Abstract
Phenibut is a glutamic acid derivative with activity on the γ-aminobutyric acid (GABA)B, A, and B-phenethylamine receptors. It is prescribed in former Communist Bloc countries for anxiolysis and related psychiatric disorders. It can be easily obtained in Western countries and is thought to have abuse potential. Abrupt discontinuation has been reported to precipitate an abstinence syndrome. A review of the literature identified 22 reported cases, many of which were notable for severe psychomotor agitation and requirements for aggressive pharmacologic treatment. Neurologic and autonomic signs and symptoms may mimic serotonin or neuroleptic malignant syndrome. Patients were typically younger and had coexisting substance abuse disorders to other drugs. Also presented is a case of a 23-year-old male with an acute phenibut abstinence syndrome. This patient exhibited severe psychomotor agitation requiring physical restraints, dexmedetomidine, lorazepam, haloperidol, diphenhydramine, cyproheptadine, melatonin, olanzapine, and baclofen for symptom control.
Keywords: Phenibut, abstinence syndrome, psychomotor agitation, serotonin syndrome, neuroleptic malignant syndrome, GABA
INTRODUCTION
Phenibut (4-amino-3-phenyl-butyric acid, PHB; also known as Anvifen, Fenibut, and Noofen) is a glutamic acid derivative with agonistic effects on the γ-aminobutyric acid (GABA)B receptor in the brain, spinal cord, and autonomic nervous systems [1-5]. PHB is a racemic mixture with the R-PHB enantiomer exerting pharmacological activity [2], and is structurally similar to gabapentin and baclofen (Figure 1) [6]. GABAB receptor activation leads to neurotransmitter inhibition, via antagonism of voltage-gated calcium channels, similar to gabapentin and pregabalin [2,5,6]. PHB action on the GABAB receptor is similar but is less potent than baclofen [1,2,6]. Furthermore, although PHB primarily acts as a GABAB agonist, it also affects the GABAA receptor responsible for the action of benzodiazepines, and antagonizes the anxiogenic β-phenethylamine receptor [1,2,4-6].
FIGURE 1.
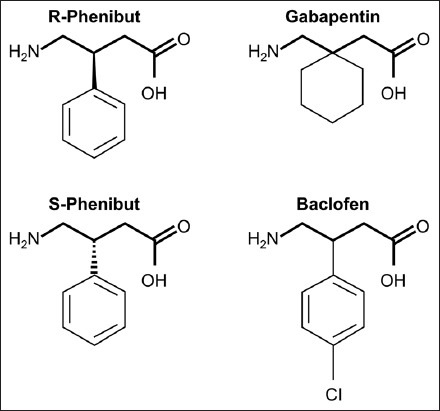
Chemical structures of R-phenibut, S-phenibut, baclofen, and gabapentin (from Zvejniece L. et al. [6]). R-phenibut binds to the alpha2-delta subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects.
PHB is prescribed in former Communist Bloc countries to treat anxiety and insomnia, as well as other psychiatric conditions (motor tics, alcohol withdrawal, restless leg syndrome, and post-traumatic stress disorder) [1,7,8]. While PHB is unavailable as a prescription in Western countries, it can be obtained via the internet or vitamin and supplement suppliers [9-11]. At this time, it is unclear how widespread the use of PHB is within the United States, and there is no laboratory or blood test to confirm its presence. As a central nervous system (CNS) depressant, PHB can be abused to induce euphoria and anxiolysis. It is also widely used as a nootropic agent [1,7,11-14]. Chronic PHB use results in tolerance, and abrupt discontinuation can lead to abstinence syndromes, some of which have been reported to be severe [3,10,11,13,15-18].
To better understand the presentations and severity of PHB abstinence syndromes as well as therapeutic approaches, we conducted a thorough review of the literature. In addition, we present an illustrative case from our institution of a 23-year-old male who required intensive care to manage withdrawal symptoms associated with acute, severe PHB abstinence.
