Abstract
Background and Aims
Analyses of Crohn’s Disease [CD] studies of anti-TNF agents, including adalimumab, have reported higher remission rates among patients with shorter disease duration. To further explore the relationship between disease duration and clinical efficacy, we analysed a larger patient cohort.
Methods
Data were pooled from 10 clinical trials in patients with moderately to severely active CD who received treatment with either adalimumab or placebo. Analyses of efficacy using Crohn’s Disease Activity Index [CDAI] endpoints [remission, clinical response [CR]-70, CR-100, patient-reported outcome [PRO] remission] or Harvey–Bradshaw Index [HBI] endpoints [remission/response] were conducted for induction and maintenance treatment periods. Logistic regression was used for comparisons between adalimumab and placebo treatment. Cochran–Armitage trend tests were used for comparisons between disease-duration subgroups [<1 year, ≥1–<2 years, 2–≤5 years, and >5 years].
Results
During induction, the proportion of patients achieving CDAI remission was higher in adalimumab- versus placebo-treated patients [p <0.001] and was highest [adalimumab: 45.8%] in the <1 year subgroup compared with longer disease-duration subgroups [≥1–<2 years: 31.0%; 2–≤5 years: 23.1%; >5 years: 23.6%, Cochran–Armitage p = 0.026]. In the majority of maintenance treatment analyses, patients with <1 year disease duration had the highest efficacy responses, with statistically significant differences in remission rates across disease-duration subgroups.
Conclusions
This analysis demonstrates that earlier initiation of adalimumab treatment shortly after diagnosis in patients with moderately to severely active CD leads to improved long-term clinical outcomes.
Keywords: Adalimumab, Crohn’s disease, disease duration
1. Introduction
Crohn’s disease [CD] is a chronic inflammatory disorder principally of the gastrointestinal tract, associated with mural and transmural inflammation.1,2 The management plan for a patient with CD should include the activity, site, and behaviour of disease, together with the views of the patient.3,4 Anti-TNF biologics have been shown to be effective in inducing and maintaining remission in patients with CD.5,6 Controlling intestinal inflammation early in the course of CD is thought to prevent structural tissue damage and disease-related complications;3,7 hence treatment with anti-TNF agents, such as adalimumab, early following diagnosis may lead to improved clinical outcomes. Indeed, in post hoc analysis of anti-TNF trials, including the CHARM trial using adalimumab, higher remission rates were observed in adalimumab-treated patients with CD with disease duration <2 years compared with longer disease duration.8,9
The objective of the current analysis was to confirm and extend on the findings in CHARM through analysis of a more extensive set of 10 adalimumab clinical trials. Specifically, we aimed to compare adalimumab with placebo clinical efficacy responses during induction treatments, and to measure adalimumab efficacy responses during maintenance treatment, determining their association with CD duration at baseline.
2. Materials and Methods
2.1. Study design
Data were pooled from 10 Phase III and IIIb adalimumab clinical trials in patients with CD. Details for each trial have been published previously: CLASSIC I10 and II,11 CHARM,12 GAIN,13 ADHERE,14 Japan CD induction and maintenance,15 EXTEND,6 CARE,16 and ACCESS.17
Across the 10 trials included in our analysis, patients eligible for inclusion were aged 18 to 75 years (except ACCESS [≥18 years] and Japan CD [>15 to <75 years]). Patients were required to have had a confirmed diagnosis of CD for ≥4 months and a Crohn’s Disease Activity Index [CDAI] of 220–450 [CLASSIC I/II, CHARM, GAIN, Japan CD, and EXTEND], >220 [ACCESS], or a Harvey–Bradshaw Index [HBI] of ≥7 [CARE and ACCESS]. Dosing in the studies included induction therapy with placebo or adalimumab [160 mg at Week 0 and 80 mg at Week 2, or 80 mg at Week 0 and 40 mg at Week 2], followed by maintenance therapy with placebo or adalimumab [40 mg every other week, or 40 mg weekly, according to study design]. CLASSIC I had an additional induction arm of adalimumab 40 mg at Week 0 and 20 mg at Week 2, but data for those patients were excluded from this analysis as this is not an approved induction dose.
This analysis was divided by induction and maintenance studies. For the induction analysis, patients randomised to double-blind placebo or adalimumab from CLASSIC I, GAIN, and Japan CD induction studies were included and compared by treatment arm and disease duration. The 1-year maintenance treatment analysis included patients from CLASSIC II, CHARM, ADHERE [for patients who entered from GAIN only], EXTEND, Japan CD, CARE, and ACCESS. For the maintenance analyses, only patients who received maintenance adalimumab were studied. No placebo comparison group was included for the maintenance analysis, and patients were compared across disease-duration groups only. This was for two reasons: some studies had an open-label induction period, whereby all patients received adalimumab before being randomised to double-blind placebo or adalimumab [CHARM and EXTEND], making comparison of the placebo-randomised patients with the adalimumab-randomised patients inappropriate; or the trials were open-label single-arm adalimumab studies without a placebo group [ADHERE, CARE, and ACCESS]. Patients were included in the maintenance analysis if they received adalimumab maintenance treatment in any of the maintenance studies mentioned above, except for patients who first received induction with double-blind placebo in CLASSIC I or Japan CD and were later re-randomised to double-blind adalimumab maintenance therapy in CLASSIC II or Japan CD. Furthermore, the maintenance analysis was not limited to patients who responded to induction therapy.
Supplementary Table 1 [available as Supplementary data at ECCO-JCC online] summarises patient numbers from each trial included in the induction and maintenance treatment analyses.
2.2. Endpoints
Efficacy data [CDAI and HBI values] were pooled from studies included in the analyses. For studies using CDAI [CLASSIC I/II, GAIN, Japan CD, CHARM, EXTEND, and ADHERE], clinical remission was defined as CDAI <150. Two definitions of clinical response were applied: CR-70, a decrease in CDAI ≥70 points relative to study baseline; and CR-100, a decrease in CDAI ≥100 points relative to study baseline. For studies using HBI [CARE and ACCESS], clinical remission was defined as HBI <5 and clinical response was defined as a decrease in HBI ≥3 points relative to study baseline. A patient-reported outcome [PRO] measure of remission, based on mean daily CDAI subscores of stool frequency [SF] and abdominal pain [AP], was also included in this analysis. PRO remission was defined as mean daily SF ≤3.0 and mean daily AP ≤1.0, neither worse than baseline. PRO remission was evaluated in patients with average daily SF ≥4.0 and/or AP ≥2.0 at baseline in studies that used CDAI. Safety was assessed by evaluation of reported adverse events.
