Abstract
Background: Liver plays a vital role in the elimination of xenobiotics that can induce hepatotoxicity in living organisms.Silver nanoparticles have evolved recently as an alternative in various industries and are used for their biomedical applications.Rhizophora apiculata is a least studied mangrove-based plant that has been used in the traditional medicine of Southeast Asia for its healing properties. It is a well-known fact that the generation of free radicals has been associated with oxidative stress.
Methods: Hence, in this study we used carbon tetrachloride as a hepatotoxin to induce liver damage. The protective effects of silver nanoparticles synthesized using Rhizophora apiculata on hepatotoxin-induced liver damage in experimental mice were assessed.
Results: The results of the assessment indicate that silver nanoparticles were effective in protecting the liver from damages induced by carbon tetrachloride.
Conclusion: Among existing literature, this is the first ever approach for hepatoprotective effect of nanoparticles derived using plant extract from mangrove ecosystem.
Keywords: mangroves, Rhizophora apiculata, silver nanoparticles, carbon tetrachloride, hepatotoxicity
Introduction
The liver plays an important role in metabolism and detoxification of compounds which enter the body and may cause hepatic injury, leading to life-threatening diseases.1 Therefore, major toxicological problems associated with several diseases have been centered around the effects on the liver.2 Usually, liver cells are affected by hepatotoxic agents through the induction of oxidative damage.3 Drugs of both synthetic and natural origin are available for treatment of liver diseases.4 Natural remedies have long been used for treatment of liver diseases. Based on this, protective effects of plant-based herbal medicines against drug-induced toxicity have reached paramount importance recently.5
Rhizophora apiculata is a member of the Rhizophoraceae family of the south-east Asian mangrove ecosystem.6 Members of Rhizophora family have been known to possess several biomedical compounds.7 R. apiculata has not been studied in depth regarding its therapeutic values, although reports suggest that they possess some biological activities when used in diseases such as cancer.8 To the best of our knowledge, among available literature, there are no reports yet on the hepatoprotective activity of R. apiculata. Therefore, the leaf extract and the extract-derived nanoparticles have been used in this study to analyze their protective effects on the liver.
Carbon tetrachloride (CCl4) is a xenobiotic released into water as waste from several industries, thereby leading to hepatotoxicity when living organisms are exposed to it.9 It is often used to induce liver disorders in various models for the screening of hepatoprotective agents.10 In the studies that involve animal models, particularly those that study the effects on the liver, CCl4 was transformed into other forms of free radicals, leading to lipid peroxidation. This may therefore result in cellular necrosis.11 Hence, in this study, we used CCl4 as the hepatotoxin to analyze the effects on the liver.
Free radicals can also be generated by normal physiological conditions.12 However, when there is an imbalance in ROS and free radical scavenging, it results in tissue damage and cell death.13 Elevated levels of ROS can determine the occurrence of oxidative stress.14 ROS can therefore have a profound effect on several infections induced by chemical and biological means.15 Therefore, we intended to test the ROS and oxidative stress-related biochemical parameters to analyze the hepatoprotective effect of the nanoparticles.
Silver nanoparticles (AgNPs) have been exploited for several therapeutic purposes because of its properties such as limited toxicity, increased biodegradability, and bioavailability.16,17 They are known to interact easily with biological systems because of their small size.18 AgNPs can be synthesized via various routes, among which, green synthesis is considered to be the need of the hour, since it possesses several advantages compared to chemical synthesis.19–21 Biosynthesis of AgNPs has been performed using several biological sources and such nanoparticles have been used in various industries.22 But recently, the synthesis of AgNPs using plants has become the most preferred method for biosynthesis of nanoparticles.23 AgNPs biosynthesized using Andrographis paniculata have been known to possess hepatoprotective properties.24 However, there are no reports on hepatoprotective activity of mangrove-derived AgNPs.
Taking this as an initiative, we intended to study the hepatoprotective activity of AgNPs in CCl4 induced mouse model. A plant leaf extract of the mangrove R. apiculata and AgNPs derived using the same plant leaf extract (shade-dried leaf extract as a reducing agent) were used as test agents. The procedure for synthesis and characterization of AgNPs using R. apiculata has been published earlier by our group.25
Materials and method
Characterization of AgNPs
UV-visible spectroscopy, Fourier transform infra-red spectroscopy and transmission electron microscopy were used for characterization of shade-dried R. apiculata leaf extract-derived AgNPs in our previously published report.25 In the present study, the AgNPs were further characterized by scanning electron microscopy (SEM), energy dispersive X-ray (EDX) analysis, inductively coupled plasma-optical emission spectrometry (ICP-OES), and X-ray photoelectron spectroscopy (XPS). SEM and EDX analysis were performed using JEOL-JSM 6390 instrument. Concentration of AgNPs was determined by ICP-OES analysis (Perkin Elmer Optima 5300 DV model, PerkinElmer Inc., Waltham, MA, USA). XPS analysis was performed with Omicron ESCA Probe spectrometer.
