Introduction
Diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state (HHS) are the most serious and life-threatening hyperglycemic emergencies in patients with diabetes. Although DKA and HHS are often discussed as separate entities, they represent points along a spectrum of hyperglycemic emergencies due to poorly controlled diabetes. Both DKA and HHS can occur in patients with type 1 and type 2 diabetes; however, DKA is more common in young people with type 1 diabetes (T1D) and HHS is more frequently reported in adult and elderly patients with type 2 diabetes (T2D). In many patients, features of the two disorders with ketoacidosis and hyperosmolality may also co-exist. The frequency of DKA has increased by 30% during the past decade, with more than 140,000 hospital admissions per year in the United States 1,2. The rate of hospital admissions for HHS is lower than for DKA, accounting for less than 1% of all diabetes-related admissions 3,4. Both disorders are characterized by insulinopenia and severe hyperglycemia. Early diagnosis and management is paramount to improve patient outcomes. The mainstays of treatment in both DKA and HHS are aggressive rehydration, insulin therapy, electrolyte replacement, and discovery and treatment of underlying precipitating events. Herein we review the epidemiology, pathogenesis, diagnosis, and provide practical recommendations for the management of patients with hyperglycemic emergencies.
Historical Review of Diabetic Comas
The first detailed clinical description of diabetic coma in an adult patient with severe polydipsia, polyuria, and a large amount of glucose in the urine followed by progressive decline in mental status and death was reported by August W. von Stosch in 1828 5. This publication was followed by several case reports describing young and adult patients, with newly diagnosed or with established diabetes, who presented with abrupt clinical course of excessive polyuria, glycosuria, coma and death 6–8. In 1874, The German physician Adolf Kussmaul reported that many cases of diabetic coma were preceded by deep and frequent respiration and severe dyspnea 9,10. Kussmaul breathing rapidly became one of the hallmarks of diabetic coma. Shortly after that, it was reported that in many of these patients, the urine contained large amounts of acetoacetic acid and β-hydroxybutyric acid 11,12. Dr. Julius Dreshfeld in 1886, was first to provide a comprehensive description of the two different categories of diabetic coma 13, one with Kussmaul breathing and positive ketones and the other, an unusual type of diabetic coma in older, well-nourished individuals, characterized by severe hyperglycemia and glycosuria but without Kussmaul breathing, fruity breath odor, or a positive urine acetone test.
Prior to the discovery of insulin in 1921, the mortality rate of patients with diabetic ketoacidosis was over 90%. The first successful case of DKA treated with insulin was reported by Banting and Best in a 14 year old boy who presented with a blood glucose of 580 mg/dL and strongly positive urinary ketones at the Toronto General Hospital in 1923 14. They reported a dramatic improvement in glycosuria along with disappearance of acetone bodies in the urine after a few doses of pancreatic extract injections14. Following the discovery of insulin, mortality rate associated with diabetic comas fell dramatically to 60% in 1923 and 25% by 1930’s 15, 7–10% in the 1970s 16,17 and is currently less than 2% in patients for DKA 1,18,19 and between 5–16% in patients with HHS 20,21.
Epidemiology
Even though DKA occurs more commonly in patients with autoimmune T1D, the cumulative number of cases of DKA reported in patients with T2D represents at least a third of all cases 22. Global epidemiological studies have reported on the incidence of DKA among patients with T1D. An analysis from the Prospective Diabetes Registry in Germany including 31,330 patients reported a DKA admission rate of 4.81/100 patient-years (95% CI, 4.51–5.14) 23. Individuals with the highest risk included those with high HbA1c, longer diabetes duration, adolescents, and girls 23. Multinational data from 49,859 children (<18 years) with T1D across three registries and five nations similarly found higher odds of DKA among females (odds ratio [OR] 1.23, 99% CI 1.10–1.37), ethnic minorities (OR 1.27, 99% CI, 1.11–1.44), and among those with HbA1c ≥7.5% (OR 2.54, 99% CI, 2.09–3.09 for HbA1c from 7.5 to <9% and OR 8.74, 99% CI, 7.18–10.63 for HbA1c ≥9.0%) 24. Data from the T1D Exchange Clinic Network including 2,561, shows that young adults (18–25 years) have the highest occurrence of DKA (~5%) defined as ≥1 event in prior 3 months 25. HHS typically occurs in older patients with type 2 diabetes 20; however, it is being recognized as an emerging problem in children and young adults 26.
Similar mortality rates have been reported in European countries, but the reported mortality continues to be higher than 10% in Indonesia and sub-Saharan African countries 27,28. HHS occurs most commonly in older patients with T2D with an intercurrent illness such as infection, surgery or ischemic events, and is associated with higher mortality rate than DKA. Mortality in patients with HHS is reported between 5 and 16%, which is about 10 times higher than the mortality in patients with DKA 20,21,29. The cause of death in patients with DKA and HHS rarely results from the metabolic complications of hyperglycemia or metabolic acidosis but relates to the underlying precipitating cause, severity of dehydration, and advanced age 1,4,30.
Treatment of patients with DKA and HHS is associated with substantial mortality and healthcare costs. DKA is the leading cause of mortality among children and young adults with T1D, accounting for ~50% of all deaths in diabetic patients younger than 24 years of age 1. In the United States, the overall inpatient DKA mortality is <1% 1,2 but a higher rate is reported among elderly patients with life-threatening illnesses 1,2,31,32. Similar mortality rates have been reported in European countries, but the reported mortality continues to be higher than 10% in countries with limited acute care resources 28. A history of recurrent DKA episodes increases substantially the long-term mortality after discharge, particularly among young, socially disadvantaged adults with very high HbA1c 33. In a retrospective review from the United Kingdom, the long-term mortality following a single episode of DKA was 5.2% (4.1 [2.8–6.0] years of follow-up) compared with 23.4% in those with recurrent DKA admissions (2.4 [2.0–3.8] years of follow-up) (HR 6.18) 33. Inpatient mortality has been reported in 5–16% of patients with HHS, a rate that is ~10-fold higher than that reported for DKA 20,21,29. The prognosis and outcome of patients with HHS is determined by the severity of dehydration, presence of co-morbidities and advanced age. In addition, patients with history of HHS are at significant risk of mortality after hospitalization, in particular those with multiple episodes. Compared to patients with diabetes without HHS, a recent study reported that after adjustment for age, sex, selected comorbidities, and monthly income, the mortality hazard ratio was 2.8 and 4.5 times higher in subjects with one episode and two or more episodes of hyperglycemic crisis, respectively 34. National data shows a decline in death related to both hyperglycemic crises with and absolute decline of 529 deaths in the period of 1990 to 2010 (2.7 fewer cases per 10,000; 95% CI, 2.4 to 3.0) 35.
Treatment of hyperglycemic crises represents a substantial economic burden, with an estimated total annual hospital cost of $2.4 billion1. In the US, it is estimated that DKA episodes represent more than $1 of every $4 spent on direct medical care for adult patients with T1D and $1 of every $2 in those patients with multiple DKA episodes 36.
Precipitating Cause
The most common precipitating causes of DKA reported in different epidemiological studies worldwide are shown in Table 1. DKA is the initial presentation of diabetes in ~15% to 20% of adults and in ~30 to 40% of children with T1D 4,37,38. Infection is the most common cause of DKA around the world; however, poor adherence to insulin treatment is the most common precipitating cause of DKA in young patients with T1D and in inner city populations in the U.S. 39–41. According to a recent report from a safety net hospital in Atlanta, insulin discontinuation accounted for 56% of patients with their first and 78% of patients with multiple DKA episodes 39. Other potential precipitants of DKA included infections (14%) and non-infectious illness (4%) 39 such as acute myocardial infarction, neurovascular accidents, alcohol use, and pancreatitis 42. Psychological risk factors including depression and eating disorders have been reported in up to 20% of recurrent episodes of ketoacidosis in young patients 39,43,44. Insulin pump malfunction has long been recognized as a cause of DKA 45,46 due to the short acting insulin formulation used in pumps; however, this is not a common event with newer improved pump technology 47,48.
