Abstract
Background
Surgical ventricular reshaping (SVR) is a treatment option for patients with severe ischaemic heart failure (HF). Recently, a new minimally invasive, hybrid technique named “less invasive ventricular enhancement” (LIVE), has been developed adopting the Reviven™ Myocardial Anchoring System (BioVentrix Inc., San Ramon, CA, USA).
Methods
Between January 2015 and November 2018, 7 patients (5 men and 2 women; mean age 72±8.9 years) underwent LIVE procedure at our institution.
Results
Procedural success was 100%. A total anchors number of 3.0±0.9 was used to reshape the left ventricle (LV). Preoperative and postoperative echocardiographic assessments showed an increase of LV ejection fraction (EF) from 22.8%±8.1% to 35%±7.2% (P=0.001) and a decrease of LV volumes in terms of LV end-systolic volume index (LVESVI), from 93.2±10.5 to 52.1±15.1 mL/m2 (P<0.001), and LV end-diastolic volume index LVEDVI, from 137.2±20.1 to 78±10.2 mL/m2 (P=0.001), respectively. In all patients functional mitral regurgitation (MR) prior to surgery decreased significantly after LIVE procedure. In 1 patient, the occurrence of right ventricle perforation required correction through a standard sternotomy. All patients survived the surgical procedure. The mean duration of intensive care unit stay was 7.8 days (range, 1–22 days), and the mean length of hospital stay was 22.1 days (range, 9–45 days). Mean follow-up (FU) time was 189.7±104.5 days. Average NYHA functional class at FU was 1.4±0.9 compared to 3.4±0.6 preoperatively (P=0.001). All patients were in satisfactory clinical condition and resumed their own daily activities. Echocardiographic monitorings at FU were stable and comparable to the above mentioned results at discharge.
Conclusions
In high-risk patients and selected cases, LIVE procedure may be advantageous both technically and clinically. Preliminary results of this novel hybrid treatment for symptomatic ischaemic cardiomyopathy are encouraging, in terms of significant improvement in LV EF, reduction in LV volumes and functional MR grade.
Keywords: Heart failure (HF), ischaemic cardiomyopathy, minimally invasive cardiac surgery, transcatheter left ventricular reshaping, hybrid operating theatre
Introduction
Currently, refractory heart failure (HF) is one of the major health care issues in the western world (1,2). Ischaemic cardiomyopathy is observed in the majority of end-stage HF patients. Left ventricular (LV) dilation and adverse ventricular remodeling occurs in 20% of infarcted patients, despite early interventional or surgical treatment (3,4), leading to functional mitral regurgitation (MR) and ventricular mechanical inefficiency (5).
Therapeutic interventions that reduce LV volume can significantly improve patients survival (6). Several pioneering surgical procedures established the concept of reverse ventricular remodeling by adopting various operative approaches aiming to ‘reshape’ and ‘restore’ the LV (7-10).
A novel, minimally invasive, hybrid technique, named “less invasive ventricular enhancement” (LIVE), has been developed using the Revivent™ Myocardial Anchoring System (BioVentrix Inc., San Ramon, CA, USA) to treat patients with aneurysms, or even akinetic scar, involving the apical-anterior-septal segments of LV (11-15). The Revivent™ system enables an off-pump, sternal sparing, LV volume reduction, thereby offering a safe and effective LV reconstruction to high-risk patients early in the treatment cycle prior to more severe onset of the disease.
Methods
Patient selection and preoperative assessment
All candidate patients suffered an ischaemic cardiomyopathy after anteroseptal myocardial infarction and consequent evolution into a severely dilated LV with an anteroseptal transmural scar.
The selection criteria of our studied patients mirror the major ones of the CONFIGURE-HF trial, a multicenter, prospective, single-armed study which has been designed to establish the efficacy and safety of the new LIVE system for LV volume reduction (11-15), thus being: (I) LV ejection fraction (EF) <0.35; (II) LV end-systolic volume index (LVESVI) >60 mL/m2 (preferably <120 mL/m2); (III) severe HF, in terms of New York Heart Association (NYHA) functional class III–IV, despite optimal medical therapy for at least 90 days; (IV) age 18 to 75 years; (V) no infarction within three months of operation; (VI) referral for ventricular reshaping operation. Major exclusion criteria, in accordance to the trial, were: (I) need for concomitant mitral valve surgery; (II) the presence of intracardiac thrombus; (III) contraindication for warfarin therapy. Additionally, we excluded a severe multiple valvular pathology, needing eventual associated valve repair or replacement, a severe preoperative right ventricle (RV) dysfunction. All patients in this study were contraindicated to standard surgery due to elevated risk (high EuroScore II) according to a pre-procedural multidisciplinary evaluation (Table 1).