2.3. Analyses and statistical methods
Induction assessments of remission and response were measured at Week 4 by placebo and adalimumab treatments. For the maintenance analysis, the CDAI efficacy endpoints were measured at Weeks 8, 12, 26, and 52/56 of treatment, and HBI remission and response were measured at Weeks 4, 8, 12, and 20/24 of treatment. All efficacy measures were analysed by disease duration at trial baseline [four subgroups: <1 year; ≥1–<2 years; ≥2–≤5 years; and >5 years].
Modified non-responder imputation [mNRI] was used for missing data, whereby patients who received open-label adalimumab every other week or every week were not imputed and patients were counted according to their observed response.
For baseline characteristics, differences between disease-duration groups were determined using a logistical regression method for categorical data and analysis of variance [ANOVA] for continuous data. For adalimumab versus placebo comparison in the induction studies, a logistic regression with treatment effect, disease duration, and treatment by disease duration was applied to assess the treatment effect. Cochran–Armitage trend tests were applied to determine statistically significant trends in efficacy across the disease-duration groups. All treatment comparisons used two-sided tests for statistical significance.
3. Results
3.1. Baseline demographics and clinical characteristics by disease duration
Adults with moderately to severely active CD who received induction (adalimumab [n = 377] or placebo [n = 263]) and maintenance (adalimumab [n = 2325]) treatment were included in the efficacy and safety analyses. PRO remission is reported for a subset of patients: 318 patients receiving induction adalimumab treatment, 218 patients receiving placebo, and 910 patients receiving adalimumab maintenance treatment. The number of patients from each study who were analysed for the induction and maintenance endpoints is shown in Supplementary Table 1. Baseline demographics and clinical characteristics by disease duration are shown for studies with CDAI (Table 1A [induction studies] and Table 1B [maintenance]) and with HBI [Table 1C] assessments. Statistically significant across-group [disease duration] differences in baseline characteristics were noted for age, CDAI, and previous anti-TNF use among patients in studies using CDAI [Table 1A and B], and for age, C-reactive protein [CRP] level, fistula presence, previous anti-TNF use, corticosteroid use, and immunosuppressant use among patients in studies using HBI [Table 1C].
Table 1.
Demographics and clinical characteristics at baseline for patients with CD treated with adalimumab or placebo by baseline disease. A] CLASSIC I, GAIN, and Japan CD [induction] clinical trials; B] CLASSIC II, CHARM, ADHERE, EXTEND, and Japan CD [maintenance] clinical trials; C] CARE and ACCESS clinical trials. Statistical significance across disease-duration groups determined by logistical regression [categorised data] or ANOVA model [continuous data]. ***, **, * statistically significant at p = 0.001, 0.01, and 0.05 level, respectively. n values for some measures smaller than total n value; from left to right, 1A: an = 13, 24, 17, 28, 58, 65, 175, 259; 1B: an = 45, 66, 195, 766; bn = 45, 66, 196, 768; 1C: an = 63, 86, 258, 814; bn = 65, 87, 264, 832; cn = 63, 85, 252, 814.
| A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD duration [years] | |||||||||
| <1 | ≥1 to <2 | ≥2 to ≤5 | >5 | ||||||
| PBO [n = 13] |
ADA [n = 24] |
PBO [n = 17] |
ADA [n = 29] |
PBO [n = 58] | ADA [n = 65] |
PBO [n = 175] |
ADA [n = 259] |
p-Value | |
| Age, mean years [SD] | 38.8 [15.5] | 31.0 [12.0] | 31.4 [9.8] | 34.7 [10.4] | 32.3 [12.6] | 34.9 [12.9] | 38.5 [11.5] | 39.2 [11.0] | <0.001*** |
| Female; n [%] | 8 [61.5] | 13 [54.2] | 11 [64.7] | 19 [65.5] | 34 [58.6] | 42 [64.5] | 92 [52.6] | 156 [60.2] | 0.621 |
| Race, White; n [%] | 11 [84.6] | 18 [75.0] | 13 [76.5] | 19 [65.5] | 51 [87.9] | 47 [72.3] | 153 [87.4] | 194 [74.9] | 0.693 |
| Serum CRP ≥1 mg/dL, n [%] | 5 [38.5] | 10 [41.7] | 11 [64.7] | 15 [51.7] | 26 [44.8] | 36 [55.4] | 71 [40.6] | 124 [47.9] | 0.316 |
| Serum CRP, mg/dL; mean [SD] | 2.4 [4.6] | 2.0 [2.4] | 4.5 [7.1] | 1.9 [1.7] | 1.9 [2.9] | 2.3 [2.8] | 1.8 [2.8] | 1.9 [2.5] | 0.147 |
| BL fistula, n [%] | 2 [15.4] | 1 [4.2] | 0 | 5 [17.2] | 4 [6.9] | 10 [15.4] | 31 [17.7] | 44 [17.0] | 0.184 |
| CDAI, mean [SD] | 293.1 [61.2] | 288.0 [63.4] | 293.5 [74.3] | 300.4 [55.1] | 295.3 [59.1] | 300.4 [55.3] | 314.5 [64.3] | 308.0 [59.6] | 0.045* |
