, exploring the mechanism of radiation resistance in bone marrow mesenchymal stem cells is of great significance for recovering the hematopoietic function after cell transplantation. This study aimed to demonstrate the miR-29b adsorption of circRNA_014511 and explore the effect of circRNA_014511 on radiosensitivity of bone marrow mesenchymal stem cells. In this study, circRNA_014511 overexpression vector was constructed and transfected into bone marrow mesenchymal stem cells, miR-29b-2-5p and P53 were found to be decreased, which could be reversed by miR29b-mimics. Dual luciferase reporter assay confirmed the binding of circRNA_014511 and mmu-miR-29b-2-5p. Flow cytometry analysis showed the apoptosis rate of bone marrow mesenchymal stem cells overexpressing circRNA_014511 was significantly decreased. In the circRNA_014511 transfection group, after cells were subjected to 6Gy irradiation, G2 phase arrest appeared, the expression of P21 and GADD45A was significantly decreased, and cyclin B1 was significantly increased. Colony formation assay showed the survival fraction of circRNA_014511 overexpression cells after irradiation was significantly higher than control group, and the radiosensitivity was decreased. In conclusion, our findings demonstrated that circRNA_014511 could inhibit the expression of P53 by binding miR-29b-2-5p, and decrease the radiosensitivity of bone marrow mesenchymal stem cells by affecting cell cycle and cell apoptosis.
Keywords: circular RNA, miRNA, bone marrow mesenchymal stem cell, radiosensitivity
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is a kind of transplantation of pluripotent hematopoietic stem cells that are usually derived from bone marrow, peripheral blood, or umbilical cord blood [1]. HSCT is commonly used in patients with certain hematological or bone marrow tumors, such as leukemia and multiple myeloma [2,3]. Total body irradiation (TBI) combined with chemotherapy is part of the preconditioning protocol that was the most commonly used before HSCT [4]. It had been reported that about 10% of autologous transplant patients and 50% of allograft patients received TBI [5]. TBI exerts uniform radiation across the whole body, and it can penetrate the central nervous system (CNS) and testis, where traditional chemotherapy is often ineffective [4,6]. TBI has three principal goals: first, to eliminate residual cancer cells; second, to provide space for stem cell transplantation through the exhaustion of diseased bone marrow; third, to prevent the rejection of donor stem cells by suppressing immunity [6]. However, in the process of destroying and clearing the diseased hematopoietic tissues, TBI preconditioning damages other normal cells in bone marrow (including bone marrow mesenchymal stem cells (BMMSCs)) to some extent [7,8]. BMMSCs are non-hematopoietic stem cells in bone marrow capable of self-renewal, pronounced proliferation, and differentiation into multiple types of other cells [9]. Recent studies have found that, in addition to their ability to support in vitro hematopoiesis and promote the restoration of hematopoietic function in vivo, BMMSCs could also reduce immunogenicity and inhibit the proliferation of allogeneic T cells [10], so reducing transplant rejection and decreasing the severity and incidence of graft versus host disease (GVHD). Maximizing the quantity and quality of BMMSCs in TBI preconditioning before transplantation is of great significance for the recovery of hematopoietic function and the prevention of GVHD after transplantation.
Circular RNA (circRNA) is a noncoding RNA present in large quantities in mammalian cells [11]. CircRNA possesses miRNA response elements (MREs), which can instantly bind or release a large quantity of miRNAs, and thus effectively regulate gene expression [12]. In one of our previous studies [13], a series of differentially expressed circRNAs in BMMSCs of TBI-treated mice were screened through circRNA microarrays, in which the expression of circRNA_014511 was significantly up-regulated. CircRNA_014511 is an exon circRNA located at chr4: 132656692-132673032. Bioinformatics analysis showed that circRNA_014511 could bind miR-29b-2-5p by pairing. MiR-29b-2-5p belongs to the miR-29 family, which is composed of miR-29a, miR-29b, and miR-29c, and there are only 2 to 3 differential bases in the sequences of the genes in this family. Park et al. [14] showed that miR-29 family could activate P53 expression and induce the P53-mediated apoptosis in Hela cells. In addition, in a variety of tumors including chronic lymphocytic leukemia, lung cancer, prostate cancer, and breast cancer, the decreased levels of P53 expression induced by the down-regulation of miR-29 were identified [15,16]. P53 is a powerful transcriptional factor and it is involved in cell cycle arrest, cell apoptosis, and cell senescence through multiple signal pathways [17]. Studies have shown that the status of the p53 gene is closely related to radiosensitivity. The wild type p53 gene is prone to induce apoptosis in response to radiation, while the mutant p53 gene mediates cell resistance to radiation-induced apoptosis. Studies also found that miR-29 family could promote cell senescence and apoptosis in BMMSCs [18].