Table1:
Precipitating causes of Diabetic Ketoacidosis by Country.
| Precipitating Causes, % | Australia | Brazil | China | Indonesia | Korea | Nigeria | Spain | Syria | Taiwan | USA |
|---|---|---|---|---|---|---|---|---|---|---|
| Newly diagnosed diabetes mellitus | 5.7 | 12.2 | NR | 3.3 | NR | NR | 12.8 | NR | 18.2 | 17.2–23.8 |
| Infection | 28.6 | 25.0 | 39.2 | 58.3 | 25.3 | 32.5 | 33.2 | 47.8 | 31.7 | 14.0–16.0 |
| Poor adherence to treatment | 40.0 | 39.0 | 24.0 | 13.3 | 32.7 | 27.5 | 30.7 | 23.5 | 27.7 | 41.0–59.6 |
| Other | 25.7 | 15.0 | 10.9 | 17.1 | 11.2 | 4.8 | 23.3 | 7.8 | 6.2 | 9.7–18.0 |
| Unknown | NA | 8.8 | 25.9 | 8.0 | 30.8 | 34.6 | NA | 20.9 | 16.2 | 3.0–4.2 |
Urinary tract infection and pneumonia are common precipitating causes of HHS 46,49, as well as acute cardiovascular events and other concomitant medical illnesses 20,50. Poor adherence to medical therapy and new diabetes onset are less common precipitating cause of HHS than in DKA49.
Several medications that altered carbohydrate metabolism may precipitate the development of DKA and HHS including glucocorticoids, beta-blockers, thiazide diuretics, certain chemotherapeutic agents 50,51, and atypical antipsychotics 52–55. One large retrospective review from the UK reported that hyperglycemic emergencies occurred at a rate of 1–2 per 1,000 person-years following initiation of antipsychotics 56. Of the antipsychotics, olanzapine and resperidone where associated with the highest risk 56.
Recently, the sodium glucose co-transporter 2 (SGLT2) inhibitors, a new class of oral antidiabetic agents that lower plasma glucose by inhibiting proximal tubular reabsorption of glucose in the kidney have been associated with DKA in patients with T1D and T2D 57,58. An atypical presentation of DKA, which can lead to delayed recognition and treatment, has been referred to as “euglycemic DKA” due to only mild to moderate elevations in blood glucose reported in many cases 59. Compiled data from randomized studies with the use of SGLT2-inhibitors reported a very low incidence of DKA in patients with T2D ~0.07% 60,61; however, the risk of ketosis and DKA is higher in patients with T1D. About 10% of patients with T1D treated with SGLT2-inhibitors develop ketosis and 5% require hospital admission for DKA 59. Potential mechanisms have been proposed, including higher glucagon levels, reduction of daily insulin requirement leading to a decrease in the suppression of lipolysis and ketogenesis, and decreased urinary excretion of ketones 62,63.
Pathophysiology
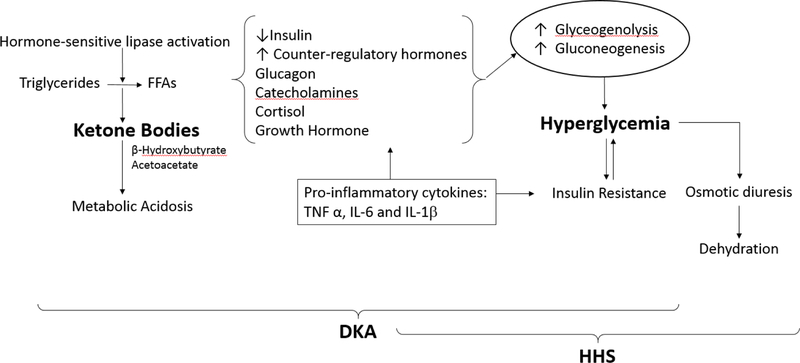
The two most important pathophysiologic mechanisms for DKA and HHS are significant insulin deficiency and increased concentration of counter-regulatory hormones such as glucagon, catecholamines, cortisol, and growth hormone, Figure 1 64,65,66. The insulin deficiency of DKA can be absolute in patients with T1D or relative as observed in patients with T2D in the presence of stress or intercurrent illness 67. Insulin deficiency coupled with increased counterregulatory hormones lead to increased hepatic glucose production due to increased hepatic gluconeogenesis and glycogenolysis 68, as well as reduced glucose utilization in peripheral tissues, in particular muscle 69. Insulinopenia also leads to activation of hormone-sensitive lipase and accelerated breakdown of triglycerides to free fatty acids (FFA)70. In the liver, FFAs are oxidized to ketone bodies, a process predominantly stimulated by glucagon 71,72 and increased glucagon/insulin ratio 73. The increased glucagon/insulin ratio lowers the activity of malonyl coenzyme A (CoA), the enzyme that modulates movement of FFA into the hepatic mitochondria where fatty acid oxidation takes place. The increased production of ketone bodies (acetoacetate and β-hydroxybutyrate), two strong acids, leads to reduction of bicarbonate and metabolic acidosis.
Figure 1.
Pathogenesis of Hyperglycemic Emergencies
Hyperglycemia and accumulation of ketones bodies result from a relative or absolute insulin deficiency and excess counter-regulatory hormones (glucagon, cortisol, catecholamines, and growth hormone).
Increased Ketone Bodies and ketoacidosis. Decrease in insulin levels combined with increased in counter-regulatory hormones, particularly epinephrine causes the activation of hormone sensitive lipase in adipose tissue and breakdown of triglyceride into glycerol and free fatty acids (FFAs). In the liver, FFAs are oxidized to ketone bodies, a process predominantly stimulated by glucagon. The two major ketone bodies are β-hydroxybutyrate and acetoacetic acid. Accumulation of ketone bodies leads to a decrease in serum bicarbonate concentration and metabolic acidosis. Higher insulin levels present in HHS inhibit ketogenesis and limit metabolic acidosis.
Increased Glucose Production in DKA and HHS. When insulin is deficient, hyperglycemia develops as a result of three processes: increased gluconeogenesis, accelerated glycogenolysis, and impaired glucose utilization by peripheral tissues. Hyperglycemia cause osmotic diuresis that lead to hypovolemia, decreased glomerular filtration rate and worsening hyperglycemia.
Several mechanisms have been proposed to explain the absence or minimal presence of ketone bodies in patients with HHS including higher levels of circulating insulin, lower levels of counter-regulatory hormones and FFAs, and inhibition of lipolysis by the hyperosmolar state (Figure 1). Of them, higher insulin secretion appears to be the most important mechanism to prevent ketosis in HHS compared to patients with DKA 66. This is due to the fact that the antilipolytic effect of insulin is about one tenth that of glucose utilization.
Oxidative stress/Inflammation.
Several experimental and clinical studies have shown that development of hyperglycemia and ketoacidosis result in an inflammatory state characterized by an elevation of pro-inflammatory cytokines and increased oxidative stress markers 74,75. Severe hyperglycemia-induced macrophage production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF α), interleukin (IL)-6 and IL-1β, and C-reactive protein, which inturn lead to impaired insulin secretion as well as reduced insulin sensitivity 75–77. Elevation in FFAs also increases insulin resistance as well as impaired nitric oxide production in endothelial cells and endothelial dysfunction 78. The increased inflammatory response, oxidative stress and generation of reactive oxygen species (ROS) can lead to capillary perturbation and cellular damage of lipids, membranes, proteins, and DNA75,79.
Diagnosis of DKA
Signs and Symptoms.
