Fosfomycin combined with other antimicrobials has shown good efficacy against multidrug-resistant (MDR) bacteria in both in vitro and clinical studies; however, the activity of fosfomycin combined with other antimicrobials against metallo-β-lactamase (MBL)-producing Pseudomonas aeruginosa strains has not been tested. The objective of this study was to determine the synergism and optimal intravenous dosing regimens of fosfomycin with meropenem against MDR and MBL-producing P. aeruginosa strains.
KEYWORDS: antimicrobial combinations, fosfomycin, MBL-producing Pseudomonas aeruginosa, meropenem, multidrug-resistant bacteria, pharmacodynamics
ABSTRACT
Fosfomycin combined with other antimicrobials has shown good efficacy against multidrug-resistant (MDR) bacteria in both in vitro and clinical studies; however, the activity of fosfomycin combined with other antimicrobials against metallo-β-lactamase (MBL)-producing Pseudomonas aeruginosa strains has not been tested. The objective of this study was to determine the synergism and optimal intravenous dosing regimens of fosfomycin with meropenem against MDR and MBL-producing P. aeruginosa strains. The MICs of both antimicrobials were determined by the checkerboard method and analyzed by two synergism tests with 19 clones of P. aeruginosa isolates, 10 of which were MBL producers. A pharmacodynamic (PD) analysis was performed for meropenem (administered at 1 g every 8 h [q8h], 1.5 g every 6 h [q6h], and 2 g q8h) and fosfomycin (administered at 4 g q8h, 4 g q6h, 6 g q8h, and 8 g q8h) regimens with a dose reduction for renal impairment by determining the probability of target attainment (PTA) for target PD indices of meropenem (the percentage of the time in a 24-h duration at which the free drug concentration remains above the MIC [fT>MIC], ≥40%) and fosfomycin (the ratio of the area under the free drug concentration-versus-time curve over 24 h and the MIC [fAUC/MIC], ≥40.8). The combination reduced the MIC50 and MIC90 by 8-fold. Seven (44%) isolates with MICs in the intermediate or resistant ranges became sensitive to meropenem. For the MBL-producing isolates, the combination resulted in 40% of isolates becoming sensitive to meropenem. The meropenem regimens reached a PTA of ≥90% (MIC = 4 μg/ml) in 6 (32%) isolates when they were used as monotherapy and 13 (68%) isolates when they were combined with fosfomycin. None of the fosfomycin monotherapy regimens reached the PTA of ≥90% (MIC = 16 μg/ml). When combined with meropenem, the fosfomycin regimens reached the PTA of ≥90% in 14 (74%) isolates. The increase in pharmacodynamic activities resulting from the synergistic action of meropenem with fosfomycin demonstrates the potential relevance of this combination to fight infections caused by MDR and MBL-producing P. aeruginosa strains.
INTRODUCTION
Being an opportunistic, nonfermenting Gram-negative bacillus, Pseudomonas aeruginosa is a common cause of nosocomial infections mainly in immunocompromised patients with various infections, including pneumonia, abscesses, meningitis, urinary tract infections, and catheter-associated infections (1). The adaptive capacity of this pathogen that allows it to survive in low-nutrient medium, the presence of several mechanisms of resistance to many antimicrobials (including intrinsic, acquired, or adaptive resistance) and virulence factors, and its capacity to form biofilms favor its survival in various media and contribute to its high pathogenicity and the high mortality rates from infections with this organism seen in infected patients (2, 3).
Among the various types of resistance produced by P. aeruginosa, the enzymatic mechanism induced by metallo-β-lactamases (MBLs) is the most challenging one to deal with because MBLs are capable of hydrolyzing carbapenems, yet there is no clinically effective antimicrobial to combat MBL-producing pathogens. MBLs belong to the class B β-lactamases, which confer drug resistance to the majority of β-lactam antimicrobials (except aztreonam) by hydrolysis through the divalent metals. Characteristics like carbapenem degradation, β-lactamase inhibitor resistance, high-capacity gene transfer through plasmids and transposons, and, most importantly, the lack of effective therapeutic options make infections caused by pathogens carrying MBLs a tremendous challenge to treat (4, 5).
Combination therapy provides an alternative to the reduced arsenals of effective antimicrobials used as monotherapy (6). Some studies have shown that use of a combination of two or more types of antimicrobial drugs results in a synergistic effect, reducing the risk of an inappropriate empirical therapy and resistance development. Currently, combination therapy is primarily recommended against carbapenemase-producing bacteria, including nonfermenting microorganisms, such as P. aeruginosa (7–10). However, the literature is still lacking on the choice of effective antimicrobial combinations to be used against MBL-producing P. aeruginosa strains. Among several potential combinations, meropenem with fosfomycin can achieve good microbiological results (10, 11). There are clinical studies that utilized antimicrobial combinations with fosfomycin against multidrug-resistant (MDR) P. aeruginosa and demonstrated a 90% therapeutic success rate (12).
Meropenem binds to penicillin binding proteins in the periplasmic space, preventing peptidoglycan biosynthesis and viable cell wall production (13). Fosfomycin binds to the enzyme UDP-N-acetylglucosamine enolpyruvyl transferase, preventing the production of peptidoglycan and inhibiting cell wall biosynthesis (14, 15). The inhibition of peptidoglycan production by meropenem and fosfomycin at different stages of its biosynthesis and the rupture of the outer membrane can theoretically result in the synergism of the activities of these two antimicrobials. Faced with the scarcity of antimicrobials effective against MBL-producing microorganisms, the aim of this study was to evaluate by Monte Carlo simulation the pharmacodynamic (PD) attainment of intravenous (i.v.) dosing regimens of fosfomycin and meropenem in combination against non-MBL- and MBL-producing P. aeruginosa clinical isolates.
RESULTS
In vitro susceptibility and test for synergism.
The collection of isolates tested consisted of 19 P. aeruginosa clinical isolates of different clones identified by pulsed-field gel electrophoresis (PFGE) or enterobacterial repetitive intergenic consensus PCR (ERIC-PCR). Ten of these isolates (9 isolates from this study and 1 isolate from another study [16]) were MBL producers, as characterized by multiplex PCR.
Table 1 presents the profiles of the sensitivities of the isolates to meropenem and fosfomycin in monotherapy or combination therapy, as well as the results of the synergism tests. The isolates showed meropenem and fosfomycin MIC values ranging from 0.25 to 1,024 μg/ml and 32 to 512 μg/ml, respectively. The MIC50 and MIC90 were 16 and 512 μg/ml, respectively, for meropenem, and 128 and 512 μg/ml, respectively, for fosfomycin.
TABLE 1.
MICs of meropenem and fosfomycin alone or in combination against non-MBL and MBL-producing Pseudomonas aeruginosa clinical isolates by FICI and Loewe additivity index analysisa
| P. aeruginosa isolate | Mero |
Fosfo |
Synergism analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) |
Fold reduction in Mero MIC | MIC (μg/ml) |
Fold reduction in Fosfo MIC | |||||||
| Mero alone | Mero in combination | Fosfo alone | Fosfo in combination | FICI | S or I based on FICI | Loewe additivity index | S or A based on Loewe additivity index | |||
| PA-73 | 4 | 0.5 | 8 | 128 | 16 | 8 | 0.25 | S | 0.75 | S |
| PA-87 | 4 | 2 | 2 | 128 | 32 | 4 | 0.75 | I | 0.25 | S |
| PA-116 | 16 | 1 | 16 | 64 | 8 | 8 | 0.19 | S | 0.81 | S |
| PA-106 | 16 | 4 | 4 | 64 | 4 | 16 | 0.50 | S | 0.69 | S |
| PA-146 | 16 | 4 | 4 | 32 | 8 | 4 | 0.50 | S | 0.50 | S |
| PA-149 | 128 | 64 | 2 | 128 | 16 | 8 | 0.62 | I | 0.38 | S |
| PA-114 | 0.50 | 0.25 | 2 | 64 | 8 | 8 | 0.62 | I | 0.38 | S |
| PA-64 | 0.25 | 0.06 | 4 | 32 | 8 | 4 | 0.49 | S | 0.51 | S |
| PA-69 | 0.50 | 0.50 | 128 | 128 | 2.00 | I | −1 | A | ||
| PA-30b | 16 | 4 | 4 | 512 | 8 | 64 | 0.26 | S | 0.73 | S |
| PA-43b | 4 | 0.25 | 8 | 512 | 1 | 512 | 0.06 | S | 0.90 | S |
| PA-314b | 8 | 2 | 4 | 128 | 4 | 32 | 0.28 | S | 0.72 | S |
| PA-524b | 8 | 1 | 8 | 64 | 16 | 4 | 0.37 | S | 0.62 | S |
| PA-13b | 512 | 128 | 4 | 64 | 16 | 4 | 0.50 | S | 0.50 | S |
| PA-525b | 1,024 | 32 | 32 | 128 | 16 | 8 | 0.16 | S | 0.85 | S |
| PA-573b | 128 | 8 | 16 | 512 | 64 | 8 | 0.19 | S | 0.81 | S |
| PA-377b | 256 | 32 | 8 | 128 | 32 | 4 | 0.37 | S | 0.63 | S |
| PA-170b | 8 | 1 | 8 | 128 | 32 | 4 | 0.37 | S | 0.63 | S |
| PA-GIMb | 512 | 64 | 8 | 256 | 16 | 16 | 0.19 | S | 0.81 | S |
| MIC50 | 16 | 2 | 128 | 16 | ||||||
| MIC90 | 512 | 64 | 512 | 64 | ||||||
Mero, meropenem; Fosfo, fosfomycin; FICI, fractional inhibitory concentration index; S, synergy; I, indifferent; A, antagonistic.