This study demonstrated for the first time that circRNA_014511 could affect the expression of P53, regulate cell apoptosis and cell cycle arrest, and influence the radiosensitivity of BMMSCs through the adsorption of miR-29b-2-5p in vitro, establishing a new theoretical basis for the development of radiation damage protective agents.
MATERIALS AND METHODS
Cell culture
Mouse BMMSCs (ScienCell, US) were cultured using the mesenchymal stem cell complete medium (5% FBS + 1% streptomycin + 1% MSCMs growth factor + 93% MSCM) (ScienCell, US) in a 37°C, 5% CO2, saturated humidity incubator (Santeng, China). The primary culture was recorded as P0. After 48 h, the medium was refreshed for the first time, and the non-adherent cells were discarded; after that, the medium was refreshed every 3 days. When the adherent cells were fused to 80–90% of the culture plate, cells were digested using trypsin (HyClone, US) and then sub-cultured at 1:2 ratio. These cells were recorded as P1. Cells were returned to the incubator for culture, and the cell growth was observed the next day.
Overexpression vector construction and cell transfection
The construction of circRNA_014511 overexpression vector (p-circRNA_014511) was performed as follows: the GV535 vector (Genechem, China) was digested with AgeI/BamHI (NEB, US) to produce the linearized vector. PCR primers were designed and homologous recombination sequences were added to the 5¢-end of the primers. The target gene was amplified using the designed primers, and the 5¢ and 3¢ terminal sequences of amplified product were identical to the corresponding sequences of the linearized vector. The shuttle vector was constructed by inserting the DNA fragment of circRNA_014511 into the multiple clone site of GV535 vector (Genechem, China). Using the AdMax adenovirus packaging system established by Professor Frank L. Graham [19], the constructed shuttle vector and the packaging plasmid (Microbix, Canada) were co-transfected into HEK293 cells (ATCC, China), where the recombinant adenovirus was packaged.
MiR29b-mimics and mimics-NC were purchased from RiboBio Co., Ltd, China. Mouse BMMSCs in exponential phase were prepared for transfection. P-circRNA_014511 was transfected using Polybrene transfection reagent (Genechem, China), and miR29b-mimics were transfected using Lipofectamine 2000 (Thermo, US) according to the manufacturer’s instructions. After transfection, the fluorescence of transfected cells was observed, and the transfection efficiency was detected by PCR.
Fluorescence quantitative PCR
Total RNAs were extracted with TRIzol Reagent (Invitrogen, US) in accordance with the manufacturer’s instructions. Here, 2 µg of total RNAs were used to synthesize cDNA by Gene Amp PCR System Hema9600 (Hema, China). Real-time PCR was performed by PIKO REAL 96 Sequence Detection System (Thermo, US) using 2 µl cDNA as templates. The reactions started at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. All experiments were repeated three times. RNA expression levels were reported relative to Actin or U6. The primers used in the present study were as follows:
mmu_circRNA_014511 forward: 5¢-GAACCTGTTTT GGCACCAGTTTGTGAAGACCGCAGAGACC-3¢, reverse: 5¢-GAACC TGTTTTG GCGGAACCGAAA GGA GGTAGTCCGTAAG-3¢; Actin forward: 5¢-ACATCCGTAA AGACCTCTATGCC-3¢, reverse: 5¢-TACTCCTGCTTGCT GATCCAC-3¢; mmu-miR-29b-2-5p: 5¢-CTGGTTTCACAT GGTGGCTTAGATT-3¢; U6 forward: 5¢-CTCG CTTCGGCA GCACA-3¢, reverse: 5¢-AACGCTTCACG AATT TGCGT -3¢.
Western blotting
Total proteins were lysed by RIPA buffer (Wellbio, China), containing protease inhibitors cocktail (Merck, Germany). After centrifugation (12,000 g for 15 min), protein concentrations were detected with BCA Protein Assay Kit (Wellbio, China). Protein extractions were separated by 10% SDS-PAGE and transferred onto nitrocellulose membrane (Roche Biosciences, Germany). After blocking with 5% non-fat milk for 1.5 h, the membranes were incubated with primary antibody overnight at 4°C. After that, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Proteintech, US) for 1.5 h at room temperature. Proteins were detected via chemiluminescence according to the manufacturer’s recommendations (ECL Pierce) (Thermo, US). The intensity of each protein band was quantified by Quantity One 4.6.2 software (Bio-Rad, China). Primary antibodies included those against P53 (dilution 1:1200, 10442-1-AP), P21 (dilution 1:800, 60214-1-Ig), cyclin B1 (dilution 1:500, 55004-1-AP), GADD45A (dilution 1:500, 13747-1-AP), Bcl-2 (dilution 1:1200, 12789-1-AP), Mcl-1 (dilution 1:500, 16225-1-AP), and β-actin (dilution 1:5000, 60008-1-Ig) from Proteintech (Chicago, IL, US).
