Abstract
Background
Left ventricular assist devices (LVAD) are increasingly used in patients with end stage heart failure. The HeartAssist 5 and aVAD LVADs offer telemetric monitoring capabilities. Here we report our initial single centre experience with the largest telemonitoring cohort of LVAD patients.
Methods
Eleven patients (9 males) received a telemonitoring-capable LVAD and were included in our telemonitoring cohort. Waveforms and alarm data were obtained from the telemonitoring system and hospital records were reviewed for clinical data.
Results
Mean age at LVAD implantation was 59±5.1 years (mean ± standard deviation). Seven patients had non-ischemic cardiomyopathy and 4 patients had ischemic cardiomyopathy. Median LVEF at implant was 16% (IQR, 15–20%). The total follow-up time was 2,438 patient-days. A total of 6,216 alarm messages were generated in 11 patients. Most common were low flow alarms due to hypovolemia, followed by low flow alarms because of suspected pump thrombosis. One patient died during follow-up, one received a cardiac transplant and one had the LVAD explanted because of pump thrombosis. Pump thrombosis was suspected in 5 patients with 8 episodes of sudden flow decreases and laboratory signs of haemolysis.
Conclusions
Real-time telemonitoring of LVAD pump flow, motor speed and power consumption is a promising tool in the follow-up of LVAD recipients. Trending pump flow over hours or days can assist in the early detection of complications, especially flow reductions due to hypovolemia and LVAD thrombosis. Further studies are warranted to delineate the impact of remote monitoring on patients’ prognosis.
Keywords: Left ventricular assist device (LVAD), heart failure, telemonitoring, hypovolemia, device thrombosis
Introduction
Left ventricular assist devices (LVADs) are increasingly used as a bridge to heart transplantation or destination therapy in end-stage heart failure patients (1-5). Post-LVAD implantation patient management is complex. Anticoagulation, fluid management and pump settings have to be carefully balanced to reduce risks of bleeding, pump thrombosis, hypovolemia and LVAD failure. Timely detection of problems during follow-up allows for early intervention and has the potential to avoid serious complications. Telemetric transmission of device and patient data was introduced over a decade ago for remote monitoring of patients with implanted electrical cardiac devices such as pacemakers, implantable cardioverter-defibrillators (ICDs) and more recently pressure sensors (6). It has been shown that such telemetric monitoring facilitates earlier detection of device failure than achieved by routine in-office follow ups (7-9). Additionally, telemetric monitoring of patients with ICDs significantly reduced the composite clinical endpoint of all-cause death, hospital admissions and deterioration of New York Heart Association (NYHA) functional class and patient global self-assessment (10).
The HeartAssist 5 LVAD and aVAD (ReliantHeart Inc., Houston, TX, USA) offer telemetric transmission of pump performance data via cellular networks and the possibility for clinicians to view the data via a secure online platform (11). The devices have been approved in Europe and are investigational in the US. Here we report our initial experience with telemonitoring in patients using the HeartAssist 5 and aVAD continuous-flow LVADs and its potential value for LVAD patient management.
Methods
Patients
All patients implanted with telemedicine capable LVADs at our institution between June 2015 and December 2016 were included in the study. Informed consent requirements for this observational study were waived by the institutional committee on human research.
Remote monitoring
The HeartAssist 5 and aVAD are both axial-flow pump devices featuring an ultrasonic flow probe on the outflow graft. The external components of these systems, which connect to the pump and probe via a percutaneous driveline, are comprised of two batteries and a controller unit which incorporates pump control, power transmission, flow signal conditioner and telemetry circuitry. Pump performance indexes measured by the controller are transmitted via a standard cellular network to a central secured server. The telemetered signals include outflow graft blood flow, pump speed and power consumption.
The remote monitoring platform (VADLink) consists of a central database and a secure website accessible to authorized medical personnel. Physicians and specialized nurses involved in LVAD patient care can log onto this website and review their patients’ transmissions. Fifteen-minute running average values for flow (L/min), pump speed (krpm) and motor power (watts) are captured and can be reviewed for any given point in time. A ten second high resolution real-time waveform snapshot can be requested for review anytime. Furthermore, an alarm is triggered by the pump controller whenever predefined thresholds (lower limits for flow and pump speed, upper limit for power consumption) are crossed. Once an alarm is triggered, a 10-second high resolution snapshot of the flow waveform is saved to the server for review on the remote monitoring platform. The system can also be configured to automatically send e-mail or text message alerts to the care team whenever an alarm is received.
Follow-up
Besides routine in-office follow-up visits, patients were monitored remotely using the secure internet access portal described above. Remote monitoring was initiated at the day of hospital discharge. Specialized nurses access the web page and evaluate LVAD parameters and alarms. Relevant findings were reviewed by the attending physician. If an intervention was deemed necessary, patients were contacted by phone.
