Abstract
Young female sex-workers (FSW) aged 18–24 are at high risk of HIV due to high numbers of sexual partners, difficulty negotiating condom use, increased risk of gender-based violence, and limited access to services. Here we describe changes in sexual behaviours among young FSW across Zimbabwe between 2013 and 2016, and risk factors for prevalent HIV in 2013 and 2016. FSW ≥ 18 years were recruited using respondent-driven sampling in 14 sites across Zimbabwe in 2013 and 2016 as part of the SAPPH-IRe trial. We collected data on socio-demographics and sexual behaviour and offered HIV testing. Statistical analyses were RDS-II weighted. Characteristics of young FSW aged 18–24 were described, stratified by age. Logistic regression was used to assess difference in sexual behaviours by reported HIV status between 2013 and 2016, and to explore associations with prevalent HIV in 2013 and 2016. 656 young FSW were recruited in 2013 and 503 in 2016. Characteristics of young FSW were similar across both surveys. HIV prevalence was similar at both time points (35% vs 36%) and rose steeply with age. Compared to young FSW in 2013, reported condom-less sex with a steady partner and condom-less sex with clients was higher in 2016 among women self-reporting HIV negative status (OR = 6.41; 95%CI: 3.40-12.09; P<0.001) and (OR = 1.69; 95%CI: 1.14-2.51, P = 0.008), respectively, but not among young FSW self-reporting HIV positive status (OR = 2.35; 95%CI: 0.57-9.76; P = 0.236) and (OR = 1.87; 95%CI: 0.74-4.74; P = 0.186). After adjusting for age in 2016, young FSW who had ever been married had increased odds of testing HIV positive (OR = 1.88; 95% CI 1.04–3.39; P = 0.036) compared with those who had never married. Young FSW who completed secondary education or higher were less likely to test HIV positive (OR = 0.41; 95% CI 0.20–0.83; P = 0.012) compared with those with primary education or less. Young FSW remain at very high risk of HIV. Strategies to identify young FSW when they first start selling and refer them into services that address their economic, social and sexual vulnerabilities are critical.
Electronic supplementary material
The online version of this article (10.1007/s10461-019-02410-1) contains supplementary material, which is available to authorized users.
Keywords: HIV prevention, Young female sex-worker, Adolescent girls and young women, Young women who sell sex, Zimbabwe
Introduction
In sub-Saharan Africa, the burden of HIV is higher among women than men, and adolescent girls and young women (AGYW) bear the greatest burden of new infections [1]. In 2016, AGYW (aged 15–24 years) accounted for more than 60% of new infections among those aged 15–24 [1]. Young female sex-workers (FSW) are at particularly heightened HIV risk. In addition to the physiological, emotional and social vulnerabilities faced by AGYW as they transition into adulthood, young FSW face added challenges related to stigma, discrimination and criminalisation [2, 3], and reduced ability to negotiate condom use with sexual partners [4]. This results in a ‘perfect storm’ of synergistic vulnerabilities that increase their susceptibility to HIV, sexually transmitted infections (STIs) and unintended pregnancy, as well as violence, poor mental health and substance use. As a consequence, young FSW are a particularly important group for comprehensive prevention interventions [5, 6].
Despite their increased risk of HIV, STIs and unplanned pregnancies, young FSW are poorly engaged with sexual health and HIV prevention and care programmes, in part because of fear of stigma and discrimination from healthcare providers, older FSW, families and friends, and the possible legal repercussions of visiting healthcare services and disclosing their engagement in sex work [5, 7, 8].
Data on socio-economic characteristics and HIV risk behaviours among young FSW remain sparse in Africa, including in Zimbabwe, due to their lack of engagement in health services [7] and difficulty in reaching them for research. The SAPPH-IRe trial (Sisters Antiretroviral Programme for Prevention of HIV, an Integrated Response PACTR201312000722390) [9, 10] tested a community intervention to improve FSW engagement with prevention and care services in Zimbabwe. Using data from this trial we explored the characteristics and sexual behaviours of young FSW aged 18–24 at two time-points prior to the roll-out of the US Government’s DREAMS (Determined, Resilient, Empowered, AIDS-free, Mentored and Safe) Partnership in Zimbabwe. Through the delivery of a combined package of HIV prevention interventions, DREAMS aims to reduce the risk of HIV among the most vulnerable AGYW, including young women who sell sex (YWSS), in ten sub-Saharan African countries [11]. We investigated trends in sexual risk behaviours over this time period to better interpret any future changes in behaviour following the introduction of DREAMS in Zimbabwe. We investigated whether these behaviours were associated with prevalent HIV in 2013 and 2016 [9]. Our hypothesis was that HIV prevalence would be higher among women reporting more years of sex work, and sexual risk behaviours would become less risky over time and differ by age. We aimed to identify characteristics and behaviours that put young FSW at high risk of HIV, which might be necessary for DREAMS and similar programmes to target among AGYW going forward.
Methods
Study Setting
The SAPPH-IRe trial was conducted in 14 communities in Zimbabwe where a national sex worker HIV prevention programme, ‘Sisters with a Voice’, had been running. The trial is described in detail elsewhere [10]. Briefly, the trial aimed to estimate the effect of a combination intervention on the proportion of all FSW who had a viral load ≥ 1000 copies/mL (reflecting whether an individual is infectious) [9]. The trial was completed in 2016, before the introduction of the DREAMS Partnership, allowing us to describe the risk environment among young FSW prior to the roll-out of DREAMS. The trial sites were purposively selected to reflect a range of settings [9]. Women were eligible to participate in the trial baseline and endline surveys if they were aged 18 years or older, had exchanged sex for money or gifts in the preceding 30 days, and had lived at the site for at least the previous 6 months.
Data Sources
In this study, we used data from the SAPPH-IRe trial baseline and endline cross-sectional surveys. The baseline survey was conducted between 13th November and 20th December 2013 (2013 survey) and the endline survey between 11th April and 6th May 2016 (2016 survey). The surveys used respondent-driven sampling (RDS) to recruit two independent samples of FSW [9, 10, 12]. In these settings, sex is not commonly sold within brothels or other sex work specific venues, making RDS the most appropriate sampling method. As described elsewhere, prior to the RDS survey, geographic and social mapping was conducted over 2 to 3 days in each site [9]. This included informal discussions with trained peer educators, healthcare staff, and community informants. The mapping defined the geographic and social typology of sex work, determined if sex-workers at that site were well networked, and allowed for identification of FSW representative of these typologies (termed ‘seeds’) to initiate the RDS recruitment chains. In each location, between 6 and 8 seeds were invited to attend the survey site to complete a questionnaire and had a dried blood spot (DBS) taken for HIV antibody testing and, if positive, HIV viral load testing. All participants were also offered free HIV testing at the survey sites [10]. Seeds were then given two recruitment coupons to pass on to FSW peers. The women receiving a coupon (‘recruits’) who attended for interview were also given two coupons to give out to FSW in that location. We used an in-house coupon manager software to track coupons and all coupons were verified and redeemed only once. In all 14 sites, five iterations (‘waves’) of this process were performed for each survey. The sample size for each site was approximately 200 women, based on trial considerations [9]. The estimated population of FSW across these 14 sites ranged from 371 to 1409 therefore a sample of 200 women per site accounted for 14–54% of the target population across sites.