Colony formation assay
Cells from the p-circRNA_014511 transfection group and the corresponding control group were irradiated with different doses (0 Gy, 2 Gy, 4Gy, 6 Gy, 8 Gy, and 10 Gy) in a linear accelerator (Varian unique, US). Cells from each group were digested with 0.25% trypsin (HyClone, US) and then suspended in complete medium (ScienCell, US). Cell suspensions were serially diluted and inoculated in 6-well plates containing 2 ml pre-warmed medium with 100 cells per well. The cells were cultured in 37°C, 5% CO2, saturated humidity incubator (Santeng, China) for 2–3 weeks, with the medium refreshed as needed. The culture was terminated when visible colonies appeared in the wells. Discarding the medium, cells were washed twice using PBS buffer (Thermo, US) and then immobilized with 3 ml of 4% paraformaldehyde (Wellbio, China) for 15 min. The fixative solution was discarded, and cells were stained with the appropriate amount of 0.5% crystal violet staining solution (Wellbio, China) at room temperature for 5 min, washed gently with running water, air-dried, and then photographed. The 6-well plate was inverted on blank white paper, and colonies with more than ten cells were counted with the naked eye. The colony formation rate was calculated as (number of clones/number of inoculated cells) × 100%.
Cell cycle assessment
When the cultured cell density of each group reached 90%, the cells were digested with 0.25% trypsin (HyClone, US), washed 2-3 times in PBS buffer (Thermo, US), and made into single cell suspension with cell number adjusted to 1×106> cells/ml. Discarding the supernatant, cells were added with pre-cooled 75% ethanol and fixed overnight at 4°C. The cells were collected by centrifugation, washed twice by PBS buffer to remove ethanol, and stained with 150 µL of propidium iodide (PI) working solution (Sigma, US) at 4°C for 30 min in darkness. The cell cycle was then detected using flow cytometry (BD, US).
Cell apoptosis assessment
Cells in each group were inoculated into 6-well plates, and then digested with 0.25% trypsin (HyClone, US) without EDTA when cell density reached 90%. 5×105> cells were counted and collected into an EP tube, then washed twice with PBS buffer (Thermo, US). After centrifugation, the cells were stained with Annexin V-APC/PI double stained cell apoptosis detection kit (KeyGEN BioTECH, China) for 15 min in darkness, then cell apoptosis was detected by flow cytometry (BD, US).
Dual luciferase reporter assay
Briefly, BMMSCs were co-transfected with psiCHECK-2-circRNA_014511-WT or psiCHECK-2-circRNA_014511-Mut and miR29b-mimics or miR-NC using Lipofectamine2000 (Thermo, US). Luciferase activity was measured at 48 h after transfection by a Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Statistical analysis
All results are presented as mean ± SEM of at least three independent experiments except where otherwise indicated. Student’s t-test was used to assess differences between two groups. P < 0.05 was considered statistically significant.
RESULTS
CircRNA_014511 could regulate P53 expression through mmu-miR-29b-2-5p in BMMSCs
To preliminary prove that circRNA_014511 could adsorb mmu-miR-29b-2-5p in BMMSCs, BMMSCs were transfected with p-circRNA_014511, miR29b-mimics, or both, and blank control and empty vector control were set simultaneously. The expression of circRNA_014511 in p-circRNA_014511 transfection group and miR29b-mimics + p-circRNA_014511 co-transfection group was significantly higher than in other groups (Figure 1A), which proved the transfection efficiency of p-circRNA_014511. After transfection with p-circRNA_014511, the mmu-miR-29b-2-5p levels in BMMSCs and BMMSCs transfected with miR29b-mimics decreased significantly, suggesting that in BMMSCs, circRNA_014511 overexpression could down-regulate the expression of mmu-miR-29b-2-5p (Figure 1B).
FIGURE 1.