Results
Patients
Between June 2015 and December 2016, 11 patients (9 male) received a telemonitoring-capable LVAD and were included in the present cohort. Mean age at LVAD implantation was 59±5.1 years (mean ± standard deviation). Seven patients had non-ischemic cardiomyopathy and 4 patients had ischemic cardiomyopathy. Median LVEF at implant was 16% (IQR, 15–20%). The total follow-up time was 2,438 patient-days. Patient characteristics are summarized in Table 1.
Table 1. Patient demographics.
| Patient | Gender | Age (years) | INTERMACS | Type of device | LVEF (%) | Cardiomyopathy | Follow-up (days) | Status |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 60 | III | HA5 | 20 | Non-ischemic | 429 | Alive, cardiac transplant |
| 2 | Male | 55 | III | HA5 | 11 | Ischemic | 393 | Deceased (intracranial bleeding) |
| 3 | Male | 58 | III | HA5 | 15 | Ischemic | 458 | Alive on LVAD |
| 4 | Male | 70 | III | HA5 | 20 | Non-ischemic | 325 | Alive on LVAD |
| 5 | Male | 62 | II | HA5 | 20 | Non-ischemic | 217 | LVAD exchange (pump thrombosis) |
| 6 | Male | 61 | III | HA5 | 15 | Ischemic | 233 | Alive on LVAD |
| 7 | Female | 61 | III | aVAD | 30 | Non-ischemic | 121 | Alive on LVAD |
| 8 | Male | 54 | III | aVAD | 15 | Non-ischemic | 108 | Alive on LVAD |
| 9 | Male | 49 | III | aVAD | 25 | Non-ischemic | 73 | Alive on LVAD |
| 10 | Male | 62 | III | aVAD | 15 | Ischemic | 52 | Alive on LVAD |
| 11 | Male | 57 | III | aVAD | 16 | Non-ischemic | 29 | Alive on LVAD |
Age, INTERMACS and LVEF are given at time of LVAD implantation. INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support Score; HA5, Heart Assist 5; LVEF, left ventricular ejection fraction.
One patient died of intracranial bleeding 13 months after LVAD implantation. Another patient received a cardiac transplant after 14 months. In one patient, the HeartAssist 5 LVAD was exchanged for a HeartWare system (HeartWare Inc., Framingham, MA, USA) because of pump thrombosis.
LVAD alarms
A total of 6,216 alarm messages were generated in 11 patients. The median number of alarms per patient-day was 0.558 (IQR, 0.307–2.60), with the minimum of 0.0686 alarm messages per day in patient 6 and the maximum of 77.59 alarm messages per day in patient 11 (Table 2).
Table 2. Number and causes of alarm messages.
| Patient | Type of alarm message | Alarms per day | ||||
|---|---|---|---|---|---|---|
| Reduced flow | Reduced motor speed | Excess current | Pump stopped | Total | ||
| 1 | 1,759 (92.14%) | 2 (0.10%) | 140 (7.33%) | 8* (0.42%) | 1,909 | 4.44 |
| 2 | 1,482 (96.23%) | 4 (0.26%) | 53 (3.44%) | 1 (0.06%) | 1,540 | 3.91 |
| 3 | 27 (44.26%) | 2 (3.28%) | 31 (50.82%) | 1 (1.64%) | 61 | 0.133 |
| 4 | 13 (8.23%) | 14 (8.86%) | 129 (81.65%) | 2 (1.27%) | 158 | 0.486 |
| 5 | 0 | 9 (47.37%) | 9 (47.37%) | 1 (5.26%) | 19 | 0.087 |
| 6 | 15 (93.75%) | 1 (6.25%) | 0 | 0 | 16 | 0.0686 |
| 7 | 59 (67.05%) | 2 (2.27%) | 27 (30.68%) | 0 | 88 | 0.727 |
| 8 | 52 (100%) | 0 | 0 | 0 | 52 | 0.481 |
| 9 | 75 (79.79%) | 19 (20.21%) | 0 | 0 | 94 | 1.28 |
| 10 | 28 (96.55%) | 0 | 1 (3.45%) | 0 | 29 | 0.557 |
| 11 | 2,244 (99.73%) | 6 (0.27%) | 0 | 0 | 2,250 | 77.59 |
| Total | 5,754 (92.57%) | 59 (0.95%) | 390 (6.27%) | 13 (0.21%) | 6,216 | |
*, all 8 of these pump stops were controller-related not pump-related.
Because every single threshold crossing triggers an alarm message, every episode of reduced flow typically generated between 3 and 200 individual alarm messages. In one patient (patient 11), 2,227 alarm messages for reduced flow were sent within 3 days during a single episode of reduced flow due to volume depletion. Remote transmission of system information was successful 74% of the time, reaching 100% in 2 patients. Reasons for loss of transmissions could not be ascertained with certainty but were most likely due to lack of cellular network coverage.