Table 1. Revivent™ case series population and outcomes.
| Patient No. | ICMP | Age (y) | Gender | NYHA pre | EuroSCORE II (%) | RV-LV anchors | LV-LV anchors | ICU stay (d) | Ventilation time (h) | Hospital stay (d) | Intraoperative AE | Outcome | FU (d) | NYHA at FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | 77 | F | III | 43.05 | 1 | 1 | 5 | 4 | 12 | – | Discharged | 274 | I |
| 2 | Yes | 60 | M | IV | 32.24 | 3 | 1 | 2 | 17 | 11 | – | Discharged | 262 | II |
| 3 | Yes | 71 | M | III | 39.44 | 2 | 1 | 1 | 4 | 9 | – | Discharged | 210 | I |
| 4 | Yes | 77 | M | IV | 43.59 | 2 | 1 | 1 | 3 | 23 | – | Discharged | 185 | I |
| 5 | Yes | 72 | M | III | 40.12 | 2 | 1 | 22 | 35 | 41 | Sternotomy for bleeding | Discharged | 165 | II |
| 6 | Yes | 69 | M | III | 38.08 | 2 | 1 | 4 | 4 | 14 | – | Discharged | 135 | I |
| 7 | Yes | 78 | F | IV | 49.76 | 2 | 1 | 20 | 18 | 45 | – | Discharged | 104 | II |
AE, adverse event; F, female; d, days; FU, follow-up; h, hours; ICMP, ischaemic cardiomyopathy; ICU, intensive care unit; y, years; M, male; LV, left ventricle; NYHA, New York Heart Association functional class; RV, right ventricle.
Preoperative assessment required Gadolinium-enhanced magnetic resonance or contrast computed tomography imaging to properly evaluate the scar region extension and morphology, as being needed at least 50% of the wall thickness to enable an effective anchor insertion. Echocardiographic imaging was additionally used to define wall motion abnormalities and global contractility, identify areas of myocardium viability, assess valvular disease, measure LV volumes and calculate the sphericity index (SI) (15).
The study was approved by our institutional review board.
Revivent TC™ system and surgical procedure
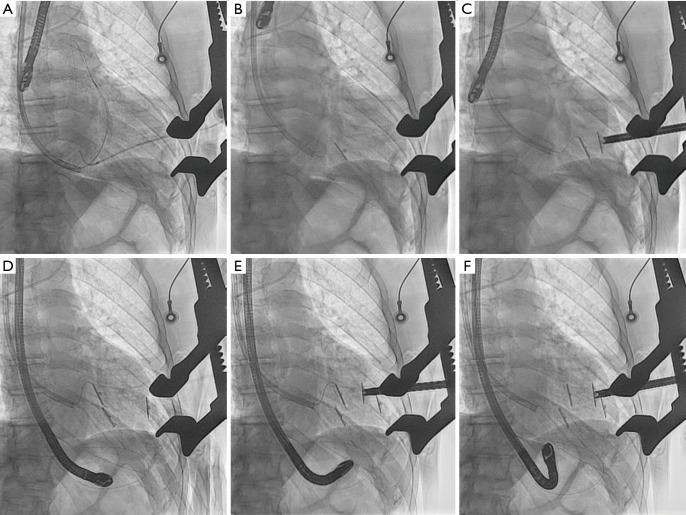
The Revivent TC™ system consists of four components: (I) a delivery system; (II) the implantable anchor elements; (III) a force gauge to control the amount of compression applied to the anchors when apposing the LV walls; and (IV) a retrieval system (11-15). The implantable anchors are composed of titanium and covered by polyester cloth; one is a hinged (“internal”) anchor that passes through a low-profile catheter to the right side of the septum, and the other, a locking (“external”) anchor deployed in the LV epicardial position (Figures 1,2).
Figure 1.