| IBDQ total score, mean [SD]a | 128.9 [33.3] | 129.8 [36.0] | 128.9 [35.5] | 123.0 [34.0] | 129.9 [27.5] | 127.3 [30.6] | 124.9 [28.0] | 127.1 [29.6] | 0.802 |
| Previous anti-TNF use, n [%] | 1 [7.7] | 2 [8.3] | 9 [52.9] | 9 [31.0] | 37 [63.8] | 29 [44.6] | 125 [71.4] | 131 [50.6] | <0.001*** |
| Concomitant therapy, n [%] | |||||||||
| CS only | 1 [7.7] | 7 [29.2] | 3 [17.6] | 10 [34.5] | 9 [15.5] | 14 [21.5] | 36 [20.6] | 46 [17.8] | 0.488 |
| Immunosuppressant only | 4 [30.8] | 3 [12.5] | 3 [17.6] | 8 [27.6] | 12 [20.7] | 19 [29.2] | 39 [22.3] | 61 [23.6] | 0.880 |
| CS and immunosuppressant | 1 [7.7] | 2 [8.3] | 6 [35.3] | 2 [6.9] | 16 [27.6] | 7 [10.8] | 34 [19.4] | 35 [13.5] | 0.506 |
| B | |||||||||
| CD duration [years] | |||||||||
| <1 | ≥1 to <2 | ≥2 to ≤5 | >5 | ||||||
|
ADA
[n = 45] |
ADA
[n = 67] |
ADA
[n = 196] |
ADA
[n = 768] |
p-Value | |||||
| Age, mean years [SD] | 35.1 [12.8] | 35.4 [11.1] | 34.4 [12.4] | 38.6 [11.3] | <0.001*** | ||||
| Female; n [%] | 27 [60.0] | 41 [61.2] | 134 [68.4] | 459 [59.8] | 0.180 | ||||
| Race, White; n [%] | 38 [84.4] | 57 [85.1] | 173 [88.3] | 670 [87.2] | 0.922 | ||||
| Serum CRP ≥1 mg/dL, n [%] | 19 [42.2] | 33 [50.0] | 96 [49.2] | 345 [45.0] | 0.618 | ||||
| Serum CRP, mg/dL; mean [SD]a | 2.3 [2.8] | 2.4 [3.9] | 2.1 [3.5] | 2.0 [2.8] | 0.065 | ||||
| BL fistula, n [%] | 7 [15.6] | 5 [7.5] | 24 [12.2] | 119 [15.5] | 0.261 | ||||
| CDAI, mean [SD] | 304.0 [56.4] | 306.8 [60.8] | 300.4 [55.7] | 312.3 [60.7] | 0.009** | ||||
| IBDQ total score, mean [SD]b | 122.4 [28.1] | 125.7 [30.0] | 126.7 [28.7] | 122.1 [28.4] | 0.100 | ||||
| Previous anti-TNF use, n [%] | 7 [15.6] | 26 [38.8] | 100 [51.0] | 451 [58.7] | <0.001*** | ||||
| Concomitant therapy, n [%] | |||||||||
| CS only | 12 [26.7] | 18 [26.9] | 42 [21.4] | 143 [18.6] | 0.226 | ||||
| Immunosuppressant only | 10 [22.2] | 22 [32.8] | 57 [29.1] | 178 [23.2] | 0.142 | ||||
| CS and immunosuppressant | 9 [20.0] | 11 [16.4] | 33 [16.8] | 149 [19.4] | 0.809 | ||||
| C | |||||||||
| CD duration [years] | |||||||||
| <1 | ≥1 to <2 | ≥2 to ≤5 | >5 | ||||||
|
ADA
[n = 65] |
ADA
[n = 87] |
ADA
[n = 264] |
ADA
[n = 833] |
p-Value | |||||
| Age, mean years [SD] | 33.0 [12.7] | 29.6 [11.7] | 32.5 [11.4] | 37.6 [10.9] | <0.001*** | ||||
| Female; n [%] | 42 [64.6] | 48 [55.2] | 152 [57.6] | 499 [59.9] | 0.610 | ||||
| Race, White; n [%] | 64 [98.5] | 85 [97.7] | 253 [95.8] | 815 [97.8] | 0.324 | ||||
| Serum CRP ≥1 mg/dL, n [%] | 33 [52.4] | 46 [53.5] | 132 [51.2] | 386 [47.4] | 0.519 | ||||
| Serum CRP, mg/dL; mean [SD]a | 2.0 [2.8] | 3.7 [11.2] | 2.3 [3.1] | 1.8 [2.5] | <0.001*** | ||||
| BL fistula, n [%]b | 8 [12.3] | 6 [6.9] | 49 [18.6] | 176 [21.2] | 0.009** | ||||
| HBI, mean [SD] | 10.8 [4.1] | 10.6 [3.6] | 10.8 [4.3] | 11.3 [4.2] | 0.150 | ||||
| IBDQ total score, mean [SD]c | 36.0 [10.7] | 36.9 [9.7] | 36.5 [11.0] | 36.3 [10.1] | 0.954 | ||||
| Previous anti-TNF use, n [%] | 9 [13.8] | 23 [26.4] | 105 [39.8] | 372 [44.7] | <0.001*** | ||||
| Concomitant therapy, n [%] | |||||||||
| CS only | 26 [40.0] | 23 [26.4] | 46 [17.4] | 204 [24.5] | 0.002** | ||||
| Immunosuppressant only | 8 [12.3] | 27 [31.0] | 87 [33.0] | 206 [24.7] | 0.003** | ||||
| CS and immunosuppressant | 15 [23.1] | 24 [27.6] | 88 [33.3] | 212 [25.5] | 0.078 |
ADA, adalimumab; BL, baseline; CRP, C-reactive protein; CS, corticosteroid; HBI, Harvey–Bradshaw Index; IBDQ, inflammatory bowel disease questionnaire; PBO, placebo, CD, Crohn’s disease; ANOVA, analysis of variance; SD, standard deviation.
3.2. Efficacy—clinical remission and response
3.2.1. Induction and maintenance endpoints—CDAI studies
At Week 4 following induction treatment, CDAI remission rates were significantly higher in adalimumab-treated patients compared with placebo-treated patients [p <0.001, logistic regression] and higher in adalimumab-treated patients with <1 year of disease duration [45.8%] compared with longer disease duration subgroups [31.0% for ≥1–<2 years, 23.1% for 2–≤5 years, and 23.6% for >5 years], with p = 0.026 in Cochran–Armitage trend test comparing disease-duration subgroups [Figure 1A]. Similarly, a significant difference between adalimumab and placebo was observed for CR-70 and CR-100 at Week 4 after induction treatment [p <0.001] and response rates were highest in the <1 year subgroup receiving adalimumab [Figure 1B and C], although there was no statistical significance by disease duration; the highest PRO remission rates were also seen in adalimumab-treated patients with <1 year disease duration, although no statistical significance was observed [Figure 1D].
Figure 1.