Key Outcomes and Measures
Data were restricted to women recruited who were aged 18–24. The primary outcome of interest was HIV prevalence among young FSW. Sexual risk behaviours explored included those related to sex work characteristics (e.g. duration of selling sex, number of clients per week), condom use at last sex, and condom-less sex with steady partners and/or clients. Condom-less sex was defined as not having used a condom with a sexual partner at least once in the last month. The questions used to assess condom-less sex were: “In the past month, how often did you use condoms with your steady partner?”; “In the past month, has there been an occasion when you did not use condoms with your steady partner?”; “In the past month, how often did you use condoms with your clients?” and “Think again about all your clients in the last month, have there been any times when you did not use condoms?” We measured common mental disorders using the validated Shona Symptom Questionnaire (SSQ-8) [13], which asks eight questions about mental health symptoms experienced in the previous 1 week. The SSQ-8 is a screening tool for common mental disorders, including depression and anxiety [13–15]. A score of six or more out of eight indicates risk of common mental disorders. Insufficient quantity of food was defined as reporting spending at least 1 day without eating in the past month because there was no food in the household.
Laboratory Methods
Blood samples were air-dried on filter papers and stored at room temperature until transported bi-weekly to the Flowcytometry Laboratory in Harare. If HIV antibodies were detected using AniLabsytems EIA kit (AniLabsystems Ltd, OyToilette 3, FIN-01720, Finland) then the sample was retested for HIV viral load using NucliSENS EasyQ HIV-1 v2.0, both to confirm HIV positive status and to quantify the viral load. For samples with a positive HIV antibody test using Anilab EIA, but an undetectable viral load, a second confirmatory ELISA was performed (Enzygnost Anti-HIV 1/2 Plus ELISA (Germany).
Data Analyses
We follow the STROBE-RDS guidelines in reporting our study by first describing the samples recruited at each survey site [16]. RDS makes a number of assumptions including that purposively selected seeds do not bias the final estimates and that the target population is networked. We generated recruitment trees to judge onward recruitment by FSW in both surveys. We assessed whether final estimates of key outcomes converged over the five waves of recruitment and whether the social networks of FSW appeared to have been disconnected (bottlenecks) using combined convergence and bottleneck plots in each site. We also assessed recruitment homophily with respect to age and prevalent HIV to understand if recruiters were more likely to recruit women of their own age and if women who tested HIV positive were more likely to recruit women who tested HIV positive, respectively.
All analyses were RDS-II weighted, with women’s responses weighted by the inverse of the reported number of sex workers they knew i.e. the number of other women that she could have recruited to the survey [17]. The rationale for RDS-II weighting has been described in detail elsewhere [12, 18]. We pooled data from across the fourteen sites and both time points and normalised the RDS-II weights by site, dropping seeds and including a fixed term for site in all regression analysis. In order to minimise the risk of duplicate enrolment in each survey, we constructed a check identifier for each participant consisting of the first letter of her first name, the last three letters of her surname and her date of birth. Comparing check identifiers and questionnaire responses between 2013 and 2016 surveys suggested that fifteen young FSW (2% of those enrolled in 2013; 3% of those enrolled in 2016) were likely to have participated in both surveys. HIV prevalence and sexual behaviour estimates did not change after excluding the fifteen individuals, therefore, in both years, all participants were retained in the analysis. Socio-demographic characteristics and sexual risk behaviours were analysed descriptively among young FSW aged 18–19 and aged 20–24. Where there were a small number of women within categories of a categorical variable, for example duration in sex work, variables were collapsed into fewer categories to overcome issues of sparse data.
Weighted logistic regression models, with sexual risk behaviour as outcome and survey year as exposure were used to assess evidence for changes in sexual risk behaviours between the 2013 and 2016 surveys, separately for young FSW who self-reported HIV negative status and those who self-reported HIV positive status. This was done separately by self-reported HIV status, to understand if adoption of safer sexual behaviours differed between women who reported knowing their HIV negative or positive status.
We intentified the socio-economic factors and sexual risk behaviours associated with prevalent HIV (rapid HIV test results) at each survey, by first using univariable logistic regression. Factors associated with prevalent HIV at P ≤ 0.10 in univariable analysis were included into multivariable regression models, firstly adjusted for age at survey, and secondly adjusted for all factors that were associated with prevalent HIV in the univariable analysis with some conceptual framework for how these variables might be related, to understand factors independently associated with prevalent HIV. Age at time of the survey was included categorically. 2013 data will be presented in the Online Appendix and 2016 data in the main paper.
Results
A total of 2722 FSW were recruited in 2013, of whom 24% (n = 656) were young FSW. In 2016, 2883 FSW were recruited, of whom 17% (n = 503) were young FSW. Extensive RDS diagnostics have been reported elsewhere [10, 19] and the findings apply to these data. Here we report RDS diagnostics of prevalent HIV and sexual risk behaviours.
In the 2013 survey, 90 FSW were seeds, 2804 FSW were examined for eligibility, and 2722 (97%) were eligible for the study. Overall, 3449 coupons were distributed of which 2714 (79%) were returned. In the 2016 survey, 92 FSW were seeds, 3025 FSW were examined for eligibility, and 2883 (95%) were eligible for the study. Overall, 3977 coupons were distributed of which 2933 (74%) were returned.
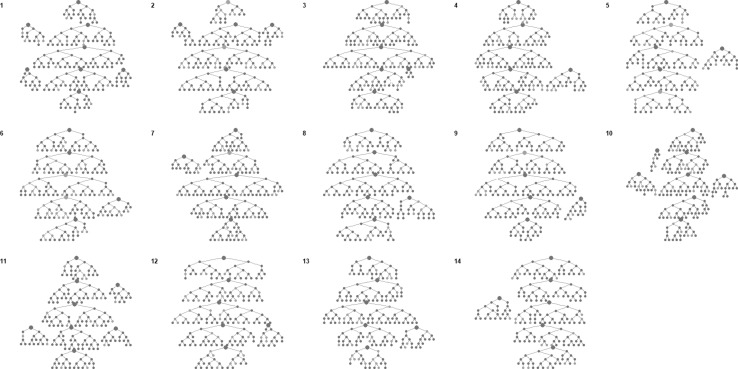
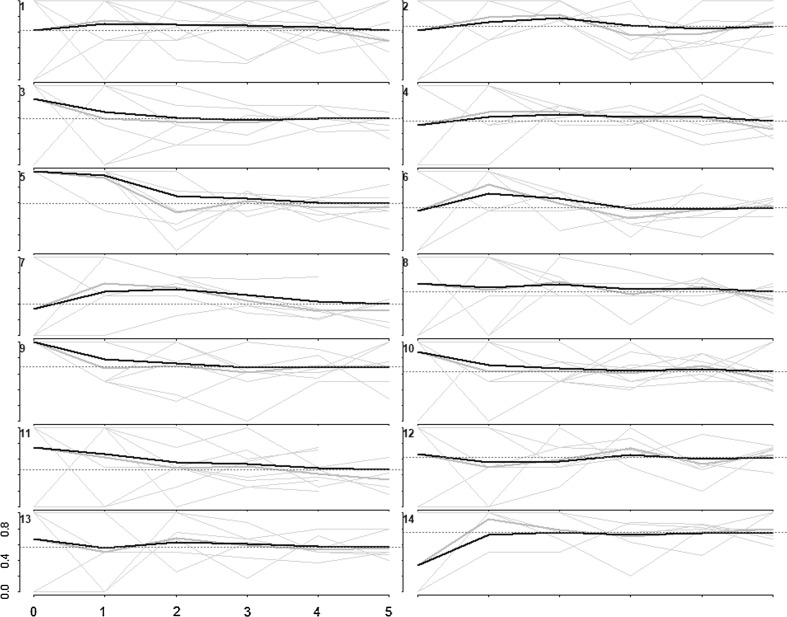
In the 2013 (Online Appendix 1) and 2016 surveys (Fig. 1), seeds were productive in recruiting FSW. In the 2013 survey, the number of recruits by seed ranged from 5 to 59 and in 2016 survey from 8 to 59 across sites. We judged that convergence appeared to have been achieved by the final sample size for HIV prevalence and that social networks of FSW in the respective sites were well connected (Fig. 2). This was true for sexual risk behaviours in 2013 and 2016 surveys (Online Appendix 1). Recruitment homophily on age and HIV prevalence was low in 2013 and 2016 surveys (Online Appendix 1).