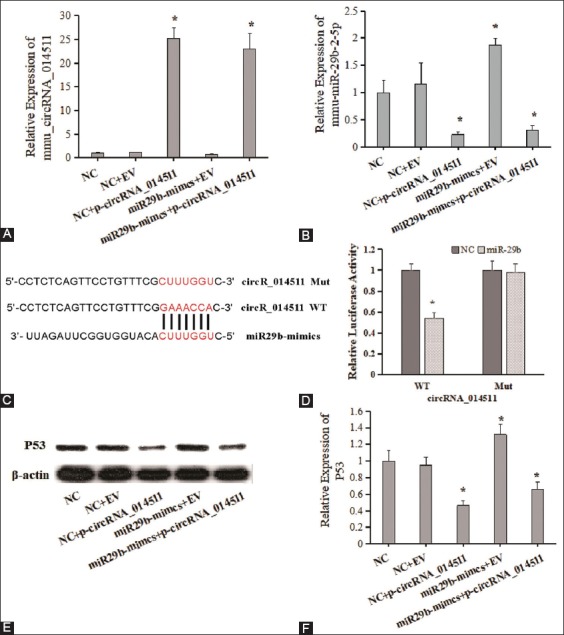
The regulation of p53 expression by the combination of circRNA_014511 and mmu-miR-29b-2-5p in BMMSCs. (A) and (B), quantitative fluorescence PCR detection of the expressions of circRNA_014511 and mmu-miR-29b-2-5p in the cells of each group. (C) and (D) results of dual luciferase reporter assay. (E) and (F) Western blot assessment of p53 expression.
In addition, the results of dual luciferase reporter assay showed that, when transfected with wild-type recombinant luciferase reporter plasmid, the luciferase activity of the mmu-miR-29b-2-5p group was significantly lower than in the NC group; however, when transfected with mutant recombinant luciferase reporter plasmid, the luciferase activity was not affected (Figure 1C, 1D). These results suggested that circRNA_014511 could bind mmu-miR-29b-2-5p.
Because the down-regulation of miR-29 family could lead to the decrease of P53 expression, the expression of P53 in each group was assessed using Western blot analysis. The results showed that P53 level in BMMSCs overexpressing p-circRNA_014511 was significantly lower, which could be complemented slightly by the co-transfection of miR29b-mimics. The P53 expression was significantly up-regulated in BMMSCs only transfected with miR29b-mimics (Figure 1E, 1F). These results suggested that circRNA_014511 could regulate P53 expression through binding mmu-miR-29b-2-5p.
Overexpression of circRNA_014511 could reduce the apoptotic rate of BMMSCs
To study the effect of circRNA_014511 on the apoptosis of BMMSCs, the expression levels of various apoptosis-related proteins in p-circRNA_014511-transfected cells, normal BMMSCs, and cells of each group receiving a moderate dose (6Gy) of irradiation were compared using Western blot analysis. The results showed that in p-circRNA_014511 transfection group, the expression level of P53 was decreased and the expressions of Bcl-2 and Mcl-1 were up-regulated. After irradiation, the P53 level of BMMSCs higher than in normal control cells, while the P53 level in p-circRNA_014511 transfected cells was significantly lower. The expression levels of Bcl-2 and Mcl-1 were significantly after irradiation than in un-irradiated cells, and the p-circRNA_014511 transfected cells had the highest expression levels (Figure 2A).
FIGURE 2.
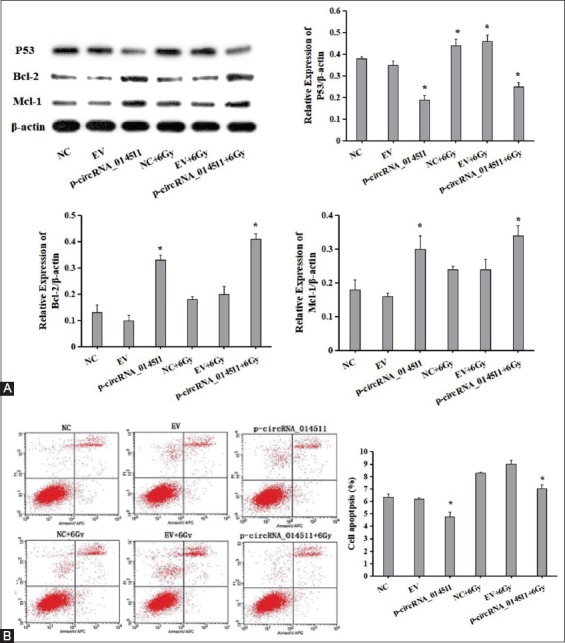
The effect of circRNA_014511 on apoptosis of BMMSCs. (A) Western blot assessment of the expressions of apoptosis-related proteins p53, Bcl-2, and Mcl-1. (B) Flow cytometry detection of apoptosis in cells of each group.
Cell apoptosis of each group was detected using flow cytometry. The results showed the rate of apoptosis rate of BMMSCs to be significantly higher after irradiation and the rate of apoptosis rate of p-circRNA_014511-transfected BMMSCs to be significantly lower thanthat that of blank control and empty vector control. The rate of apoptosis of p-circRNA_014511-transfected BMMSCs increased after irradiation, but it was significantly lower than that of the irradiated control cells (Figure 2B). These results suggested that overexpression of circRNA_014511 could reduce the rate of apoptosis of BMMSCs.