Chemicals and laboratory animals
Silymarin and CCl4 were obtained from Ranbaxy Laboratories and Qualigens respectively. Fifty- four male Swiss albino mice weighing around 30–35 g, bought from Kerala Veterinary and animal Sciences University, Thrissur, Kerala, India, were used for the study. The animals were confined in polypropylene cages and fed with standard pellet and water ad libitum. A temperature of 23°C–25°C and humidity of 50%–60% were maintained throughout the experiment with a 12-hour light–dark cycle. Standard pellet diet was purchased from Sai Enterprisei, Chennai, India. Permission for the experiment was obtained from the Institutional Animal Ethics Committee, and CPCSEA regulations were followed during the entire course of the study (Ethics Committee number: BDU/IAEC/42/2013/09.04.2013).
Experimental plan
Fifty-four Swiss albino male mice were divided into nine groups. Each group consisted of six mice. Group I animals were normal. Animals intoxicated with CCl4 intraperitoneally(1:1 with olive oil, 0.5 mL/kg of body weight [BW]) for 2 days at the start of the experiment were Group II (control). Group III (low dose [LD] – 1.75 mg/kg BW), Group IV (medium dose [MD] – 2.5 mg/kg BW), and Group V (high dose [HD] – 3.25 mg/kg BW) were CCl4-intoxicated mice treated with the specified doses of aqueous leaf extract of R. apiculata. Groups VI (1.75 mg/kg BW – LD), VII (2.5 mg/kg BW – MD), and VIII (3.25 mg/kg BW – HD) were CCl4-intoxicated mice treated with the specified doses of R. apiculata-derived AgNPs. Group IX animals were treated with silymarin (standard drug, 20 mg/kg BW) after intoxication with CCl4. The groups involved in the study are explained in Table 1. Treatment was initiated orally from day 3 of the induction of hepatotoxicity. The experiment was continued for 7 more days. Serum was collected at the end of the experiment by euthanizing the animals. Clear serum obtained after centrifuging at 2,500 rpm for 15 minutes was used for further analyses.
Table 1.
Experimental groups involved in analysis of hepatoprotective activity of silver nanoparticles
| Groups (6 animals each) |
Carbon tetrachloride mixed 1:1 with olive oil | R. apiculata leaf extract | R. apiculata-derived silver nanoparticles | Silymarin |
|---|---|---|---|---|
| I | - | - | - | - |
| II | 0.5 mL/kg body weight (BW) | - | - | - |
| III | 0.5 mL/kg BW | Low dose - 1.75 mg/kg BW | - | - |
| IV | 0.5 mL/kg BW | Medium dose - 2.5 mg/kg BW | - | - |
| V | 0.5 mL/kg BW | High dose - 3.25 mg/kg BW | - | - |
| VI | 0.5 mL/kg BW | - | Low dose - 1.75 mg/kg BW | - |