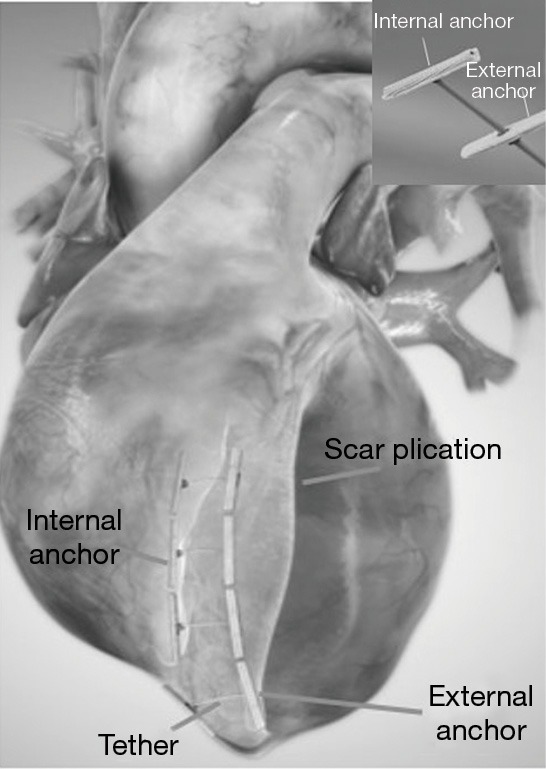
View of implantable anchors (internal and external), scar plication and tether of Revivent™ BioVentrix system (courtesy of BioVentrix Inc.).
Figure 2.
Step-by-step Revivent™ hybrid surgical procedure scheme. (A) Intra-operative fluoroscopy image showing straight needle and .032” guidewire in the right ventricle (antero-posterior projection); (B) the internal anchor (IA) tether is loaded into a .014” guidewire and the tether-guidewire combination is advanced through the 14-Fr introducer; (C) IA at right ventricle septum and external anchor (EA) on left ventricle epicardium (antero-posterior projection); (D,E) the procedure is repeated for another anchor pair; (F) two implanted anchor pairs.
Plication and exclusion of LV scar is achieved using paired, above mentioned, microanchors, fixed by a PEEK (Poly-Ether-Ether-Ketone) tether to provide a longitudinal line of apposition between the LV anterolateral wall and the anterior septum (Figures 1-4) (15). Thus, one anchor is implanted surgically and directly onto the scarred portion of the LV epicardium while the other is deployed through a percutaneously placed catheter onto, endovascularly, the right side of the interventricular septum (via internal jugular vein approach). The most apical part of the LV (beyond the right ventricle apex) is excluded by an epicardial-only anchor pair. An external-only approach can also be offered to patients with large apical-anterior-inferior scars but lacking septal involvement, and in case of difficult or not feasible endovascular approach.
Figure 3.
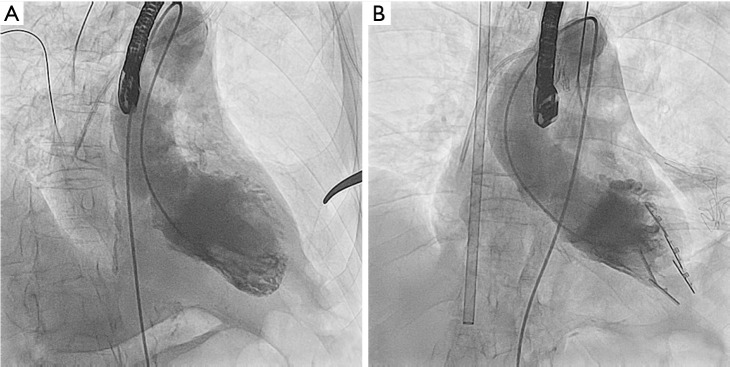
Intra-operative angiographic view before (Panel A) and after (Panel B) Revivent™ anchors placement.
Figure 4.
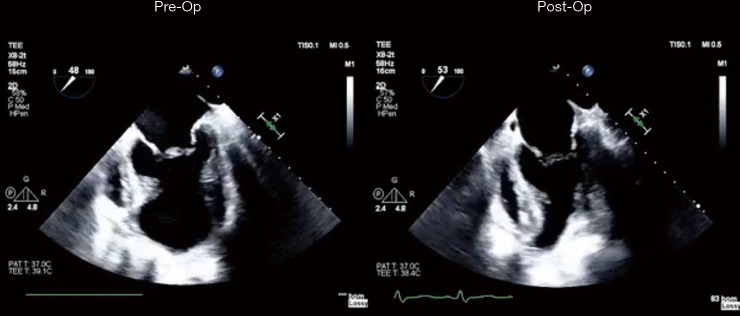
Pre-operative and post-operative echocardiographic assessment of LIVE procedure. LIVE, less invasive ventricular enhancement.