Metallo-β-lactamase-producing isolate.
Only three (16%) isolates (PA-114, PA-64, PA-69) were sensitive to meropenem as monotherapy; of the MBL-producing isolates, none were sensitive to meropenem, according to the breakpoints of the Clinical and Laboratory Standards Institute (CLSI). The antimicrobials in combination significantly reduced the MIC values of 18 (95%) isolates up to 1/32 and 1/512 of the monotherapy MICs for meropenem and fosfomycin, respectively, as well as reduced the MIC50 and MIC90 by 1/8. The reduction in the MIC values resulting from use of the antimicrobials in combination was quite pronounced, such that it shifted the MICs for isolates previously classified as resistant and intermediate in monotherapy to within the sensitive range. Of the 16 isolates with MIC values greater than the susceptible breakpoints for meropenem, 7 (44%) isolates presented MIC values less than or equal to the susceptible breakpoint values, when the two antimicrobials were combined. The MBL-producing isolates showed a greater reduction of MIC values with the two-agent combination than the non-MBL-producing isolates, resulting in a reduction of MIC values of up to 32- and 512-fold for meropenem and fosfomycin, respectively, against the MBL-producing isolates. However, among the non-MBL-producing isolates, the maximum MIC decrease was only 16-fold for the two antimicrobials.
The combination of meropenem and fosfomycin exhibited synergistic action against the majority of the MBL and non-MBL producers, as evaluated by the use of two criteria for synergism. For the 19 isolates, analysis of the fractional inhibitory concentration index (FICI) showed synergism for 15 (79%) isolates, indifference for 4 (21%) isolates, and antagonism for none of the isolates, while use of the Loewe additivity index criterion resulted in synergism for 18 (95%) isolates and antagonism for only 1 isolate.
Pharmacokinetic (PK)-pharmacodynamic simulation.
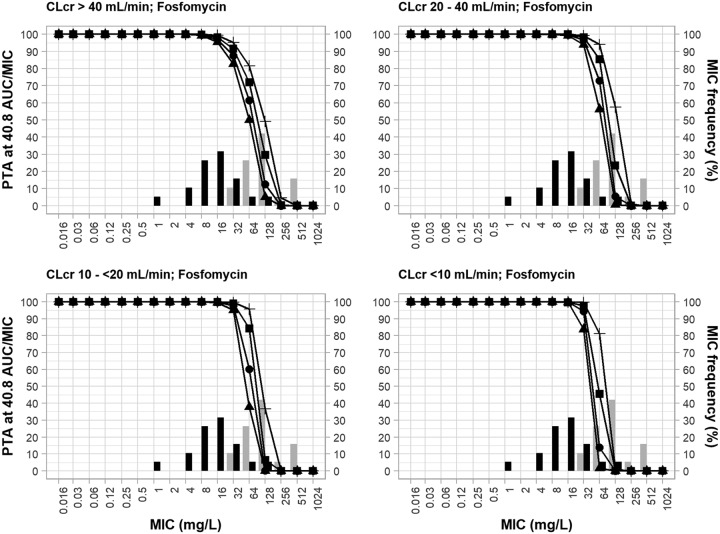
Figure 1 shows the probability of target attainment (PTA) (the area under the free drug concentration-versus-time curve over 24 hours [ƒAUC]/MIC ratio ≥ 40.8) achieved by the 4 different fosfomycin intravenous regimens (4 g every 8 h [q8h], 4 g every 6 h [q6h], 6 g q8h, 8 g q8h) and the reduced doses used according to the stage of renal impairment based on the creatinine clearance (CLCR) range (20 to 40 ml/min, 10 to <20 ml/min, and <10 ml/min [resulting dose reductions, 30%, 50%, and 70%, respectively]) against the MIC values and the respective frequencies of the isolates in both monotherapy and combination therapy.
FIG 1.
MIC frequency of 19 P. aeruginosa clinical isolates (non-MBL producing and MBL producing) in incremental fosfomycin MICs in monotherapy and in combination therapy with meropenem and the probability of target attainment of an fAUC/MIC of ≥40.8 for fosfomycin dosing regimens. PTA, probability of target attainment; AUC, area under the concentration-time curve; CLCR, creatinine clearance; black bars, fosfomycin MICs for monotherapy; gray bars, fosfomycin MICs for combination therapy. The fosfomycin dosing regimens are represented by lines and symbols, as follows: for CLCRs of >40 ml/min, ▲, 4 g q8h; ●, 4 g q6h; ■, 6 g q8h; +, 8 g q8h; for CLCRs of 20 to 40 ml/min, ▲, 2.8 g q8h; ●, 2.8 g q6h; ■, 4.2 g q8h; +, 5.6 g q8h; for CLCRs of 10 to <20 ml/min, ▲, 2 g q8h; ●, 2 g q6h; ■, 3 g q8h; +, 4 g q8h; for CLCRs of <10 ml/min, ▲, 1.2 g q8h; ●, 1.2 g q6h; ■, 1.8 g q8h; +, 2.4 g q8h.
For patients with a CLCR of >40 ml/min, all fosfomycin regimens reached the PTA of ≥90% at an MIC of 16 μg/ml, and the two biggest fosfomycin monotherapy dosing regimens achieved the PTA of ≥90% at an MIC of 32 μg/ml. The synergistic action of the combined fosfomycin and meropenem regimens was evident. The fosfomycin regimens (6 g q8h, 8 g q8h) as monotherapy achieved the PTA of ≥90% at an MIC of 32 μg/ml against only 2 (11%) isolates; however, when combined with meropenem, the same regimens achieved the PTA of ≥90% at an MIC of 32 μg/liter against 17 (89%) isolates. Reductions of the fosfomycin doses in patients with renal impairment (CLCR ≤ 40 ml/min) did not reduce the MIC values to reach PTAs of ≥90%; rather, higher MIC breakpoints were achieved. As an example, the fosfomycin regimen of 8 g q8h for a CLCR of >40 ml/min reached the PTA of >90% at an MIC of 32 μg/ml; when the doses were reduced by 30% and 50% for patients with CLCR ranges of 20 to 40 ml/min and 10 to <20 ml/min, respectively, the PTA of ≥90% was achieved at an MIC of 64 μg/ml.
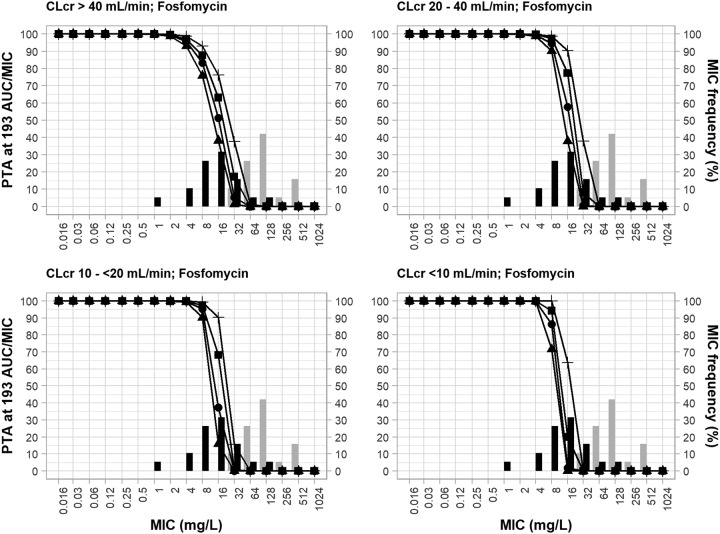
Figure 2 shows the PTA achieved by fosfomycin in a worst-case scenario (fAUC/MIC ≥ 193) with the same fosfomycin regimens and reduced daily doses in patients in each CLCR category used in the experiment whose results are presented in Fig. 1 in both the monotherapy and combination settings. Considering that the evaluated target was almost 5 times higher than the original target (fAUC/MIC ≥ 40.8), the PTA achieved in this scenario was much lower, as expected. For patients with a CLCR of >40 ml/min, the fosfomycin regimens reached the PTA of ≥90% only at an MIC value of 4 μg/ml, and only the higher-dose regimen (fosfomycin at 8 g q8h) achieved the PTA of ≥90% at an MIC of 8 μg/ml. The coverage of the monotherapy or combination therapy regimens, including the doses adjusted for lower CLCR ranges, against the isolates was also significantly reduced.
FIG 2.
MIC frequency of 19 P. aeruginosa clinical isolates (non-MBL producing and MBL producing) in incremental fosfomycin MICs in monotherapy and in combination therapy with meropenem and probability of target attainment of an fAUC/MIC of ≥193 (worst-case scenario) for fosfomycin dosing regimens. PTA, probability of target attainment; AUC, area under the concentration-time curve; CLCR, creatinine clearance; black bars, MICs for fosfomycin monotherapy; gray bars, MICs for fosfomycin combination therapy. The fosfomycin dosing regimens are represented by lines and symbols, as follows: for CLCRs of >40 ml/min, ▲, 4 g q8h; ●, 4 g q6h; ■, 6 g q8h; +, 8 g q8h; for CLCRs of 20 to 40 ml/min, ▲, 2.8 g q8h; ●, 2.8 g q6h; ■, 4.2 g q8h; +, 5.6 g q8h; for CLCRs of 10 to <20 ml/min, ▲, 2 g q8h; ●, 2 g q6h; ■, 3 g q8h; +, 4 g q8h; for CLCRs of <10 ml/min, ▲, 1.2 g q8h; ●, 1.2 g q6h; ■, 1.8 g q8h; +, 2.4 g q8h.