| VII | 0.5 mL/kg BW | - | Medium dose - 2.5 mg/kg BW | - |
| VIII | 0.5 mL/kg BW | - | High dose - 3.25 mg/kg BW | - |
| IX | 0.5 mL/kg BW | - | - | 20 mg/kg BW |
Biochemical parameters
Liver function test was performed by analyzing markers such as SGOT, SGPT, and ALP.26 Levels of enzymatic and non-enzymatic antioxidants such as GPx and GSH were estimated.27 Cellular metabolite LDH was studied to analyze tissue injury.28 MDA was studied to analyze lipid peroxidation.29
Histopathology
Livers were collected after euthanizing the mice at the end of the experimental period. After processing with formalin and staining with H&E, the histopathological changes in the livers were observed under a microscope at 40× using paraffin sections of 5 μm thickness.30
Statistics
Results obtained were expressed as mean ± SD. SPSS version 17 was applied to perform one-way ANOVA.31
Results and discussion
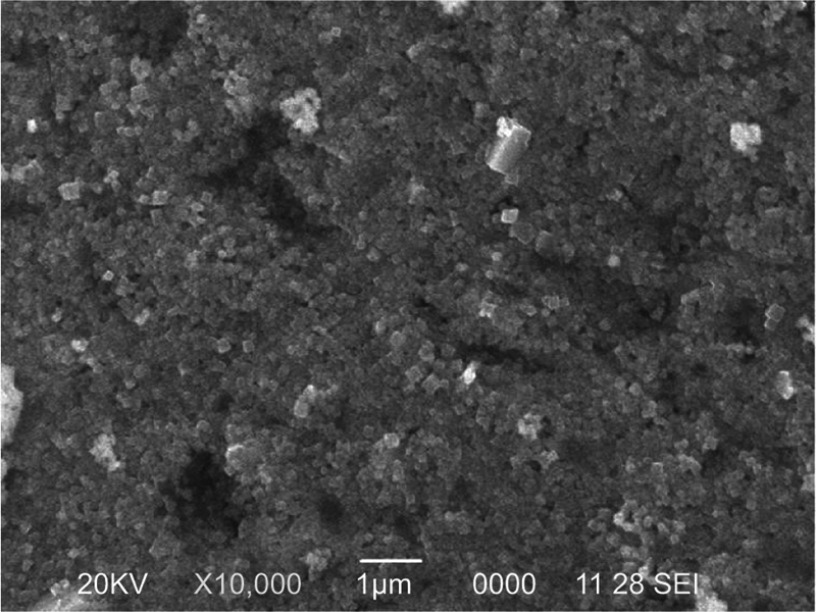
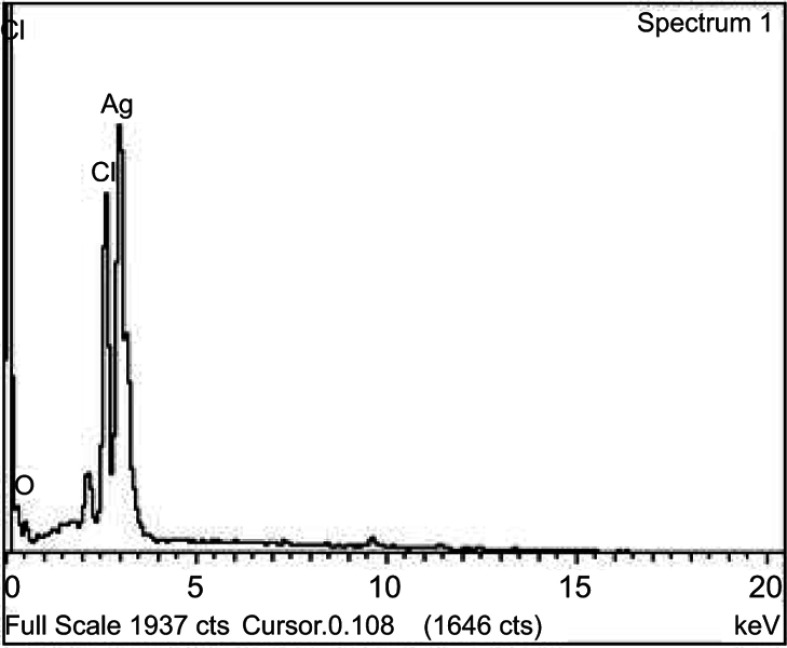
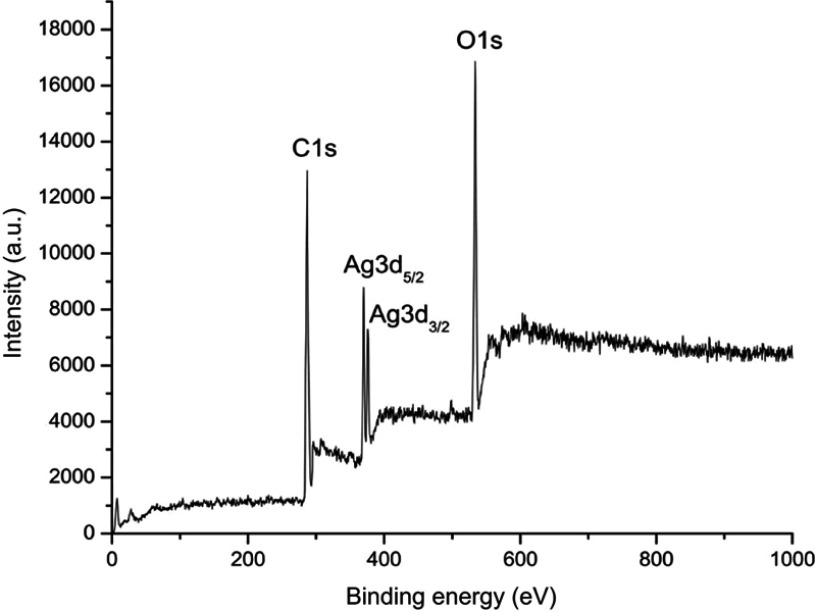
Figure 1 shows the SEM micrograph of the biosynthesized AgNPs. Silver was the foremost element (69.8%) in comparison with chlorine (21.81%) and oxygen (8.39%), as observed by EDX spectrum. The weaker peaks of O and Cl in the EDX spectrum are probably due to X-ray emissions from proteins or sugars.32 No peaks for compounds of silver were observed (Figure 2). This confirms that silver nitrate was reduced to AgNPs. The spectrum around 3 KeV stands as a strong signal for the presence of AgNPs due to surface plasmon resonance.33 The total concentration of AgNPs, as calculated by ICP-OES, a widely used method to detect metal ions, was 19.51 mg/L.34 Strong peaks of C1s, O1s, and Ag3d core levels were found in XPS analysis. There was a major C1s peak at 286.8 eV. Considering the carbon shift from 284.8 eV, the O1s value was centered at 532 eV. Ag3d5/2 and Ag3d3/2 peaks occurred at binding energies of 367.8 and 374.0 eV, respectively (Figure 3). This suggests the oxidation state at Ag.35 The Auger core level value stood at 358.5 eV. The metallic state of Ag was further confirmed by Wagner plot. The Auger parameter between 726.00 and 726.40 corresponds to the state of pure silver. Values in the range of 722.80–724.80 correspond to the presence of silver salts. Auger parameter was calculated by the sum of Auger core value (358.5 eV) and the Ag3d5/2 value (367.8 eV). This resulted in a sum of 726.3, the Auger parameter specific for metallic Ag.36 This protocol again suggests that the plant extract acted as reductant and converted the silver nitrate into AgNPs.