Fig. 1.
Recruitment tree diagrams. Participants are depicted by circles with their recruits shown as the connected circles below them. The larger circles denote seeds. Red circles represent women aged 18–24 and blue circles represent women aged ≥ 25 (Colour figure online)
Fig. 2.
Convergence of the proportion of HIV positive FSW. The heavy black lines indicate the cumulative RDS-II weighted estimate overall for each site, while the grey lines are unweighted proportions for each seed, by sample wave (Colour figure online)
The socio-demographic and behavioural characteristics of young FSW were similar across both surveys (Table 1). Two thirds of young FSW had some secondary education or higher. In 2013, 63% of young FSW aged 18–19 had never married versus 49% in 2016. Among young FSW aged 20–24, a similar proportion had never married in 2013 and 2016 (32% vs 31%). Overall, around a quarter of young FSW reported initiating sex work before the age of 18 in both surveys. In 2013, 27% of young FSW aged 18–19 reported ≥ 10 clients in the last week compared to 35% in 2016. Among young FSW aged 20–24, a similar proportion reported ≥10 clients in the last week in 2013 and 2016 (27% vs 30%). A higher proportion of young FSW aged 18–19 were aware of their of HIV positive status in 2016 compared to 2013 (54% vs 45%) and this was true for young FSW aged 20–24.
Table 1.
Socio-demographic and sexual behavioural characteristics of young FSW by age, 2013 (N = 656) and 2016 (N = 503) (RDS-II weighted)
| 2013 (N = 656) | 2016 (N = 503) | |||||||
|---|---|---|---|---|---|---|---|---|
| 18–19 years | 20–24 years | 18–19 years | 20–24 years | |||||
| N = 109 | N = 547 | N = 51 | N = 452 | |||||
| n | (%) | n | (%) | N | (%) | n | (%) | |
| Marital status | ||||||||
| Married/ living together as if married | 0 | 0.0 | 3 | 0.5 | 1 | 0.8 | 3 | 1.6 |
| Divorced/ separated | 45 | 37.5 | 343 | 64.9 | 19 | 49.5 | 294 | 66.4 |
| Widowed | 0 | 0.0 | 19 | 2.8 | 1 | 0.8 | 8 | 1.0 |
| Never been married | 64 | 62.6 | 182 | 31.7 | 30 | 49.0 | 147 | 31.0 |
| Highest level of education | ||||||||
| None | 1 | 1.4 | 8 | 1.9 | 0 | 0.0 | 6 | 2.1 |
| Primary level | 38 | 34.8 | 106 | 21.3 | 13 | 30.4 | 87 | 22.1 |
| Some secondary | 52 | 49.0 | 229 | 44.3 | 28 | 55.8 | 178 | 39.2 |
| Complete secondary or higher | 17 | 14.8 | 203 | 32.5 | 10 | 13.9 | 181 | 36.6 |
| Tribe | ||||||||
| Shona | 85 | 68.3 | 427 | 74.0 | 41 | 81.3 | 334 | 71.9 |
| Ndebele | 18 | 24.3 | 47 | 11.4 | 7 | 10.5 | 48 | 11.2 |
| Kalanga | 0 | 0.0 | 11 | 1.4 | 0 | 0.0 | 1 | 0.1 |
| Other | 6 | 7.4 | 62 | 13.3 | 3 | 8.1 | 68 | 16.8 |
| Religion | ||||||||
| Christian | 56 | 57.2 | 267 | 49.0 | 32 | 62.1 | 263 | 53.2 |
| Muslim | 0 | 0.0 | 2 | 0.4 | 1 | 0.8 | 3 | 1.1 |
| African traditional | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 |
| Other | 11 | 14.6 | 35 | 6.2 | 8 | 10.5 | 54 | 13.8 |
| No religion | 42 | 28.1 | 241 | 44.1 | 10 | 26.6 | 131 | 31.7 |
| Number of children | ||||||||
| 0 | 46 | 46.4 | 152 | 30.6 | 23 | 43.2 | 119 | 30.3 |
| 1–2 | 52 | 47.4 | 329 | 59.3 | 26 | 54.4 | 268 | 54.9 |
| 3 + | 11 | 6.2 | 66 | 10.2 | 2 | 2.4 | 65 | 14.8 |
| No food for one day in the past month | ||||||||
| No | 70 | 63.0 | 325 | 60.0 | 36 | 67.4 | 308 | 67.7 |
| Yes | 39 | 37.0 | 222 | 40.0 | 15 | 32.6 | 143 | 32.3 |
| Age at start of sex work | ||||||||
| ≤ 15 | 19 | 16.2 | 26 | 5.6 | 9 | 18.6 | 21 | 5.9 |
| 16–17 | 56 | 51.8 | 63 | 9.6 | 32 | 69.7 | 68 | 12.6 |
| 18–24 | 34 | 32.0 | 458 | 84.7 | 10 | 11.7 | 362 | 81.5 |
| Duration in sex work (years) | ||||||||
| 0–2 | 82 | 79.7 | 300 | 60.5 | 36 | 71.4 | 204 | 46.7 |
| 3–4 | 22 | 15.6 | 165 | 26.0 | 14 | 28.2 | 161 | 34.5 |
| ≥ 5 | 5 | 4.7 | 82 | 13.5 | 1 | 0.4 | 86 | 18.8 |
| Number of clients in the last week | ||||||||
| 0–4 | 47 | 55.8 | 225 | 46.4 | 21 | 42.7 | 181 | 41.4 |
| 5–9 | 24 | 17.7 | 141 | 26.8 | 16 | 22.6 | 136 | 29.2 |
| ≥ 10 | 38 | 26.5 | 181 | 26.9 | 14 | 34.7 | 134 | 29.5 |
| Relationship with other female sex-workers in one’s location | ||||||||
| Good | 63 | 56.8 | 323 | 62.1 | 36 | 59.4 | 295 | 63.1 |
| Neither good nor bad | 31 | 28.6 | 174 | 28.7 | 12 | 31.8 | 132 | 33.0 |
| Bad | 8 | 6.5 | 32 | 5.8 | 1 | 0.3 | 15 | 2.2 |
| No relationship | 7 | 8.1 | 18 | 3.3 | 2 | 8.5 | 9 | 1.7 |
| No. of female sex-workers who are close friends | ||||||||
| ≤ 1 | 30 | 20.6 | 156 | 26.6 | 19 | 34.2 | 169 | 35.2 |
| 2–3 | 58 | 61.6 | 291 | 55.9 | 24 | 57.2 | 188 | 44.6 |
| ≥ 4 | 21 | 17.8 | 100 | 17.5 | 8 | 8.6 | 94 | 20.2 |
| Alcohol consumption in the past 12 months | ||||||||
| Never | 42 | 47.4 | 184 | 36.1 | 19 | 30.7 | 131 | 31.4 |
| Once a month or less | 8 | 5.9 | 59 | 12.5 | 3 | 7.4 | 35 | 6.4 |
| 2–4 times per month | 14 | 9.2 | 77 | 13.7 | 4 | 2.3 | 51 | 11.8 |
| 2–3 times per week | 23 | 20.4 | 79 | 13.1 | 13 | 27.1 | 116 | 27.2 |
| 4 or more times per week | 22 | 16.9 | 148 | 24.6 | 12 | 32.4 | 118 | 23.2 |
| Symptoms of common mental disorder | ||||||||
| No | 58 | 53.3 | 268 | 51.4 | 41 | 84.8 | 311 | 68.2 |
| Yes | 51 | 46.7 | 276 | 48.6 | 10 | 15.2 | 138 | 31.8 |
| Experience of physical violence from steady partner | ||||||||
| No | 63 | 62.7 | 305 | 59.5 | 27 | 50.4 | 242 | 58.3 |
| Yes | 46 | 37.3 | 242 | 40.5 | 24 | 49.6 | 209 | 41.7 |
| Experience of physical violence from client | ||||||||
| No | 82 | 77.1 | 397 | 76.2 | 29 | 52.6 | 327 | 75.9 |
| Yes | 27 | 22.9 | 150 | 23.8 | 22 | 47.4 | 124 | 24.1 |
| Ever forced to have sexual intercourse | ||||||||