Interpretation of representative wave forms
Myocardial contractility and aortic valve opening
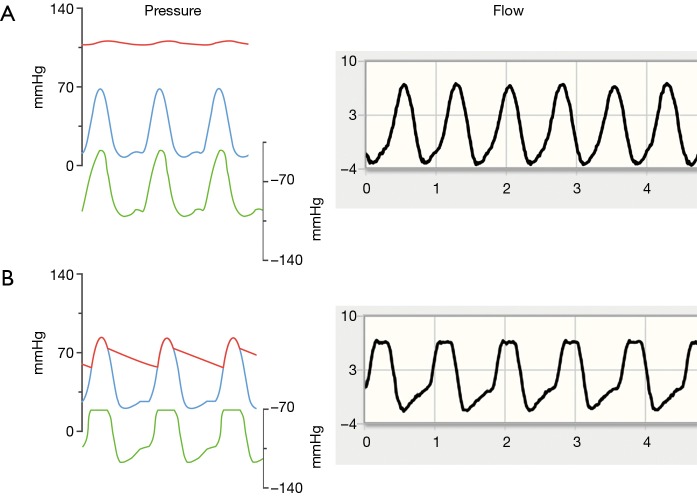
Blood flow through continuous-flow axial blood pumps such as the HeartAssist 5 and aVAD is inversely related to the pressure gradient between the pump inlet and outlet (12). The details of this relationship also vary with rotational speed (RPM); more flow is generated for a given pressure gradient at higher RPMs. At a given RPM, blood flow waveforms therefore reflect the instantaneous pressure gradient between the left ventricle and the ascending aorta. In a full support scenario, i.e., complete absence of native cardiac output, the aortic valve does not open. While the LV pressure waveform resembles a sinusoidal curve in this situation, aortic blood pressure shows negligible pulsatility, indicated by the red and blue waveforms in Figure 1A (13). The instantaneous pressure difference between LV and aorta (green waveform in Figure 1A) thus exhibits a peaked maximum without any plateau phase, as does pump flow.
Figure 1.
Representative wave forms of myocardial contractility and aortic valve opening. Left: pressure waveforms for the ascending aorta (red), left ventricle (blue) and difference left ventricle—aorta (green; negative values, scale offset). Right: representative flow waveforms from the telemonitoring system. (A) Aortic valve not opening; (B) aortic valve opening. Pressure curves (on the left) modified from Burkhoff et al. (13) with permission.
In a partial support scenario, where the native heart contributes to total cardiac output and the aortic valve opens on every beat, the pressure difference between inflow (LV) and outflow (aorta) is minimized during the entire ejection period (Figure 1B). This results in a characteristic plateau in the pressure head faced by the pump with an associated plateau in the flow curve. It is therefore possible to detect aortic valve opening remotely from the transmitted flow waveform. Figure 1B shows representative flow waveforms.
Fluid status and suction events
Alarms due to reduced pump flow were by far (92.57%) the most common type of alarm encountered and these resolved spontaneously in most cases. The differential diagnosis of acutely reduced pump flow includes hypovolemia, pump thrombosis and inflow or outflow obstruction. In our cohort, there were 9 episodes of persistent low flow refractory to increased fluid intake by the patient. In 8 of these episodes, thrombus was suspected based on laboratory evidence of haemolysis (elevated LDH and free haemoglobin) and the patients were treated with intravenous argatroban.
Hypovolemia was suspected in patients with a gradual decrease in flow without signs of haemolysis. During follow-up, 21 clusters of clinically significant low-flow alarms occurred that were attributed to hypovolemia. The waveform is generally characterized by lower pulsatility mainly due to an increase of minimum flow and little change in peak flow (Figure 2A). In addition, the rising and falling phases appear faster than normal, resulting in a peaked waveform.