Regulation of the cell cycle by circRNA_014511 overexpression
The cell cycle was assessed using flow cytometry. After receiving 6Gy irradiation, the control cells showed more G1 phase cells and fewer S phase cells, indicating that G1 phase arrest had taken place. However, in circRNA_014511 overexpressed BMMSCs, the number of G1 phase cells showed little change, while the G2 phase cells increased and the S phase cells decreased, indicating that G2 phase arrest occurred (Figure 3A). Compared with the control group, the expression levels of P21 and GADD45A in circRNA_014511 overexpressed cells decreased significantly, and the expression of Cyclin B1 increased significantly. The expression levels of P21, GADD45A and Cyclin B1 of each group increased after irradiation (Figure 3B).
FIGURE 3.
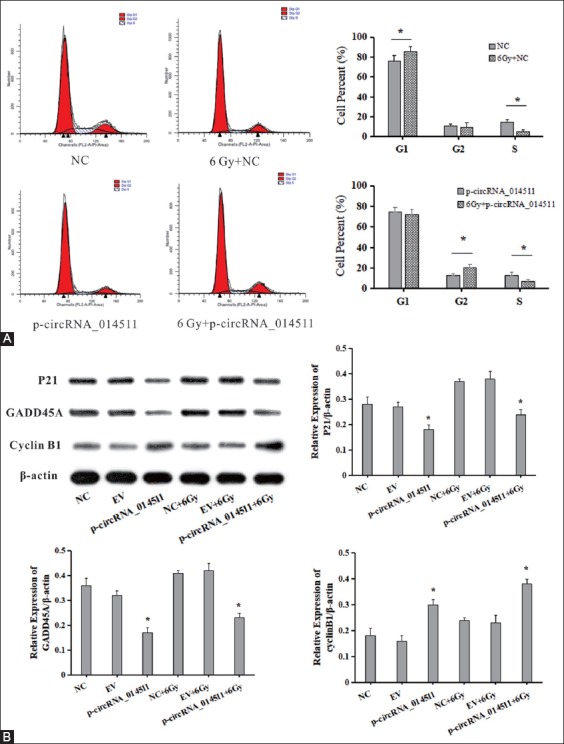
The effect of circRNA_014511 on cell cycle of BMMSCs. (A) Flow cytometry assessment of cell cycle in cells of each group.(B) Western blot assessment of the expressions of cell cycle-related proteins P21, GADD45A, and Cyclin B1.
Overexpression of circRNA_014511 could reduce radiosensitivity of BMMSCs
The effect of circRNA_014511 on radiosensitivity of BMMSCs was studied using colony formation assay. The results showed that the survival fraction of BMMSCs decreased as the radiation dose increased, but for a given dose of irradiation, the survival fraction of cells overexpressing circRNA_014511 was higher than that of blank control and empty vector control, suggesting that overexpression of circRNA_014511 could effectively reduce the radiosensitivity of BMMSCs in vitro (Figure 4).
FIGURE 4.
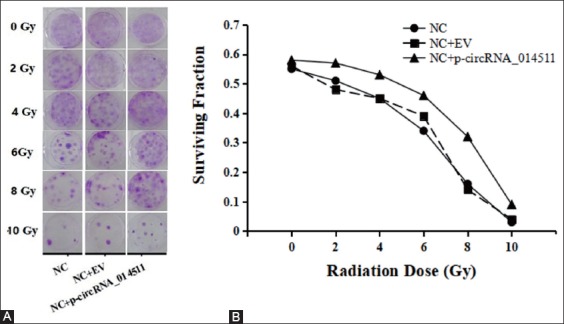
Effect of circRNA_014511 on the radiosensitivity of BMMSCs.
DISCUSSION
TBI is an important step in the preconditioning of bone marrow transplantation [20]. However, many patients sustain long-term impairment of the hematopoietic function of BM after receiving moderate or high doses of TBI, and may even suffer a failure of hematopoietic function, which threatens life [21,22]. The main causes of this fatal pathological damage are radiation-induced damage to two types of stem cells in bone marrow, namely hematopoietic stem cells (HSCs) and BMMSCs. The radiosensitivity of HSCs is very strong, so almost all HSCs are killed by radiation; while BMMSCs have stronger radiation resistance [23,24]. Dickhut et al. [25] confirmed that all the long-term effective BMMSCs after allogeneic stem cell transplantation came from the recipient, which indicates that protecting and restoring the function of endogenous BMMSCs is very important to resistance to radiation damage. Further studies on the mechanism underlying resistance to radiation damage in BMMSCs could provide new ideas for the restoration of hematopoietic function, healing of injuries, and the prevention and treatment of side effects of radiotherapy after radiation damage to bone marrow.