Figure 1.
Scanning electron microscopy analysis of the synthesized nanoparticles.
Figure 2.
Energy dispersive X-ray analysis of the synthesized nanoparticles.
Figure 3.
X-ray photoelectron spectroscopy analysis of synthesized silver nanoparticles.
CCl4 is a well-known hepatotoxin used in animal models to test liver injury. The major reasons behind CCl4-induced hepatopathies are lipid peroxidation, decreased antioxidant enzyme activity, and the generation of free radicals.37–39 Hepatotoxins give rise to lipid peroxides by attacking the fatty acids of membranes, which can cause a loss in functional integrity of hepatic mitochondria, leading to liver damage.40 Reducing the generation of free radicals or creating an antioxidant effect are effective ways to treat hepatopathies.41 Alternative medicines such as herbal medicines rich in antioxidants are therefore the need of the hour due to toxicity issues associated with certain medications.42,43 In this context, a large number of plants have been utilized in several countries to treat liver diseases and to enhance liver functions.44 The use of plants for treatment of injuries related to the liver has been credited to the involvement of antioxidants as a supplement.3
Among the biochemical analyses performed in this experiment, LDH is a prominent indicator and a diagnostic tool for tissue injury.45 LDH levels were elevated in the control group, but were effectively restored to normal in groups treated with test (AgNPs) and standard (silymarin) agents (Table 2). HD treatment of both AgNPs and plant extract were effective in restoring LDH. This indicates the positive effect on injured hepatic cells. Following the analysis of LDH, MDA levels were tested. It is a prominent indicator of lipid peroxidation and oxidative stress which can interact at both genomic and proteomic levels.46–48 The MDA level was increased in CCl4-treated group, which was restored to near normal levels in treatment groups III–IX (Table 2). LD treatment of plant extract (Group III) and HD treatment of AgNPs (Group VIII) were effective in revival compared to the other treatment groups. These results indicate that the AgNPs had a positive effect on the liver with regard to lipid peroxides. Treatment with HD AgNPs was effective in attenuating the CCl4-induced injuries.3
Table 2.
Biochemical parameters associated with this study
| Parameters | Group I |
Group II |
Group III |
Group IV |
Group V |
Group VI |
Group VII |
Group VIII |
Group IX |
|---|---|---|---|---|---|---|---|---|---|
| Cellular metabolites | |||||||||
| LDH (IUdL−1) | 217.74±13.87e | 347.74±13.47 a | 291.72±20.99b | 263.33±22.65cd | 251.79±9.69d | 297.55±13.41b | 278.34±13.46bc | 228.23±16.95e | 231.22±13.28e |
| Lipid peroxidation marker | |||||||||
| MDA (nmoles/g) | 20.04±4.85bcd | 28.78±3.63a | 19.45±4.44 bcd | 24.93±4.28ab | 15.35±1.84de | 16.25±3.08cde | 12.69±4.13e | 20.34±3.49bcd | 21.45±4.17bc |
| Enzymatic antioxidant | |||||||||
| GPx (IU/mg) | 18.97±5.27c | 14.98±4.05c | 19.01±3.51c | 28.33±4.42b | 17.69±4.14c | 35.42±4.89a | 19.76±6.36c | 18.69±5.94c | 19.88±7.55c |
| Non-enzymatic antioxidant | |||||||||
| GSH (IU/mg) | 24.16±6.98b | 18.53±2.73b | 21.72±5.81b | 26.2±5.12ab | 20±6.53b | 32.99±3.42a | 20.41±4.37b | 23.16±7.50b | 25.11±3.98b |
| Liver enzymes | |||||||||
| SGOT (IU/l) | 152.2±9.94ab | 163.16±18.14a | 117.8±33.74cd | 138.95±17.17bc | 134.2±8.91bc | 108±11.67d | 99.4±10.64d | 132±15.91bc | 137.3±18.58bc |
| SGPT (IU/l) | 85.75±6.03a | 91.98±9.45a | 88.8±8.82a | 74.27±6.70c | 54.86±7.51c | 39.49±1.57d | 33.652±3.35d | 82.88±8.87ab | 83.82±7.26a |
| ALP (IU/l) | 107.41±11.20cd | 138.71±13.30b | 258.79±24.69a | 127.78±10.27b | 135.49±4.57b | 92.09±10.90d | 102.88±9.43cd | 106.93±9.78cd | 111.12±6.09c |
Notes: Results are expressed as mean ± SD (n=6). Each lower case letter above the SD indicates the significant (p<0.05) difference between the groups. Similar letter represents insignificant change. Different letters determine significant changes among the groups.