Patients with DKA often present with a short clinical course characterized by fatigue and classic symptoms of hyperglycemia: polyuria, polydipsia, and weight loss. Gastrointestinal complaints are common with diffuse abdominal pain reported in 46% of patients and nausea and vomiting in up to two-third of patients 42. About half of the patients present with lethargy and stupor, but less than 25% present with loss of consciousness 1. On physical examination, patients often present with signs of dehydration with dry mucous membranes and poor skin turgor, tachycardia or hypotension. Patients in DKA may exhibit Kussmaul respirations and a classic fruity (acetone) breath odor (Table 2).
Table 2:
Clinical Features of Hyperglycemic Emergencies
| Condition | Symptoms | Signs | Presentation |
|---|---|---|---|
| DKA | Polydipsia | Hypothermia | Acute onset (hours-days) |
| Polyuria | Tachycardia | More common in T1D than T2D | |
| Weakness | Tachypnea | ||
| Weight loss | Kussmaul breathing | ||
| Nausea | Ileus | ||
| Vomiting | Acetone breath | ||
| Abdominal pain | Altered sensorium | ||
| HHS | Polydipsia | Hypothermia | Insidious onset (days-weeks) |
| Polyuria | Hypotension | Older age | |
| Weakness | Tachycardia | More common in T2D than T1D | |
| Weight loss | Altered sensorium |
Laboratory Findings.
The syndrome of DKA consists of the triad of hyperglycemia, ketonemia and metabolic acidosis (Table 3). The American Diabetes Association classifies DKA by severity as mild, moderate, or severe depending on the degree of acidosis (along with decrease in bicarbonate) and altered sensorium 1. Most patients with DKA present with mild to moderate DKA with blood glucose > 250 mg/dL, bicarbonate between 10 and <18 mEq/L, arterial pH < 7.3, high ketones in urine or blood, and increased anion gap metabolic acidosis > 12.
Table 3:
Diagnostic Criteria for DKA and HHS
| Measure | DKA | HHS | ||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Plasma glucose (mg/dl) | >250 | >250 | >250 | >600 |
| Arterial pH | 7.25–7.30 | 7.00 to <7.24 | <7.00 | >7.30 |
| Serum bicarbonate (mEq/L) | 15–18 | 10 to < 15 | < 10 | >18 |
| Urine or Serum Ketones* | Positive | Positive | Positive | Small |
| Urine or Serum β-hydroxybutyrate (mmol/L) | >3.0 | >3.0 | >3.0 | <3.0 |
| Effective serum osmolalityƮ | Variable | Variable | Variable | >320 mOsm/kg |
| Anion gap | >10 | >12 | >12 | Variable |
| Mental Status | Alert | Alert/drowsy | Stupor/coma | Stupor/Coma |
Nitroprusside reaction
Effective serum osmolality: 2[measured Na+ (mEq/L)] + glucose (mg/dL)/18
Modified by permission of Diabetes Care from the American Diabetes Association Consensus Statement on Hyperglycemic Crises, 2009 1.
Anion gap is calculated with the formula = sodium [Na+] – (chloride [Cl-] + [HCO3−]. Although the majority of patients present with plasma glucose levels > 250 mg/dL, some patients exhibit only mild elevations in plasma glucose levels (termed ‘euglycemic DKA’) 80. This phenomenon has been reported during pregnancy, in patients with prolonged starvation, alcohol intake, pregnancy, partially treated patients receiving insulin, and more recently in the setting of SGLT-2 inhibitor use 59,81,82.
The key diagnostic criterion is an elevation in circulating total blood ketone and high anion gap metabolic acidosis >12. Assessment of ketonemia can be performed by the nitroprusside reaction in urine or serum, which provides a semi-quantitative estimation of acetoacetate and acetone levels. The nitroprusside test is highly sensitive, but it can underestimate the severity of ketoacidosis because this assay does not recognize the presence of β-hydroxybutyrate, the main metabolic product in ketoacidosis 69,83. Therefore, direct measurement of serum β-hydroxybutyrate is preferred for diagnosis 84.
Diagnosis of HHS
Symptoms and Signs.
The majority of patients with HHS present with a history of polyuria, polydipsia, weakness, blurred vision, and progressive decline in mental status 50,85. The typical patient with HHS is older than 60 years of age with an infection or acute illness who has delayed seeking medical attention. On physical examination, similar to DKA, patients with HHS frequently have clear signs of dehydration, dry mucous membranes and poor skin turgor, or hypotension 50.
Laboratory Findings.
The diagnostic criteria for HHS includes a plasma glucose of over 600 mg/dl, and effective osmolality >320 mOsm/kg, and the absence of ketoacidosis 1. Effective osmolality is calculated with the formula = sodium ion (mEq/L) x 2 + glucose (mg/dL)/18. Although by definition, HHS is characterized by a pH > 7.3, bicarbonate > 18 mEq/L, and negative ketone bodies, mild to moderate ketonemia may be present. Patients with HHS have an increased anion gap metabolic acidosis as the result of concomitant ketoacidosis and/or to an increase in serum lactate levels or renal failure 21
Common Laboratory Pitfalls.
Patients with DKA frequently present with significant leukocytosis with white cell counts in the 10,000–15,000 mm3 range. A leukocyte count greater than 25,000 mm3 or the presence of greater than 10% neutrophil bands is seldom seen in the absence of bacterial infection 66,86. In ketoacidosis, leukocytosis is attributed to stress, dehydration, and demargination of leukocytes.
The admission serum sodium may be low because of the osmotic flux of water from the intracellular to the extracellular space in the presence of hyperglycemia. To assess the severity of sodium and water deficit, serum sodium may be corrected by adding 1.6 mg/dL to the measured serum sodium for each 100 mg/dL of glucose above 100 mg/dL 1. An increase in serum sodium concentration in the presence of severe hyperglycemia indicates a profound degree of dehydration and water loss.
The admission serum potassium concentration is usually elevated in patients with DKA and HHS66. In a several studies 1,39,87, the mean serum potassium in patients with DKA and HHS was 5.6 mEq/l and 5.7 mEq/L, respectively. These high levels occur because of a shift of potassium from the intracellular to the extracellular space due to insulin deficiency and hypertonicity as well as academia in DKA 88. It is important to keep in mind that during insulin treatment and fluid administration, potassium levels decrease due to a shift back to the intracellular space, which may result in hypokalemia.
Similarly, serum phosphate levels in patients with DKA do not reflect the actual body deficit that uniformly exists, as phosphate shifts from the intracellular to the extracellular space due to insulin deficiency, hypertonicity, and catabolic state. Dehydration also can lead to increases in total serum protein, albumin, amylase, and creatinine phosphokinase concentration in patients with hyperglycemic crises.
Not all patients who present with ketoacidosis have DKA. Patients with chronic ethanol abuse with a recent binge culminating in nausea, vomiting and acute starvation may present with alcoholic ketoacidosis. The key diagnostic feature that differentiates diabetic and alcohol-induced ketoacidosis is the concentration of blood glucose 89. The presence of ketoacidosis without hyperglycemia in an alcoholic patient is virtually diagnostic of alcoholic ketoacidosis. In addition, some patients with decreased food intake and caloric intake lower than 500 calories/day for several days may present with starvation ketosis. Patients with starvation ketosis rarely present with a serum bicarbonate concentration less than 18 mEq/L because of the slow onset of ketosis that allows increased ketone clearance by peripheral tissue (brain and muscle) and enhancement of the kidney’s ability to excrete ammonia to compensate for the increased acid production 90.