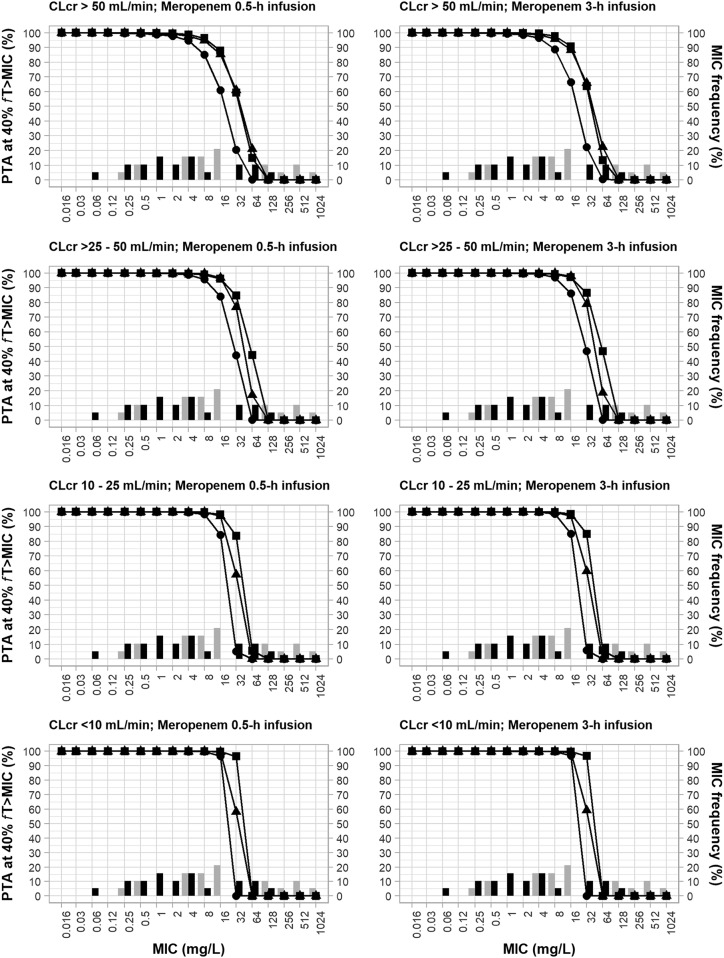
Figure 3 shows the PTAs achieved by the different meropenem regimens used for the respective CLCR ranges (1 g q8h, 1.5 g q6h, and 2 g q8h for CLCRs of >50 ml/min; 1 g every 12 h [q12h], 1 g q6h, and 2 g q12h for CLCRs of >25 to 50 ml/min; 0.5 g q12h, 0.5 g q6h, and 1 g q12h for CLCRs of 10 to 25 ml/min; and 0.5 g every 24 h (q24h), 0.5 g q12h, and 1 g q24h for CLCRs of <10 ml) infused over 0.5 h or 3 h against the incremental MIC values and the respective frequency of the isolates in both monotherapy and combination therapy.
FIG 3.
MIC frequency of 19 P. aeruginosa clinical isolates (non-MBL producing and MBL producing) in incremental meropenem MICs in monotherapy and in combination therapy with fosfomycin and probability of target attainment of an fT>MIC of 40% for meropenem dosing regimens. (Left) 0.5-h infusion regimens; (right) 3-h infusion regimens. PTA, probability of target attainment; fT>MIC, the percentage of the time in a 24-h duration at which the free drug concentration remains above the MIC; CLCR, creatinine clearance; black bars, MICs for meropenem monotherapy; gray bars, MICs for meropenem combination therapy. The meropenem dosing regimens are represented by lines and symbols, as follows: for CLCRs of >50 ml/min, ●, 1 g q8h; ▲, 1.5 g q6h; ■, 2 g q8h; for CLCRs of >25 to 50 ml/min, ●, 1 g q12h; ▲, 1 g q6h; ■, 2 g q12h; for CLCRs of 10 to 25 ml/min, ●, 0.5 g q12h; ▲, 0.5 g q6h; ■, 1 g q12h; for CLCRs of <10 ml/min, ●, 0.5 g q24h; ▲, 0.5 g q12h; ■, 1 g q24h.
In patients with CLCRs of >50 ml/min, in whom a dose reduction was not required, all evaluated regimens achieved the PTA of ≥90% at an MIC of 4 μg/ml, which is considered an intermediate breakpoint according to CLSI criteria. The two highest-dose regimens administered as a 0.5-h or a 3-h infusion achieved the PTA of ≥90% at an MIC of 8 μg/ml (the resistance breakpoint, according to CLSI), and the meropenem regimen of 1.5 g q6h given as a 3-h infusion achieved the PTA of ≥90% at an MIC of 16 μg/ml, which is considered a high MIC value. The synergism of the combination meropenem regimens was also evidenced in these pharmacodynamic analyses. All meropenem monotherapy regimens achieved the PTA of ≥90% against only 6 (32%) isolates at an MIC of 4 μg/ml; however, when combined with fosfomycin, these regimens reached the PTA of ≥90% at the same MIC value against 13 (68%) isolates. The meropenem dose regimens (1.5 g q6h, 2 g q8h) as monotherapy achieved the PTA of ≥90% at MICs of up to 8 μg/ml against 9 (47%) isolates; however, when combined, they achieved the PTA of ≥90% at the same MIC value against 14 (74%) isolates.
The reduction in the daily dose of meropenem in patients with CLCRs of ≤50 ml/min did not result in a decrease in pharmacodynamic coverage; instead, there was even some improvement. Of the meropenem regimens infused over 0.5 h evaluated in patients with a CLCR of >50 ml/min, the PTA of ≥90% was achieved for a maximum MIC of 8 μg/ml; however, in patients with a CLCR of <10 ml/min, for whom daily doses of meropenem infused over 0.5 h were significantly reduced, all regimens nevertheless achieved the PTA of ≥90% at an MIC of 16 μg/ml.
Table 2 shows the PTAs achieved by the monotherapy and combination therapy regimens for the pharmacodynamic indices for fosfomycin of an fAUC/MIC of 40.8 (CLCR > 40 ml/min) and for meropenem of a percentage of the time in a 24-h duration at which the free drug concentration remains above the MIC (fT>MIC) of 40% (CLCR >50 ml/min) at the MIC50 and MIC90 for the collection of multidrug-resistant P. aeruginosa isolates. None of the monotherapy regimens reached the appropriate PTA (≥90%) evaluated at the MIC50 and MIC90. However, in combination, the three highest-dose fosfomycin regimens and all meropenem regimens evaluated at the MIC50 achieved PTAs of ≥90%. No fosfomycin or meropenem regimen evaluated at the MIC90 achieved the PTA of >90%.
TABLE 2.
PTAs at PD surrogate indices for fosfomycin and meropenem against non-MBL- and MBL-producing P. aeruginosa clinical isolates by dosing regimen for monotherapy and combination therapya
| Antimicrobial and regimen | Total daily dose (g) | MIC50/MIC90 (μg/ml) |
PTA (%) |
||||
|---|---|---|---|---|---|---|---|
| Monotherapy |
Combination therapy |
||||||
| Monotherapy | Combination therapy | MIC50 | MIC90 | MIC50 | MIC90 | ||
| Fosfomycin | 128/512 | 16/64 | |||||
| 4 g i.v. q8h | 12 | 0 | 0 | 89 | 16 | ||
| 4 g i.v. q6h | 16 | 0.4 | 0 | 94 | 33 | ||
| 6 g i.v. q8h | 18 | 3.1 | 0 | 96 | 46 | ||
| 8 g i.v. q8h | 24 | 16 | 0 | 98 | 64 | ||
| Meropenem (0.5-h infusion) | 16/512 | 2/64 | |||||
| 1 g q8h | 3 | 61 | 0 | 98 | 2.5 | ||
| 1.5 g q6h | 6 | 87 | 0 | 99 | 14 | ||
| 2 g q8h | 6 | 85 | 0 | 99 | 21 | ||
| Meropenem (3-h infusion) | |||||||
| 1 g q8h | 3 | 67 | 0 | 99 | 4.1 | ||
| 1.5 g q6h | 6 | 91 | 0 | 100 | 13 | ||
| 2 g q8h | 6 | 88 | 0 | 100 | 22 | ||
The pharmacodynamic surrogate index for fosfomycin was an fAUC/MIC of 40.8 for a CLCR of >40 ml/min, and that for meropenem was an ƒT>MIC of 40% for a CLCR of >50 ml/min.