| No | 100 | 90.4 | 528 | 96.4 | 46 | 90.7 | 412 | 89.9 |
| Yes | 9 | 9.6 | 19 | 3.6 | 5 | 9.3 | 39 | 10.1 |
| Self-reported HIV status | ||||||||
| Negative | 80 | 85.0 | 403 | 82.8 | 43 | 86.8 | 354 | 74.7 |
| Positive | 12 | 15.0 | 100 | 17.2 | 5 | 13.2 | 88 | 25.3 |
| Rapid HIV test result | ||||||||
| Negative | 80 | 73.3 | 339 | 63.0 | 39 | 79.3 | 290 | 62.0 |
| Positive | 28 | 26.7 | 208 | 37.0 | 12 | 20.7 | 161 | 38.0 |
| Knowledge of HIV positive statusa | ||||||||
| No | 18 | 55.4 | 116 | 61.0 | 9 | 45.7 | 77 | 38.1 |
| Yes | 10 | 44.6 | 92 | 39.0 | 3 | 54.3 | 84 | 61.9 |
aProportion reported HIV positive among those who tested HIV positive during the survey
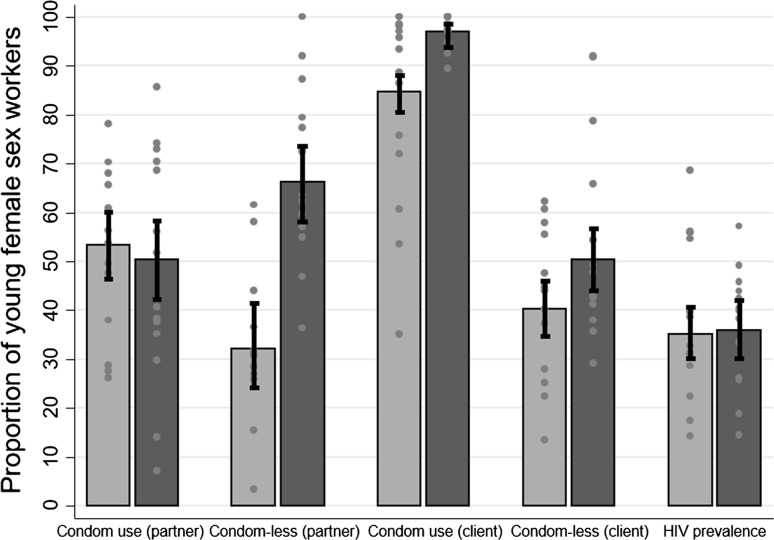
Between 2013 and 2016, reported sexual behaviours changed most among women who self-reported their HIV negative status. In 2016, there was evidence that a higher proportion of young FSW who self-reported their HIV negative status were in sex work for ≥ 3 years relative to 2013 (48% vs 35%, respectively; OR = 1.68; 95% CI 1.13–2.49; P = 0.010) (Table 2). In 2016, young FSW reporting HIV negative status were more likely to report having a steady partner compared to 2013 (OR = 1.63; 95% CI 1.07–2.47; P = 0.022). Compared to young FSW participating in the 2013 survey, condom-less sex with a steady partner in the past month was higher in 2016 among women self-reporting HIV negative status (31% vs 70%, respectively; OR = 6.41; 95% CI 3.40–12.09; P<0.001) but not among women self-reporting HIV positive status (OR = 2.35; 95% CI 0.57–9.76; P = 0.236), and condom-less sex with clients in the past month evidently increased among women who reported being HIV negative (OR = 1.69; 95% CI 1.14–2.51, P = 0.008) but there was no evidence of an increase among those who reported knowing their HIV positive status (OR = 1.87; 95% CI 0.74–4.74; P = 0.186). Figure 3 shows the overall changes in condom variables and HIV prevalence between 2013 and 2016, with site specific values denoted by dots to show the variability of proportions and prevalence across sites. There was evidence that ever being forced to have sex was higher in 2016 relative to 2013 among women who reported being HIV negative (11.1% vs 4.5%, respectively; OR = 2.63; 95% CI 1.03–6.75; P = 0.044).
Table 2.
Comparison of sexual risk behaviour characteristics among young FSW, by self-reported HIV status, 2013 (N = 656) and 2016 (N = 503)
| Characteristic | 2013 | 2016 | 2016 vs 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18–24 years (N = 656) | 18–24 years (N = 503) | 18–24 years | ||||||||||
| HIV-negative | HIV-positive | HIV-negative | HIV-positive | HIV-negative | HIV-positive | |||||||
| N = 544 | N = 112 | N = 409 | N = 93 | |||||||||
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | OR (95% CI)a | P-valueb | OR (95% CI)a | P-valueb | |
| Duration in sex work (years) | ||||||||||||
| 0–2 | 321 | 64.6 (58.6–70.1) | 61 | 60.3 (47.3–72.0) | 201 | 52.5 (45.7–59.3) | 39 | 40.7 (27.8–55.0) | – | – | ||
| 3+ | 223 | 35.4 (29.9–41.4) | 51 | 39.7 (28.0–52.7) | 208 | 47.5 (40.7–54.3) | 54 | 59.3 (45.0–72.2) | 1.68 (1.13–2.49) | 0.010 | 1.88 (0.83–4.22) | 0.126 |
| Number of clients in the last week | ||||||||||||
| 0–4 | 231 | 49.5 (43.4–55.5) | 41 | 39.9 (27.9–53.4) | 169 | 43.9 (37.3–50.8) | 33 | 33.6 (21.8–47.9) | – | – | ||
| 5+ | 313 | 50.5 (44.5–56.6) | 71 | 60.1 (46.6–72.1) | 240 | 56.1 (49.2–62.7) | 60 | 66.4 (52.1–78.2) | 1.27 (0.87–1.86) | 0.213 | 1.19 (0.55–2.60) | 0.656 |
| Has a steady partner | ||||||||||||
| No | 194 | 36.5 (30.7–42.6) | 43 | 35.6 (24.0–49.3) | 123 | 27.0 (21.8–33.0) | 32 | 42.3 (28.7–57.1) | – | – | ||
| Yes | 350 | 63.5 (57.4–69.3) | 69 | 64.4 (50.7–76.0) | 286 | 73.0 (67.0–78.2) | 61 | 57.7 (42.9–71.3) | 1.63 (1.07–2.47) | 0.022 | 0.89 (0.39–2.06) | 0.791 |
| Condom use at last sex with steady partner | ||||||||||||
| No | 154 | 47.0 (39.5–54.6) | 30 | 44.8 (29.5–61.3) | 141 | 51.7 (42.7–60.6) | 24 | 41.3 (24.4–60.6) | – | – | ||
| Yes | 178 | 53.0 (45.4–60.5) | 33 | 55.2 (38.7–70.5) | 114 | 48.3 (39.4–57.3) | 35 | 58.7 (39.4–75.6) | 0.88 (0.54–1.44) | 0.614 | 1.45 (0.44–4.73) | 0.535 |
| Condom–less sex with steady partner in the past month | ||||||||||||
| No | 124 | 68.6 (58.3–77.3) | 21 | 63.3 (39.9–81.7) | 68 | 29.7 (22.0–38.7) | 26 | 48.9 (31.2–67.0) | – | – | ||
| Yes | 54 | 31.4 (22.7–41.7) | 12 | 36.7 (18.3–60.1) | 187 | 70.3 (61.3–78.0) | 33 | 51.1 (33.0–68.8) | 6.41 (3.40–12.09) | <0.001 | 2.35 (0.57–9.76) | 0.236 |
| Condom use at last sex with client | ||||||||||||
| No | 94 | 15.2 (11.5–19.9) | 18 | 15.3 (7.9–27.5) | 10 | 2.8 (1.2–6.3) | 2 | 3.5 (0.7–15.4) | – | – | ||
| Yes | 450 | 84.8 (80.1–88.5) | 94 | 84.7 (72.5–92.1) | 399 | 97.2 (93.7–98.8) | 90 | 96.5 (84.6–99.3) | 8.15 (2.56–25.93) | <0.001 | 9.34 (1.84–47.33) | 0.007 |
| Condom-less sex with client in the past month | ||||||||||||
| No | 300 | 60.8 (54.5–66.7) | 51 | 53.7 (40.1–66.9) | 200 | 48.4 (41.4–55.5) | 44 | 53.8 (38.9–68.0) | – | – | ||
| Yes | 199 | 39.2 (33.3–45.5) | 49 | 46.3 (33.1–59.9) | 185 | 51.6 (44.5–58.6) | 42 | 46.2 (32.0–61.1) | 1.69 (1.14–2.51) | 0.008 | 1.87 (0.74–4.74) | 0.186 |
| Failed to use a condom with client as a result of own drinking during the past 12 months | ||||||||||||
| No | 254 | 79.0 (73.0–84.0) | 50 | 69.8 (54.5–81.6) | 234 | 83.5 (76.4–88.7) | 57 | 87.2 (73.0–94.5) | – | – | ||
| Yes | 101 | 21.0 (16.0–27.0) | 21 | 30.2 (18.4–45.5) | 49 | 16.5 (11.3–23.6) | 11 | 12.8 (5.5–27.0) | 0.79 (0.45–1.37) | 0.400 | 0.37 (0.11–1.30) | 0.122 |