Management of hyperglycemic crises
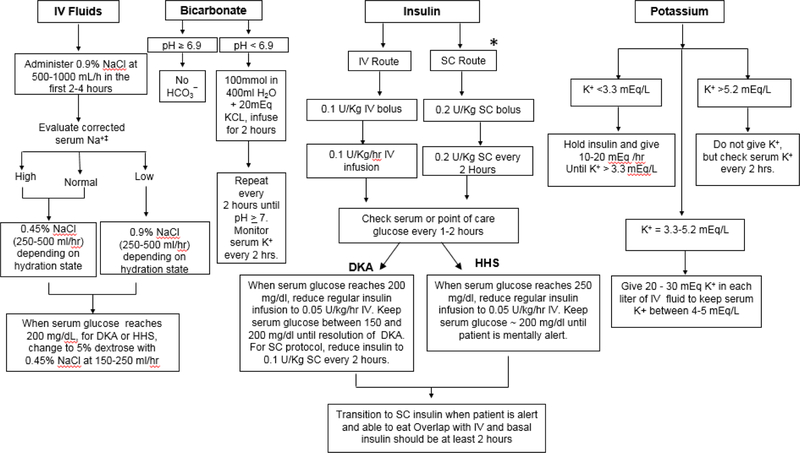
The American Diabetes Association algorithm for the management of hyperglycemic emergencies is shown in Figure 2 1. Similar therapeutic measures are recommended for the treatment of DKA and HHS. In general, treatment goals include correction of dehydration, hyperglycemia and hyperosmolality, electrolyte imbalance, increased ketonemia, and identification and treatment of precipitating event(s). The average time to resolution between 10–18 hours for DKA 91,92 and ~9–11 hours for HHS 4. During treatment, frequent monitoring of vital signs, volume and rate of fluid administration, insulin dosage, and urine output are needed to assess response to medical treatment. In addition, laboratory measurements of glucose and electrolytes, venous pH, bicarbonate, and anion gap should be repeated every 2–4 hours 93.
Figure 2.
Management of Hyperglycemic Emergencies
*Subcutaneous Insulin Protocol has not been validated for HHS
Modified by permission of Diabetes Care from the American Diabetes Association Consensus Statement on Hyperglycemic Crises, 2009 1.
Most patients with uncomplicated DKA can be treated in the emergency department or in step-down units, if close nursing supervision and monitoring is available. Several studies have failed to demonstrate clear benefits in treating DKA patients in the ICU compared to step-down units 94–96. The mortality rate, length of hospital stay, or time to resolve ketoacidosis are similar between patients treated in ICU and non-ICU settings. In addition, ICU admission has been associated with more laboratory testing and higher hospitalization cost in patients with DKA 36,94. Patients with mild to moderate DKA can be safely managed in the emergency department or in step-down units, and only patients with severe DKA or those with a critical illness as precipitating cause (i.e., myocardial infarction, gastrointestinal bleeding, sepsis) 1,97 should be treated in the ICU. Because patients with HHS frequently present with altered mental status and have significantly higher mortality than patients with DKA, we recommend that patients with HHS should be managed in the ICU.
Fluid Therapy
Intravenous (IV) fluids are a critical aspect of treatment of hyperglycemic emergencies. Treatment with IV fluids alone expands intravascular volume, restores renal perfusion and reduces insulin resistance by decreasing circulating counter-regulatory hormone levels 64. Isotonic saline (0.9% NaCl) is the preferred solution and is given at an initial rate of 500–1000 mL/hour during the first 2–4 hours. A study comparing two IV fluid regimens with sodium chloride and lactate ringers found no significant difference in time to resolution of DKA, but the time to correct hyperglycemia was significantly longer in the lactate ringers group 98. After intravascular volume depletion has been corrected, the rate of normal saline infusion should be reduced to 250 mL/h or changed to 0.45% saline (250–500 mL/h) depending upon the serum sodium concentration and state of hydration 1. Once the plasma glucose level reaches ~200 mg/dL (11.1 mosm/L), replacement fluids should contain 5–10% of dextrose to allow continued insulin administration until ketonemia is corrected, while avoiding hypoglycemia 99. Adequate fluid resuscitation is of particular importance in management of HHS, as many of them, may see improvement in or resolution of mental status changes with correction of fluid deficits 85.
Potassium
Metabolic acidosis and insulin deficiency both lead to extracellular movement of potassium. Thus, although serum potassium levels may be normal or elevated in DKA, patients are actually total body depleted. Similarly, HHS is associated with total body potassium depletion due to lack of insulin and increased plasma osmolality 20,88. The total-body potassium deficit has been estimated to be ~3–5 mEq/kg 87,100. Insulin therapy lowers serum potassium levels by promoting the movement of potassium back into the intracellular compartment. Thus, potassium replacement should be started when the serum concentration is < 5.2 mEq/L to maintain a level of 4–5 mEq/L. The administration of 20–30 mEq of potassium per liter of fluids is sufficient for most patients; however, lower doses are required for patients with acute or chronic renal failure. Among patients with admission hypokalemia, with serum potassium levels < 3.3 mEq/L, insulin administration may result in severe symptomatic hypokalemia with muscle weakness and increased risk of cardiac arrhythmias. In such patients, potassium replacement should begin at a rate of 10–20 mEq/h and insulin therapy should be delayed until the potassium level rises above 3.3 mEq/L.
Bicarbonate
Routine administration of bicarbonate has not been shown to improve clinical outcomes such as time to resolution, length of hospital stay, or mortality in patients with DKA 101–104 and is generally only recommended in patients with life threatening acidosis with pH <6.9. Bicarbonate therapy may increase the risk of hypokalemia and cerebral edema 105,106. Although no studies have looked at the effect of bicarbonate therapy in patients with severe acidosis, because of the potential risk of reduced cardiac contractility and arrhythmias, clinical guidelines recommend the administration of 50–100 mmol of sodium bicarbonate as an isotonic solution (in 400 mL of water) until pH is > 6.9. In patients with mild DKA with pH >7.0 or with HHS, bicarbonate therapy is not indicated.
Insulin regimens
Insulin administration is the mainstay of DKA therapy as it lowers the serum glucose by inhibiting endogenous glucose production and increasing peripheral utilization. Insulin also inhibits lipolysis, ketogenesis, and glucagon secretion, thereby decreasing the production of ketoacidosis.
Continuous IV infusion of regular insulin is the treatment of choice. Most treatment protocols recommend the administration of 0.1 unit/kg body weight bolus followed by continuous insulin infusion at 0.1 u/kg/hr until blood glucose is ~ 200 mg/dL (Figure 2). At this point, the dose is reduced by half (0.05 u/kg/hr) and rate is adjusted between 0.02–0.05 u/kg/hr, along with the addition of 5% dextrose, to maintain glucose concentrations between 140 and 200 mg/dL until resolution of ketoacidosis 1.
Several studies have demonstrated that the administration of subcutaneous doses of rapid-insulin analogs (lispro and aspart) every 1–2 hours is an effective alternative to the IV infusion of regular insulin in terms of time to resolution of DKA 107–109. Patients are treated with an initial bolus of 0.2 – 0.3 U/kg followed by 0.1 – 0.2 U/kg every 1– 2 hours, respectively until glucose is < 250 mg/dl. The dose is then reduced by half to 0.05 U/kg every 1 hour or 0.01 U/kg every two hours until resolution of DKA 91,107. Using scheduled subcutaneous insulin allows for safe and effective treatment in the emergency room and step-down units without the need for ICU care. The use of intramuscular injections of rapid-acting insulin is also effective in the treatment of DKA, but this route tends to be more painful than subcutaneous injection and might increase the risk of bleeding among patients receiving anticoagulation therapy 99,110. It is important to keep in mind that the use of rapid-acting subcutaneous insulin analogues is not recommended for patients with arterial hypotension, severe and complicated DKA, or with HHS.
Transition to maintenance insulin regimen
Resolution of DKA is defined when glucose levels are lower than 250 mg/dl, venous pH > 7.30, normal anion gap, and serum bicarbonate ≥18 mEq/L 1. HHS resolution is achieved when effective serum osmolality < 310 mOsm/kg, glucose level ≤ 250 mg/dL (13.8 mmol/l) in a patient who has recovered mental alertness and regaining of mental status 1,99.