ROS levels are maintained by the enzymatic (GPx) and non-enzymatic antioxidants (GSH) as they regulate the production and removal of free radicals under normal conditions.49 Both these antioxidants can scavenge free radicals such as H2O2 and can defend against oxidative stress.50 The enzymatic antioxidant of the study (GPx) was depleted in CCl4-treated groups. This was effectively restored to near normal in HD plant extract-treated and HD AgNPs-treated groups (Table 2). MD plant extract (Group IV) and HD AgNPs (Group VIII) were effective in restoring GSH, the non-enzymatic antioxidant of the study, to near normal levels (Table 2). Biosynthesized AgNPs have been known to possess antioxidant and free radical scavenging properties.51,52 This indicates that the AgNPs had increased the antioxidant potential or, by creating a change in enzymatic system, had caused a significant effect on the revival of the antioxidant system. This may be due to the prevention of the release of free radicals or a protective effect toward the generation of such free radicals.
Increase in serum transaminases is an indication of the structural damage to the liver.53 The subcellular structures affected by CCl4 exposure are the plasma membrane, endoplasmic reticulum, mitochondria, and golgi apparatus.54 This toxic effect releases enzymes of cytoplasmic origin that enter the circulation during liver damage.55 Hence, both cell membrane permeability and increased enzyme activity contribute to hepatic structural injury.56,57 Increase in circulating levels of liver enzymes is a result of leakage of serum enzymes as a consequence of lipid peroxidation.58 In our study, there was an increase in level of serum enzymes such as SGOT, SGPT, and ALP, denoting hepatic damage. Reduction of serum transaminases to near normal levels by the treatment with HD AgNPs (Group VIII), indicates a possible regeneration of hepatocytes and a possible healing effect on the hepatic parenchyma.59 The overall revival observed among treatment groups III–IX denotes the curative effect of plant extract and AgNPs. Treatment with HD AgNPs (3.25 mg/kg BW, Group VIII) was effective in treatment considering all the biochemical parameters tested (Table 2).
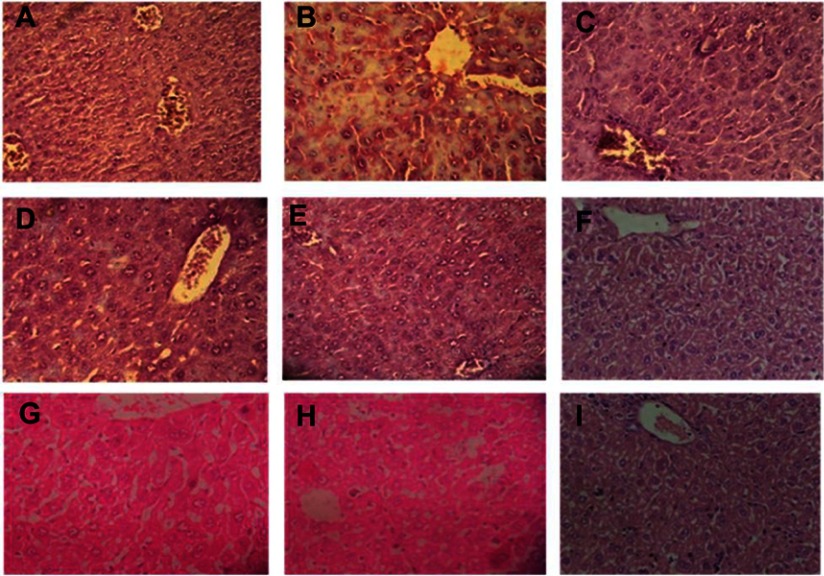
Histopathological sections of livers of normal animals indicated normal hepatic architecture with distinct hepatocytes and sinusoidal spaces. In the CCl4-intoxicated group, the hepatic architecture was disrupted, indicating an abnormality among hepatic cells. Tissue necrosis and cytoplasmic vacuolization were observed around the central vein. The cell membranes and central vein were disintegrated. The sinusoidal spaces were dilated. This phenomenon in the intoxicated group was brought back to near normal hepatic architecture in the plant extract- and nanoparticles-treated groups, indicating significant protection of the liver (Figure 4).
Figure 4.
Livers of mice were dissected (after they were euthanized at the end of the experiment), fixed, stained with H&E, and observed at 40× magnification. (A) Normal, (B) control, (C) CCl4+LD PE, (D) CCl4+MD PE, (E) CCl4+HD PE, (F) CCl4+LD AgNPs, (G) CCl4+MD AgNPs, (H) CCl4+HD AgNPs, (I) CCl4+silymarin.
Abbreviations: CCl4, carbon tetrachloride; LD, low dose; PE, plant extract; MD, medium dose; HD, high dose; AgNPs, silver nanoparticles.