The surgical approach relies on a 5-cm left-sided minithoracotomy after properly assessing the position of LV apex. For safety, arterial and venous sheaths are placed into both femoral artery and vein, respectively, thus being ready for a quick cannulas insertion in the case of need of extracorporeal circulation installation.
Our preferred setting for LIVE procedure is the hybrid operating room (OR) (11-15) which provides advanced imaging capabilities in terms of fluoroscopy and echocardiography guidance.
After surgery, the patients are treated with warfarin for 3 months, in terms of anticoagulation management, followed by cardioaspirin mantainance only, thereafter.
Statistical analysis
All statistics were undertaken with SPSS statistics version 24 (IBM Corp., Armonk, NY, USA). Categorical variables are described as frequencies and percentages and compared using the χ2 test. Continuous data are expressed as mean ± standard deviation and compared using the Wilcoxon signed-rank test. A P value <0.05 was considered statistically significant.
Results
Between January 2015 and November 2018, 7 patients (5 men and 2 women; mean age 72±8.9 years), with a body surface area (BSA) of 1.9±0.8, underwent LIVE procedure at our institution (Table 1).
Procedural success was fully accomplished. In all patients, a reshaping with total exclusion of the aneurysmatic LV was achieved. A total anchors number of 3.0±0.9 was used (Table 1). Mean skin-to-skin surgical time was 195±115 minutes with a fluoroscopy time of 60:60±45:35 and a dosage (mGy/cm2) of 2,685±2,455 (approximate effective radiation dose of 10 mSv).
Preoperative and postoperative echocardiographic assessments (Figure 4) showed an increase of LV EF from 22.8%±8.1% to 35%±7.2% (P=0.001) and a decrease of LV volumes in terms of LV end-systolic volume index (LVESVI), from 93.2±10.5 to 52.1±15.1 mL/m2 (P<0.001), and LV end-diastolic volume index LVEDVI, from 137.2±20.1 to 78±10.2 mL/m2 (P=0.001), respectively (Table 2). The sphericity index (SI) slightly changed from 0.5±0.1 to to 0.4±0.1 (P=0.621). In all patients functional MR prior to surgery decreased significantly after LIVE procedure.
Table 2. Preoperative and postoperative echocardiographic assessment of Revivent™ patients population.
| Patient No. | LVEF pre (%) | LVEDVI pre (mL/m2) | LVESVI pre (mL/m2) | MR pre | TR pre | SI | RVSP pre (mmHg) | LVEF post (%) | LVEDVI post (mL/m2) | LVESVI post (mL/m2) | MR post | TR post | SI | RVSP post (mmHg) | FU days |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | 152 | 121 | Mild | Mild | 0.5 | 25 | 44 | 93 | 71 | Mild | Mild | 0.4 | 20 | 274 |
| 2 | 20 | 162 | 125 | Moderate | Mild | 0.4 | 70 | 33 | 80 | 53 | Mild | Mild | 0.3 | 45 | 236 |
| 3 | 18 | 114 | 77 | Moderate | Moderate | 0.5 | 60 | 28 | 83 | 55 | Mild | Mild | 0.5 | 40 | 192 |
| 4 | 18 | 150 | 114 | Moderate | None | 0.5 | 20 | 26 | 97 | 63 | Mild | None | 0.4 | 20 | 161 |
| 5 | 28 | 120 | 64 | Severe | Moderate | 0.6 | 80 | 34 | 62 | 35 | Mild | Moderate | 0.5 | 60 | 143 |
| 6 | 25 | 138 | 85 | Severe | Mild | 0.6 | 70 | 40 | 63 | 51 | Moderate | Mild | 0.5 | 60 | 125 |
| 7 | 25 | 125 | 67 | Moderate | Mild | 0.4 | 65 | 40 | 68 | 37 | Mild | Mild | 0.4 | 55 | 94 |
FU, follow-up; LVEF, left ventricular ejection fraction; LA, left atrium; LVEDVI, Left Ventricular End Diastolic Volume Index; LVESVI, Left Ventricular End Systolic Volume Index; MR, mitral regurgitation; RVSP, Right ventricular systolic pressure; SI, sphericity index; TR, tricuspid regurgitation.