Because of the short half-life of insulin (< 10 minutes) 111, abrupt cessation of the insulin may result is rebound hyperglycemia, ketogenesis, and recurrent metabolic acidosis. Subcutaneous basal insulin (NPH, glargine, detemir, degludec), should be given at least 2 hours before discontinuing the IV insulin infusion 1. Earlier initiation 3–4 hours before discontinuation of insulin drip should be considered when using basal insulin analogs (glargine, determir, degludec), which have a longer delay in onset of action than NPH insulin. One randomized controlled trial evaluated the effect of co-administration of IV insulin with subcutaneous glargine shortly after the onset of treatment of DKA compared to IV insulin alone 112. Patients who received glargine had slightly shorter time to resolution of DKA (based on closure of anion gap) and shorter hospital stay; however, these differences were not statistically significant 112. Another study found that the administration of glargine early in the course of treatment reduced the frequency of rebound hyperglycemia after transition off of insulin drip 113.
For insulin naïve patients, a starting total daily insulin dose of 0.5–0.6 units/kg may be started (half as basal and half as bolus) 1. Patients with poor oral intake should receive basal insulin alone or, alternatively, may be continued on insulin drip until they are able to eat. Patients with known diabetes can be restarted on their previous insulin regimens, however an adjustment of the previous regimen should be considered if there is a history of frequent hypoglycemia, or significantly uncontrolled hyperglycemia before admission, as indicated by admission HbA1c. Multi-dose insulin regimens with basal insulin and prandial rapid-acting insulin analogs are the preferred insulin regimen for patients with T1D and DKA, and for most patients with HHS. A randomized controlled trial in DKA patients compared transition regimens of NPH and regular insulin twice daily versus glargine once daily and glulisine before meals found similar glycemic control between the two groups; however, the NPH/regular insulin group had more than double the rate of hypoglycemia (< 70 mg/dl) compared to the glargine/glulisine group 114.
Complications
Hypoglycemia is the most common complication during treatment, reported in 5–25% of patients with DKA 1,4,107. Lack of frequent monitoring, and the failure to reduce insulin infusion rate and/or to use dextrose-containing solutions when blood glucose levels are < 200 mg/dL are the most important risk factors associated with hypoglycemia during insulin treatment. Many patients with hypoglycemia do not experience adrenergic manifestations of sweating, nervousness, fatigue, hunger and tachycardia, thus frequent blood glucose monitoring every 1–2 hours is mandatory 99. Acute adverse outcomes of hypoglycemia include seizures, arrhythmias and cardiovascular events. Clinicians should be aware that recurrent episodes of hypoglycemia might be associated with a state of hypoglycemia unawareness (loss of perception of warning symptoms of developing hypoglycemia), which may complicate diabetes management after resolution of hyperglycemic crises.
Hypokalemia is the second most common complication during DKA and HHS treatment 4. Although the admission serum potassium concentration is commonly elevated, during insulin treatment, plasma concentration of potassium will invariably decrease due increased cellular potassium uptake in peripheral tissues 1. Thus, to prevent hypokalemia, replacement with IV potassium when concentration is < 5.2 mEq/l is indicated. In patients admitted with reduced serum potassium < 3.3 mEq/L, IV potassium replacement should begin immediately and insulin therapy should be held until serum potassium is ≥ 3.3 mEq/L to avoid severe hypokalemia.
Cerebral edema is rare in adults, but is reported in ~1 percent of children with DKA with a mortality rate between 20–40% 105,115. The pathogenesis of cerebral edema is incompletely understood. Evidence for disruption of the blood–brain barrier has been found in cases of fatal cerebral edema 105,116. The degree of edema formation during DKA in children correlates with the degree of dehydration and hyperventilation at presentation, but it does not correlate with initial osmolality, osmotic changes during treatment, or rate of fluid or sodium administration 117. Clinically significant cerebral edema usually develops 4–12 h after treatment has started, but it can occur as late as 24–48 h after the start of treatment. Clinical criteria includes altered mentation or fluctuating level of consciousness, abnormal motor or verbal response to pain, decorticate or decerebrate posturing, cranial nerve palsy (especially III, IV, and VI), abnormal neurogenic respiratory pattern (e.g., grunting, tachypnea, Cheyne–Stokes respiration. Recommended treatment includes the administration of mannitol 0.5–1 g/kg IV over 20 min and repeat if there is no initial response in 30 min 118,119. Hypertonic saline (3%), 5–10 mL/kg over 30 min, may be an alternative to mannitol, especially if there is no initial response to mannitol 120. After treatment for cerebral edema has been started, a cranial CT scan should be obtained to rule out other possible intracerebral causes of neurologic deterioration (≈10% of cases), especially thrombosis and cerebral infarction, hemorrhage, or dural sinus thrombosis, which may benefit from specific therapy 115,121–123. Corticosteroid and diuretic therapy are of no proven benefits on the treatment of cerebral edema in DKA patients 124.
Rhabdomyolysis may occur in patients with DKA and more commonly with HHS resulting in increased risk of acute kidney failure. The classic symptom triad of rhabdomyolysis includes myalgia, weakness, and dark urine, and monitoring creatine kinase concentrations every 2 to 3 h is recommended for early detection.
Prevention
Medication non-compliance is a leading cause of diabetic ketoacidosis among both newly diagnosed and recurrent episodes of DKA 39–41. The mean cost of hospitalization is about $7,500 40. In half of such episodes, patients report inability to afford medication or to pay for transportation as the reason why medication was discontinued 41. Development of system wide changes such as assistance programs to provide insulin to patients and reduce lapses in treatment may be a cost-effective way to reduce the rate of hospitalization for hyperglycemic emergencies. This could include implementation of safety nets such as assistance programs to provide insulin to patients and reduce lapses in treatment.
Multidisciplinary approaches with the use of clinical diabetes educators in close contact with and easily accessible to the patients has been shown to reduce the number of hospitalizations related to hyperglycemic emergencies 125. Systems-based methods to reduce preventable causes of hyperglycemic emergencies may represent an important next step in reducing costs and improving patient care.
References
- 1.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32(7):1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Mortality due to Hyperglycemic crises http://www.cdc.gov/diabetes/statistics/complications_national.htm. 11/19/2013. Accessed on 9/2/2016.
- 3.Ennis ED, Stahl EJVB, Kreisberg RA. The hyperosmolar hyperglycemic syndrome. Diabetes Rev 1994;2:115–126. [Google Scholar]
- 4.Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med 1997;157(6):669–675. [PubMed] [Google Scholar]
- 5.von Stosch A Versuch einer Pathologie und Therapie des Diabetes Mellitus. Berlin, Duncker und Humblot, 1828. [in German].