| Failed to use a condom with client as a result of client’s drinking during the past 12 months | ||||||||||||
| No | 456 | 86.4 (81.8–89.9) | 91 | 76.4 (61.6–86.6) | 383 | 92.1 (87.0–95.3) | 85 | 90.3 (78.0–96.1) | – | – | ||
| Yes | 86 | 13.6 (10.1–18.2) | 21 | 23.6 (13.4–38.4) | 26 | 7.9 (4.7–13.0) | 8 | 9.7 (3.9–22.0) | 0.57 (0.29–1.10) | 0.092 | 0.26 (0.07–1.02) | 0.053 |
| Ever forced to have sexual intercourse | ||||||||||||
| No | 522 | 95.5 (91.8–97.5) | 106 | 94.5 (86.7–97.8) | 371 | 88.9 (83.9–92.4) | 87 | 93.7 (84.2–97.7) | – | – | ||
| Yes | 22 | 4.5 (2.5–8.2) | 6 | 5.5 (2.2–13.3) | 38 | 11.1 (7.6–16.1) | 6 | 6.3 (2.3–15.8) | 2.63 (1.03–6.75) | 0.044 | 1.68 (0.41–6.92) | 0.472 |
aIncluding fixed term for site
bWald P-value
Fig. 3.
Condom use at last sex and condom-less sex with steady partner, condom use at last sex and condom-less sex with client, and HIV prevalence overall among young female sex-workers (aged 18–24) and by site (grey spots = sites; light blue bars = 2013 survey; dark blue bars = 2016 survey) (Colour figure online)
In univariable analysis of factors associated with prevalent HIV in 2013 (Online Appendix 2), there was evidence that age and marital status were associated with prevalent HIV. However, there was no evidence of an association after adjusting for age at time of the survey, marital status and number of FSW who are close friends. In 2016, there was evidence that age, marital status, educational attainment and reported number of clients in the previous week were crudely associated with prevalent HIV (Table 3), with no evidence that HIV prevalence was higher among women selling sex for ≥ 5 years relative to women selling sex for 0–2 years (28.4% vs 38.1%; OR = 0.66; 95% CI 0.32–1.35; P = 0.302) and little evidence of an association with condom use variables, experience of violence or alcohol use.
Table 3.
Factors associated with prevalent HIV among young FSW aged 18–24 in Zimbabwe, 2016 (N = 503)
| Characteristic | N (%) | Number of young female sex-workers tested HIV-positive during the survey (n = 173) n (%) | Crude OR (95% CI) | P-value | Age adjusted OR (95% CI) | P-value | Adjusteda OR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|
| Age at time of survey | 0.029 | 0.035 | ||||||
| 18–19 | 51 (12.5) | 12 (20.7) | 1 | 1 | ||||
| 20–24 | 451 (87.5) | 161 (38.0) | 3.06 (1.12–8.38) | 3.09 (1.09–8.81) | ||||
| Marital status | 0.013 | 0.036 | 0.060 | |||||
| Never married | 176 (33.0) | 41 (23.7) | 1 | 1 | 1 | |||
| Ever married | 326 (67.0) | 132 (41.9) | 2.10 (1.17–3.77) | 1.88 (1.04–3.39) | 1.77 (0.98–3.21) | |||
| Highest level of education | 0.025 | 0.012 | 0.031 | |||||
| Primary school or less | 105 (24.7) | 47 (47.4) | 1 | 1 | 1 | |||
| Some secondary school | 206 (41.4) | 76 (36.3) | 0.66 (0.33–1.30) | 0.68 (0.34–1.34) | 0.69 (0.35–1.36) | |||
| Complete secondary or higher | 191 (33.9) | 50 (26.9) | 0.45 (0.22–0.90) | 0.41 (0.20–0.83) | 0.46 (0.22–0.94) | |||
| Number of children | 0.462 | |||||||
| 0 | 141 (31.7) | 54 (41.9) | 1 | |||||
| 1–2 | 294 (55.0) | 96 (32.1) | 0.61 (0.34–1.10) | |||||
| ≥ 3 | 67 (13.3) | 23 (37.3) | 0.89 (0.37–2.14) | |||||
| Duration in sex work | 0.302 | |||||||
| 0–2 | 240 (49.8) | 83 (38.1) | 1 | |||||
| 3–4 | 175 (33.7) | 56 (36.2) | 0.92 (0.51–1.67) | |||||
| ≥ 5 | 87 (16.5) | 34 (28.4) | 0.66 (0.32–1.35) | |||||
| Number of clients in the last week | 0.068 | 0.043 | 0.090 | |||||
| 0–4 | 202 (41.5) | 56 (28.9) | 1 | 1 | 1 | |||
| 5–9 | 152 (28.3) | 55 (37.7) | 1.60 (0.84–3.05) | 1.58 (0.82–3.04) | 1.45 (0.74–2.85) | |||
| ≥ 10 | 148 (30.2) | 62 (43.8) | 1.79 (0.94–3.44) | 1.88 (1.01–3.51) | 1.70 (0.90–3.22) | |||
| Condom use at last sex with steady partner | 0.635 | |||||||
| No | 165 (49.5) | 54 (29.1) | 1 | |||||
| Yes | 149 (50.5) | 48 (32.5) | 1.18 (0.59–2.38) | |||||
| Condom-less sex with steady partner in the past month | 0.165 | |||||||
| No | 94 (33.7) | 33 (37.3) | 1 | |||||
| Yes | 220 (66.3) | 69 (27.6) | 0.58 (0.27–1.25) | |||||
| Condom use at last sex with client | 0.96 | |||||||
| No | 12 (3.0) | 3 (33.6) | 1 | |||||
| Yes | 489 (97.0) | 169 (35.6) | 1.05 (0.15–6.03) | |||||
| Condom-less sex with client in the past month | 0.277 | |||||||
| No | 244 (49.6) | 77 (36.6) | 1 | |||||
| Yes | 227 (50.4) | 80 (32.5) | 0.73 (0.41–1.29) | |||||
| Experience of physical violence from steady partner | 0.917 | |||||||
| No | 269 (57.3) | 87 (36.2) | 1 | |||||
| Yes | 233 (42.7) | 86 (35.4) | 0.97 (0.58–1.63) | |||||
| Experience of physical violence from client | 0.944 | |||||||
| No | 356 (73.0) | 114 (35.7) | 1 | |||||
| Yes | 146 (27.0) | 59 (36.4) | 1.08 (0.54–1.77) | |||||
| Ever forced to have sexual intercourse | 0.523 | |||||||
| No | 458 (90.0) | 154 (35.5) | 1 | |||||
| Yes | 44 (10.0) | 19 (39.4) | 1.32 (0.57–3.06) | |||||
| Alcohol use in the past 12 months | 0.699 | |||||||
| Never | 150 (31.3) | 46 (36.0) | 1 | |||||
| Once a month or less | 38 (6.5) | 12 (45.0) | 1.61 (0.50–5.17) | |||||
| 2–4 times per month | 55 (10.6) | 16 (20.2) | 0.50 (0.20–1.24) | |||||
| 2–3 times per week | 129 (27.2) | 47 (35.4) | 1.09 (0.54–2.22) | |||||
| 4 or more times per week | 130 (24.4) | 52 (40.4) | 1.20 (0.58–2.48) | |||||
| No food for one day in the past month | 0.376 | |||||||
| No | 344 (67.6) | 120 (37.4) | 1 | |||||
| Yes | 158 (32.4) | 53 (32.7) | 0.78 (0.45–1.35) | |||||
| Relationship with other FSW in one’s location | 0.499 | |||||||
| Good | 331 (62.7) | 110 (35.2) | 1 | |||||