Table 3 shows the cumulative fraction of the response (CFR; defined as the sum of the frequency of isolates at each MIC multiplied by the PTA) for each dosing regimen of fosfomycin at an fAUC/MIC of 40.8 (CLCR > 40 ml/min) and meropenem at an fT>MIC of 40% (CLCR > 50 ml/min) as monotherapy and combination therapy. The meropenem or fosfomycin regimens evaluated as monotherapy provided a probability of a CFR of no more than 65%. However, all combined fosfomycin regimens provided a CFR of ≥81%; the regimen of 8 g q8h resulted in a CFR of 91%. The four meropenem regimens (1.5 g q6h and 2 g q8h as a 0.5-h infusion, 1.5 g q6h and 2 g q8h as a 3-h infusion) resulted in a CFR of ≥79%, based on the distribution of MIC values in the combination with fosfomycin.
TABLE 3.
CFR for fosfomycin and meropenem against non-MBL and MBL-producing P. aeruginosa clinical isolates by dosing regimens in monotherapy and combination therapya
| Antimicrobial and regimen | Total daily dose (g) | CFR (%) |
|
|---|---|---|---|
| Monotherapy | Combination therapy | ||
| Fosfomycin | |||
| 4 g i.v. q8h | 12 | 11 | 81 |
| 4 g i.v. q6h | 16 | 17 | 86 |
| 6 g i.v. q8h | 18 | 22 | 88 |
| 8 g i.v. q8h | 24 | 33 | 91 |
| Meropenem (0.5-h infusion) | |||
| 1 g q8h | 3 | 53 | 72 |
| 1.5 g q6h | 6 | 63 | 79 |
| 2 g q8h | 6 | 62 | 79 |
| Meropenem (3-h infusion) | |||
| 1 g q8h | 3 | 56 | 73 |
| 1.5 g q6h | 6 | 65 | 80 |
| 2 g q8h | 6 | 64 | 80 |
The pharmacodynamic surrogate index for fosfomycin was an fAUC/MIC of 40.8 for a CLCR of >40 ml/min, and that for meropenem was an ƒT>MIC of 40% for a CLCR of >50 ml/min.
Table 4 shows the PTA (in percent) provided by the meropenem regimens at an fT>MIC of 40% according to the MICs, the duration of intravenous infusion (0.5 h or 3 h), and creatinine clearance. For example, when the meropenem regimen of 1 g q8h as a 0.5-h infusion was increased to 1.5 g q6h as a 3-h infusion in patients with a CLCR of >50 ml/min, a significant increase in PTA from 61.6% to 90.7% against isolates for which the MIC was 16 μg/ml was seen. However, it was noticeable that the effect of a prolonged infusion on PTA became less important with a reduction in CLCR values.
TABLE 4.
PTA surrogate índices for meropenem at fT>MIC of 40% relative to MIC, duration of i.v. infusion (0.5 h and 3 h), and CLCR
| CLCR (ml/min) | Dose regimen | PTA (%) at the following MIC and infusion duration: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 μg/ml |
1 μg/ml |
2 μg/ml |
4 μg/ml |
8 μg/ml |
16 μg/ml |
||||||||
| 0.5 h | 3 h | 0.5 h | 3 h | 0.5 h | 3 h | 0.5 h | 3 h | 0.5 h | 3 h | 0.5 h | 3 h | ||
| ≥50 | 1 g q8h | 99.5 | 99.8 | 99 | 99.4 | 97.8 | 98.7 | 94.9 | 96.5 | 85.2 | 88.9 | 61.6 | 66.6 |
| 1.5 g q6h | 99.9 | 100 | 99.8 | 100 | 99.6 | 99.9 | 98.9 | 99.5 | 96.4 | 97.2 | 87.8 | 90.7 | |
| 2 g q8h | 99.8 | 99.9 | 99.6 | 99.8 | 99.2 | 99.4 | 97.8 | 98.5 | 94.8 | 95.9 | 85.3 | 88.4 | |
| 25 to <50 | 1 g q12h | 99.7 | 100 | 99.9 | 99.9 | 99.6 | 99.8 | 98.9 | 99.4 | 95.8 | 97.2 | 84.1 | 86.2 |
| 1 g q6h | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.8 | 99.8 | 96.8 | 97.9 | |
| 2 g q12h | 100 | 100 | 99.9 | 100 | 99.8 | 99.9 | 99.6 | 99.7 | 99 | 99.4 | 96.1 | 97.2 | |
| 10 to <25 | 0.5 g q12h | 100 | 100 | 100 | 100 | 100 | 100 | 99.8 | 99.9 | 98.3 | 98.6 | 84.4 | 85.2 |
| 0.5 g q6h | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.9 | 97.1 | 97.1 | |
| 1 g q12h | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.8 | 99.8 | 98.4 | 98.6 | |
| <10 | 0.5 g q24h | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.9 | 100 | 96.8 | 99.9 |
| 0.5 g q12h | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.5 | 99.4 | |
| 1 g q24h | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.9 | 100 | |
DISCUSSION
MBL producers are not inhibited by tazobactam, sulbactam, clavulanic acid, avibactam, or vaborbactam, and MBLs have the capacity to enzymatically degrade carbapenems, which are the antibiotic of last resort (17, 18). These properties make MBL producers a major concern for health care professionals. This study is the first to evaluate the presence of synergism between meropenem and fosfomycin against MBL- and non-MBL-producing P. aeruginosa isolates, including the pharmacodynamic action achieved by the antimicrobial combination, which strongly suggested the synergism of their activities.
Although evaluation of the FICI is one of the most common methods to evaluate the in vitro synergism of antimicrobial combinations, we also analyzed the synergic action of the combination by the Loewe additivity index method, which is based on the theory of the surface model (19, 20). The purpose of evaluating the in vitro synergism of the antimicrobial combination by two mathematical methods was to provide more veracity and robustness to the results of the synergism tests, since it is still a scientific challenge to classify the synergistic activities of two or more drugs (8, 21). The two methods showed comparable results, demonstrating that the activity of the combination of meropenem with fosfomycin is synergistic against the majority of isolates by both methods.
In three isolates (PA-87, PA-149, PA-114), the activity of the antimicrobial combination was classified indifferent by the FICI and synergistic by the Loewe additivity index (19, 20). If we exclude the three meropenem-sensitive isolates (PA-114, PA-64, PA-69), which were included in the study to provide a greater variability of sensitivity but which would not be treated clinically with the antimicrobial combination, the percentages of synergism would be even better, reaching 100% and 87.5% by use of the Loewe additivity index and the FICI, respectively.
The combination exhibited synergism against all isolates harboring MBLs by the two tests used; however, among the non-MBL producers, 4 isolates were indifferent by use of the FICI test and 1 isolate was antagonistic by use of the Loewe additivity index. Numerically, the most pronounced synergistic action of the combination was against MBL-producing P. aeruginosa isolates, which demonstrated a median MIC reduction of 8-fold, whereas the reduction was only 4-fold for the non-MBL-producing isolates. In view of the limited number of new antimicrobial agents and the rapid rise in bacterial resistance, combination therapy with two or more agents in optimized regimens using the PK/PD approach has become a viable alternative to rescue these old antimicrobials and to combat infections caused by multidrug-resistant Gram-negative bacteria (8, 22, 23).
The current study is an important addition to the experience of the use of fosfomycin in combination against infections caused by MBL producers. All fosfomycin dose regimens evaluated achieved a pharmacodynamic index (AUC/MIC ≥ 40.8) with a PTA of ≥90% at an MIC of 16 μg/ml, thus suggesting that this value would have potential for determination of the PK/PD breakpoint for sensitivity against P. aeruginosa, since neither CLSI nor the European Committee on Antimicrobial Susceptibility Testing (EUCAST) provides breakpoints for fosfomycin against P. aeruginosa. Coincidentally, in an in vitro study evaluating the synergism of fosfomycin in combination with other antimicrobials against multidrug-resistant P. aeruginosa strains, Okazaki et al. used an MIC of ≤16 μg/ml as the breakpoint for sensitivity for fosfomycin, but the authors did not explain why they chose this value (24). The higher pharmacodynamic reach provided by higher dosing regimens (6 g q6h, 8 g q8h) to achieve a PTA of >90% at MICs of 32 μg/ml, which was also shown by our study, suggests a possible better coverage with higher fosfomycin dosing regimens.
Although our results are close to the EUCAST breakpoint for susceptibility to fosfomycin for the Enterobacteriaceae (≤32 μg/ml), we need to be careful to evaluate them before proposing any recommendations. The pharmacodynamic index of fosfomycin used in our evaluation (AUC/MIC ≥ 40.8) originated from a study that used a neutropenic mouse infection model and only one clinical isolate of P. aeruginosa which did not harbor MBLs. The same study evaluated 5 isolates of Escherichiacoli and provided a wide range of AUC/MIC ratios (27.5 to 193) (25). Thus, we performed another pharmacodynamic evaluation using the AUC/MIC ratio achieving the worst result against E. coli (AUC/MIC ≥ 193), and the maximum reach by all fosfomycin regimens with a PTA of ≥90% was at an MIC of 4 μg/ml, and only fosfomycin at a dose of 8 g q8h reached a PTA of ≥90% at an MIC of 8 μg/ml. This variability demonstrates that we need new studies and more robust data to propose a PK/PD breakpoint for fosfomycin against P. aeruginosa.