Current studies have shown circRNAs to be involved in the occurrence and development of multiple diseases (such as atherosclerosis, myotonic dystrophy, and prion disease) as miRNA sponges, and were closely related to nervous system diseases (such as Alzheimer’s disease and Parkinson’s disease) [26,27]. CircRNAs play important roles in tumor development and drug resistance, and they are expected to be suitable molecular markers for tumor diagnosis and therapeutic targets [28]. However, little is known about the effects of circRNA on radiation tolerance. In our previous study [13], a mouse model of TBI-induced bone marrow damage was successfully established. The BMMSCs of the model were analyzed by circRNA microarray, and a series of differentially expressed circRNAs were screened by comparison with BMMSCs from normal mice. These included of up-regulated expression of circRNA_014511. Bioinformatics analysis showed that circRNA_014511 had mmu-miR-29b-2-5p response element. In this study, the changes of mmu-miR-29b-2-5p after circRNA_014511 transfection into BMMSCs and the dual luciferase reporter assay confirmed that circRNA_014511 could adsorb mmu-miR-29b-2-5p in BMMSCs. This study further confirmed that the up-regulation of mmu-miR-29b-2-5p could promote the expression of P53 in BMMSCs, but the co-transfection of circRNA_014511 could reverse the increased expression of P53, which resulted in down-regulation of P53. These results indicated that circRNA_014511 could affect the expression of P53 by absorbing mmu-miR-29b-2-5p.
The present work showed that overexpression of circRNA_014511 in both normal and irradiated BMMSCs could decrease the rate of apoptosis in BMMSCs. In circRNA_014511 overexpression cells, the expression of P53 was decreased, while the expressions of Bcl-2 and Mcl-1 were increased. P53 can induce apoptosis, while Bcl-2 and Mcl-1 are the main members of Bcl-2 protein family, which block the changes of mitochondrial permeability and the release of apoptosis-inducing factors in mitochondria by binding the pro-apoptotic members of Bcl-2 protein family, and thus play the role of anti-apoptosis [29,30]. P53 could inhibit the transcription of Bcl-2 gene. Studies found that [31] overexpression of P53 in M1 mouse myeloid leukemia cells could down-regulate the Bcl-2 expression in mRNA and protein levels. Increased expression of Bcl-2 protein was detected in the tissues of p53 knockout mice. This study demonstrated for the first time that circRNA_014511 inhibited P53 by adsorbing miR-29b in BMMSCs, and up-regulated the expression of Bcl-2 and Mcl-1, which could inhibit cell apoptosis.
The cell cycle is an important factor in determining the radiation resistance of cells. In this study, G1 phase arrest occurred in normal BMMSCs after irradiation, while G2 phase arrest occurred in BMMSCs overexpressing circRNA_014511 after irradiation due to the significant down-regulation of P53. P53 could keep the cells in G1/S checkpoint by activating the DNA repair protein and inhibiting cell growth [32]. Some studies had shown that [33] when exposed to irradiation, G1 phase arrest always occurred in P53 wild-type cells, while G2 phase arrest was mainly found in p53 knockout cells. Except for down-regulation of P53, the overexpression of circRNA_014511 also lead to the decreased expressions of cell cycle-related proteins P21 and GADD45A, and the increased expression of Cyclin B1.
The inhibition of apoptosis and the changes in cell cycle after overexpression of circRNA_014511 led to radiation resistance in BMMSCs. In a colony formation assay, the survival fraction of cells overexpressing circRNA_014511 after irradiation was significantly higher than in the control group, indicating that circRNA_014511 could reduce the radiosensitivity of BMMSCs in vitro, suggesting that circRNA_014511 might be a new target for the protection and repair of radiation damage in patients.
To sum up, through in vitro experiments in mouse BMMSCs, this study demonstrated that circRNA_014511 could inhibit the expression of P53 through the endogenous competitive combination of mmu-miR-29b-2-5p, and induced the changes in multiple apoptosis-related proteins and cell-cycle-related proteins, which decreased the radiosensitivity of the cells. This might help to reduce the BMMSCs loss in bone marrow of HSCT patients receiving TBI, and it might play a positive role in hematopoietic reconstitution and reducing the GVHD occurrence after transplantation. However, this effect needed to be further demonstrated by in vivo experiments. In this study, the effects of circRNA_014511 on radiosensitivity of BMMSCs and its possible mechanisms were established.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 81602801 and 81573091).