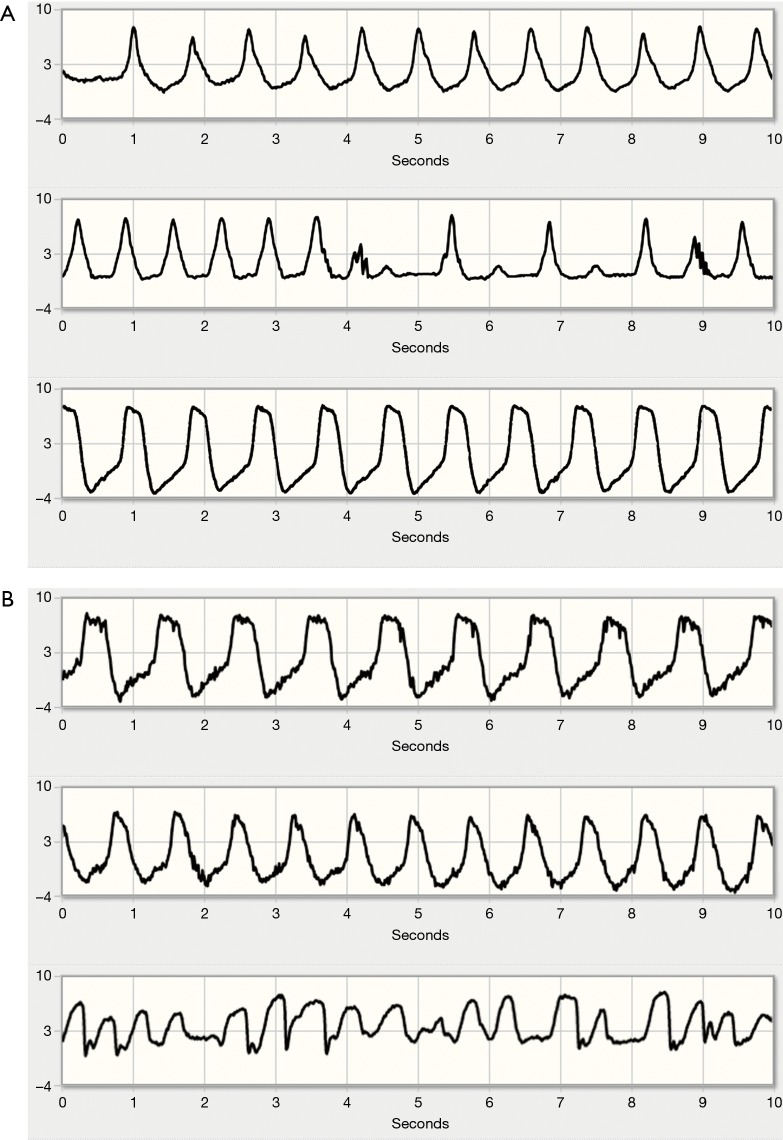
Figure 2.
Representative waveforms of hypovolemia. (A) Hypovolemic episode in the telemonitoring system. Top: typical hypovolemic flow waveform. Note the fast undulations in flow; Centre: additional mild suction artefacts at 4 and 9 seconds and significant beat-to-beat variation in flow, suggesting severe hypovolemia; Bottom: normalization of the flow waveform after sufficient fluid intake. (B) Hypovolemia with suction events, as represented in the telemonitoring system. Top: baseline flow waveform; Centre: hypovolemic flow waveform; Bottom: suction event with sudden, steep decreases in flow.
Further preload reduction results in progressive ventricular unloading, partial or complete ventricular collapse and suction of the ventricular wall over the inflow cannula. Suction events presented as sudden, rapid decreases in flow during a beat (Figure 2B). In such cases, patients were instructed to increase fluid intake and reduce diuretic doses, if applicable, obviating the need for hospital admission in most cases.
Arrhythmias, atrial fibrillation
Although the VADlink telemonitoring system offers no specific arrhythmia detection capabilities, it is possible to infer certain changes in heart rhythm from the flow waveform. Since atrial fibrillation or non-sustained VT does not cause significant reductions in pump flow and because waveforms are only transmitted in case of an alarm, most arrhythmias were incidental findings in transmissions for other reasons. Comprehensive rhythm surveillance was thus not possible. The most common arrhythmias detected were atrial fibrillation and premature contractions. Non-sustained VT was suspected in 2 flow waveforms transmitted. Examples of waveforms obtained during such arrhythmias are shown in Figure 3.