Evaluation of the PTA of fosfomycin alone or in combination therapy with meropenem clearly demonstrates the synergistic action between the two antimicrobials. None of the fosfomycin monotherapy regimens reached the PTA of ≥90% at an MIC of 16 μg/ml for any tested isolates; however, the three major regimens combined with meropenem achieved the PTA of ≥90% for 14 (74%) isolates, thus demonstrating greater coverage when combination therapy is used. These results are consistent with those of other in vitro and clinical studies demonstrating the increased action of fosfomycin in combination with other classes of antimicrobials due to their different mechanisms of action, supporting the clinical adoption of the use of other antibiotics in combination with fosfomycin (10, 11, 26, 27). The pharmacodynamic assessment also showed that the reach of the PTA was maintained or even increased, even with a reduction in the daily dose of fosfomycin for patients with CLCRs of ≤40 ml/min. Therefore, the regimens proposed by the manufacturer for use in patients with renal dysfunction are well determined to achieve a PTA comparable to that achieved with regimens for patients without renal dysfunction; the reduced regimens do not expose patients with renal impairment to subtherapeutic doses.
The reason for recommending the use of fosfomycin in combination with another agent for the treatment of severe infections is due to its great capacity for synergism with other agents, as demonstrated in this study, and the possibility of resistance development during treatment when it is used as a single agent (28). There are several resistance mechanisms capable of reducing the sensitivity of fosfomycin (29). Hamou-Segarra et al. evaluated P. aeruginosa mutant isolates defective in several components of the peptidoglycan recycling system exposed to fosfomycin and imipenem alone or in combination and showed that the hyperproduction of AmpC significantly increased susceptibility to fosfomycin (30). Using a hollow-fiber infection model, Drusano et al. evaluated the activity of meropenem and fosfomycin against P. aeruginosa at a high inoculum (8.18 log10 CFU/ml) and demonstrated that the combination resulted in significant synergism and was also able to contain the amplification of the subpopulation of mutants resistant to both antimicrobials; the authors speculated that the different mechanisms of action of the agents and the AmpC hyperproduction, commonly expressed in the presence of carbapenems, could be responsible for these positive effects (31).
Analysis of the PTA for meropenem monotherapy showed that the evaluated regimens reached MIC values higher than the CLSI breakpoint for sensitivity (MIC ≤ 2 μg/ml). The fact that all meropenem dose regimens in monotherapy achieved a PTA of ≥90% at an MIC of 4 μg/ml (an intermediate breakpoint by CLSI) and the regimens of 1.5 g q6h and 2 g q8h administered as a 0.5- or 3-h infusion achieved the PTA of ≥90% at an MIC of 8 μg/ml (the resistance breakpoint of CLSI) demonstrates the possible existence of greater margins for the meropenem regimens to be exploited against isolates for which MICs are higher and may also justify the higher value of the resistance breakpoint (MIC > 8 μg/ml) defined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for this carbapenem (32). In addition, PK/PD simulations can be used to determine individualized meropenem regimens against infections caused by MDR strains, for example, using a prolonged infusion duration (33, 34).
To maximize the antimicrobial activity of time-dependent agents (percent fT>MIC) with a short half-life, such as meropenem and other beta-lactams, the following strategies are normally used: increasing the conventional doses by maintaining the intervals between doses, keeping conventional doses or even reducing them but administering them more frequently, increasing the intravenous infusion time, or a combination of these approaches. These changes in the conventional regimens, called optimization, are beneficial because they maintain the free concentration of antimicrobial above the MIC, thus providing greater bacterial killing power, less chance of selecting resistant isolates, lower antimicrobial and treatment costs, and better therapeutic results, as demonstrated by in vitro, animal, and clinical studies (35, 36).
The three strategies were used in our pharmacodynamic evaluation. The positive effects of optimization favoring the increase of the PTA were clearly noticed in patients with CLCRs of >50 ml/min and against strains with less susceptible MICs; however, this advantage decreased with increasing renal impairment because the drug half-life is increased in this patient population, in which fAUC/MIC becomes the predominant pharmacodynamic characteristic (37). Results similar to ours were demonstrated by Zelenitsky et al., who compared bolus administration and extended infusions of piperacillin-tazobactam (another beta-lactam) and who found that prolonged infusion is advantageous only for the treatment of infections caused by less susceptible pathogens (MIC ≥ 32 μg/ml) (38); Isla et al., evaluating meropenem regimens in patients with different ranges of renal function by Monte Carlo simulation, observed in patients with a CLCR of 100 ml/min that a 0.5-h infusion of the drug at 2 g q8h and a 3-h infusion at 2 g q8h provided an fT>MIC of 40% at MICs of 8 and 16 μg/ml, respectively, and for patients with a CLCR of 35 ml/min, both infusion durations provided comparable coverage at the same MIC (32 μg/ml) (39). These results suggest that the extended infusion should not be used in a generalized way and should be preceded by careful evaluation of the factors involved, which may contribute to therapeutic success against less susceptible pathogens and with empirical treatments.
Comparison of the PTAs of meropenem as monotherapy and combination therapy regimens demonstrates the importance of the addition of fosfomycin to potentiate the action of meropenem, thus recovering the utility of the carbapenem. Considering a PTA of >90% at an MIC of 8 μg/ml, the combined regimens of meropenem of 1.5 g q6h and 2 g q8h as 0.5-h and 3-h infusions resulted in a 57% increase in the coverage of the isolates compared to that of monotherapy.
Fosfomycin should also be combined with another antimicrobial for the treatment of complicated upper urinary tract infections (e.g., pyelonephritis) and complicated systemic infections in order to prevent the development of resistance during treatment (22). Fosfomycin activity is also enhanced in an acidic environment (40). Okazaki et al. analyzed the in vitro synergy of the activity of fosfomycin combined with 8 antimicrobials in pairs against 30 isolates of multidrug-resistant P. aeruginosa by an efficacy time index assay and showed that the combination of fosfomycin with meropenem obtained the second-best result in 76.6% of the isolates, a value only 0.1% lower than the best result (24). Kastoris et al. surveyed in vitro studies of the synergism of fosfomycin combined with other antimicrobials against P. aeruginosa, and in one of these studies, fosfomycin showed synergism against 70% of the isolates when it was combined with either aztreonam, cefepime, ceftazidime, gentamicin, imipenem, or levofloxacin (41). In a clinical study, Mirakhur et al. followed 15 patients with pulmonary exacerbations due to cystic fibrosis colonized by multidrug-resistant P. aeruginosa treated with fosfomycin at 5 g q8h in combination with other antimicrobials in pairs, including meropenem, and showed clinical improvement in 93% of the patients (42). In a clinical case, Seija et al. reported the successful treatment of a patient with sepsis caused by a New Delhi MBL-producing Morganella morganii isolate sensitive to fosfomycin alone using a combined regimen of meropenem at 2 g q8h and fosfomycin at 4 g q8h (43).
The results of the pharmacodynamic evaluations, including the MICs, provided by the use of the combination of these agents indicate a potential to restore the activities of these antimicrobials. The shift in the MICs of the drugs in the combination resulted in meropenem sensitivity in 44% of the isolates, allowing the PTA of ≥90% to be achieved for all meropenem regimens and for the three highest-dose fosfomycin regimens at the MIC50. The estimate of the marginal PTA of meropenem against P. aeruginosa in the current study was conservative, given that the process of selection of the bacteria for use in the study had a propensity toward the selection of isolates with MIC values higher than the actual distribution of MICs in the general population. In fact, the higher clinical dose used in the combination of meropenem and fosfomycin would provide a PTA that exceeds the PTA for more common P. aeruginosa infections. The result reinforces the potential to restore the utility of these antimicrobial agents against infections caused by MDR bacteria through the use of a combination strategy (44). Although the methods of evaluation of synergism in vitro sometimes present controversial clinical reproducibility, Jean et al., evaluating the combination of tigecycline and imipenem-cilastatin in 28 patients with ventilator-associated pneumonia due to extensively drug-resistant (XDR) Acinetobacter baumannii, demonstrated a good correlation between the results of the checkerboard test and the lowest 30-day mortality for the patients treated with this combination (45). Comparing the synergistic action of antimicrobial combinations against extended-drug-resistant A. baumannii, Bremmer et al. also demonstrated by the checkerboard method and a clinical study that the combination consisting of minocycline with colistin was superior to meropenem with colistin, with microbiological eradication being achieved in 88% and 30% of the patients (P = 0.025), respectively (46). These studies support the use of information from the checkboard method in the PTA determination to optimize combination dosing regimens in clinical settings where no monotherapy option is available for MDR infections.
The limitation of this study is the small number of clinical isolates evaluated. However, we started with a large initial number of isolates (547 units) collected from two large health centers in the southern part of Brazil, and those 19 isolates selected were from different clones analyzed molecularly. In addition, 10 of the 19 isolates were MBL producers, as identified by molecular analysis, which made it possible to compare behavioral synergistic differences between MBL-producing and non-MBL-producing isolates.
In conclusion, favorable results for the combination of meropenem and fosfomycin were observed through synergism analysis and PK/PD evaluation. The current study demonstrates that the use of the two antimicrobials in combination through dose optimization has the potential to combat infections caused by carbapenem-resistant P. aeruginosa, even MBL-producing isolates.
MATERIALS AND METHODS
Microorganisms.