The ability of a drug to attenuate injuries or to preserve the normal physiological function of the liver after induction of toxicity is the index of its hepatoprotective effect.60 Plant extracts used to synthesize AgNPs can lead to synergistic effects, including an antioxidant effect.61 Results obtained, considering the biochemical and histological parameters, signify that AgNPs were effective in protection against CCl4-intoxication. Failure in antioxidant system can lead to an increase in lipid peroxidation and loss of normal cellular functioning in the liver.29 Thus, the antioxidant and free radical scavenging effects of AgNPs might play a crucial role in the revival of the biochemical and histological parameters to near normal in the treatment groups.62 This makes AgNPs a valuable candidate for several therapeutic purposes, including protective effects on the liver.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no 81704169 and 81503306), Natural Science Foundation of Jiangsu Province (grant no BK20171067) and The Project of Jiangsu Administration of Traditional Chinese Medicine (grant no FY201803).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rane J, Jadhao R, Bakal R. Liver diseases and herbal drugs:-A review. J Innov Pharm Biol Sci. 2016;3(2):24–36. [Google Scholar]
- 2.Ramappa V, Aithal GP. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol. 2013;3(1):37–49. doi: 10.1016/j.jceh.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh D, Cho WC, Upadhyay G. Drug-induced liver toxicity and prevention by herbal antioxidants: an overview. Front Physiol. 2016;6:363. doi: 10.3389/fphys.2015.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong M, Li S, Tan HY, Wang N, Tsao S-W FY. Current status of herbal medicines in chronic liver disease therapy: the biological effects, molecular targets and future prospects. Int J Mol Sci. 2015;16(12):28705–28745. doi: 10.3390/ijms161226126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong F, Guan Y-S. Cautiously using natural medicine to treat liver problems. World J Gastroenterol. 2017;23(19):3388–3395. doi: 10.3748/wjg.v23.i19.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saenger P, West PW. Determinants of some leaf characteristics of Australian mangroves. Bot J Linean Soc. 2016;180(4):530–541. doi: 10.1111/boj.2016.180.issue-4 [DOI] [Google Scholar]
- 7.Hamzah TNT, Lee SY, Hidayat A, Terhem R, Faridah-Hanum I, Diversity MR. Characterization of endophytic fungi isolated from the tropical mangrove species, rhizophora mucronata, and identification of potential antagonists against the soil-borne fungus, fusarium solani. Front Microbiol. 2018;9:1707. doi: 10.3389/fmicb.2018.01707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prabhu VV, Guruvayoorappan C. Anti-inflammatory and anti-tumor activity of the marine mangrove Rhizophora apiculata. J Immunotoxicol. 2012;9(4):341–352. doi: 10.3109/1547691X.2012.660997 [DOI] [PubMed] [Google Scholar]
- 9.Acharya K, Chatterjee S, Biswas G, Chatterjee A, Saha GK. Hepatoprotective effect of a wild edible mushroom on carbon tetrachloride-induced hepatotoxicity in mice. Int J Pharm Pharm Sci. 2012;4(3):285–288. [Google Scholar]
- 10.Mahmoodzadeh Y, Mazani M, Rezagholizadeh L. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol Rep. 2017;4:455–462. doi: 10.1016/j.toxrep.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahmat AA, Dar FA, Choudhary IM. Protection of CCl4-induced liver and kidney damage by phenolic compounds in leaf extracts of cnestis ferruginea (de Candolle). Pharmacognosy Res. 2014;6(1):19–28. doi: 10.4103/0974-8490.122913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Ndian J Clin Biochem. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nita M ,Grzybowski AThe role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev. 2016;2016:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts RA, Smith RA, Safe S, Szabo C, Tjalkens RB, Robertson FM. Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology. 2010;276(2):85–94. doi: 10.1016/j.tox.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanmuganathan R, MubarakAli D, Prabakar D, et al. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Pollut Res. 2018;25(11):10362–10370. doi: 10.1007/s11356-017-9367-9 [DOI] [PubMed] [Google Scholar]
- 17.Saravanan M, Barik SK, MubarakAli D, Prakash P, Pugazhendhi A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog. 2018;116:221–226. doi: 10.1016/j.micpath.2018.01.038 [DOI] [PubMed] [Google Scholar]
- 18.Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int J Pharm. 2018;539(1):104–111. doi: 10.1016/j.ijpharm.2018.01.034 [DOI] [PubMed] [Google Scholar]
- 19.Saratale RG, Saratale GD, Shin HS, et al. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ Sci Pollut Res. 2018;25(11):10164–10183. doi: 10.1007/s11356-017-9912-6 [DOI] [PubMed] [Google Scholar]