REVIEW OF THE LITERATURE
A review of the literature was performed on November 19, 2018, using Embase, Medline/PUBMED, Scopus and Web of Science databases for citations in English and the following terms: “4 amino 3 phenylbutyric acid 216”, “phenyl gamma aminobutyric acid”, “phenyl-gaba”, “amino 3 phenylbutanoic acid”, “beta phenyl 4 aminobutyric acid”, “beta phenyl gaba”, “beta phenyl gamma aminobutyric acid”, “fenibut”, “fenigam”, “fenigama”, “phenibut”, “phenigam”, “phenigama”, “phenybut”, “phenygam”, “phgaba”, crossed with “abstinen*” or “withdraw*” or “dependen”. Bibliographies were reviewed for additional reports. Identified reports were reviewed for relevancy.
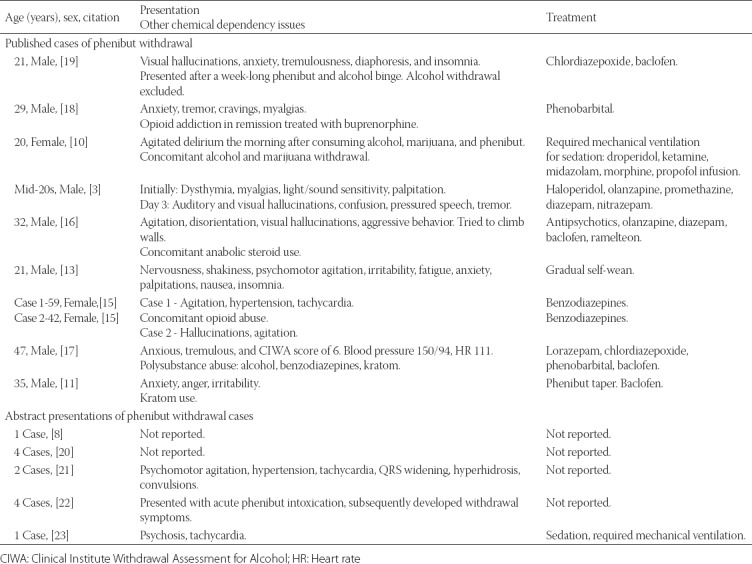
Our literature search identified nine reports of 10 cases of PHB withdrawal (Table 1) [3,10,11,13,15-19]. In addition, there were five abstracts presented at meetings reporting 12 additional cases (Table 1) [8,20-23].
TABLE 1.
Reported cases of phenibut withdrawal
CASE REPORT
A 23-year-old male with an extensive polysubstance abuse history (cannabis, cocaine, opioids, amphetamines, benzodiazepines, and hallucinogens) and anxiety/depression managed with the selective serotonin reuptake inhibitor, sertraline, presented to the emergency department with progressive hallucinations and psychomotor agitation. A year prior, he had completed a drug rehabilitation program, but relapsed after a few months by abusing PHB. At the time of his emergency department presentation, he was using 4000 mg of PHB every six hours. He abruptly discontinued PHB two days prior. He denied other current drug abuse.
His urine toxicology panel (PHB screening was not included) was negative. He was admitted to the psychiatry service with the differential diagnosis of PHB withdrawal and substance-induced psychosis. However, his symptoms were recalcitrant to multiple pharmacological treatments including lorazepam, haloperidol, diphenhydramine, melatonin, and olanzapine. His physical symptoms worsened and he developed tachycardia, increased muscular tone, and inducible clonus. Laboratory evaluation revealed elevated creatine kinase at 867 U/L, though he remained afebrile. He was subsequently transferred to the intensive care unit for worsening agitation. Physical restraints, a dexmedetomidine infusion, and additional doses of lorazepam were required for severe agitation. Out of concern he was developing rhabdomyolysis, baclofen was administered and aggressive intravenous hydration begun; however, his kidney function was not adversely affected (creatinine levels remained between 0.7 mg/dl and 0.8 mg/dl throughout hospital stay). Cyproheptadine, a first-generation antihistamine with anticholinergic and antiserotonergic properties, was administered to empirically treat serotonin syndrome (patient was on sertraline) or neuroleptic malignant syndrome, without improvement.