A total of 547 isolates of P. aeruginosa were consecutively recovered over 2 years from different patients from two teaching hospitals (HU/AHC complex, affiliates of the State University of Londrina, Paraná, Brazil) which have a total of 540 beds and constitute a medical reference center that provides health care services to a metropolitan region of more than 1 million in population. Nineteen of these clinical isolates were included in the study on the basis of the following criteria: the presence or absence of the different MBL enzymes and the presence of different molecular types and different meropenem MICs (from sensitive to highly resistant). Thus, the group of isolates selected showed a high degree of variability. The process of selection of 18 of these isolates from the total of 547 initial isolates identified by use of the MicroScan WalkAway automated system (Dade Behring Inc, West Sacramento, CA, USA) started with 141 isolates resistant to ceftazidime and sensitive or nonsensitive to carbapenems (meropenem or imipenem). Of these isolates, 45 expressed MBL enzymes (43 isolates expressed blaSPM-1, and 2 isolates expressed blaIMP-16), as determined by phenotypic tests (double-disk synergy test; Etest MBL), as confirmed by a multiplex PCR (47–49). These MBL-positive isolates were analyzed by pulsed-field gel electrophoresis (PFGE), which demonstrated the presence of nine different clones. In order to increase the diversity among the samples analyzed, one isolate from each clone was selected, resulting in seven blaSPM-1 producers and two blaIMP-16 producers (50). The 96 non-MBL-producing isolates were analyzed by the enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) method and subsequently evaluated by BioNumerics (version 7.6.1) software; nine different clones with different MICs for meropenem (from sensitive to resistant) were selected from this group (51). One strain of P. aeruginosa encoding the gene blaGIM-1 (isolate 73-5671) was also included (16). In view of the large number of non-MBL-producing isolates (96 isolates), the ERIC-PCR method was chosen to analyze them, since it presents a low cost, has a shorter analysis time than PFGE, and has an efficiency comparable to that of the PFGE method.
Evaluation of combined antimicrobial activity.
Meropenem (AstraZeneca, Cotia, São Paulo, Brazil) was donated by the State University of Maringa Hospital. Fosfomycin (Sigma-Aldrich, St. Louis, MO, USA) was purchased from Lab Company (Londrina, Paraná, Brazil). Fosfomycin was dissolved in water at 10 mg/ml and stored at 20°C, and meropenem solutions were prepared at the same concentration on the day of experimentation.
The antimicrobial activity of the combination of meropenem and fosfomycin against each isolate was evaluated by the checkerboard method. Each P. aeruginosa isolate was analyzed in triplicate using a sterile 96-well microdilution plate containing cation-adjusted Mueller-Hinton broth with several concentrations of meropenem and fosfomycin alone or in combination. A standard 0.5 McFarland inoculum was dispensed into each well, which gave a final concentration of 5 × 105 CFU/ml, and the plate was incubated at 37°C for 24 h. The MICs of the antimicrobials alone or in combination were determined for each isolate. For the interpretation of sensitivity, the MIC results for meropenem were determined using the breakpoint values for P. aeruginosa according to CLSI (susceptible, ≤ 2 μg/ml; intermediate, 4 μg/ml; resistant, ≥ 8 μg/ml). For quality control testing, the P. aeruginosa ATCC 27853 strain (American Type Culture Collection, Manassas, VA, USA) was used. The concentration ranges of meropenem and fosfomycin tested were 0.015 to 2,048 and 0.5 to 2,048 μg/ml, respectively.
The fractional inhibitory concentration index (FICI), determined on the basis of the results of the checkerboard method, was used to classify the antimicrobial efficacy of the combination and was calculated as follows: FICI = (MIC of meropenem in the combination/MIC of meropenem alone) + (MIC of fosfomycin in the combination/MIC of fosfomycin alone).
Classification of the antimicrobial effect of the combination according to the FICI is as follows: an FICI of ≤0.5 indicates synergism, an FICI of >0.5 and ≤4 indicates indifference, and an FICI of >4 indicates antagonism (19). The MIC50 and MIC90 of the antimicrobials as monotherapy or in combination were the MICs required to inhibit 50% and 90% of the 19 isolates, respectively.
The results of the checkerboard method were used in the analysis of the synergism of two antimicrobials by the Loewe additivity method, which was determined using the following equation: 1 = (MIC of meropenem in the combination/MIC of meropenem alone) + (MIC of fosfomycin in the combination/MIC of fosfomycin alone) + α.
Classification of the effects of the antimicrobial combination is based on the value of the Loewe additivity index, represented by α in the equation. If the value of α is 0, >0, or <0, the antimicrobial combination is classified as additive, synergistic, or antagonistic, respectively (20).
Pharmacodynamics of meropenem and fosfomycin antimicrobial activities.
PK/PD studies have shown that the antimicrobial activities of meropenem and fosfomycin are best characterized by the following pharmacodynamic indices: the percentage of time in a 24-h duration at which the free drug concentration remains above the MIC (percent fT>MIC) and the ratio of the area under the free drug concentration-versus-time curve over 24 h and the MIC (fAUC/MIC), respectively. Their target values were an fT>MIC of ≥40% for meropenem and an fAUC/MIC of ≥40.8 for fosfomycin (25, 52).
The meropenem and fosfomycin regimens evaluated in the pharmacodynamic analysis and the ranges of renal function were as follows: for meropenem, regimens of 1 g q8h, 1.5 g q6h, and 2 g q8h for creatinine clearances (CLCRs) of >50 ml/min; 1 g q12h, 1 g q6h, and 2 g q12h for CLCRs of >25 to 50 ml/min; 500 mg q12h, 500 mg q6h, and 1 g q12h for CLCRs of 10 to 25 ml/min; and 500 mg q24h, 500 mg q12h, and 1 g q24h for CLCRs of <10 ml/min for two infusion durations (0.5 h or 3 h); for fosfomycin, regimens of 4 g q6h, 4 g q8h, and 8 g q8h for CLCRs of >40 ml/min; a 30% dose reduction for CLCRs of 20 to 40 ml/min, a 50% dose reduction for CLCRs of 10 to 20 ml/min, and a 70% dose reduction for CLCRs of <10 ml/min (53) as a 0.5-h infusion. The evaluations were performed for monotherapy or combined therapy at the MIC50 and MIC90 of the isolates. For meropenem, which is a time-dependent antimicrobial, the goal is to find a regimen that maintains the drug concentration above the MIC for a longer period of time when it is administered in fractional doses and by extended infusion, thus providing better therapeutic results. Therefore, the more frequent meropenem dosing regimen of q6h and a prolonged infusion duration of 3 h were included in the analysis (54, 55). For fosfomycin, whose pharmacodynamic index is characterized by AUC/MIC and which is not affected by the duration of infusion, a 0.5-h infusion time was used for all dosing regimens, as recommended by the manufacturer.
Pharmacokinetic model and exposure of meropenem and fosfomycin.
The height and body weight (WT) relationship for a virtual population with a 50/50 ratio of adult males to adult females consisting of approximately 10,000 individuals per renal function category was simulated as described previously (56–58). CLCR was simulated by assuming a uniform distribution (for meropenem, >50 ml/min, >25 to 50 ml/min, 10 to 25 ml/min, and <10 ml/min; for fosfomycin, >40 ml/min, 20 to 40 ml/min, 10 to <20 ml/min, and <10 ml/min) (59). The corresponding serum creatinine concentration (SCr) was subsequently back-calculated from the creatinine clearance values by use of the Cockcroft-Gault equation (60).
The population pharmacokinetic model for meropenem was the one-compartment model described by Muro et al. (61), which was previously shown to best predict free meropenem drug concentrations in critically ill patients (62). The relationship between the serum creatinine concentration (SCr) and meropenem clearance (CL; in liters per hour) is such that CL is equal to 11.1 × (SCr/0.7)−1 with a coefficient of variation (CV) of 52.1%. The average volume of distribution (V) was 33.6 liters. Protein binding for meropenem was 2%.
The population pharmacokinetic model for fosfomycin was a two-compartment model parameterized on CL, the volume of the central (compartment VC), the volume of the peripheral compartment (VP), and intercompartmental clearance (Q). The population pharmacokinetic model of fosfomycin in critically ill patients described by Parker et al. (63) was used to simulate virtual patient profiles. Their model reported seven interoccasion CL parameters. For the purpose of simulation, the highest CL value was used in order to avoid predicting a high fosfomycin concentration. Both CLCR and body weight (WT) were influential covariates. The equations for the population CL (in liters per hour) and VC (in liters) incorporated these two covariates and were as follows: CL = 5.57 × (CLCR/90) and VC = 26.5 × (WT/70)0.75. VP and Q were 22.3 liters and 19.8 liters/h, respectively. Interindividual variability was incorporated into CL and VC, assuming a log-normal distribution of both parameters, with a CV of 91.9% and 39%, respectively. Fosfomycin has negligible plasma protein binding (64, 65).
For meropenem, the percentage of the 10,000 virtual concentration-time profiles that achieved an fT>MIC of 40% was determined for the three meropenem dosing regimens for infusion durations of 0.5 h or 3 h in the computation of the probability of target attainment (PTA). For fosfomycin, PTA was computed for 10,000 areas under the concentration-time curves (AUCs) computed from the simulated profiles for each dosing regimen and evaluated by determination of the percentage of virtual profiles that reached an fAUC/MIC of ≥40.8. The cumulative fraction of the response (CFR) was defined as the sum of the frequency of isolates at each MIC multiplied by the PTA.
The simulation of population pharmacokinetic models was carried out using the RxODE package (66). AUC was computed using the trapz function in the caTools package (67). Computations of PTA and CFR were performed with user-defined codes. All simulations, integrations, and probability computations were carried out in R (version 3.5.0) (68).