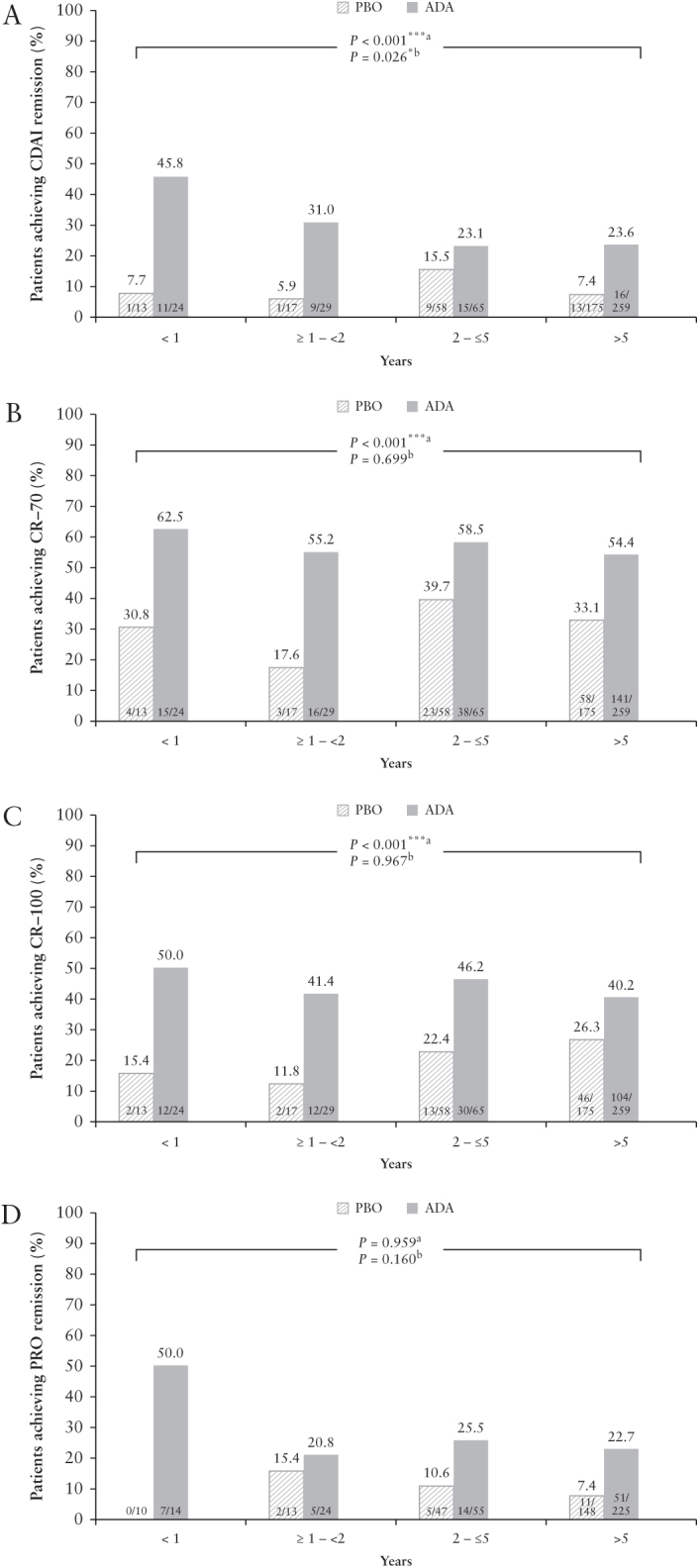
Efficacy at Week 4 of treatment by baseline disease duration, mNRI analysis: [A] CDAI remission, [B] CR-70, [C] CR-100, and [D] PRO remission. Analysis from CLASSIC I, GAIN, and Japan CD clinical trials; ap-values from logistic regression analysis for comparisons between adalimumab versus placebo treatment; bp-values from Cochran–Armitage trend tests comparing disease-duration subgroups ***, *, statistically significant at p = 0.001 and p = 0.05 levels, respectively. ADA, adalimumab; CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CR, clinical response; mNRI, modified non-responder imputation; PBO, placebo; PRO, patient-reported outcome.
In the maintenance analyses [presented as the total number of weeks of treatment], at Week 52/56 patients with <1 year disease duration had the highest rates of CDAI remission [51.1%], CR-70 [57.8%], CR-100 [53.3%], and PRO remission [48.6%] compared with the longer disease-duration subgroups [p = 0.002, 0.139, 0.074, and 0.016, respectively, Cochran–Armitage trend test] [Figure 2A–D]. For CDAI and PRO remission, the highest remission rates were achieved in patients with <1 year disease duration compared with longer disease-duration groups at all time points assessed [Weeks 8–52/56], achieving statistical significance in the Cochran–Armitage trend test at Week 26 and Week 52/56 for both measures [Figure 2A and D]. Also, patients with <1 year disease duration achieved the highest proportions of CR-70 at Weeks 12, 26, and 52/56 and for CR-100 at all time points compared with the longer disease-duration groups, although these trends were not statistically significant other than for CR-100 at Week 26 [Figure 2B and C].
Figure 2.