The patient spent three days in intensive care where the dexmedetomidine infusion was slowly discontinued and gabapentin initiated and titrated. Baclofen and lorazepam were continued as needed for ongoing tremulousness, insomnia, nausea/vomiting, anxiety, and psychomotor agitation. After his acute episode of withdrawal resolved, he was transferred to the psychiatry service for further management of anxiety and insomnia. The patient was discharged from the psychiatry service six days later with scheduled outpatient follow-up.
DISCUSSION
Our case illustrates the serious nature of PHB withdrawal as well as complex management needed to prevent potentially life-threatening sequelae. In our patient, PHB withdrawal posed substantial management challenges and protracted treatment in the intensive care unit. Further, our case also demonstrates the diagnostic conundrum related to similarity between PHB withdrawal and serotonin syndrome. Only a few cases of PHB withdrawal are available in the literature (Table 1). However, patients were consistently reported to display signs of psychomotor agitation, oftentimes severe [3,10,11,13,15-19,21,23]. Two reports of withdrawal described patients who required such degree of sedation that mechanical ventilation was required [10,23]. In addition, the patient demographics including young to middle age adults and concomitant substance abuse were typically present [10,11,15-19].
PHB toxicity
Acute PHB intoxication typically results in depressive symptoms including a decreased level of consciousness, muscle tone, and stupor. Physical symptoms of acute intoxication can include depressed respiratory drive, altered consciousness, temperature dysregulation, and alterations in hemodynamics including hyper/hypotension and tachycardia [7,8,10-12,15,16,20,24-27]. Paradoxically, symptoms can include psychomotor agitation, hallucinations, seizures, and delirium [7,8,10-12,15,16,20,24-27]. There are three reports where PHB intoxication was so severe that patients required intravenous sedation to the point where they required tracheal intubation and mechanical ventilation [25,27]. Rhabdomyolysis secondary to extreme agitation has also been reported with intoxication [25].
Treatment of PHB intoxication
Management of intoxication is largely supportive with focus on respiratory drive, airway protection, and providing sedation if paradoxical agitation, seizures and delirium are present [7,8,10-12,15,16,20,24-27]. PHB is minimally metabolized in the liver and secreted largely unchanged in the urine, according to animal models. Little is known about the potential drug-drug interactions or alterations in metabolism in humans [1].
PHB withdrawal
Because chronic use of PHB downregulates GABAB and A receptors, its abrupt discontinuation induces withdrawal symptoms including psychomotor agitation, psychosis, hallucinations, seizures, nausea and vomiting, and tachycardia [3,10,11,13,15-19]. Furthermore, rigidity, hyperreflexia, autonomic instability, fever and myoclonus [3,10,11,13,15-19] may resemble serotonin or neuroleptic malignant syndrome. Our patient manifested with tachycardia, increased muscular tone and rigidity, and inducible clonus. PHB intoxication, as well as withdrawal, can result in rhabdomyolysis leading to acute kidney injury [25]. As seen in our case, despite aggressive and prompt anxiolytic and antipsychotic treatment, the patient developed a moderate increase of serum creatine kinase but did not develop kidney impairment, as assessed from unchanged serum creatinine concentrations. Severe hypertension requires aggressive blood pressure management and agitation and rebound anxiety requires anxiolytic therapy [3,10,11,13,15-19]. In extreme situations, these patients may harm self and/or others [3,16].
Treatment of PHB withdrawal
The pharmacologic treatment of PHB withdrawal is largely directed towards the management of withdrawal symptoms (Table 2). In cases of severe agitation, both chemical and physical restraints may be required [25], as well as admission to the intensive care unit. During the acute withdrawal, sedatives including dexmedetomidine, benzodiazepines, olanzapine, phenobarbital and haloperidol have been used; although it remains unclear which agent is optimal [3,10,11,13,15-19]. Once the patient is able to take oral medications, clonidine, gabapentin, baclofen, benzodiazepines, phenobarbital and pregabalin may be used. Gabapentin, baclofen, benzodiazepines, and pregabalin may be particularly useful due to their similar interaction with GABAB and A receptors as PHB [2,6]. Patients who take PHB will often need continued outpatient management of underlying psychiatric and substance abuse issues with the transition to safer medications.