ACKNOWLEDGMENTS
We thank the laboratory assistants at the microbiology laboratory of the State University of Londrina, Paraná, Brazil, for performing part of the microbiological analyses presented in this work.
Maria Cristina Bronharo Tognim received funding from the Coordination Improvement of Higher Level Personnel (CAPES) and Foundation Araucaria. As these government funds are designed to encourage higher education training in Brazil, they only cover the cost of laboratory materials.
We have no conflicts of interest to declare.
REFERENCES
- 1.Liu Q, Li X, Li W, Du X, He JQ, Tao C, Feng Y. 2015. Influence of carbapenem resistance on mortality of patients with Pseudomonas aeruginosa infection: a meta-analysis. Sci Rep 5:11715. doi: 10.1038/srep11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee M, Anju CP, Biswas L, Anil Kumar V, Gopi Mohan C, Biswas R. 2016. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int J Med Microbiol 306:48–58. doi: 10.1016/j.ijmm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 3.El Zowalaty ME, Al Thani AA, Webster TJ, El Zowalaty AE, Schweizer HP, Nasrallah GK, Marei HE, Ashour HM. 2015. Pseudomonas aeruginosa: arsenal of resistance mechanisms, decades of changing resistance profiles, and future antimicrobial therapies. Future Microbiol 10:1683–1706. doi: 10.2217/fmb.15.48. [DOI] [PubMed] [Google Scholar]
- 4.Cornaglia G, Giamarellou H, Rossolini GM. 2011. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect Dis 11:381–393. doi: 10.1016/S1473-3099(11)70056-1. [DOI] [PubMed] [Google Scholar]
- 5.Poole K. 2011. Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oo C, Sy S. 2018. Fixed-dose combinations: a potential means to boost drug development for selected drugs. Drug Discov Today 23:457–459. doi: 10.1016/j.drudis.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, Isgri S. 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 8.Tamma PD, Cosgrove SE, Maragakis LL. 2012. Combination therapy for treatment of infections with Gram-negative bacteria. Clin Microbiol Rev 25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangden T. 2014. Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Ups J Med Sci 119:149–153. doi: 10.3109/03009734.2014.899279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassetti M, Giacobbe DR, Giamarellou H, Viscoli C, Daikos GL, Dimopoulos G, De Rosa FG, Giamarellos-Bourboulis EJ, Rossolini GM, Righi E, Karaiskos I, Tumbarello M, Nicolau DP, Viale PL, Poulakou G, Critically Ill Patients Study Group of the European Society of Clinical Microbiology and Infectious Disease (ESCMID), Hellenic Society of Chemotherapy (HSC), Societá Italiana di Terapia Antinfettiva (SITA). 2018. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect 24:133–144. doi: 10.1016/j.cmi.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Albiero J, Sy SK, Mazucheli J, Caparroz-Assef SM, Costa BB, Alves JL, Gales AC, Tognim MC. 2016. Pharmacodynamic evaluation of the potential clinical utility of fosfomycin and meropenem in combination therapy against KPC-2-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 60:4128–4139. doi: 10.1128/AAC.03099-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falagas ME, Kastoris AC, Karageorgopoulos DE, Rafailidis PI. 2009. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and clinical studies. Int J Antimicrob Agents 34:111–120. doi: 10.1016/j.ijantimicag.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepe JA, Torres MJ, Smani Y, Parra-Millan R, Pachon J, Vazquez-Barba I, Aznar J. 2014. In vitro and intracellular activities of fosfomycin against clinical strains of Listeria monocytogenes. Int J Antimicrob Agents 43:135–139. doi: 10.1016/j.ijantimicag.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi I, Sakurai M, Karato A, Ichiki M, Sekine I, Ishikawa T, Shiotani J, Yoshida T, Niida M, Ogawa M. 1994. Laboratory and clinical studies on combined effects of fosfomycin plus sulbactam/cefoperazone for mixed infections of MRSA and Pseudomonas aeruginosa. Jpn J Antibiot 47:991–1005. (In Japanese.) [PubMed] [Google Scholar]
- 16.Castanheira M, Toleman MA, Jones RN, Schmidt FJ, Walsh TR. 2004. Molecular characterization of a beta-lactamase gene, blaGIM-1, encoding a new subclass of metallo-beta-lactamase. Antimicrob Agents Chemother 48:4654–4661. doi: 10.1128/AAC.48.12.4654-4661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotondo CM, Wright GD. 2017. Inhibitors of metallo-beta-lactamases. Curr Opin Microbiol 39:96–105. doi: 10.1016/j.mib.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Palzkill T. 2013. Metallo-beta-lactamase structure and function. Ann N Y Acad Sci 1277:91–104. doi: 10.1111/j.1749-6632.2012.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillai SK, Moellering R, Eliopoulos GM. 2005. Antimicrobial combinations, 5th ed, p 365–440. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 20.Greco WR, Bravo G, Parsons JC. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 47:331–385. [PubMed] [Google Scholar]
- 21.Tang J, Wennerberg K, Aittokallio T. 2015. What is synergy? The Saariselka agreement revisited. Front Pharmacol 6:181. doi: 10.3389/fphar.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavascki AP, Bulitta JB, Landersdorfer CB. 2013. Combination therapy for carbapenem-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther 11:1333–1353. doi: 10.1586/14787210.2013.845523. [DOI] [PubMed] [Google Scholar]
- 23.Kostyanev T, Bonten MJ, O'Brien S, Steel H, Ross S, Francois B, Tacconelli E, Winterhalter M, Stavenger RA, Karlen A, Harbarth S, Hackett J, Jafri HS, Vuong C, MacGowan A, Witschi A, Angyalosi G, Elborn JS, deWinter R, Goossens H. 2016. The Innovative Medicines Initiative's New Drugs for Bad Bugs programme: European public-private partnerships for the development of new strategies to tackle antibiotic resistance. J Antimicrob Chemother 71:290–295. doi: 10.1093/jac/dkv339. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki M, Suzuki K, Asano N, Araki K, Shukuya N, Egami T, Higurashi Y, Morita K, Uchimura H, Watanabe T. 2002. Effectiveness of fosfomycin combined with other antimicrobial agents against multidrug-resistant Pseudomonas aeruginosa isolates using the efficacy time index assay. J Infect Chemother 8:37–42. doi: 10.1007/s101560200004. [DOI] [PubMed] [Google Scholar]
- 25.Lepak AJ, Zhao M, VanScoy B, Taylor DS, Ellis-Grosse E, Ambrose PG, Andes DR. 2017. In vivo pharmacokinetics and pharmacodynamics of ZTI-01 (fosfomycin for injection) in the neutropenic murine thigh infection model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e00476-17. doi: 10.1128/AAC.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reffert JL, Smith WJ. 2014. Fosfomycin for the treatment of resistant gram-negative bacterial infections. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 34:845–857. doi: 10.1002/phar.1434. [DOI] [PubMed] [Google Scholar]
- 27.Pontikis K, Karaiskos I, Bastani S, Dimopoulos G, Kalogirou M, Katsiari M, Oikonomou A, Poulakou G, Roilides E, Giamarellou H. 2014. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents 43:52–59. doi: 10.1016/j.ijantimicag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver LL. 2017. Fosfomycin: mechanism and resistance. Cold Spring Harb Perspect Med 7:a025262. doi: 10.1101/cshperspect.a025262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamou-Segarra M, Zamorano L, Vadlamani G, Chu M, Sanchez-Diener I, Juan C, Blazquez J, Hattie M, Stubbs KA, Mark BL, Oliver A. 2017. Synergistic activity of fosfomycin, beta-lactams and peptidoglycan recycling inhibition against Pseudomonas aeruginosa. J Antimicrob Chemother 72:448–454. doi: 10.1093/jac/dkw456. [DOI] [PubMed] [Google Scholar]
- 31.Drusano GL, Neely MN, Yamada WM, Duncanson B, Brown D, Maynard M, Vicchiarelli M, Louie A. 2018. The combination of fosfomycin plus meropenem is synergistic for Pseudomonas aeruginosa PAO1 in a hollow-fiber infection model. Antimicrob Agents Chemother 62:e01682-18. doi: 10.1128/AAC.01682-18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Committee on Antimicrobial Susceptibility Testing. 2018. Breakpoint tables for interpretation of MICs and zone diameters, version 8.0. http://www.eucast.org.