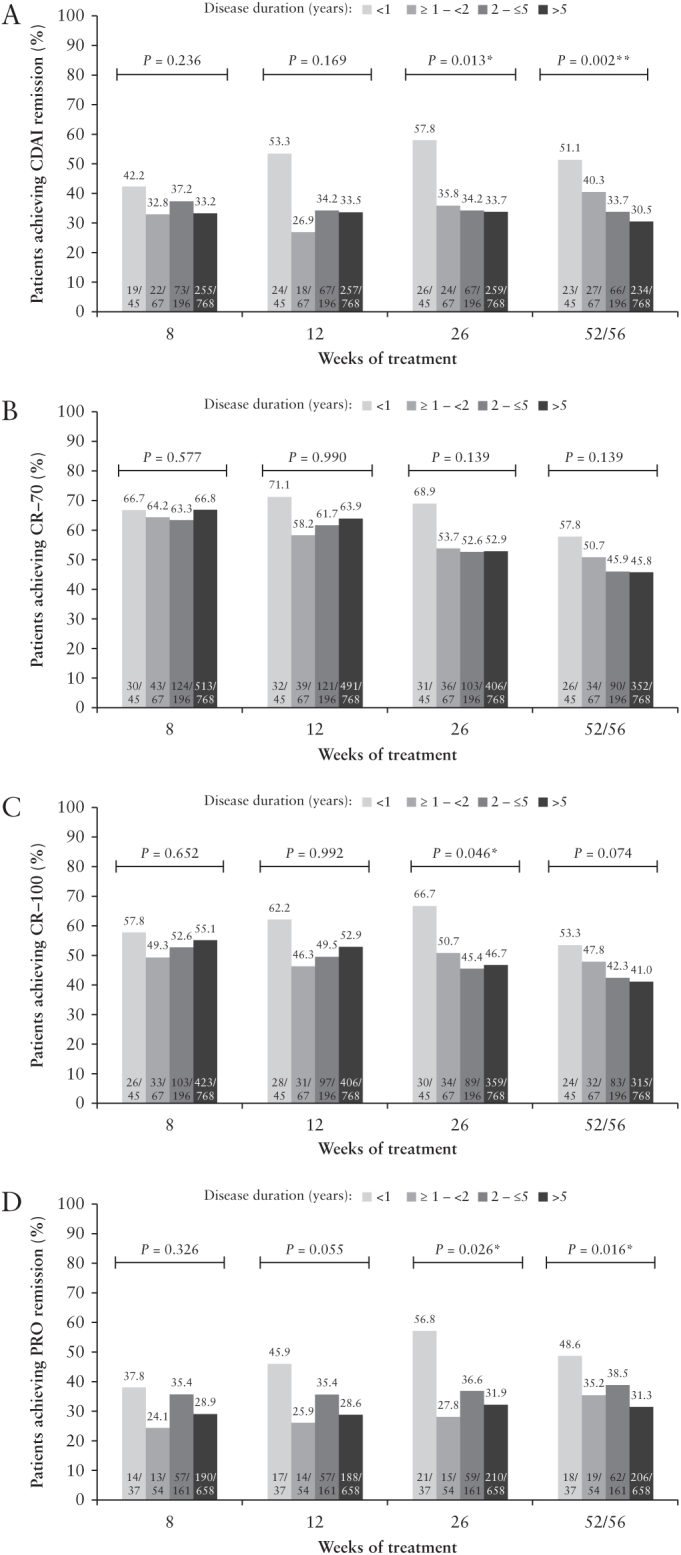
Efficacy [CDAI] over time by baseline disease duration, mNRI analysis: [A] CDAI remission, [B] CR-70 response, [C] CR-100 response, and [D] PRO remission. Analysis from CLASSIC II, CHARM, ADHERE, EXTEND, and Japan CD clinical trials. Last time point: Week 56 in CHARM, otherwise Week 52; p-values from Cochran–Armitage trend tests comparing disease-duration subgroups **, *, statistically significant at p = 0.01 level and p = 0.05 level, respectively. CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CR, clinical response; mNRI, modified non-responder imputation; PRO, patient-reported outcome.
3.2.2. HBI studies
At Weeks 8, 12, and 20/24 of adalimumab treatment, HBI remission was highest in patients with disease duration <1 year (50.8, 58.5, and 64.6% of patients, respectively [Figure 3A]). A statistically significant difference between disease-duration groups was seen from Week 4 of adalimumab treatment through Week 20/24 [Figure 3A]. HBI response was numerically highest in patients with disease duration <1 year at all time points, although a statistically significant difference between disease-duration groups was only observed at Week 4 [Figure 3B].
Figure 3.
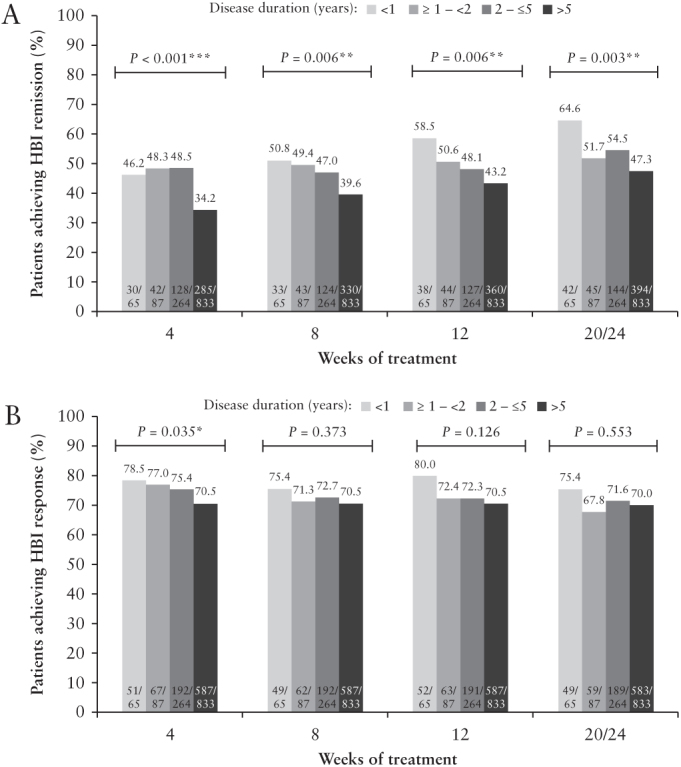
Efficacy [HBI] over time by baseline disease duration, mNRI analysis: [A] HBI remission and [B] HBI response. Analysis from CARE and ACCESS clinical trials. Last time point: Week 20 in CARE, Week 24 in ACCESS; p-values from Cochran–Armitage trend tests comparing disease-duration subgroups ***, **, *, statistically significant at p = 0.001, p = 0.01 level, and p = 0.05 level, respectively. HBI, Harvey–Bradshaw Index; mNRI, modified non-responder imputation.
3.3. Safety
Detailed safety data have previously been reported for each of the trials included in our analysis6,10–17 and in review form.18 The adverse-event analysis captured for induction periods and maintenance periods across all trials is shown in Table 2A and Table 2B, respectively. For both induction and maintenance treatment sets, rates of any adverse event and any infection were similar for patients across disease-duration subgroups, whereas the rates of serious adverse events and serious infections were numerically higher in the longer disease-duration subgroups compared with <1 year subgroup.
Table 2.
Treatment-emergent adverse events by disease duration. A] AEs during placebo or adalimumab treatment in induction studies [CLASSIC I, GAIN, and Japan CD [induction] clinical trials]; B] AEs during adalimumab maintenance studies [CLASSIC II, CHARM, ADHERE, EXTEND, Japan CD [maintenance] clinical trials], CARE, and ACCESS clinical trials.