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Morris EC, Thomson KJ. Bone marrow and stem cell transplantation. Medicine (Baltimore) 2009;37(3):168–171. https://doi.org/10.1016/j.mpmed.2009.01.001. [Google Scholar]
- 2.Garcia IN. Role of Hematopoietic Stem Cell Transplantation in Multiple Myeloma. Clin Lymphoma, Myeloma Leuk. 2015;15(2):86–91. doi: 10.1016/j.clml.2014.07.007. https://doi.org/10.1016/j.clml.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28(23):3730–3738. doi: 10.1200/JCO.2010.28.8852. https://doi.org/10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamidieh AA, Monzavi SM, Kaboutari M, Behfar M, Esfandbod M. Outcome Analysis of Pediatric Patients with Acute Lymphoblastic Leukemia Treated with Total Body Irradiation-Free Allogeneic Hematopoietic Stem Cell Transplantation:Comparison of Patients with and Without Central Nervous System Involvement. Biol Blood Marrow Transplant. 2017;23(12):2110–2117. doi: 10.1016/j.bbmt.2017.08.036. https://doi.org/10.1016/j.bbmt.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Heinzelmann F, Ottinger H, Müller C-H, Allgaier S, Faul C, Bamberg M, et al. Total-Body Irradiation Role and Indications. Strahlentherapie und Onkol. 2006;182(4):222–230. doi: 10.1007/s00066-006-1468-1. https://doi.org/10.1007/s00066-006-1468-1. [DOI] [PubMed] [Google Scholar]
- 6.Sayan M, Cassidy RJ, Butker EE, Nanda RH, Krishnamurti L, Khan MK, et al. Gonadal shielding technique to preserve fertility in male pediatric patients treated with total body irradiation for stem cell transplantation. Bone Marrow Transplant. 2016;51(7):997–998. doi: 10.1038/bmt.2016.25. https://doi.org/10.1038/bmt.2016.25. [DOI] [PubMed] [Google Scholar]
- 7.Eapen M, Rubinstein P, Zhang M-J, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia:a comparison study. Lancet. 2007;369(9577):1947–1954. doi: 10.1016/S0140-6736(07)60915-5. https://doi.org/10.1016/s0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Zhu G, Wang J, Chen J. Irradiation alters the differentiation potential of bone marrow mesenchymal stem cells. Mol Med Rep. 2016;13(1):213–223. doi: 10.3892/mmr.2015.4539. https://doi.org/10.3892/mmr.2015.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R Liu, W Chang, H Wei, K Zhang. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from Bone Marrow and Skin. Stem Cells Int. 2016;2016:1–12. doi: 10.1155/2016/3658798. https://doi.org/10.1155/2016/3658798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schu S, Nosov M, O'Flynn L, Shaw G, Treacy O, Barry F, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16(9):2094–2103. doi: 10.1111/j.1582-4934.2011.01509.x. https://doi.org/10.1111/j.1582-4934.2011.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonizzato A, Gaffo E, Te Kronnie G, Bortoluzzi S. CircRNAs in hematopoiesis and hematological malignancies. Blood Cancer J. 2016;6(10):e483–12. doi: 10.1038/bcj.2016.81. https://doi.org/10.1038/bcj.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993. https://doi.org/10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Jiang J, Huang R, Wang Y, Nie X, Gui R. Circular RNA expression profiles are significantly altered in mice bone marrow stromal cells after total body irradiation. Leuk Res. 2018;70:67–73. doi: 10.1016/j.leukres.2018.05.010. https://doi.org/10.1016/j.leukres.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85?and CDC42. Nat Struct Mol Biol. 2009;16(1):23–29. doi: 10.1038/nsmb.1533. https://doi.org/10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 15.Merkel O, Asslaber D, Piñón JD, Egle A, Greil R. Interdependent regulation of p53 and miR-34a in chronic lymphocytic leukemia. Cell Cycle. 2010;9(14):2764–2768. https://doi.org/10.4161/cc.9.14.12267. [PubMed] [Google Scholar]