TABLE 2.
Mechanisms of action of medications used for phenibut withdrawal treatment

CONCLUSION
The clinical presentation of our patient suggests that severe PHB withdrawal can be difficult to manage and refractory to multiple standard interventions. Neurologic and autonomic signs and symptoms may mimic serotonin or neuroleptic malignant syndrome. Medical providers must be familiar with the complex mechanisms by which PHB may affect various CNS receptors in order to treat patients with PHB withdrawal.
DECLARATION OF INTERESTS
Dr. Weingarten serves as a consultant to Medtronic in the role as chairman of the Clinical Endpoint Committee for the Prodigy Trial and has received unrestricted investigator-initiated grants from Merck for research unrelated to the current study. The other authors declare no conflict of interests.
REFERENCES
- 1.Lapin I. Phenibut (beta-phenyl-GABA):A tranquilizer and nootropic drug. CNS Drug Rev. 2001;7(4):471–81. doi: 10.1111/j.1527-3458.2001.tb00211.x. https://doi.org/10.1111/j.1527-3458.2001.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dambrova M, Zvejniece L, Liepinsh E, Cirule H, Zharkova O, Veinberg G, et al. Comparative pharmacological activity of optical isomers of phenibut. Eur J Pharmacol. 2008;583(1):128–34. doi: 10.1016/j.ejphar.2008.01.015. https://doi.org/10.1016/j.ejphar.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Hogberg L, Szabo I, Ruusa J. Psychotic symptoms during phenibut (beta-phenyl-gamma-aminobutyric acid) withdrawal. J Subst Use. 2013;18(4):335–8. https://doi.org/10.3109/14659891.2012.668261. [Google Scholar]
- 4.Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. https://doi.org/10.1016/S0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- 5.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, et al. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386(6622):239–46. doi: 10.1038/386239a0. https://doi.org/10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 6.Zvejniece L, Vavers E, Svalbe B, Veinberg G, Rizhanova K, Liepins V, et al. R-phenibut binds to the α2-δ subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects. Pharmacol Biochem Behav. 2015;137:23–9. doi: 10.1016/j.pbb.2015.07.014. https://doi.org/10.1016/j.pbb.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Sankary S, Canino P, Jackson J. Phenibut overdose. Am J Emerg Med. 2017;35(3):516.e1–2. doi: 10.1016/j.ajem.2016.08.067. https://doi.org/10.1016/j.ajem.2016.08.067. [DOI] [PubMed] [Google Scholar]
- 8.Goertemoeller S, Behrman A, Goetz R, Spiller H. Retrospective review of phenibut exposures reported to Ohio poison control centers. Clin Toxicol. 2015;53(7):764. [Google Scholar]
- 9.O'Connell CW, Schneir AB, Hwang JQ, Cantrell FL. Phenibut, the appearance of another potentially dangerous product in the United States. Am J Med. 2014;127(8):e3–4. doi: 10.1016/j.amjmed.2014.03.029. https://doi.org/10.1016/j.amjmed.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Downes MA, Berling IL, Mostafa A, Grice J, Roberts MS, Isbister GK, et al. Acute behavioural disturbance associated with phenibut purchased via an internet supplier. Clin Toxicol (Phila) 2015;53(7):636–8. doi: 10.3109/15563650.2015.1059945. https://doi.org/10.3109/15563650.2015.1059945. [DOI] [PubMed] [Google Scholar]
- 11.Samokhvalov AV, Paton-Gay CL, Balchand K, Rehm J. Phenibut dependence. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-008381. https://doi.org/10.1136/bcr-2012-008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deyo Z, Stuppy S, Breden E, Moran J. Nootropic-induced psychosis:A case report. J Pharm Pract. 2010;23(2):185. [Google Scholar]