- 6.Parsons J Case of Infantile Diabetes. Prov Med Surg J 1849;13(13):342–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hindle R Case of Acute Diabetes. Prov Med Surg J 1845;9(29):452–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favell CF. Cases of Diabetes. Prov Med J Retrosp Med Sci 1843;6(153):467–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kussmaul A Zur lehre vom diabetes mellitus. Dtsch Arch Klin Med 1874;14:1–46 [in German]. [Google Scholar]
- 10.Kussmaul Adolf (1822–1902)--Country Doctor to Clinical Professor. JAMA 1964;189:58–59. [DOI] [PubMed] [Google Scholar]
- 11.Stadelmann E Ueber die Ursachen der pathologischen ammoniakausscheidung beim diabetes mellitus und des coma diabeticum. Arch Exp Pathol und Pharmakol 1883;17:419–444 [in German]. [Google Scholar]
- 12.Külz E Ueber eine neue linksdrehende saure (pseudo-oxybuttersaure). Zeitschr f Biologie 1884;20:165–178 [in German]. [Google Scholar]
- 13.Dreschfeld J The Bradshawe Lecture on Diabetic Coma. Br Med J 1886;2(1338):358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic Extracts in the Treatment of Diabetes Mellitus: Preliminary Report. Can Med Assoc J 1962;87(20):1062–1067. [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinowitch IM. Diabetic Coma and Diabetic Mortality Rates. Can Med Assoc J 1929;21(5):583–586. [PMC free article] [PubMed] [Google Scholar]
- 16.Clements RS Jr., Vourganti B. Fatal diabetic ketoacidosis: major causes and approaches to their prevention. Diabetes Care 1978;1(5):314–325. [DOI] [PubMed] [Google Scholar]
- 17.Felig P Diabetic ketoacidosis. N Engl J Med 1974;290(24):1360–1363. [DOI] [PubMed] [Google Scholar]
- 18.Graves EJ, Gillium BS. Detailed diagnosis and procedures: National Discharge Survey, 1995. National Center for Health Statistics. Vital Health Stat 1997;13((no. 133)). [PubMed] [Google Scholar]
- 19.Wagner A, Risse A, Brill HL, et al. Therapy of severe diabetic ketoacidosis. Zero-mortality under very-low-dose insulin application. Diabetes Care 1999;22(5):674–677. [DOI] [PubMed] [Google Scholar]
- 20.Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 2014;37(11):3124–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadini GP, de Kreutzenberg SV, Rigato M, et al. Characteristics and outcomes of the hyperglycemic hyperosmolar non-ketotic syndrome in a cohort of 51 consecutive cases at a single center. Diabetes Res Clin Pract 2011;94(2):172–179. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZH, Kihl-Selstam E, Eriksson JW. Ketoacidosis occurs in both Type 1 and Type 2 diabetes--a population-based study from Northern Sweden. Diabet Med 2008;25(7):867–870. [DOI] [PubMed] [Google Scholar]
- 23.Karges B, Rosenbauer J, Holterhus PM, et al. Hospital admission for diabetic ketoacidosis or severe hypoglycemia in 31,330 young patients with type 1 diabetes. Eur J Endocrinol 2015;173(3):341–350. [DOI] [PubMed] [Google Scholar]
- 24.Maahs DM, Hermann JM, Holman N, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care 2015;38(10):1876–1882. [DOI] [PubMed] [Google Scholar]
- 25.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38(6):971–978. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbloom AL. Hyperglycemic hyperosmolar state: an emerging pediatric problem. J Pediatr 2010;156(2):180–184. [DOI] [PubMed] [Google Scholar]
- 27.Savage MW, Dhatariya KK, Kilvert A, et al. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabet Med 2011;28(5):508–515. [DOI] [PubMed] [Google Scholar]
- 28.Otieno CF, Kayima JK, Omonge EO, Oyoo GO. Diabetic ketoacidosis: risk factors, mechanisms and management strategies in sub-Saharan Africa: a review. East Afr Med J 2005;82(12 Suppl):S197–203. [DOI] [PubMed] [Google Scholar]
- 29.Bhowmick SK, Levens KL, Rettig KR. Hyperosmolar hyperglycemic crisis: an acute life-threatening event in children and adolescents with type 2 diabetes mellitus. Endocr Pract 2005;11(1):23–29. [DOI] [PubMed] [Google Scholar]
- 30.Wachtel TJ, Silliman RA, Lamberton P. Prognostic factors in the diabetic hyperosmolar state. J Am Geriatr Soc 1987;35(8):737–741. [DOI] [PubMed] [Google Scholar]
- 31.Basu A, Close CF, Jenkins D, Krentz AJ, Nattrass M, Wright AD. Persisting mortality in diabetic ketoacidosis. Diabet Med 1993;10(3):282–284. [DOI] [PubMed] [Google Scholar]
- 32.Malone ML, Gennis V, Goodwin JS. Characteristics of diabetic ketoacidosis in older versus younger adults. J Am Geriatr Soc 1992;40(11):1100–1104. [DOI] [PubMed] [Google Scholar]
- 33.Gibb FW, Teoh WL, Graham J, Lockman KA. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia 2016. [DOI] [PMC free article] [PubMed]
- 34.Huang CC, Weng SF, Tsai KT, et al. Long-term Mortality Risk After Hyperglycemic Crisis Episodes in Geriatric Patients With Diabetes: A National Population-Based Cohort Study. Diabetes care 2015;38(5):746–751. [DOI] [PubMed] [Google Scholar]
- 35.Gregg EW, Williams DE, Geiss L. Changes in diabetes-related complications in the United States. N Engl J Med 2014;371(3):286–287. [DOI] [PubMed] [Google Scholar]
- 36.Javor KA, Kotsanos JG, McDonald RC, Baron AD, Kesterson JG, Tierney WM. Diabetic ketoacidosis charges relative to medical charges of adult patients with type I diabetes. Diabetes Care 1997;20(3):349–354. [DOI] [PubMed] [Google Scholar]
- 37.Klingensmith GJ, Tamborlane WV, Wood J, et al. Diabetic ketoacidosis at diabetes onset: still an all too common threat in youth. J Pediatr 2013;162(2):330–334 e331. [DOI] [PubMed] [Google Scholar]
- 38.Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 2014;133(4):e938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randall L, Begovic J, Hudson M, et al. Recurrent diabetic ketoacidosis in inner-city minority patients: behavioral, socioeconomic, and psychosocial factors. Diabetes Care 2011;34(9):1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maldonado MR, Chong ER, Oehl MA, Balasubramanyam A. Economic impact of diabetic ketoacidosis in a multiethnic indigent population: analysis of costs based on the precipitating cause. Diabetes Care 2003;26(4):1265–1269. [DOI] [PubMed] [Google Scholar]
- 41.Musey VC, Lee JK, Crawford R, Klatka MA, McAdams D, Phillips LS. Diabetes in urban African-Americans. I. Cessation of insulin therapy is the major precipitating cause of diabetic ketoacidosis. Diabetes Care 1995;18(4):483–489. [DOI] [PubMed] [Google Scholar]
- 42.Umpierrez G, Freire AX. Abdominal pain in patients with hyperglycemic crises. J Crit Care 2002;17(1):63–67. [DOI] [PubMed] [Google Scholar]
- 43.Barnard KD, Skinner TC, Peveler R. The prevalence of co-morbid depression in adults with Type 1 diabetes: systematic literature review. Diabet Med 2006;23(4):445–448. [DOI] [PubMed] [Google Scholar]
- 44.Canadian Diabetes Association Clinical Practice Guidelines Expert C, Goguen J, Gilbert J. Hyperglycemic emergencies in adults. Can J Diabetes 2013;37 Suppl 1:S72–76. [DOI] [PubMed] [Google Scholar]
- 45.Garg SK, Walker AJ, Hoff HK, D’Souza AO, Gottlieb PA, Chase HP. Glycemic parameters with multiple daily injections using insulin glargine versus insulin pump. Diabetes Technol Ther 2004;6(1):9–15. [DOI] [PubMed] [Google Scholar]
- 46.Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995;18(3):361–376. [DOI] [PubMed] [Google Scholar]
- 47.Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 2013;310(12):1240–1247. [DOI] [PubMed] [Google Scholar]