| Neither good nor bad | 144 (32.8) | 53 (35.5) | 1.12 (0.62–2.02) | |||||
| Bad or no relationship | 27 (4.5) | 10 (47.2) | 1.94 (0.64–5.91) | |||||
| No. of FSW who are close friends | 0.201 | |||||||
| ≤ 1 | 188 (35.1) | 70 (36.8) | 1 | |||||
| 2–3 | 212 (46.1) | 77 (40.8) | 1.26 (0.68–2.31) | |||||
| ≥ 4 | 102 (18.8) | 26 (22.0) | 0.47 (0.21–1.05) | |||||
| Symptoms of common mental disorder | 0.795 | |||||||
| No | 352 (70.3) | 121 (36.3) | 1 | |||||
| Yes | 148 (29.7) | 51 (34.8) | 0.93 (0.52–1.65) |
aAdjusted for age, marital status, highest level of education and number of clients in the last week
After adjusting for age, there was strong evidence that marital status, educational attainment and reported number of clients in the previous week were associated with prevalent HIV. Young FSW who had ever been married had a higher prevalence of HIV (adjOR = 1.88; 95% CI 1.04–3.39; P = 0.036) compared with those never married. Young FSW with complete secondary education or higher were less likely to test HIV positive compared to women with primary education or less (adjOR = 0.41; 95% CI 0.20–0.83; P = 0.012). Young FSW reporting ≥ 10 clients in the last week (adjOR = 1.88; 95% CI 1.01–3.51; P = 0.043) were more likely to be HIV positive compared to those reporting 0–4 clients in the last week.
Among women aged 20–24, HIV prevalence was 38.0% compared to 20.7% among women aged 18–19 and after adjusting for marital status, educational attainment and reported number of clients in the previous week, there was evidence that HIV prevalence differed by age (adjOR = 3.09; 95% CI 1.09–8.81; P = 0.035).
Discussion
We used data from a completed trial to explore the characteristics and sexual behaviours of young FSW aged 18 to 24 at two time-points separated by 30 months, and investigated whether these factors were associated with prevalent HIV in 2013 and 2016. In both surveys, over one-third of the women tested HIV positive, confirming that young FSW in Zimbabwe are at consistently high risk of infection. A lower proportion were aware of their HIV positive status in 2013. As seen among other young women in southern Africa, HIV prevalence was higher amongst those young FSW who were divorced or separated [20]. Young FSW reported high numbers of clients in the previous week and high rates of condom-less sex in the past month with a steady partner or client. In 2016, we found that women who were ever married, had less education, and who reported a higher number of clients in the past week were more likely to test HIV-positive but found no evidence that duration of selling sex or reported condom use were associated with HIV.
In 2016, HIV prevalence among young FSW aged 20–24 was approximately four-times higher than the HIV prevalence of 10% among women aged 20–24 in the general population [21]. It was also 1.5 times higher than in young FSW aged 18–19, suggestive of high HIV incidence. This hypothesis is supported by a cohort analysis of the Sisters with a Voice programmatic data, which estimated an HIV incidence of 11% per year among women aged < 26 between 2009 and 2013 [22].
We hypothesised that HIV prevalence would be higher among women reporting more years of sex work but found no association; in 2016 HIV prevalence seemed to be decreasing with increased duration in sex work. Prevalent HIV was higher among women reporting more clients in the past week, highlighting the risk associated with “high activity” sex work. We also found an association with reporting ever being married, possibly because it is difficult for married women to use condoms with their husbands. Alternatively it is possible that women widowed by an HIV-positive husband or separated from their partner because of their HIV-positive status are more likely to transition into sex work, although we were not able to explore this in our survey data. A study among sex-workers attending a clinic in Kenya found that younger women were at higher risk of HIV infection, and that women in sex work for < 2 years at enrolment had 2.3-fold higher risk of HIV than women in sex work for > 2 years [23]. An earlier study in India among brothel-based sex-workers found that HIV prevalence was higher among sex-workers aged ≤20 compared to sex-workers aged 21 or older (12.5% vs 5.4%) [24]. A better understanding of the complex relationship between HIV risk and entry into sex work is required to effectively target HIV prevention and social protection services based on the needs of different groups of women.
Despite our hypothesis that sexual risk behaviours would become less risky between 2013 and 2016, analysis of sexual behaviours found that sexual risky behaviours actually became more risky within this period, particularly among women who reported to be HIV negative. This upward change in some risky behaviours was also seen in Thailand among men who have sex with men, an equally most-at-risk population [25]. Condom-less sex with steady partners increased in 2016 compared to 2013 but the magnitude of increase was higher among women who reported being HIV negative compared to those who reported their HIV status as positive. This might suggest that women aware of their HIV-positive status may adopt safer behaviours to reduce the risk of onward transmission to clients and partners, and points to the need for additional targeting of HIV negative young women. Or possibly that more women have steady partners who are aware of their HIV negative status.