In a single patient, the occurrence of RV perforation required correction through a standard sternotomy (Table 2). All patients survived the surgical procedure. The mean duration of intensive care unit stay was 7.8 days (range, 1–22 days), and the mean length of hospital stay was 22.1 days (range, 9–45 days), with no negative sequelae. At discharge, no leakage between the excluded scar and the LV chamber was noticed (Figures 3,4).
Mean follow-up (FU) time was 189.7±104.5 days. Average NYHA functional class at FU was 1.4±0.9 compared to 3.4±0.6 preoperatively (P=0.001). All patients were in satisfactory clinical condition and resumed their own daily activities Echocardiographic monitorings at FU were stable and comparable to the above mentioned results at discharge.
Discussion
Recently, results from the Surgical Treatment for Ischaemic Heart Failure (STICH) randomized trial showed that patients with a postoperative LVESVI of 70 mL/m2 or less after coronary bypass grafting (CABG) plus SVR resulted to have better outcomes if compared with CABG alone (9,10).
The gold standard treatment for LV adverse remodeling due to ischaemic cardiomyopathy remains the conventional surgical repair (7-10). The Revivent TC™ procedure may be considered an alternative solution for patients desiring early intervention or with a high surgical risk profile and major associated comorbidities, as it does not require LV incision or extracorporeal circulation and is a potentially more rapid procedure. Furthermore, it has potential advantages regarding standard linear resection, as the septum is included in the repair (11-15).
According to the data documented in the LIVE CE (Communauté européenne) marking study (11-15), there is an improvement of LV efficiency with significant reduction of LV volume, increase of LV EF and, in some cases, reduction/resolution of secondary MR owing to re-alignment of the papillary muscles, at mid- and long-term follow-up (6, 12 and 24 months) (14,15). Additionally, a clinical improvement in exercise tolerance and a significant reduction of NYHA functional class is documented (11-15).
Our early results in a limited cohort of patients confirmed that the procedure is effective and results in a significant improvement of LV EF and reduction in LV volumes. We found a significant reduction of LVESVI (Figure 4), and moreover, in all patients, a post-LIVE LVESVI of <60 mL/m2 was achieved thus following the effective goals of SVR as reported by Di Donato et al. (16) and the STICH trial, as well (9,10,15).
However after a surgical reduction in LV volume, which is generally considered beneficial, a significant reduction in the diastolic function (less distensibility) occurs (15,16). This potential negative impact on the diastolic function has to be evaluated in further studies.
Concerning the LV reconstruction, we found no significant change in the SI, similarly to the results reported by the RESTORE group (16,17). This proves that LIVE procedure, as reported by Klein et al. (15), may potentially play a role in reducing both LV long- and short-axis with resulting similar preoperative SI.
Furthermore, important functional MR could be reduced by LIVE procedure since the improvement of both LV systolic function and reduction of LV volumes, as showed significantly in our studied cohort of patients.
Despite all above mentioned promising effects and results, the weak points of LIVE procedure remain the need of a sufficient transmural anteroseptal scar extension for a safe and effective transseptal anchor placement and plication, and the avoidance of a pre-operative small RV, which may lead to a RV restrictive pattern (15).
Additionally, it is important to emphasize the potentially related intraoperative LIVE major adverse events (11-15). Sustained ventricular arrhythmias are often caused by instrumentation of healthy myocardium during anchors implantation thus a primed cardiopulmonary bypass (CPB) machine or extracorporeal membrane oxygenation (ECMO) system ready to be rapidly employed is recommended. Other reported complications are RV perforation, stroke, ventricular septal defect, and tricuspid valve chordae or leaflets damage (15). All above issues may be related to the significant learning curve needed by clinicians and surgeons when initially approaching the procedure.
This is why the Revivent™ procedure requires a well trained and dedicated multidisciplinary ‘heart team’, which includes an interventional cardiologist, a cardiac surgeon, and an echo-cardiographer, experienced in structural interventions (11-15).
Thereafter, we do still have to wait for the results of the ongoing CONFIGURE-HF and the upcoming REVIVE-HF trials (14,15) with longer FU data are necessary to confirm both the durable safety of this novel procedure and the long-term LV effective remodeling, aiming the complete exclusion of the septal scar and dyskinetic apex as only partially demonstrated by the cardiac magnetic resonance studies of small series currently reported (11-15).