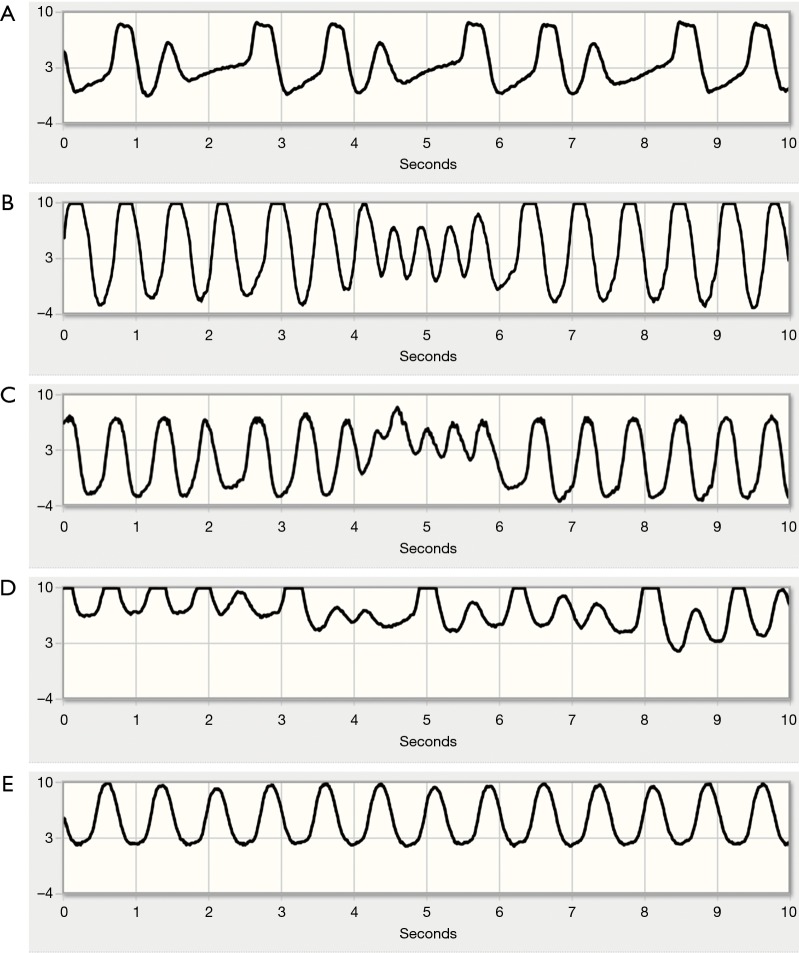
Figure 3.
Representative waveforms of arrhythmias in our cohort. (A) Premature contractions (ventricular or atrial). Note that the aortic valve does not open in these short coupled premature contractions. (B,C) Non sustained tachycardia. Both waveforms are from the same patient. Tachycardia cycle length is approximately 390 ms with haemodynamic relevance (note reduced pulsatility). Although ventricular tachycardia is probable, supraventricular tachycardia cannot be ruled out. (D) Atrial fibrillation. Note the irregularly irregular timing of waveform peaks and the beat-to-beat variation in pulsatility, resulting from underlying beat-to-beat changes in ventricular pressure generation characteristic of atrial fibrillation. (E) Same patient one hour later after spontaneous conversion to normal sinus rhythm.
Pump thrombosis or obstruction
Pump thrombosis was suspected in 5 patients with 8 episodes of sudden flow decreases and laboratory signs of haemolysis. All patients were out of the hospital at the time of the alarm and were asked to present to the LVAD clinic for urgent evaluation and subsequent hospital admission. Echocardiography was performed in all cases but demonstrated thrombus at the inflow cannula in only one. Computed tomography (CT) was performed in 2 cases but was unable to detect thrombotic material in both, probably reflecting the overall low sensitivity of imaging in the detection of LVAD thrombosis.
In 7 of the episodes (in 4 patients) flow was restored after administration of intravenous argatroban. In one patient the LVAD was exchanged for a HeartWare device.
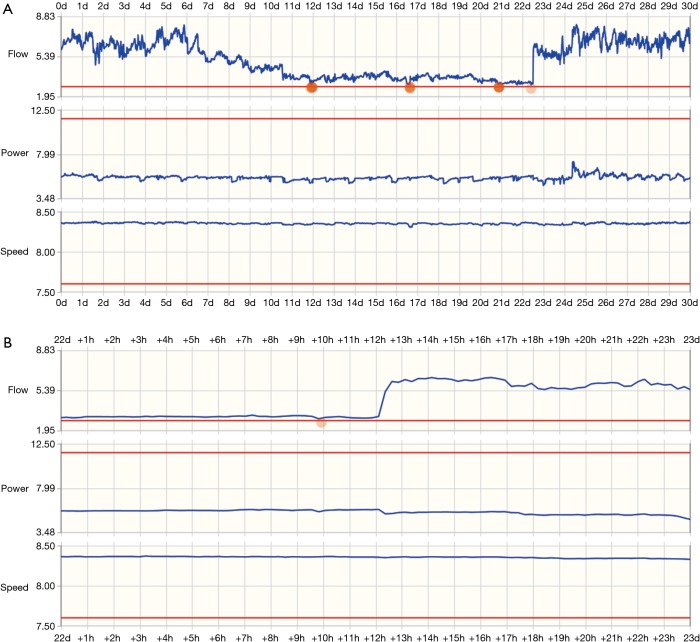
Pump flow trends can be reviewed over longer time periods to address potential issues. Figure 4 shows a telemonitored waveform for suspected LVAD thrombosis: the top panel shows a 30 day waveform summary, the bottom panel shows 24 hours of data. There is a slight increase in power consumption at the same time as a sudden, significant drop in flow, suggesting the wash-in of a pre-formed thrombus into the pump. The obstruction was resolved following argatroban administration.
Figure 4.