Both reported condom use at last sex and condom-less sex in the previous month with clients increased between 2013 and 2016, which is contradictory. Reported condom use is subject to social desirability bias, so while it is possible these changes may reflect changes in actual condom use they could also reflect biases in reporting. Poor validity of self-reported sexual behaviours, particularly condom use, is well recognised [26, 27], with condom use at last sex considered at particular risk of over-reporting [28]. Similar to studies among FSW in India [29] and Cambodia [30], our analysis of factors associated with prevalent HIV found no association between condom use variables and HIV. HIV may have been acquired some time prior to the survey. Further, women may or may not be using condoms because they know their HIV status and/or their partners’ HIV status, or if they know that ART treatment is effective at preventing HIV transmission. Despite the complexities and limitations in understanding the relationship between recent condom use and prevalent HIV, reported condom-less sex in the past month was also high among HIV-negative young FSW. Although an important prevention tool, condom provision alone may be insufficient to protect young FSW from HIV, particularly without specific interventions to increase risk perception and improve condom negotiation skills. In recent years, trials have proven the effectiveness of oral pre-exposure prophylaxis (PrEP) in reducing HIV among women adherent to PrEP [31, 32]. The availability of PrEP provides an important alternate prevention tool for women, and an opportunity to reduce HIV incidence among this population of women.
After adjusting for age at the time of the survey in 2016, lower educational attainment was strongly associated with HIV. This finding is similar to trends observed in some, but not all, sub-Saharan African countries [33]. Studies among young women in the general population have shown that staying in school can reduce HIV risk in some settings [34, 35] and that educational attainment has a protective effect against HIV [36, 37]. Low educational attainment has also been shown to be associated with initiating selling sex, likely contributing to increased HIV risk [5, 38]. Our findings emphasise the need to identify female adolescents who are at risk of dropping out of school to either support them to remain in school or provide a safety net to reduce their future vulnerability to HIV [39]. Interventions such as the DREAMS partnership which address the structural drivers of HIV are likely important for preventing HIV in this population [11].
Our study is subject to limitations. The women participating in the survey were recruited through RDS. Due to the nature of RDS, we cannot empirically explore whether the sample of women participating in the survey is representative of FSW in the study sites. However, extensive diagnostic testing of the RDS data suggests little evidence of bias [10, 19], although, as we have reported elsewhere, there is some suggestion that women who engaged with the Sisters with a Voice programme were more likely to participate in the 2016 survey [19] which might explain social desirability bias in reporting of condom use at last sex because women are taught about the importance of condom use at programme visit. The surveys were cross-sectional and therefore we cannot explore the temporal relationship between risk factors and HIV. The check identifier we used to minimise the risk of duplicate enrolment in each survey was subject to women using different names and/or giving different date of birth. However, many participants aged 22 to 24 in 2013 (43%) would have aged out of eligibility by 2016, and additionally those aged 18 to 20 in 2016 (23%) would have been ineligible to take part in the 2013 survey. Despite these limitations, both surveys include a sizeable proportion of young FSW recruited using the same RDS procedures in 14 sites nationally. The findings provide critical insights into the vulnerability and HIV risk among this understudied population of women.
In conclusion, our findings confirm that young FSW in Zimbabwe are at a very high risk of HIV. The multicomponent DREAMS Partnership aims to reach YWSS, providing an important opportunity to offer these women a package of services to improve their health and well-being, tailored to address the findings from this research, such as condom negotiation, access to education, PrEP, and sexual and reproductive health services more generally. As part of DREAMS, PrEP is being offered to YWSS. To maximise the HIV prevention impact of PrEP, programmes will need to address structural factors such as costs and social factors such as stigma likely to hinder PrEP access and adherence among young FSW, which DREAMS partnership is designed to provide [11]. To support effective targeting of future strategies to reduce HIV risk, a better understanding of whether FSW’s higher HIV risk is due to a high risk when they first initiate sex work, or reflects higher risk prior to transitioning into sex work, is needed. Such information would help identify strategies to target young women when they are most at risk of HIV, and refer them into appropriate socioeconomic services and HIV-prevention and related health interventions.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank CeSHHAR staff and peer educators who were involved in the SAPHH-IRe trial as well as the women who participated in the surveys.
Funding
The SAPPH-Ire trial was funded by UNFPA via Zimbabwe’s Integrated Support Programme which receives funds from DfID, Irish Aid and Swedish SIDA. A small amount of funding for survey work was from GIZ. USAID supported the cost of PSI Zimbabwe to provide ART and PrEP to sex workers as part of the trial. We received a donation of Truvada for PrEP use for the trial from Gilead. Data analyses for this manuscript was funded by the Bill and Melinda Gates Foundation (OPP1136774).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The SAPPH-IRe 2013 and 2016 surveys were reviewed and approved by the Medical Research Council of Zimbabwe, University College London, the London School of Hygiene and Tropical Medicine, and RTI International before the initiation of research activities. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNAIDS . Global AIDS update. Geneva: UNAIDS; 2016. [Google Scholar]
- 2.Inter-Agency Working Group on Key Populations . HIV and young key populations: technical briefs. Geneva: UNAIDS; 2015. [Google Scholar]
- 3.Busza J, Mtetwa S, Chirawu P, Cowan F. Triple jeopardy: adolescent experiences of sex work and migration in Zimbabwe. Health Place. 2014;28:85–91. doi: 10.1016/j.healthplace.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Busza J, Mtetwa S, Mapfumo R, Wong-Gruenwald R, Hanisch D, Cowan F. Underage and underserved: reaching young women who sell sex in Zimbabwe. AIDS Care. 2016. [DOI] [PMC free article] [PubMed]
- 5.UNAIDS. HIV and young people who sell sex: a technical brief. Geneva, Switzerland; 2014.
- 6.Napierala Mavedzenge S, Fearon E, Hargreaves J, Mushati P, Mtetwa S, Chiyaka T, et al., editors. Estimating engagement in HIV care among young female sex workers in Zimbabwe. IAS 2015; 2015; Vancouver, Canada. [DOI] [PubMed]
- 7.Napierala S, Chabata ST, Fearon E, Hargreaves JR, Busza J, Mushati P, et al. Engagement in HIV care among young female sex workers in Zimbabwe. J Aquir Immune Defic Syndr. 2018;79(3):358–366. doi: 10.1097/QAI.0000000000001815. [DOI] [PubMed] [Google Scholar]
- 8.Oringanje C, Meremikwu MM, Eko H, Esu E, Meremikwu A, Ehiri JE. Interventions for preventing unintended pregnancies among adolescents. Cochrane Database Syst Rev. 2016;2:CD005215. doi: 10.1002/14651858.CD005215.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hargreaves JR, Fearon E, Davey C, Phillips A, Cambiano V, Cowan FM. Statistical design and analysis plan for an impact evaluation of an HIV treatment and prevention intervention for female sex workers in Zimbabwe: a study protocol for a cluster randomised controlled trial. Trials. 2016;17:6. doi: 10.1186/s13063-015-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan FM, Davey CB, Fearon E, Mushati P, Dirawo J, Cambiano V, et al. The HIV care cascade among female sex workers in Zimbabwe: results of a population-based survey from the sisters antiretroviral therapy programme for prevention of HIV, an integrated response (SAPPH-IRe) trial. J Acquir Immune Defic Syndr. 2017;74(4):375–382. doi: 10.1097/QAI.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 11.Abdool Karim Q, Baxter C, Birx D. Prevention of HIV in adolescent girls and young women: key to an AIDS-free generation. J Acquir Immune Defic Syndr. 2017;75:S17–S26. doi: 10.1097/QAI.0000000000001316. [DOI] [PubMed] [Google Scholar]
- 12.Cowan FM, Mtetwa S, Davey C, Fearon E, Dirawo J, Wong-Gruenwald R, et al. Engagement with HIV prevention treatment and care among female sex workers in Zimbabwe: a respondent driven sampling survey. PLoS ONE. 2013;8(10):e77080. doi: 10.1371/journal.pone.0077080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chingono R, Chibanda D, Chabata ST, Maringwa G, Mupambireyi Z, Simms V, et al., editors. Validation of the 8-item Shona Symptom Questionnaire, as a measure of common mental disorders in a population with high HIV prevalence in Zimbabwe. 13th International AIDSImpact Conference; 2017; Cape Town, South Africa.