- 13.Magsalin RM, Khan AY. Withdrawal symptoms after internet purchase of phenibut (β-phenyl-γ-aminobutyric acid HCl) J Clin Psychopharmacol. 2010;30(5):648–9. doi: 10.1097/JCP.0b013e3181f057c8. https://doi.org/10.1097/JCP.0b013e3181f057c8. [DOI] [PubMed] [Google Scholar]
- 14.Neumyvakin IP, Krupina TN, Polevoĭ LG, Semeĭkina LA. Principles for making up pharmaceutical kits to supply cosmonauts with drug packs. Kosm Biol Aviakosm Med. 1978;12(3):27–31. [Article in Russian] [PubMed] [Google Scholar]
- 15.Marraffa J, Nacca N, Stork C, Hodgman M. Phenibut:One poison center's experience. Clin Toxicol. 2014;52(7):736–7. [Google Scholar]
- 16.Joshi YB, Friend SF, Jimenez B, Steiger LR. Dissociative intoxication and prolonged withdrawal associated with phenibut:A case report. J Clin Psychopharmacol. 2017;37(4):478–80. doi: 10.1097/JCP.0000000000000731. https://doi.org/10.1097/JCP.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rod W, Kudryk A, Brunetti L, Sun N, Nguyen M. Phenibut withdrawal management in the setting of concomitant kratom and alcohol dependence. Crit Care Med. 2018;46(Supplement 1):451. https://doi.org/10.1097/01.ccm.0000528942.37373.7a. [Google Scholar]
- 18.Brunner E, Levy R. Case report of physiologic phenibut dependence treated with a phenobarbital taper in a patient being treated with buprenorphine. J Addict Med. 2017;11(3):239–40. doi: 10.1097/ADM.0000000000000303. https://doi.org/10.1097/ADM.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 19.Ahuja T, Mgbako O, Katzman C, Grossman A. Phenibut (β-phenyl-γ-aminobutyric acid) dependence and management of withdrawal:Emerging nootropics of abuse. Case Rep Psychiatry. 2018;2018:9864285. doi: 10.1155/2018/9864285. https://doi.org/10.1155/2018/9864285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huntington S, Vohra R, Lewis J, Iknoian J, Dougherty B. GABA GABA hey (I wanna be sedated):Phenybut exposures reported to a statewide poison control system. Clin Toxicol. 2015;53(7):721–2. [Google Scholar]
- 21.Koppen A, Van Riel A, Roelen C, De Vries I, Meulenbelt J. Reports of phenibut usage to the Dutch Poisons Information Center (DPIC) Clin Toxicol. 2015;53(7):716–7. [Google Scholar]
- 22.McCabe D, Bangh S, Arens A, Cole J. Phenibut exposures reported to a regional poison center. J Med Toxicol. 2018;14(1):16. doi: 10.1016/j.ajem.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 23.Odujebe OA, Hoffman RS, Nelson LS. Phenibut withdrawal - A novel “nutritional supplement”. Clin Toxicol. 2008;46(7):605. [Google Scholar]
- 24.Elamin M, Dunn M, Hill S, Thomas S. Convulsions associated with analytically confirmed phenibut ingestion. Clin Toxicol. 2016;54(4):448. [Google Scholar]
- 25.Li W, Madhira B. Phenibut (beta-phenyl-gamma-aminobutyric acid) psychosis. Am J Ther. 2017;24(5):e639–40. doi: 10.1097/MJT.0000000000000618. https://doi.org/10.1097/MJT.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 26.Teter C, Varney S, Kruse C. Phenibut:High school vital sign lows, patient woes, and poison center kudos. Clin Toxicol. 2017;55(7):845. [Google Scholar]
- 27.Wong A, Little M, Caldicott D, Easton C, Andres D, Greene S. Multiple intensive care admissions associated with analytically confirmed recreational use of phenibut (beta-phenyl-g-amniobutyric acid) purchased over the internet. Clin Toxicol. 2015;53(4):303–4. doi: 10.3109/15563650.2015.1059944. [DOI] [PubMed] [Google Scholar]