- 48.Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia 2013;56(11):2392–2400. [DOI] [PubMed] [Google Scholar]
- 49.Wachtel TJ, Tetu-Mouradjian LM, Goldman DL, Ellis SE, O’Sullivan PS. Hyperosmolarity and acidosis in diabetes mellitus: a three-year experience in Rhode Island. J Gen Intern Med 1991;6(6):495–502. [DOI] [PubMed] [Google Scholar]
- 50.Gerich JE, Martin MM, Recant L. Clinical and metabolic characteristics of hyperosmolar nonketotic coma. Diabetes 1971;20(4):228–238. [DOI] [PubMed] [Google Scholar]
- 51.Ben Salem C, Fathallah N, Hmouda H, Bouraoui K. Drug-induced hypoglycaemia: an update. Drug Saf 2011;34(1):21–45. [DOI] [PubMed] [Google Scholar]
- 52.Caro JJ, Ward A, Levinton C, Robinson K. The risk of diabetes during olanzapine use compared with risperidone use: a retrospective database analysis. J Clin Psychiatry 2002;63(12):1135–1139. [DOI] [PubMed] [Google Scholar]
- 53.Buse JB, Cavazzoni P, Hornbuckle K, Hutchins D, Breier A, Jovanovic L. A retrospective cohort study of diabetes mellitus and antipsychotic treatment in the United States. J Clin Epidemiol 2003;56(2):164–170. [DOI] [PubMed] [Google Scholar]
- 54.Gianfrancesco F, Grogg A, Mahmoud R, Wang RH, Meletiche D. Differential effects of antipsychotic agents on the risk of development of type 2 diabetes mellitus in patients with mood disorders. Clin Ther 2003;25(4):1150–1171. [DOI] [PubMed] [Google Scholar]
- 55.Ananth J, Parameswaran S, Gunatilake S. Side effects of atypical antipsychotic drugs. Curr Pharm Des 2004;10(18):2219–2229. [DOI] [PubMed] [Google Scholar]
- 56.Lipscombe LL, Austin PC, Alessi-Severini S, et al. Atypical antipsychotics and hyperglycemic emergencies: multicentre, retrospective cohort study of administrative data. Schizophr Res 2014;154(1–3):54–60. [DOI] [PubMed] [Google Scholar]
- 57.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care 2015. [DOI] [PMC free article] [PubMed]
- 58.Taylor SI, Blau JE, Rother KI. Perspective: SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015:jc20151884. [DOI] [PMC free article] [PubMed]
- 59.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care 2015;38(9):1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erondu N, Desai M, Ways K, Meininger G. Diabetic Ketoacidosis and Related Events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes care 2015;38(9):1680–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang H, Li D, Wang T, Zhai S, Song Y. Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Diabetic Ketoacidosis Among Patients With Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. Diabetes care 2016;39(8):e123–124. [DOI] [PubMed] [Google Scholar]
- 62.Kibbey RG. SGLT-2 inhibition and glucagon: Cause for alarm? Trends Endocrinol Metab 2015;26(7):337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. The Journal of clinical endocrinology and metabolism 2015;100(8):2849–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waldhausl W, Kleinberger G, Korn A, Dudczak R, Bratusch-Marrain P, Nowotny P. Severe hyperglycemia: effects of rehydration on endocrine derangements and blood glucose concentration. Diabetes 1979;28(6):577–584. [DOI] [PubMed] [Google Scholar]
- 65.Chupin M, Charbonnel B, Chupin F. C-peptide blood levels in keto-acidosis and in hyperosmolar non-ketotic diabetic coma. Acta Diabetol Lat 1981;18(2):123–128. [DOI] [PubMed] [Google Scholar]
- 66.Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes care 2001;24(1):131–153. [DOI] [PubMed] [Google Scholar]
- 67.Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab 2003;88(11):5090–5098. [DOI] [PubMed] [Google Scholar]
- 68.Miles JM, Rizza RA, Haymond MW, Gerich JE. Effects of acute insulin deficiency on glucose and ketone body turnover in man: evidence for the primacy of overproduction of glucose and ketone bodies in the genesis of diabetic ketoacidosis. Diabetes 1980;29(11):926–930. [DOI] [PubMed] [Google Scholar]
- 69.Foster DW, McGarry JD. The metabolic derangements and treatment of diabetic ketoacidosis. N Engl J Med 1983;309(3):159–169. [DOI] [PubMed] [Google Scholar]
- 70.Laffel L Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 1999;15(6):412–426. [DOI] [PubMed] [Google Scholar]
- 71.Miles JM, Haymond MW, Nissen SL, Gerich JE. Effects of free fatty acid availability, glucagon excess, and insulin deficiency on ketone body production in postabsorptive man. J Clin Invest 1983;71(6):1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGarry JD, Foster DW. Effects of exogenous fatty acid concentration on glucagon-induced changes in hepatic fatty acid metabolism. Diabetes 1980;29(3):236–240. [DOI] [PubMed] [Google Scholar]
- 73.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem 1980;49:395–420. [DOI] [PubMed] [Google Scholar]
- 74.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 2011;50(5):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, Huang M, Shen X. The association of oxidative stress and pro-inflammatory cytokines in diabetic patients with hyperglycemic crisis. J Diabetes Complications 2014;28(5):662–666. [DOI] [PubMed] [Google Scholar]
- 76.Vaarala O, Yki-Jarvinen H. Diabetes: Should we treat infection or inflammation to prevent T2DM? Nat Rev Endocrinol 2012;8(6):323–325. [DOI] [PubMed] [Google Scholar]
- 77.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27(3):813–823. [DOI] [PubMed] [Google Scholar]
- 78.Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol 2005;25(5):989–994. [DOI] [PubMed] [Google Scholar]
- 79.Chaudhuri A, Umpierrez GE. Oxidative stress and inflammation in hyperglycemic crises and resolution with insulin: implications for the acute and chronic complications of hyperglycemia. J Diabetes Complications 2012;26(4):257–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jenkins D, Close CF, Krentz AJ, Nattrass M, Wright AD. Euglycaemic diabetic ketoacidosis: does it exist? Acta diabetologica 1993;30(4):251–253. [DOI] [PubMed] [Google Scholar]
- 81.Bas VN, Uytun S, Torun YA. Diabetic euglycemic ketoacidosis in newly diagnosed type 1 diabetes mellitus during Ramadan fasting. J Pediatr Endocrinol Metab 2015;28(3–4):333–335. [DOI] [PubMed] [Google Scholar]
- 82.Guo RX, Yang LZ, Li LX, Zhao XP. Diabetic ketoacidosis in pregnancy tends to occur at lower blood glucose levels: case-control study and a case report of euglycemic diabetic ketoacidosis in pregnancy. J Obstet Gynaecol Res 2008;34(3):324–330. [DOI] [PubMed] [Google Scholar]
- 83.Stephens JM, Sulway MJ, Watkins PJ. Relationship of blood acetoacetate and 3-hydroxybutyrate in diabetes. Diabetes 1971;20(7):485–489. [DOI] [PubMed] [Google Scholar]
- 84.Sheikh-Ali M, Karon BS, Basu A, et al. Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care 2008;31(4):643–647. [DOI] [PubMed] [Google Scholar]
- 85.Arieff AI, Carroll HJ. Nonketotic hyperosmolar coma with hyperglycemia: clinical features, pathophysiology, renal function, acid-base balance, plasma-cerebrospinal fluid equilibria and the effects of therapy in 37 cases. Medicine (Baltimore) 1972;51(2):73–94. [DOI] [PubMed] [Google Scholar]
- 86.Slovis CM, Mork VG, Slovis RJ, Bain RP. Diabetic ketoacidosis and infection: leukocyte count and differential as early predictors of serious infection. Am J Emerg Med 1987;5(1):1–5. [DOI] [PubMed] [Google Scholar]
- 87.Beigelman PM. Potassium in severe diabetic ketoacidosis. The American journal of medicine 1973;54(4):419–420. [DOI] [PubMed] [Google Scholar]