- 14.Patel V, Simunyu E, Gwanzura F, Lewis G, Mann A. The Shona Symptom Questionnaire: the development of an indigenous measure of common mental disorders in Harare. Acta Psych Scand. 1997;95:469–475. doi: 10.1111/j.1600-0447.1997.tb10134.x. [DOI] [PubMed] [Google Scholar]
- 15.Chibanda D, Verhey R, Gibson LJ, Munetsi E, Machando D, Rusakaniko S, et al. Validation of screening tools for depression and anxiety disorders in a primary care population with high HIV prevalence in Zimbabwe. J Affect Disord. 2016;198:50–55. doi: 10.1016/j.jad.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 16.White RG, Hakim AJ, Salganik MJ, Spiller MW, Johnston LG, Kerr L, et al. Strengthening the reporting of observational studies in epidemiology for respondent-driven sampling studies: & “STROBE-RDS” statement. J Clin Epidemiol. 2015;68(12):1463–1471. doi: 10.1016/j.jclinepi.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volz E, Heckathorn DD. Probability based estimation theory for respondent driven sampling. J Off Stat. 2008;24(1):79–97. [Google Scholar]
- 18.Heckathorn DD. Extensions of respondent-driven sampling: analyzing continuous variables and controlling for differential recruitment. Sociol Methodol. 2007;37(1):151–207. doi: 10.1111/j.1467-9531.2007.00188.x. [DOI] [Google Scholar]
- 19.Cowan FM, Davey C, Fearon E, Mushati P, Dirawo J, Chabata ST, et al. Targeted combination prevention to support female sex workers in Zimbabwe accessing and adhering to antiretrovirals for treatment and prevention of HIV (SAPPH-IRe): a cluster-randomised trial. Lancet HIV. 2018;5(8):e417–e426. doi: 10.1016/S2352-3018(18)30111-5. [DOI] [PubMed] [Google Scholar]
- 20.Cowan F, Pascoe SJS, Langhaug LF, Mavhu W, Chidiya S, Jaffar S, et al. The Regai Dzive Shiri Project: results of a randomised trial of an HIV prevention intervention for Zimbabwean youth. AIDS. 2010;24:2541–2542. doi: 10.1097/QAD.0b013e32833e77c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimbabwe National Statistics Agency and ICF International. 2016. Zimbabwe Demographic and Health Survey 2015: Final Report. Rockville, Maryland, USA: Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International.
- 22.Hargreaves JR, Mtetwa S, Davey C, Dirawo J, Chidiya S, Benedikt C, et al. Cohort analysis of programme data to estimate HIV incidence and uptake of HIV-related services among female sex workers in Zimbabwe, 2009-14. J Acquir Immune Defic Syndr. 2016;72(1):e1–e8. doi: 10.1097/QAI.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 23.McKinnon L, Izulla P, Nagelkerke N, Munyao J, Wanjiru T, Shaw S, et al. Risk factors for HIV acquisition in a prospective nairobi-based female sex worker cohort. AIDS Behav. 2015;19(12):2204–2213. doi: 10.1007/s10461-015-1118-7. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar K, Bal B, Mukherjee R, Saha MK, Chakraborty S, Niyogi SK, et al. Young age is a risk factor for HIV among female sex workers: an experience from India. J Infect. 2006;53(4):255–259. doi: 10.1016/j.jinf.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 25.van Griensven F, Varangrat A, Wimonsate W, Tanpradech S, Kladsawad K, Chemnasiri T, et al. Trends in HIV prevalence, estimated HIV incidence, and risk behavior among men who have sex with men in Bangkok, Thailand, 2003–2007. J Acquir Immune Defic Syndr. 2010;53(2):234–239. doi: 10.1097/QAI.0b013e3181c2fc86. [DOI] [PubMed] [Google Scholar]
- 26.Zenilman JM, Weisman CS, Rompalo AM, Ellish N, Upchurch DM, Hook EWI, et al. Condom use to prevent incident STDs: the validity of self-reported condom use. Sex Transm Dis. 1995;22(1):15–21. doi: 10.1097/00007435-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Evans JL, Couture MC, Stein ES, Sansothy N, Maher L, Page K. Biomarker validation of recent unprotected sexual intercourse in a prospective study of young women engaged in sex work in Phnom Penh, Cambodia. Sex Transm Dis. 2013;40(6):462–468. doi: 10.1097/OLQ.0b013e318286db8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Morisky DE, Lin X, Ma E, Jiang B, Yin Y. Bias in Self-Reported Condom Use: Association Between Over-Reported Condom Use and Syphilis in a Three-Site Study in China. AIDS Behav. 2016;20(6):1343–1352. doi: 10.1007/s10461-015-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medhi GK, Mahanta J, Paranjape RS, Adhikary R, Laskar N, Ngully P. Factors associated with HIV among female sex workers in a high HIV prevalent state of India. AIDS Care. 2012;24(3):369–376. doi: 10.1080/09540121.2011.608787. [DOI] [PubMed] [Google Scholar]
- 30.Ohshige K, Morio S, Mizushima S, Kitamura K, Tajima K, Suyama A, et al. Behavioural and serological human immunodeficiency virus risk factors among female commercial sex workers in Cambodia. Int J Epidemiol. 2000;29(2):344–354. doi: 10.1093/ije/29.2.344. [DOI] [PubMed] [Google Scholar]
- 31.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–1983. doi: 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hargreaves JR, Bonell CP, Boler T, Boccia D, Birdthistle I, Fletcher A, et al. Systematic review exploring time trends in the association between educational attainment and risk of HIV infection in sub-Saharan Africa. AIDS. 2008;22(3):403–414. doi: 10.1097/QAD.0b013e3282f2aac3. [DOI] [PubMed] [Google Scholar]
- 34.Handa S, Halpern CT, Pettifor A, Thirumurthy H. The Government of Kenya’s Cash Transfer Program reduces the risk of sexual debut among young people age 15–25. PLoS ONE. 2014;9(1):e85473. doi: 10.1371/journal.pone.0085473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoner MCD, Pettifor A, Edwards JK, Aiello AE, Halpern CT, Julien A, et al. The effect of school attendance and school dropout on incident HIV and HSV-2 among young women in rural South Africa enrolled in HPTN 068. AIDS. 2017;31(15):2127–2134. doi: 10.1097/QAD.0000000000001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramjee G, Daniels B. Women and HIV in Sub-Saharan Africa. AIDS Res Ther. 2013;10(1):30. doi: 10.1186/1742-6405-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettifor AE, Levandowski BA, MacPhail C, Padian NS, Cohen MS, Rees HV. Keep them in school: the importance of education as a protective factor against HIV infection among young South African women. Int J Epidemiol. 2008;37(6):1266–1273. doi: 10.1093/ije/dyn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekker L-G, Hosek S. HIV and adolescents: focus on young key populations. J Int AIDS Soc. 2015;18(2 Suppl 1):20076. [Google Scholar]
- 39.Delany-Moretlwe S, Cowan FM, Busza J, Bolton-Moore C, Kelley K, Fairlie L. Providing comprehensive health services for young key populations: needs, barriers and gaps. J Int AIDS Soc. 2015;18(2 Suppl 1):19833. doi: 10.7448/IAS.18.2.19833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.