- 20.Saha J, Begum A, Mukherjee A, Kumar S. A novel green synthesis of silver nanoparticles and their catalytic action in reduction of methylene blue dye. Sustainable Environ Res. 2017;27(5):245–250. doi: 10.1016/j.serj.2017.04.003 [DOI] [Google Scholar]
- 21.Pugazhendhi A, Prabakar D, Jacob JM, Karuppusamy I, Saratale RG. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb Pathog. 2018;114:41–45. doi: 10.1016/j.micpath.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 22.Zhang X-F, Liu Z-G, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain S, Mehata MS. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep. 2017;7(1):15867. doi: 10.1038/s41598-017-15724-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suriyakalaa U, Antony JJ, Suganya S, et al. Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf B Biointerfaces. 2013;102:189–194. doi: 10.1016/j.colsurfb.2012.06.039 [DOI] [PubMed] [Google Scholar]
- 25.Antony JJ, Sivalingam P, Siva D, et al. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf B Biointerfaces. 2011;88(1):134–140. doi: 10.1016/j.colsurfb.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 26.Chandrashekhar, VM, Muchandi AA, Sudi SV, Ganapty S. Hepatoprotective activity of Stereospermum suaveolens against CCl4-induced liver damage in albino rats. Pharm Biol. 2010;48(5):524–528. doi: 10.3109/13880200903173601 [DOI] [PubMed] [Google Scholar]
- 27.Karthikeyan R, Anantharaman P, Chidambaram N, Balasubramanian T, Somasundaram S. Padina boergessenii ameliorates carbon tetrachloride induced nephrotoxicity in Wistar rats. J King Saud Univ Sci. 2012;24(3):227–232. doi: 10.1016/j.jksus.2011.03.002 [DOI] [Google Scholar]
- 28.Wang T, Sun NL, Zhang WD, et al. Protective effects of dehydrocavidine on carbon tetrachloride-induced acute hepatotoxicity in rats. J Ethnopharmacol. 2008;117(2):300–308. doi: 10.1016/j.jep.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 29.Nagalekshmi R, Menon A, Chandrasekharan DK, Nair CKK. Hepatoprotective activity of Andrographis Paniculata and Swertia Chirayita. Food Chem Toxicol. 2011;49(12):3367–3373. doi: 10.1016/j.fct.2011.09.026 [DOI] [PubMed] [Google Scholar]
- 30.Ye X, Feng Y, Tong Y, et al. Hepatoprotective effects of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced acute liver hepatotoxicity in rats. J Ethnopharmacol. 2009;124(1):130–136. [DOI] [PubMed] [Google Scholar]
- 31.Zar J. Biostastical Analysis. Engle Cliffs: Prentice-Hall, NJ; 1984. [Google Scholar]
- 32.Devi JS, Bhimba BV, Peter DM. Production of biogenic silver nanoparticles using Sargassum longifolium and its applications. Indian J Geo-Mar Sci. 2013;42(1):125–130. [Google Scholar]
- 33.Govindappa M, Hemashekhar B, Arthikala M-K, Ravishankar Rai V, Ramachandra YL. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results in Physics. 2018;9:400–408. doi: 10.1016/j.rinp.2018.02.049 [DOI] [Google Scholar]
- 34.Teng X, Niu Y, Xie Z, Cai Q Synthesis and application of acrylamide-maleic anhydride copolymer for solid phase extraction. Paper presented at: IOP Conference Series: Materials Science and Engineering, 2018. doi: 10.1088/1757-899X/322/2/022013 [DOI] [Google Scholar]
- 35.Li S, Shen Y, Xie A, et al. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007;9(8):852–858. doi: 10.1039/b615357g [DOI] [Google Scholar]
- 36.Ramstedt M, Franklyn P. Difficulties in determining valence for Ag0 nanoparticles using XPS—characterization of nanoparticles inside poly (3‐sulphopropyl methacrylate) brushes. Surf Interface Anal. 2010;42(6–7):855–858. doi: 10.1002/sia.v42:6/7 [DOI] [Google Scholar]
- 37.Poli G. Liver damage due to free radicals. Br Med Bull. 1993;49(3):604–620. [DOI] [PubMed] [Google Scholar]
- 38.Ighodaro OM, Akinloye OA. Sapium ellipticum (Hochst) Pax leaf extract: antioxidant potential in CCl4-induced oxidative stress model. Bull Fac Pharm Cairo Univ. 2018;56(1):54–59. doi: 10.1016/j.bfopcu.2017.11.001 [DOI] [Google Scholar]
- 39.Lu Y, Chen J, Ren D, Yang X, Zhao Y. Hepatoprotective effects of phloretin against CCl4-induced liver injury in mice. Food Agric Immunol. 2017;28(2):211–222. doi: 10.1080/09540105.2016.1258546 [DOI] [Google Scholar]
- 40.Akhtar MS, Asjad HMM, Bashir S, et al. Evaluation of antioxidant and hepatoprotective effects of Khamira Gaozaban Ambri Jadwar Ood Saleeb Wala (KGA). Bangladesh J Pharmacol. 2013;8(44):e8. doi: 10.3329/bjp.v8i1.13183 [DOI] [Google Scholar]