- 16.Avasarala S, Van Scoyk M, Wang J, Sechler M, Vandervest K, Brzezinski C, et al. hsa-miR29b, a critical downstream target of non-canonical Wnt signaling, plays an anti-proliferative role in non-small cell lung cancer cells via targeting MDM2 expression. Biol Open. 2013;2(7):675–685. doi: 10.1242/bio.20134507. https://doi.org/10.1242/bio.20134507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu S, Shao S, Wang L, Liu H, Hou H, Wang Y, et al. USP3 stabilizes p53 protein through its deubiquitinase activity. Biochem Biophys Res Commun. 2017;492(2):178–183. doi: 10.1016/j.bbrc.2017.08.036. https://doi.org/10.1016/j.bbrc.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Shang J, Yao Y, Fan X, Shangguan L, Li J, Liu H, et al. MiR-29c-3p promotes senescence of human mesenchymal stem cells by targeting CNOT6 through p53-p21 and p16-pRB pathways. Biochim Biophys Acta - Mol Cell Res. 2016;1863(4):520–532. doi: 10.1016/j.bbamcr.2016.01.005. https://doi.org/10.1016/j.bbamcr.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Ng P, Parks RJ, Cummings DT, Evelegh CM, Graham FL. An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Hum Gene Ther. 2000;11(5):693–699. doi: 10.1089/10430340050015590. https://doi.org/10.1089/10430340050015590. [DOI] [PubMed] [Google Scholar]
- 20.Paix A, Antoni D, Waissi W, Ledoux MP, Bilger K, Fornecker L, et al. Total body irradiation in allogeneic bone marrow transplantation conditioning regimens:A review. Crit Rev Oncol Hematol. 2018;123:138–148. doi: 10.1016/j.critrevonc.2018.01.011. https://doi.org/10.1016/j.critrevonc.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Zhai Z, Wang Y, Zhang J, Wu H, Wang Y, et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med. 2013;54:40–50. doi: 10.1016/j.freeradbiomed.2012.10.530. https://doi.org/10.1016/j.freeradbiomed.2012.10.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Zhuo-Jing, Chen B, Wang X, Chen L, Luo Z. Molecular Targeting Regulation of Proliferation and Differentiation of the Bone Marrow-Derived Mesenchymal Stem Cells or Mesenchymal Stromal Cells. Current Drug Targets. 2012;13(4):561–571. doi: 10.2174/138945012799499749. https://doi.org/10.2174/138945012799499749. [DOI] [PubMed] [Google Scholar]
- 23.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. https://doi.org/10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Zhang J, Liu C-Y, Hayashi Y, Kao WW-Y. Bone marrow mesenchymal stem cells can differentiate and assume corneal keratocyte phenotype. J Cell Mol Med. 2012;16(5):1114–1124. doi: 10.1111/j.1582-4934.2011.01418.x. https://doi.org/10.1111/j.1582-4934.2011.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickhut A, Schwerdtfeger R, Kuklick L, Ritter M, Thiede C, Neubauer A, et al. Mesenchymal stem cells obtained after bone marrow transplantation or peripheral blood stem cell transplantation originate from host tissue. Ann Hematol. 2005;84(11):722–727. doi: 10.1007/s00277-005-1067-8. https://doi.org/10.1007/s00277-005-1067-8. [DOI] [PubMed] [Google Scholar]
- 26.Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7 doi: 10.1038/ncomms12429. https://doi.org/10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, et al. Circular RNA:A new star of noncoding RNAs. Cancer Lett. 2015;365(2):141–148. doi: 10.1016/j.canlet.2015.06.003. https://doi.org/10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Mo Y, Gong Z, Yang X, Yang M, Zhang S, et al. Circular RNAs in human cancer. Mol Cancer. 2017;16(1):1–9. doi: 10.1186/s12943-017-0598-7. https://doi.org/10.1186/s12943-017-0598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang S, Hu J, Meng Q, Dong X, Wang K, Qi Y, et al. Daidzein Induced Apoptosis via Down-Regulation of Bcl-2/Bax and Triggering of the Mitochondrial Pathway in BGC-823 Cells. Cell Biochem Biophys. 2013;65(2):197–202. doi: 10.1007/s12013-012-9418-2. https://doi.org/10.1007/s12013-012-9418-2. [DOI] [PubMed] [Google Scholar]
- 30.Martinou JC, Youle RJ. Mitochondria in Apoptosis:Bcl-2 Family Members and Mitochondrial Dynamics. Dev Cell. 2011;21(1):92–101. doi: 10.1016/j.devcel.2011.06.017. https://doi.org/10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9(6):1799–1805. [PubMed] [Google Scholar]
- 32.Jeong JH, Chang YC. Ascochlorin, an isoprenoid antibiotic, induces G1 arrest via downregulation of c-Myc in a p53-independent manner. Biochem Biophys Res Commun. 2010;398(1):68–73. doi: 10.1016/j.bbrc.2010.06.037. https://doi.org/10.1016/j.bbrc.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 33.Kriegs M, Gurtner K, Can Y, Brammer I, Rieckmann T, Oertel R, et al. Radiosensitization of NSCLC cells by EGFR inhibition is the result of an enhanced p53-dependent G1 arrest. Radiother Oncol. 2015;115(1):120–127. doi: 10.1016/j.radonc.2015.02.018. https://doi.org/10.1016/j.radonc.2015.02.018. [DOI] [PubMed] [Google Scholar]