Trends can be reviewed over longer time periods to detect and correct potential issues. Long and short-term trends of flow, power consumption and rotor speed during an episode of suspected pump thrombosis, as represented in the telemonitoring system. (A) 30 days; (B) 24 hours. Note that the time scale goes back from a set date, i.e., oldest data points are to the right, and time-course is from right to left. There is a sudden decrease in flow from approx. 6 to 3.2 L/min near the 22 days mark, with only a slight increase in power consumption not easily recognized in the curve.
Discussion
This report summarizes our experience in monitoring patients implanted with LVADs capable of transmitting blood flow and power waveforms via a custom telemonitoring system. The experience validates the feasibility and clinical benefits of long term remote monitoring in LVAD recipients. Specifically, we demonstrated timely recognition of abnormalities (LVAD thrombosis and hypovolemia) which lead to early intervention and obviated the need for LVAD exchanges and hospital admissions. We also described how waveform analysis can inform the clinical care team about arrhythmias, volume depletion, suction events and aortic valve opening which can guide in patient management.
Telemonitoring is well-established and recommended in the care of patients with cardiac implantable devices such as ICDs and pacemakers (6,14). In such settings, remote monitoring has been shown to be effective in the early detection of device malfunctions (7,8). ICD-based heart failure telemonitoring systems use electrical surrogate parameters such as thoracic impedance, amount of pacing and arrhythmia burden to detect impending cardiac decompensation (15,16). Recently, implanted pressure sensors have been shown to detect clinical status changes that lead to exacerbations and urgent hospitalizations in heart failure patients (17). Pulmonary artery pressure telemonitoring has been shown to be associated with a 30% reduction in heart failure hospitalization rates (17). Telemonitoring of directly measured LVAD flow can potentially have a similar impact on LVAD patient management (9).
Aortic valve (AoV) opening is of major interest in patients on LVAD support and is recommended in the guidelines for LVAD rotational speed adjustment (18). It has been shown that LVAD patients without AoV opening on echocardiography are at higher risk of thromboembolic events and pump thrombosis (19). It is, however, unclear whether deliberate pump speed adjustment to ensure AoV opening is beneficial. The automated, real time assessment of AoV dynamics on LVAD support is under investigation (20,21). All of these studies used pump speed or motor current to estimate flow, thus requiring additional assumptions and conversions. The correlation between motor current and flow differs highly in accuracy between pump models and also depends on patient variables such as blood viscosity (12). Both HeartAssist 5 and aVAD overcome these uncertainties by measuring flow directly.
In addition to AoV dynamics, patient fluid status can be inferred from flow data. When venous return is reduced while LVAD flow is largely constant, ventricular collapse and suction of the ventricular wall can occur. Suction conditions have been studied and modelled extensively in animal models and in silico (22,23). The continuum of waveform characteristics in our cohort corroborates the modelled flow patterns as volume status declines from normal to reduced pulsatility with initial reductions in fluid status, followed by abbreviated peaked flow waveforms with moderate fluid reduction, to outright episodes of suction with downward going flow pulses (23). These characteristic waveform appearances generate suspicion of declining volume status which can be addressed by instructing patients to increase their fluid intake or decreasing diuretic doses.
Ventricular and supraventricular arrhythmias are common in end-stage heart failure. Although data for LVAD patients is limited, approximately 50% of LVAD recipients had a history of atrial fibrillation (AF) in one series (24). Heart failure patients with a history of AF have a worse prognosis and benefitted most from telemonitoring in one study, but the prognostic relevance of AF in LVAD patients seems to be less important (10,25). In accordance with this observation, the occurrence of AF itself did not cause flow reductions in our cohort. However, arrhythmias are still part of the differential diagnosis in case of persistent low flow. Therefore, analysis of the underlying rhythm from the flow waveform should always be attempted. Automatic continuous rhythm analysis based on the flow waveform has yielded promising results in a proof-of-concept study and warrants further investigation (26).
In our series, there were 8 occurrences of suspected pump thrombosis in 5 patients detected by telemetric monitoring. In one instance, device exchange was necessary. The remaining 7 episodes were successfully managed by increased anticoagulation. The value of echocardiography in LVAD thrombus detection is limited by ultrasound artefacts and reflection from the device itself. The sensitivity of contrast CT in the prior detection of surgically confirmed thrombus has recently been shown to be as low as 13% (27). As outlined in the published algorithm for the management of suspected pump thrombosis, early detection of possible thrombosis and optimized anticoagulation are paramount (28,29). In our cohort, pump flow reduction was more pronounced and more easily detected than a concurrent increase in power consumption, probably leading to earlier detection of device thrombosis. In a recent retrospective series 67% of patients with suspected thrombosis had their device explanted after failure of conservative measures (27). With 7 out of 8 cases of suspected thrombosis in our cohort being successfully treated by intravenous anticoagulants, telemonitoring of device flow seems to facilitate timely detection of LVAD thrombosis and early initiation of thrombolytic measures to avoid LVAD exchange. However, our initial experience suggesting earlier detection of LVAD thrombosis warrants further investigation in a controlled study before definite conclusions can be drawn.