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD duration [years] | ||||||||
| <1 | ≥1 to <2 | ≥2 to ≤5 | >5 | |||||
| PBO [n = 13] n [%] |
ADA [n = 24] n [%] |
PBO [n = 17] n [%] |
ADA [n = 29] n [%] |
PBO [n = 58] n [%] | ADA [n = 65] n [%] |
PBO [n = 175] n [%] |
ADA [n = 259] n [%] |
|
| Any AE | 9 [69.2] | 14 [58.3] | 14 [82.4] | 16 [55.2] | 44 [75.9] | 42 [64.6] | 121 [69.1] | 162 [62.5] |
| Serious AE | 1 [7.7] | 0 | 1 [5.9] | 1 [3.4] | 2 [3.4] | 1 [1.5] | 11 [6.3] | 9 [3.5] |
| AE leading to study drug discontinuation | 1 [7.7] | 0 | 1 [5.9] | 1 [3.4] | 1 [1.7] | 2 [3.1] | 5 [2.9] | 4 [1.5] |
| AE leading to death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection | 1 [7.7] | 0 | 4 [23.5] | 3 [10.3] | 15 [25.9] | 12 [18.5] | 32 [18.3] | 44 [17.0] |
| Serious infection | 0 | 0 | 1 [5.9] | 0 | 1 [1.7] | 1 [1.5] | 2 [1.1] | 3 [1.2] |
| Opportunistic infectiona | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tuberculosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any malignancy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection-site pain | 4 [30.8] | 5 [20.8] | 1 [5.9] | 4 [13.8] | 5 [8.6] | 4 [6.2] | 12 [6.9] | 26 [10.0] |
| B | ||||||||
| CD duration [years] | ||||||||
|
<1
[n = 110] [PY = 64.6] |
≥1 to <2
[n = 154] [PY = 88.1] |
≥2 to ≤5
[n = 460] [PY = 269.0] |
>5
[n = 1601] [PY = 987.5] |
|||||
| Events [events/100 PY] | ||||||||
| Any AE | 540 [835.9] | 879 [997.7] | 2356 [875.8] | 9061 [917.6] | ||||
| Serious AE | 18 [27.9] | 29 [32.9] | 112 [41.6] | 405 [41.0] | ||||
| AE leading to study drug discontinuation | 13 [20.1] | 20 [22.7] | 64 [23.8] | 260 [26.3] | ||||
| AE leading to death | 0 | 0 | 0 | 2 [0.2] | ||||
| Infection | 79 [122.3] | 151 [171.4] | 424 [157.6] | 1551 [157.1] | ||||
| Serious infection | 3 [4.6] | 2 [2.3] | 24 [8.9] | 74 [7.5] | ||||
| Opportunistic infectiona | 0 | 0 | 2 [0.7] | 7 [0.7] | ||||
| Tuberculosis | 0 | 0 | 0 | 2 [0.2] | ||||
| Any malignancy | 0 | 1 [1.1] | 1 [0.4] | 9 [0.9] | ||||
| Injection-site pain | 14 [21.7] | 21 [23.8] | 44 [16.4] | 168 [17.0] |
aExcluding oral candidiasis and tuberculosis.
CD, Crohn’s disease; AE, adverse event; ADA, adalimumab; PY, patient-years; PBO, placebo.
4. Discussion
Previous trials have shown that adalimumab is more effective than placebo in inducing and maintaining remission in patients with moderately to severely active CD.10–12,19 Consistent with this, in our pooled analyses of induction treatments the proportion of patients in CDAI remission was statistically higher in adalimumab- versus placebo-treated patients. The main aim of this analysis was to determine the relationship between efficacy [remission] and disease duration. Following induction treatment, the highest remission rates were observed in the adalimumab-treated patient group with the shortest disease duration [<1 year], and a statistically significant difference was observed between disease-duration groups. During the maintenance adalimumab treatment periods, the benefit observed during early treatment was maintained with continued adalimumab therapy, with statistically significant differences between disease-duration subgroups observed at most time points. Similar numerical trends were observed for CDAI response.
Early use of anti-TNF therapy in the course of CD has previously been advocated, based on increased chances of achieving clinical remission and mucosal healing, and of preserving bowel integrity.3,20 Overall, our data support the notion that earlier treatment with adalimumab soon after diagnosis is associated with improved efficacy outcomes compared with initiating adalimumab several years following diagnosis. Furthermore, the safety profile for adalimumab appears to be relatively more favourable in patients with the shortest disease duration, with the trend for a numerically higher incidence of serious adverse events and serious infections with longer disease duration. This likely reflects the inability to control inflammation and disease severity in a timely manner.
This post hoc analysis of adalimumab clinical trials stratified patients by disease duration at study entry. It is important to note that patients with shorter disease duration did not necessarily have early disease [less progressive disease], as all patients enrolled in these trials had moderate to severe CD and failed conventional therapy to meet the inclusion criteria. However, an association between disease duration and disease progression in CD has been reported. For example, a study by Pariente et al.21 showed that disease progression [scored using the Lémann Index] increased from a mean score of 6.3 for disease duration <2 years to a mean score of 19.0 for a duration ≥10 years.
In line with the growing evidence of CD being a chronically progressive destructive disease, our data not surprisingly showed that patients with longer disease duration at baseline were older and had higher rates of previous anti-TNF use. Because rates of remission with adalimumab have been shown to be lower in patients with previous anti-TNF use, and because older patients are, by virtue of their age, more likely to experience adverse events such as infections, it is possible that our efficacy and safety findings are influenced by the higher proportion of treatment-refractory patients and older age in the longer duration subgroups.
Our pooled analyses add to the findings in a previously published post hoc analysis9 reporting increased clinical remission rates and reduced incidence of serious adverse events in adalimumab-treated patients with early disease [duration <2 years] compared with longer-established CD. That analysis was limited to one trial and 777 patients, which contrasts with the more extensive analyses we report here capturing data from across 10 clinical trials and featuring 640 patients receiving induction [adalimumab, n = 377; placebo, n = 263] and 2325 patients receiving adalimumab maintenance.
Recent acknowledgement that CD is a progressive destructive disease has extended the focus of therapeutic strategies from control of symptoms and improved quality of life to new goals, such as mucosal healing and bowel preservation.3,20Post hoc analysis of the EXTEND study [one of the 10 trials evaluated in our pooled analysis], evaluating the effect of adalimumab on endoscopic healing in 129 patients with moderately to severely active CD, revealed that deep remission [defined as absence of mucosal ulceration and CDAI <150] was seen most frequently for patients receiving adalimumab with disease duration <2 years at Weeks 12 and 52 compared with those who had longer disease duration.22 Importantly for patients, the benefits of deep remission included fewer treatment adjustments, fewer hospitalisations and CD-related surgeries, less activity impairment, and better quality of life and physical function. In addition, recent results from CALM,3 containing a study arm featuring timely escalation with anti-TNF therapy based on clinical symptoms combined with biomarkers in patients with early CD [mean disease duration 0.9 years], support the notion that patients with recent onset of disease benefit from early biologic treatment.