- 33.Sy SK, Zhuang L, Derendorf H. 2016. Pharmacokinetics and pharmacodynamics in antibiotic dose optimization. Expert Opin Drug Metab Toxicol 12:93–114. doi: 10.1517/17425255.2016.1123250. [DOI] [PubMed] [Google Scholar]
- 34.Sy SKB, Zhuang L, Sy S, Derendorf H. 11 August 2018. Clinical pharmacokinetics and pharmacodynamics of ceftazidime-avibactam combination: a model-informed strategy for its clinical development. Clin Pharmacokinet. doi: 10.1007/s40262-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 35.Mattoes HM, Kuti JL, Drusano GL, Nicolau DP. 2004. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin Ther 26:1187–1198. doi: 10.1016/S0149-2918(04)80001-8. [DOI] [PubMed] [Google Scholar]
- 36.Rizk NA, Kanafani ZA, Tabaja HZ, Kanj SS. 2017. Extended infusion of beta-lactam antibiotics: optimizing therapy in critically-ill patients in the era of antimicrobial resistance. Expert Rev Anti Infect Ther 15:645–652. doi: 10.1080/14787210.2017.1348894. [DOI] [PubMed] [Google Scholar]
- 37.Kristoffersson AN, David-Pierson P, Parrott NJ, Kuhlmann O, Lave T, Friberg LE, Nielsen EI. 2016. Simulation-based evaluation of PK/PD indices for meropenem across patient groups and experimental designs. Pharm Res 33:1115–1125. doi: 10.1007/s11095-016-1856-x. [DOI] [PubMed] [Google Scholar]
- 38.Zelenitsky S, Nash J, Weber Z, Iacovides H, Ariano R. 2016. Targeted benefits of prolonged-infusion piperacillin-tazobactam in an in vitro infection model of Pseudomonas aeruginosa. J Chemother 28:390–394. doi: 10.1080/1120009X.2016.1140858. [DOI] [PubMed] [Google Scholar]
- 39.Isla A, Canut A, Arribas J, Asín-Prieto E, Rodríguez-Gascón A. 2016. Meropenem dosing requirements against Enterobacteriaceae in critically ill patients: influence of renal function, geographical area and presence of extended-spectrum beta-lactamases. Eur J Clin Microbiol Infect Dis 35:511–519. doi: 10.1007/s10096-015-2568-6. [DOI] [PubMed] [Google Scholar]
- 40.Fedrigo NH, Mazucheli J, Albiero J, Shinohara DR, Lodi FG, Machado A, Sy SKB, Tognim M. 2017. Pharmacodynamic evaluation of fosfomycin against Escherichia coli and Klebsiella spp. from urinary tract infections and the influence of pH on fosfomycin activities. Antimicrob Agents Chemother 61:e02498-16. doi: 10.1128/AAC.02498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kastoris AC, Rafailidis PI, Vouloumanou EK, Gkegkes ID, Falagas ME. 2010. Synergy of fosfomycin with other antibiotics for Gram-positive and Gram-negative bacteria. Eur J Clin Pharmacol 66:359–368. doi: 10.1007/s00228-010-0794-5. [DOI] [PubMed] [Google Scholar]
- 42.Mirakhur A, Gallagher MJ, Ledson MJ, Hart CA, Walshaw MJ. 2003. Fosfomycin therapy for multiresistant Pseudomonas aeruginosa in cystic fibrosis. J Cyst Fibros 2:19–24. doi: 10.1016/S1569-1993(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 43.Seija V, Medina Presentado JC, Bado I, Papa Ezdra R, Batista N, Gutierrez C, Guirado M, Vidal M, Nin M, Vignoli R. 2015. Sepsis caused by New Delhi metallo-beta-lactamase (blaNDM-1) and qnrD-producing Morganella morganii, treated successfully with fosfomycin and meropenem: case report and literature review. Int J Infect Dis 30:20–26. doi: 10.1016/j.ijid.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Decker B, Masur H. 2015. Bad bugs, no drugs: are we part of the problem, or leaders in developing solutions? Crit Care Med 43:1153–1155. doi: 10.1097/CCM.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 45.Jean SS, Hsieh TC, Hsu CW, Lee WS, Bai KJ, Lam C. 2016. Comparison of the clinical efficacy between tigecycline plus extended-infusion imipenem and sulbactam plus imipenem against ventilator-associated pneumonia with pneumonic extensively drug-resistant Acinetobacter baumannii bacteremia, and correlation of clinical efficacy with in vitro synergy tests. J Microbiol Immunol Infect 49:924–933. doi: 10.1016/j.jmii.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Bremmer DN, Bauer KA, Pouch SM, Thomas K, Smith D, Goff DA, Pancholi P, Balada-Llasat JM. 2016. Correlation of checkerboard synergy testing with time-kill analysis and clinical outcomes of extensively drug-resistant Acinetobacter baumannii respiratory infections. Antimicrob Agents Chemother 60:6892–6895. doi: 10.1128/AAC.00981-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Walsh TR, Bolmstrom A, Qwarnstrom A, Gales A. 2002. Evaluation of a new Etest for detecting metallo-beta-lactamases in routine clinical testing. J Clin Microbiol 40:2755–2759. doi: 10.1128/JCM.40.8.2755-2759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Picao RC, Andrade SS, Nicoletti AG, Campana EH, Moraes GC, Mendes RE, Gales AC. 2008. Metallo-beta-lactamase detection: comparative evaluation of double-disk synergy versus combined disk tests for IMP-, GIM-, SIM-, SPM-, or VIM-producing isolates. J Clin Microbiol 46:2028–2037. doi: 10.1128/JCM.00818-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silbert S, Pfaller MA, Hollis RJ, Barth AL, Sader HS. 2004. Evaluation of three molecular typing techniques for nonfermentative Gram-negative bacilli. Infect Control Hosp Epidemiol 25:847–851. doi: 10.1086/502307. [DOI] [PubMed] [Google Scholar]
- 52.Nicolau DP. 2008. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis 47:S32–S40. doi: 10.1086/590064. [DOI] [PubMed] [Google Scholar]
- 53.Sandoz GmbH. 2 April 2019, accession date. Fosfomycin “Sandoz” 4 g i.v. - trockensubstanz zur infusionsbereitung. Sandoz GmbH, Kundi, Austria: https://medikamio.com/de-at/medikamente/fosfomycin-sandoz-4-g-iv-trockensubstanz-zur-infusionsbereitung/pil. [Google Scholar]
- 54.Lodise TP Jr, Lomaestro B, Drusano GL. 2007. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 44:357–363. doi: 10.1086/510590. [DOI] [PubMed] [Google Scholar]
- 55.Fehér C, Rovira M, Soriano A, Esteve J, Martínez JA, Marco F, Carreras E, Martínez C, Fernández-Avilés F, Suárez-Lledó M, Mensa J. 2014. Effect of meropenem administration in extended infusion on the clinical outcome of febrile neutropenia: a retrospective observational study. J Antimicrob Chemother 69:2556–2562. doi: 10.1093/jac/dku150. [DOI] [PubMed] [Google Scholar]
- 56.Sy SK, Asin-Prieto E, Derendorf H, Samara E. 2014. Predicting pediatric age-matched weight and body mass index. AAPS J 16:1372–1379. doi: 10.1208/s12248-014-9657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sy SKB, Zhuang L, Xia H, Beaudoin ME, Schuck VJ, Nichols WW, Derendorf H. 2018. A mathematical model-based analysis of the time-kill kinetics of ceftazidime/avibactam against Pseudomonas aeruginosa. J Antimicrob Chemother 73:1295–1304. doi: 10.1093/jac/dkx537. [DOI] [PubMed] [Google Scholar]
- 58.Sy SKB, Zhuang L, Xia H, Schuck VJ, Nichols WW, Derendorf H. 28 October 2018. A model-based analysis of pharmacokinetic-pharmacodynamic (PK/PD) indices of avibactam against Pseudomonas aeruginosa. Clin Microbiol Infect. doi: 10.1016/j.cmi.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 59.AstraZeneca do Brasil Ltda. 2013. Meropenem package insert. AstraZeneca do Brasil Ltda, Cotia, SP, Brazil: http://www.anvisa.gov.br/datavisa/fila_bula/frmVisualizarBula.asp?pNuTransacao=6501872013&pIdAnexo=1733955. [Google Scholar]
- 60.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 61.Muro T, Sasaki T, Hosaka N, Umeda Y, Takemoto S, Yamamoto H, Kamimura H, Higuchi S, Karube Y. 2011. Population pharmacokinetic analysis of meropenem in Japanese adult patients. J Clin Pharm Ther 36:230–236. doi: 10.1111/j.1365-2710.2010.01171.x. [DOI] [PubMed] [Google Scholar]
- 62.Wong G, Farkas A, Sussman R, Daroczi G, Hope WW, Lipman J, Roberts JA. 2015. Comparison of the accuracy and precision of pharmacokinetic equations to predict free meropenem concentrations in critically ill patients. Antimicrob Agents Chemother 59:1411–1417. doi: 10.1128/AAC.04001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parker SL, Frantzeskaki F, Wallis SC, Diakaki C, Giamarellou H, Koulenti D, Karaiskos I, Lipman J, Dimopoulos G, Roberts JA. 2015. Population pharmacokinetics of fosfomycin in critically ill patients. Antimicrob Agents Chemother 59:6471–6476. doi: 10.1128/AAC.01321-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goto M, Sugiyama M, Nakajima S, Yamashina H. 1981. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob Agents Chemother 20:393–397. doi: 10.1128/AAC.20.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker S, Lipman J, Koulenti D, Dimopoulos G, Roberts JA. 2013. What is the relevance of fosfomycin pharmacokinetics in the treatment of serious infections in critically ill patients? A systematic review. Int J Antimicrob Agents 42:289–293. doi: 10.1016/j.ijantimicag.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 66.Wang W, Hallow KM, James DA. 2016. A tutorial on RxODE: simulating differential equation pharmacometric models in R. CPT Pharmacometrics Syst Pharmacol 5:3–10. doi: 10.1002/psp4.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tuszynski J. 2018. caTools: tools moving window statistics. R package version 1.17.1.1. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 68.R Core Team. 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]