- 88.Adrogue HJ, Lederer ED, Suki WN, Eknoyan G. Determinants of plasma potassium levels in diabetic ketoacidosis. Medicine (Baltimore) 1986;65(3):163–172. [DOI] [PubMed] [Google Scholar]
- 89.Umpierrez GE, DiGirolamo M, Tuvlin JA, Isaacs SD, Bhoola SM, Kokko JP. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis. J Crit Care 2000;15(2):52–59. [DOI] [PubMed] [Google Scholar]
- 90.Cahill GF Jr. Starvation in man. The New England journal of medicine 1970;282(12):668–675. [DOI] [PubMed] [Google Scholar]
- 91.Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care 2004;27(8):1873–1878. [DOI] [PubMed] [Google Scholar]
- 92.Hara JS, Rahbar AJ, Jeffres MN, Izuora KE. Impact of a hyperglycemic crises protocol. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2013;19(6):953–962. [DOI] [PubMed] [Google Scholar]
- 93.Kitabchi AE, Umpierrez GE, Murphy MB, et al. Hyperglycemic crises in diabetes. Diabetes Care 2004;27 Suppl 1:S94–102. [DOI] [PubMed] [Google Scholar]
- 94.May ME, Young C, King J. Resource utilization in treatment of diabetic ketoacidosis in adults. Am J Med Sci 1993;306(5):287–294. [DOI] [PubMed] [Google Scholar]
- 95.Moss JM. Diabetic ketoacidosis: effective low-cost treatment in a community hospital. South Med J 1987;80(7):875–881. [DOI] [PubMed] [Google Scholar]
- 96.Umpierrez GE, Latif KA, Cuervo R, Karabell A, Freire AX, Kitabchi AE. Subcutanbeous aspart insulin: a safe and cost effective treatment of diabetic ketoacidosis. Diabetes 2003;52(Suppl 1):584A. [DOI] [PubMed] [Google Scholar]
- 97.Glaser NS, Ghetti S, Casper TC, Dean JM, Kuppermann N, Pediatric Emergency Care Applied Research Network DKAFSG. Pediatric diabetic ketoacidosis, fluid therapy, and cerebral injury: the design of a factorial randomized controlled trial. Pediatr Diabetes 2013;14(6):435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis--Ringer’s lactate versus normal saline: a randomized controlled trial. QJM : monthly journal of the Association of Physicians 2012;105(4):337–343. [DOI] [PubMed] [Google Scholar]
- 99.Umpierrez G, Korytkowski M. Diabetic emergencies - ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol 2016;12(4):222–232. [DOI] [PubMed] [Google Scholar]
- 100.Abramson E, Arky R. Diabetic acidosis with initial hypokalemia. Therapeutic implications. JAMA : the journal of the American Medical Association 1966;196(5):401–403. [PubMed] [Google Scholar]
- 101.Lever E, Jaspan JB. Sodium bicarbonate therapy in severe diabetic ketoacidosis. Am J Med 1983;75(2):263–268. [DOI] [PubMed] [Google Scholar]
- 102.Green SM, Rothrock SG, Ho JD, et al. Failure of adjunctive bicarbonate to improve outcome in severe pediatric diabetic ketoacidosis. Ann Emerg Med 1998;31(1):41–48. [DOI] [PubMed] [Google Scholar]
- 103.Latif KA, Freire AX, Kitabchi AE, Umpierrez GE, Qureshi N. The use of alkali therapy in severe diabetic ketoacidosis. Diabetes Care 2002;25(11):2113–2114. [DOI] [PubMed] [Google Scholar]
- 104.Gamba G, Oseguera J, Castrejon M, Gomez-Perez FJ. Bicarbonate therapy in severe diabetic ketoacidosis. A double blind, randomized, placebo controlled trial. Rev Invest Clin 1991;43(3):234–238. [PubMed] [Google Scholar]
- 105.Glaser N, Barnett P, McCaslin I, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med 2001;344(4):264–269. [DOI] [PubMed] [Google Scholar]
- 106.Fraley DS, Adler S. Correction of hyperkalemia by bicarbonate despite constant blood pH. Kidney Int 1977;12(5):354–360. [DOI] [PubMed] [Google Scholar]
- 107.Umpierrez GE, Latif K, Stoever J, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Am J Med 2004;117(5):291–296. [DOI] [PubMed] [Google Scholar]
- 108.Ersoz HO, Ukinc K, Kose M, et al. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. International journal of clinical practice 2006;60(4):429–433. [DOI] [PubMed] [Google Scholar]
- 109.Karoli R, Fatima J, Salman T, Sandhu S, Shankar R. Managing diabetic ketoacidosis in non-intensive care unit setting: Role of insulin analogs. Indian J Pharmacol 2011;43(4):398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med 1976;84(6):633–638. [DOI] [PubMed] [Google Scholar]
- 111.Hipszer B, Joseph J, Kam M. Pharmacokinetics of intravenous insulin delivery in humans with type 1 diabetes. Diabetes Technol Ther 2005;7(1):83–93. [DOI] [PubMed] [Google Scholar]
- 112.Doshi P, Potter AJ, De Los Santos D, Banuelos R, Darger BF, Chathampally Y. Prospective randomized trial of insulin glargine in acute management of diabetic ketoacidosis in the emergency department: a pilot study. Acad Emerg Med 2015;22(6):657–662. [DOI] [PubMed] [Google Scholar]
- 113.Hsia E, Seggelke S, Gibbs J, et al. Subcutaneous administration of glargine to diabetic patients receiving insulin infusion prevents rebound hyperglycemia. J Clin Endocrinol Metab 2012;97(9):3132–3137. [DOI] [PubMed] [Google Scholar]
- 114.Umpierrez GE, Jones S, Smiley D, et al. Insulin analogs versus human insulin in the treatment of patients with diabetic ketoacidosis: a randomized controlled trial. Diabetes Care 2009;32(7):1164–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wolfsdorf J, Glaser N, Sperling MA, American Diabetes A. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes care 2006;29(5):1150–1159. [DOI] [PubMed] [Google Scholar]
- 116.Hoffman WH, Stamatovic SM, Andjelkovic AV. Inflammatory mediators and blood brain barrier disruption in fatal brain edema of diabetic ketoacidosis. Brain Res 2009;1254:138–148. [DOI] [PubMed] [Google Scholar]
- 117.Glaser NS, Wootton-Gorges SL, Buonocore MH, et al. Subclinical cerebral edema in children with diabetic ketoacidosis randomized to 2 different rehydration protocols. Pediatrics 2013;131(1):e73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shabbir N, Oberfield SE, Corrales R, Kairam R, Levine LS. Recovery from symptomatic brain swelling in diabetic ketoacidosis. Clin Pediatr (Phila) 1992;31(9):570–573. [DOI] [PubMed] [Google Scholar]
- 119.Roberts MD, Slover RH, Chase HP. Diabetic ketoacidosis with intracerebral complications. Pediatr Diabetes 2001;2(3):109–114. [DOI] [PubMed] [Google Scholar]
- 120.Kamat P, Vats A, Gross M, Checchia PA. Use of hypertonic saline for the treatment of altered mental status associated with diabetic ketoacidosis. Pediatr Crit Care Med 2003;4(2):239–242. [DOI] [PubMed] [Google Scholar]
- 121.Marcin JP, Glaser N, Barnett P, et al. Factors associated with adverse outcomes in children with diabetic ketoacidosis-related cerebral edema. J Pediatr 2002;141(6):793–797. [DOI] [PubMed] [Google Scholar]
- 122.Roe TF, Crawford TO, Huff KR, Costin G, Kaufman FR, Nelson MD Jr. Brain infarction in children with diabetic ketoacidosis. Journal of diabetes and its complications 1996;10(2):100–108. [DOI] [PubMed] [Google Scholar]
- 123.Keane S, Gallagher A, Ackroyd S, McShane MA, Edge JA. Cerebral venous thrombosis during diabetic ketoacidosis. Arch Dis Child 2002;86(3):204–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosenbloom AL. Intracerebral crises during treatment of diabetic ketoacidosis. Diabetes care 1990;13(1):22–33. [DOI] [PubMed] [Google Scholar]
- 125.Deeb A, Yousef H, Abdelrahman L, et al. Implementation of a Diabetes Educator Care Model to Reduce Paediatric Admission for Diabetic Ketoacidosis. J Diabetes Res 2016;2016:3917806. [DOI] [PMC free article] [PubMed] [Google Scholar]