The impact of disease duration on efficacy has also been studied for other biologics, including other anti-TNFs, although the findings have been inconsistent and the studies included smaller numbers of patients than the present analysis.8,19,23–29 An association of infliximab therapeutic efficacy with disease duration was supported by post hoc analysis of SONIC, which concluded that deep remission is achievable, particularly in combination with azathioprine, in a population of patients with relatively short duration of CD [median = 2.3 years].23 Early intervention with infliximab combination therapies has been associated with beneficial effects on corticosteroid use,3,23–25 the need for surgical intervention,23,25 and on mucosal ulceration.23–25 In contrast, post hoc analyses of other infliximab studies have not found a relationship between clinical outcome and disease duration.27,29 A mixed set of findings also applies for another anti-TNF, certolizumab pegol. Schreiber et al.8 reported that patients with CD treated with certolizumab pegol for <1 year had significantly higher response rates versus patients with disease duration of ≥5 years. However, the data for clinical remission which followed this trend did not reach statistical significance, probably due to the limited sample size. Two other certolizumab pegol studies which, however, either failed the primary endpoint or had other limitations in the overall strength of the clinical signal, did not demonstrate an association between short-term clinical remission and disease duration19,26 although disease duration was reported as a predictor of long-term maintenance of remission from one of these studies.26
In summary, the findings we report here demonstrate an association between initiating adalimumab therapy early after a CD diagnosis and improved clinical benefit. The concept of target-based decisive earlier treatment features is included in the latest European Crohn’s and Colitis Organisation [ECCO] guideline consensus paper for treatment of patients with CD4 and in recommendations from the Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE] programme.30 The ECCO guidelines highlight that induction of clinical remission is a desirable target for every patient, to be coupled with a need to consider how remission will be maintained over the longer term.4 The STRIDE recommendations include treat-to-target clinical management strategies in CD, indicating that in future the term ‘remission’ should consider resolution of both symptoms and mucosal inflammation.30
There are some limitations of our study design and analytical methods that should be noted. First, we could not measure deep remission in this pooled analysis because only the EXTEND trial had endoscopic data.22 Second, the analysis was not limited to patients who responded to induction treatment. This may have resulted in an overall reduced estimate of efficacy, as patients in clinical practice who do not respond to any therapy within the first several months do not typically continue to receive that therapy. Third, baseline characteristics, such as age and previous anti-TNF use, differed across disease-duration subgroups. Although these differences are expected, given the progressive nature of CD, they may have also influenced treatment responsiveness in favour of patients with shorter disease duration, as discussed above. Fourth, because the designs of the studies included in this analysis differed [some studies had an open-label adalimumab induction period, were open-label in nature, or lacked a placebo comparator arm], the disease-duration comparisons for the maintenance periods could not be analysed by randomised treatment group; hence observations of statistical significance between the adalimumab disease-duration groups should be interpreted with caution.
In addition, our analysis covers a relatively short treatment time frame, up to 1 year across the studies, and therefore does not provide data on long-term impact of treatment on bowel damage and disability. In contrast, the preceding study of CHARM by Schreiber et al.9 included longer-term remission rates [3 years]. A further limitation of this analysis is that time to diagnosis was not collected in the trials, so any difference between the time of diagnosis and trial baseline may have underestimated the actual duration of disease in each subgroup. Also, the majority of patients in this analysis had disease duration >5 years, and therefore the shorter-duration subgroups are relatively small. Finally, our study is a post hoc analysis with data pooled across multiple clinical studies, and the impact of early therapy remains to be demonstrated in prospective studies.
In conclusion, our analysis in patients with moderately to severely active CD shows an association between improved efficacy and the introduction of adalimumab therapy shortly following diagnosis.
Funding
This work was supported by AbbVie, who funded the studies and the analysis, provided medical writing support, and reviewed and approved the publication.
Conflict of Interest
RP reports consultant and/or lecture fees from AbbVie, Amgen, AstraZeneca, Axcan Pharma [now Aptalis], Biogen Idec, Bristol-Myers Squibb, Centocor, ChemoCentryx, Eisai Medical Research Inc., Elan Pharmaceuticals, Ferring, Genentech, GlaxoSmithKline, Janssen, Merck Sharp & Dohme Corp., Millennium Pharmaceuticals Inc. [now Takeda], Ocera Therapeutics Inc., Otsuka America Pharmaceutical, Pfizer, Shire Pharmaceuticals, Prometheus Laboratories, Schering-Plough Corporation, Synta Pharmaceuticals Corp., Teva, UCB Pharma, and Warner Chilcott. RL has received honoraria for lectures or consultation from Abbott, Asahi-Kasei, AstraZeneca, Celltech, Centocor, Connexion, Cosmo, Elan, InDex Pharmaceuticals, Meda, Otsuka, Pharmacia-Pfizer, Schering-Plough, Schering AG, Serono, and UCB; and was partly supported by a research grant from Sophiahemmet and the Stichting af Jochnick Foundation. PR reports grants and/or personal fees from AbbVie, Bristol-Myers Squibb, Centocor, Merck, Takeda, UCB Pharma, and MSD. WJS reports consulting fees from the University of Western Ontario [owner of Robarts Clinical Trials, Inc.], AbbVie, Akros Pharma, Allergan, Ambrx Inc., Amgen, Ardelyx, Arena Pharmaceuticals, Atlantic Pharmaceuticals, Avaxia, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Conatus, Cosmo Technologies, Escalier Biosciences, Ferring, Ferring Research Institute, Forward Pharma, Galapagos, Genentech, Gilead Sciences, Immune Pharmaceuticals, Index Pharmaceuticals, Janssen, Kyowa Hakko Kirin Pharma, Lilly, Medimmune, Mesoblast, Miraca Life Sciences, Nivalis Therapeutics, Novartis, Nutrition Science Partners, Oppilan Pharma, Otsuka, Palatin, Paul Hastings, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Qu Biologics, Regeneron, Ritter Pharmaceuticals, Robarts Clinical Trials, Salix, Seattle Genetics, Seres Therapeutics, Shire, Sigmoid Biotechnologies, Takeda, Theradiag, Theravance, TiGenix, Tillotts Pharma, UCB Pharma, Vascular Biogenics, and Vivelix; research grants from Atlantic Healthcare Ltd, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen, Takeda, Lilly, and Celgene/Receptos; payments for lectures/speakers’ bureau from AbbVie, Janssen, and Takeda; and holds stock/stock options in Escalier Biosciences, Oppilan Pharma, Precision IBD, Progenity, and Ritter Pharmaceuticals. SS reports personal fees from AbbVie, Falk Pharma, Ferring, Genentech, GSK, MSD, Pfizer, Shire, and Takeda. JFC reports receiving research grants from AbbVie, Janssen Pharmaceuticals, and Takeda; receiving payment for lectures from AbbVie, Amgen, Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Celgene Corp., Celltrion, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Eli Lilly, Medimmune, Merck, Novartis, Pfizer, Protagonist Therapeutics, Sandoz, Second Genome, Seres Therapeutics, Shire, Takeda, Theradiag, and Theravance Biopharma; and holds stock options in Intestinal Biotech Development and Genfit. SB, JFM, JP, and AMR are AbbVie employees and may own AbbVie stock and/or options.