- 41.Al-Dbass AM, Al- Daihan SK, Bhat RS. Agaricus blazei Murill as an efficient hepatoprotective and antioxidant agent against CCl4-induced liver injury in rats. Saudi J Biol Sci. 2012;19(3):303–309. doi: 10.1016/j.sjbs.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chao J, Liao J-W, Peng W-H, Lee M-S, Pao L-H, Cheng H-Y. Antioxidant, analgesic, anti-inflammatory, and hepatoprotective effects of the ethanol extract of Mahonia oiwakensis stem. Int J Mol Sci. 2013;14(2):2928–2945. doi: 10.3390/ijms14022928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alok S, Jain SK, Verma A, Kumar M, Mahor A, Sabharwal M. Herbal antioxidant in clinical practice: a review. Asian Pac J Trop Biomed. 2014;4(1):78–84. doi: 10.1016/S2221-1691(14)60213-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukazayire M-J, Allaeys V, Calderon PB, Stévigny C, Bigendako M-J, Duez P. Evaluation of the hepatotoxic and hepatoprotective effect of Rwandese herbal drugs on in vivo (guinea pigs barbiturate-induced sleeping time) and in vitro (rat precision-cut liver slices, PCLS) models. Exp Toxicol Pathol. 2010;62(3):289–299. doi: 10.1016/j.etp.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 45.Neal JL, Lowe NK, Corwin EJ. Serum lactate dehydrogenase profile as a retrospective indicator of uterine preparedness for labor: a prospective, observational study. BMC Pregnancy Childbirth. 2013;13:128. doi: 10.1186/1471-2393-13-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrera G, Pizzimenti S, Daga M, et al. Lipid peroxidation-derived aldehydes, 4-Hydroxynonenal and malondialdehyde in aging-related disorders. Antioxidants (Basel, Switzerland). 2018;7(8):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Upur H, Yusup A, Baudrimont I, et al. Inhibition of cell growth and cellular protein, DNA and RNA synthesis in human hepatoma (HepG2) cells by ethanol extract of abnormal savda munziq of traditional uighur medicine. Evid Based Complement Alternat Med. 2011;2011:9. doi: 10.1155/2011/196190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Chen SY, Hsu T, Santella RM. Immunohistochemical detection of malondialdehyde-DNA adducts in human oral mucosa cells. Carcinogenesis. 2002;23(1):207–211. [DOI] [PubMed] [Google Scholar]
- 49.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44(2):532–553. doi: 10.1159/000485089 [DOI] [PubMed] [Google Scholar]
- 50.Panda V, Ashar H, Srinath S. Antioxidant and hepatoprotective effect of Garcinia indica fruit rind in ethanol-induced hepatic damage in rodents. Interdiscip Toxicol. 2012;5(4):207–213. doi: 10.2478/v10102-012-0034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohanta YK, Panda SK, Jayabalan R, Sharma N, Bastia AK, Mohanta TK. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Front Mol Biosci. 2017;4:14. doi: 10.3389/fmolb.2017.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc. 2014;18(4):356–363. doi: 10.1016/j.jscs.2013.09.011 [DOI] [Google Scholar]
- 53.Tian Z, Liu H, Su X, et al. Role of elevated liver transaminase levels in the diagnosis of liver injury after blunt abdominal trauma. Exp Ther Med. 2012;4(2):255–260. doi: 10.3892/etm.2012.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boll M, Weber LW, Becker E, Stampfl A. Hepatocyte damage induced by carbon tetrachloride: inhibited lipoprotein secretion and changed lipoprotein composition. Z Naturforsch C. 2001;56(3–4):283–290. doi: 10.1515/znc-2001-3-419 [DOI] [PubMed] [Google Scholar]
- 55.Suja SR, Latha PG, Pushpangadan P, Rajasekharan S. Assessment of hepatoprotective and free radical scavenging effects of rhinacanthus nasuta (Linn.) Kurz in wistar rats. Journal of Natural Remedies. 2004;2004:7. doi: 10.1126/sageke.2004.5.pe5 [DOI] [Google Scholar]
- 56.Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol. 2015;63(4):1023–1037. doi: 10.1016/j.jhep.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Contreras-Zentella ML, Hernández-Muñoz R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxid Med Cell Longev. 2016;2016:3529149. doi: 10.1155/2016/3529149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abou Seif HS. Physiological changes due to hepatotoxicity and the protective role of some medicinal plants. Beni-Suef Univ J Basic Appl Sci. 2016;5(2):134–146. doi: 10.1016/j.bjbas.2016.03.004 [DOI] [Google Scholar]
- 59.Chatterjee S, Dey A, Dutta R, Dey S, Acharya K. Hepatoprotective effect of the ethanolic extract of Calocybe indica on mice with CCl4 hepatic intoxication. Int J PharmTech Res. 2011;3(4):2162–2168. [Google Scholar]
- 60.Yadav N, Dixit V. Hepatoprotective activity of leaves of Kalanchoe pinnata Pers. J Ethnopharmacol. 2003;86(2–3):197–202. [DOI] [PubMed] [Google Scholar]
- 61.Nagaich U, Gulati N, Chauhan S. Antioxidant and antibacterial potential of silver nanoparticles: biogenic synthesis utilizing apple extract. J Pharm. 2016;2016:7141523. doi: 10.1155/2016/7141523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keshari AK, Srivastava R, Singh P, Yadav VB, Nath G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J Ayurveda Integr Med. 2018. doi: 10.1016/j.jaim.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]