Data transmission was technically successful in the great majority of attempts but could be lower in regions with less than perfect cellular network coverage. Patients and their healthcare providers need to be aware that telemonitoring should not be solely relied upon when close monitoring is critical. No interaction between the LVAD and other implanted devices in the same patient was seen in our cohort. However, interference between different types of cardiac implantable devices has been observed in the past and clinicians should consider these interactions as new devices become available (30,31).
We observed a large number of alarms, particularly early in our clinical experience with this system. This underscores the importance of deliberate trigger settings so as not to overburden the clinical care team. In contrast to power and pump speed, flow is highly volatile and subject to daily variations in hemodynamic loading conditions. We therefore chose more lenient low flow alarm limits as we gained experience in order to enhance specificity, leading to a greatly reduced alarm burden in most patients. Careful programming of alarm settings is mandatory and threshold re-evaluation in the chronic phase of LVAD therapy allows for adaption to the individual patients’ hemodynamics (9). Development of advanced software algorithms, such as single reporting of clusters of similar events within short time periods, could reduce the number of alerts sent to the clinical team.
LVAD recipients are usually followed in a tertiary referral centre, placing a considerable burden of travel on these patients. While we were able to demonstrate the feasibility of LVAD remote monitoring, further research is warranted to determine if this safely replaces in-office follow-up visits. Telemonitoring has the potential to become the cornerstone in a multi-disciplinary approach to deliver LVAD care in the community.
Limitations
The main limitation of this observational study is the small patient cohort. However, with the HeartAssist 5 and aVAD being the only telemonitoring-enabled LVADs currently approved, patient numbers are still limited. In addition, we did not perform invasive hemodynamic measurements to prove correlations between changes in flow waveforms and volume status, nor are electrocardiograms available to prove flow waveform correlations with arrhythmias. Nevertheless, in all cases, the changes matched predictions of in silico modelling, leading to high degree of certainty of the conclusions.
Conclusions
LVAD patient telemonitoring offers the possibility for remote device function monitoring and facilitates early detection of clinical complications such as pump thrombosis, certain arrhythmias and changes in volume status. We have reported our initial experience which represents the largest cohort of LVAD patients with remote monitoring to date. More research is warranted to test whether telemonitoring improves outcomes in LVAD patients.
Acknowledgements
None.
Ethical Statement: This observational study was approved by the institutional review board of the Hannover Medical School. Informed consent requirements for this study were waived.
Footnotes
Conflicts of Interest: B Lynch is an employee of Reliant Heart Inc., Houston, Texas. The other authors have no conflicts of interest to declare.
References
- 1.Mancini D, Colombo PC. Left Ventricular Assist Devices: A Rapidly Evolving Alternative to Transplant. J Am Coll Cardiol 2015;65:2542-55. 10.1016/j.jacc.2015.04.039 [DOI] [PubMed] [Google Scholar]
- 2.Netuka I, Sood P, Pya Y, et al. Fully Magnetically Levitated Left Ventricular Assist System for Treating Advanced HF: A Multicenter Study. J Am Coll Cardiol 2015;66:2579-89. 10.1016/j.jacc.2015.09.083 [DOI] [PubMed] [Google Scholar]
- 3.Schmitto JD, Hanke JS, Rojas S, et al. Circulatory support exceeding five years with a continuous-flow left ventricular assist device for advanced heart failure patients. J Cardiothorac Surg 2015;10:107. 10.1186/s13019-015-0306-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitto JD, Zimpfer D, Fiane AE, et al. Long-term support of patients receiving a left ventricular assist device for advanced heart failure: a follow-up analysis of the Registry to Evaluate the HeartWare Left Ventricular Assist System. Eur J Cardiothorac Surg 2016;50:834-8. 10.1093/ejcts/ezw224 [DOI] [PubMed] [Google Scholar]
- 5.Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. 10.2174/1573403X1103150514151750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slotwiner D, Varma N, Akar JG, et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015;12:e69-100. 10.1016/j.hrthm.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 7.Varma N, Pavri BB, Stambler B, et al. Same-day discovery of implantable cardioverter defibrillator dysfunction in the TRUST remote monitoring trial: influence of contrasting messaging systems. Europace 2013;15:697-703. 10.1093/europace/eus410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guédon-Moreau L, Kouakam C, Klug D, et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial. J Cardiovasc Electrophysiol 2014;25:763-70. 10.1111/jce.12405 [DOI] [PubMed] [Google Scholar]