Limitations
This is a retrospective observational study. Although our cohort consists of seven patients, it is one of the largest single centre clinical case series with a significant FU time to report on such a LIVE procedure, so far. Further investigations are still required to conclusively confirm the mid- to long-term benefits of this novel technique. However, early efficacy of LIVE procedure was demonstrated in a high-risk series of patients with clear contraindication to traditional surgery correction (Table 1).
Conclusions
LIVE procedure is an encouraging novel treatment option for patients with severe ischaemic cardiomyopathy. Preliminary results demonstrate significant improvement in LV EF, reduction in LV volumes and functional MR grade in the early postoperative period.
Acknowledgements
None.
Ethical Statement: The study was approved by our institutional review board.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA 2002;287:628-40. 10.1001/jama.287.5.628 [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med 2003;348:2007-18. 10.1056/NEJMra021498 [DOI] [PubMed] [Google Scholar]
- 3.Solomon SD, Glynn RJ, Greaves S, et al. Recovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early afterload reducing therapy study. Ann Intern Med 2001;134:451-8. 10.7326/0003-4819-134-6-200103200-00009 [DOI] [PubMed] [Google Scholar]
- 4.Solomon SD, Skali H, Anavekar NS, et al. Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation 2005;111:3411-9. 10.1161/CIRCULATIONAHA.104.508093 [DOI] [PubMed] [Google Scholar]
- 5.Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48. 10.1016/j.jacc.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 6.Kramer DG, Trikalinos TA, Kent DM, et al. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol 2010;56:392-406. 10.1016/j.jacc.2010.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooley DA, Collins HA, Morris GC, Jr, et al. Ventricular aneurysm after myocardial infarction: surgical excision with use of temporary cardiopulmonary bypass. J Am Med Assoc 1958;167:557-60. 10.1001/jama.1958.02990220027008 [DOI] [PubMed] [Google Scholar]
- 8.Athanasuleas CL, Buckberg GD, Stanley AW, et al. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation J Am Coll Cardiol 2004;44:1439-45. 10.1016/j.jacc.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 9.Dor V, Civaia F, Alexandrescu C, et al. Favorable effects of left ventricular reconstruction in patients excluded from the Surgical Treatments for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg 2011;141:905-16. 10.1016/j.jtcvs.2010.10.026 [DOI] [PubMed] [Google Scholar]
- 10.Michler RE, Rouleau JL, Al-Khalidi HR, et al. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg 2013;146:1139-1145.e6. 10.1016/j.jtcvs.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wechsler AS, Sadowski J, Kapelak B, et al. Durability of epicardial ventricular restoration without ventriculotomy. Eur J Cardiothorac Surg 2013;44:e189-92. 10.1093/ejcts/ezt292 [DOI] [PubMed] [Google Scholar]
- 12.Faria R, Melica B, Pires-Morais G, et al. New less invasive ventricular reconstruction technique in the treatment of ischemic heart failure. Rev Port Cardiol 2014;33:469.e1-469.e5. 10.1016/j.repc.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 13.Schmitz C, Biffi M, Sievert H, et al. Clinical benefits of less-invasive, device–based Left Ventricular Reconstruction: A hybrid option for patients with ischemic cardiomyopathy. J Am Coll Cardiol 2018;68:B330 10.1016/j.jacc.2016.09.906 [DOI] [Google Scholar]
- 14.Clinical data published on: http://bioventrix.com/index.php/en/main. Online access, November 10th, 2018.
- 15.Klein P, Agostoni P, van Boven WJ, et al. Transcatheter and minimally invasive surgical left ventricular reconstruction for the treatment of ischaemic cardiomyopathy: preliminary results. Interact Cardiovasc Thorac Surg 2019;28:441-6. 10.1093/icvts/ivy259 [DOI] [PubMed] [Google Scholar]
- 16.Di Donato M, Castelvecchio S, Menicanti L. End-systolic volume following surgical ventricular reconstruction impacts survival in patients with ischaemic dilated cardiomyopathy. Eur J Heart Fail 2010;12:375-81. 10.1093/eurjhf/hfq020 [DOI] [PubMed] [Google Scholar]
- 17.Di Donato M, Dabic P, Castelvecchio S, et al. Left ventricular geometry in normal and post-anterior myocardial infarction patients: sphericity index and ‘new’ conicity index comparisons. Eur J Cardiothorac Surg 2006;29:S225-30. 10.1016/j.ejcts.2006.03.002 [DOI] [PubMed] [Google Scholar]