Author Contributions
RP, RL, PR, WJS, SS, and JFC were involved in acquisition of data. RP, RL, PR, WJS, SS, JFC, SB, JFM, JP, and AMR were involved in the concept and design of the study. RP, RL, PR, WJS, SS, JFC, SB, JFM, JP, and AMR analysed and interpreted the study data. All authors critically reviewed the content of this manuscript and approved final submission.
Supplementary Material
Acknowledgements
Medical writing support for development of this manuscript was provided by Kevin Hudson, PhD, of 2 the Nth funded by AbbVie.
References
- 1. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 3. Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology 2017;152:351–61.e5. [DOI] [PubMed] [Google Scholar]
- 4. Gomollón F, Dignass A, Annese V, et al. ; ECCO. Third European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016. Part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 5. Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology 2015;148:344–54.e5. [DOI] [PubMed] [Google Scholar]
- 6. Rutgeerts P, Van Assche G, Sandborn WJ, et al. ; EXTEND Investigators. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012;142:1102–11.e2. [DOI] [PubMed] [Google Scholar]
- 7. Allen PB, Olivera P, Emery P, et al. Review article: moving towards common therapeutic goals in Crohn’s disease and rheumatoid arthritis. Aliment Pharmacol Ther 2017;45:1058–72. [DOI] [PubMed] [Google Scholar]
- 8. Schreiber S, Colombel JF, Bloomfield R, et al. ; PRECiSE 2 Study Investigators. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 2010;105:1574–82. [DOI] [PubMed] [Google Scholar]
- 9. Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohns Colitis 2013;7:213–21. [DOI] [PubMed] [Google Scholar]
- 10. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody [adalimumab] in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006;130:323–33; quiz 591. [DOI] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007;56:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 2007;146:829–38. [DOI] [PubMed] [Google Scholar]
- 14. Panaccione R, Colombel JF, Sandborn WJ, et al. Adalimumab maintains remission of Crohn’s disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther 2013;38:1236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watanabe M, Hibi T, Lomax KG, et al. ; Study Investigators. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. J Crohns Colitis 2012;6:160–73. [DOI] [PubMed] [Google Scholar]
- 16. Löfberg R, Louis EV, Reinisch W, et al. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn’s disease: results from CARE. Inflamm Bowel Dis 2012;18:1–9. [DOI] [PubMed] [Google Scholar]
- 17. Panaccione R, Loftus EV Jr, Binion D, et al. Efficacy and safety of adalimumab in Canadian patients with moderate to severe Crohn’s disease: results of the Adalimumab in Canadian SubjeCts with ModErate to Severe Crohn’s DiseaSe [ACCESS] trial. Can J Gastroenterol 2011;25:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colombel JF, Sandborn WJ, Reinisch W, et al. Long-term safety of adalimumab in clinical trials in adult patients with Crohn’s disease or ulcerative colitis. Aliment Pharmacol Ther 2018;47:219–28. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, Abrue MT, D’Haens G, et al. Predictors of response and remission to certolizumab pegol in patients with Crohn’s disease: data from the WELCOME study. Gastroenterology 2010;138:(5) (Supp1):S164. https://www.gastrojournal.org/article/S0016-5085(10)60750-0/fulltext [Google Scholar]
- 20. Feagan BG, Lémann M, Befrits R, et al. Recommendations for the treatment of Crohn’s disease with tumor necrosis factor antagonists: an expert consensus report. Inflamm Bowel Dis 2012;18:152–60. [DOI] [PubMed] [Google Scholar]
- 21. Pariente B, Mary JY, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology 2015;148:52–63.e3. [DOI] [PubMed] [Google Scholar]
- 22. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:414–22.e5. [DOI] [PubMed] [Google Scholar]
- 23. Colombel JF, Reinisch W, Mantzaris GJ, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn’s disease - a SONIC post hoc analysis. Aliment Pharmacol Ther 2015;41:734–46. [DOI] [PubMed] [Google Scholar]
- 24. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 25. D’Haens G, Baert F, van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660–7. [DOI] [PubMed] [Google Scholar]
- 26. Sandborn WJ, Melmed GY, McGovern DP, et al. Clinical and demographic characteristics predictive of treatment outcomes for certolizumab pegol in moderate to severe Crohn’s disease: analyses from the 7-year PRECiSE 3 study. Aliment Pharmacol Ther 2015;42:330–42. [DOI] [PubMed] [Google Scholar]
- 27. Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut 2009;58:492–500. [DOI] [PubMed] [Google Scholar]
- 28. Schreiber S. Efficacy of natalizumab in Crohn’s patients with disease duration less than three years. Gastroenterology 2007;132:A509. [Google Scholar]
- 29. Vermeire S, Louis E, Carbonez A, et al. ; Belgian Group of Infliximab Expanded Access Program in Crohn’s Disease. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor [infliximab] treatment in Crohn’s disease. Am J Gastroenterol 2002;97:2357–63. [DOI] [PubMed] [Google Scholar]
- 30. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease [STRIDE]: determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110: 1324–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