- 9.Reiss N, Schmidt T, Müller-von Aschwege F, et al. Telemonitoring and Medical Care of Heart Failure Patients Supported by Left Ventricular Assist Devices - The Medolution Project. Stud Health Technol Inform. 2017;236:267-74. [PubMed] [Google Scholar]
- 10.Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583-90. 10.1016/S0140-6736(14)61176-4 [DOI] [PubMed] [Google Scholar]
- 11.Schmitto JD, Hanke JS, Dogan G, et al. First Implantation of a Novel Left Ventricular Assist Device: The ReliantHeart aVAD. Ann Thorac Surg 2017;104:e311-3. 10.1016/j.athoracsur.2017.04.049 [DOI] [PubMed] [Google Scholar]
- 12.Moazami N, Fukamachi K, Kobayashi M, et al. Axial and centrifugal continuous-flow rotary pumps: a translation from pump mechanics to clinical practice. J Heart Lung Transplant 2013;32:1-11. 10.1016/j.healun.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 13.Burkhoff D, Sayer G, Doshi D, et al. Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol 2015;66:2663-74. 10.1016/j.jacc.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 14.Duncker D, Michalski R, Müller-Leisse J, et al. Devicebasiertes Telemonitoring. Herzschrittmacherther Elektrophysiol 2017;28:268-78. 10.1007/s00399-017-0521-3 [DOI] [PubMed] [Google Scholar]
- 15.Böhm M, Drexler H, Oswald H, et al. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J 2016;37:3154-63. 10.1093/eurheartj/ehw099 [DOI] [PubMed] [Google Scholar]
- 16.Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Hear. J Am Coll Cardiol 2010;55:1803-10. 10.1016/j.jacc.2009.11.089 [DOI] [PubMed] [Google Scholar]
- 17.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658-66. 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 18.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. 10.1016/j.healun.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 19.Dobarro D, Urban M, Booth K, et al. Impact of aortic valve closure on adverse events and outcomes with the HeartWare ventricular assist device. J Heart Lung Transplant 2017;36:42-9. 10.1016/j.healun.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 20.Hayward C, Lim CP, Schima H, et al. Pump Speed Waveform Analysis to Detect Aortic Valve Opening in Patients on Ventricular Assist Device Support. Artif Organs 2015;39:704-9. 10.1111/aor.12570 [DOI] [PubMed] [Google Scholar]
- 21.Granegger M, Schima H, Zimpfer D, et al. Assessment of aortic valve opening during rotary blood pump support using pump signals. Artif Organs 2014;38:290-7. 10.1111/aor.12167 [DOI] [PubMed] [Google Scholar]
- 22.Salamonsen RF, Lim E, Moloney J, et al. Anatomy and Physiology of Left Ventricular Suction Induced by Rotary Blood Pumps. Artif Organs 2015;39:681-90. 10.1111/aor.12550 [DOI] [PubMed] [Google Scholar]
- 23.Voigt O, Benkowski RJ, Morello GF. Suction detection for the MicroMed DeBakey Left Ventricular Assist Device. ASAIO J 2005;51:321-8. 10.1097/01.mat.0000169118.21639.da [DOI] [PubMed] [Google Scholar]
- 24.Brenyo A, Rao M, Koneru S, et al. Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients. J Cardiovasc Electrophysiol 2012;23:515-20. 10.1111/j.1540-8167.2011.02223.x [DOI] [PubMed] [Google Scholar]
- 25.Enriquez AD, Calenda B, Gandhi PU, et al. Clinical impact of atrial fibrillation in patients with the HeartMate II left ventricular assist device. J Am Coll Cardiol 2014;64:1883-90. 10.1016/j.jacc.2014.07.989 [DOI] [PubMed] [Google Scholar]
- 26.Moscato F, Granegger M, Edelmayer M, et al. Continuous monitoring of cardiac rhythms in left ventricular assist device patients. Artif Organs 2014;38:191-8. 10.1111/aor.12141 [DOI] [PubMed] [Google Scholar]
- 27.Tran BC, Nijjar PS. Role of contrast CT for the diagnosis and the prognosis of suspected LVAD thrombosis. J Card Surg 2017;32:162-5. 10.1111/jocs.13094 [DOI] [PubMed] [Google Scholar]
- 28.Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013;32:667-70. 10.1016/j.healun.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 29.Schmitto JD, Avsar M, Haverich A. Increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:1463-4. 10.1056/NEJMc1401768 [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer TJ, König T, Duncker D, et al. Subcutaneous Implantable Cardioverter-Defibrillator Shocks After Left Ventricular Assist Device Implantation. Circ Arrhythm Electrophysiol 2016;9:e004633. 10.1161/CIRCEP.116.004633 [DOI] [PubMed] [Google Scholar]
- 31.Duncker D, König T, Müller-Leisse J, et al. Electric smog: telemetry interference between ICD and LVAD. Herzschrittmacherther Elektrophysiol 2017;28:257-9. 10.1007/s00399-017-0509-z [DOI] [PubMed] [Google Scholar]