Isavuconazole is the newest medical azole. We investigated EUCAST MICs for isavuconazole and seven comparators against 1,498 contemporary isolates (2016 to 2017).
KEYWORDS: Aspergillus, Candida, EUCAST, Mucorales, antifungal susceptibility testing, azoles
ABSTRACT
Isavuconazole is the newest medical azole. We investigated EUCAST MICs for isavuconazole and seven comparators against 1,498 contemporary isolates (2016 to 2017). EUCAST susceptibility testing was performed. Isavuconazole MICs >2 dilution steps above the modal MIC were regarded as non-wild type for species without EUCAST epidemiological cutoff values (ECOFFs). CYP51A sequencing was performed when relevant. Pearson correlation analysis was adopted for comparing activity. Aspergillus accounted for 90% of mold and Candida accounted for 97% of yeast isolates. Thirty (9.3%) Aspergillus fumigatus isolates were classified as resistant, and 10 (3.1%) were classified as non-wild type. Thirteen (4%) were cross-resistant to other mold-active azoles. Target gene alterations were found in 10 (76.9%) isolates, including 4 (30.8%) of environmental origin (TR34/L98H [n = 3] and Trip343/L98H [n = 1]). Six Aspergillus terreus isolates were resistant, including two (17%) with MICs of >2 mg/liter and M217I alterations. Modal MICs/MIC50s (milligrams per liter) against Candida spp. were ≤0.004/≤0.004 for C. albicans and C. dubliniensis, 0.008/0.008 for C. tropicalis, 0.016/0.016 for C. parapsilosis, 0.06/0.06 for C. glabrata, and 0.125/0.125 for C. krusei. A non-wild-type phenotype was observed for 6.6% of isolates (C. glabrata [11.8%] and C. tropicalis [12.3%], specifically). All of these isolates were nonsusceptible/non-wild type to fluconazole (96.1%) or voriconazole (86.2%). Low MICs were found for several other species, except Scedosporium apiospermum and Fusarium. The best correlation was found between isavuconazole and voriconazole overall but for A. terreus and Mucorales to itraconazole and posaconazole, respectively. Isavuconazole displayed broad in vitro activity. Acquired resistance was infrequent except in A. terreus, C. glabrata, and C. tropicalis and, when present, was associated with cross-resistance to other azoles. Revising the EUCAST breakpoints for A. fumigatus (defining an MIC of 2 mg/liter as intermediate [“I”]) would minimize major errors.
INTRODUCTION
Isavuconazole is the newest medical azole with activity against a broad range of yeast and molds. It was licensed in Europe and the United States in 2015 by the EMA and FDA for the treatment of adults with invasive aspergillosis and also for mucormycosis although by the EMA only in patients for whom amphotericin B is inappropriate. In the same year, EUCAST clinical breakpoints were established for three Aspergillus species (A. fumigatus, A. nidulans, and A. terreus), and epidemiological cutoff values (ECOFFs) for these as well as for A. flavus and A. niger were determined (1). Isavuconazole given daily or weekly has also been found efficacious and noninferior to fluconazole for uncomplicated esophageal candidiasis in a randomized, double-blind, multicenter phase 2 trial, where Candida albicans was the most common cause of infection, accounting for 94.8% (2). In line with this in vivo efficacy, isavuconazole has potent in vitro activity against particularly C. albicans, C. parapsilosis, and C. tropicalis, with an MIC of ≤0.03 mg/liter for 91.5% to 96.0% of such isolates (3). MICs against C. glabrata and C. krusei are higher, with modal MICs of between 0.03 mg/liter and 0.5 mg/liter in most data sets (3–6). Unfortunately, EUCAST ECOFFs and breakpoints have not been established for Candida species due to significant interlaboratory variability (3).
Previous studies have shown a correlation between the susceptibility to the azoles and, particularly, the susceptibility to voriconazole and isavuconazole for A. fumigatus (7, 8). Similarly, studies have shown that isavuconazole susceptibility was lower for Candida isolates with resistance or non-WT (wild-type) susceptibility to fluconazole and voriconazole (9).
Denmark is a high-incidence country for candidemia, with a high use of antifungal compounds, including azoles, in a Nordic perspective (10). Hence, it is of utmost importance to monitor the susceptibility profiles of clinically relevant fungal isolates. In this study, we investigated and compared the in vitro activities of isavuconazole and seven comparators against a large contemporary clinical collection of mold and yeast isolates received at the Danish mycology reference center during the years 2016 to 2017. MICs were interpreted by applying recently established EUCAST clinical breakpoints and ECOFFs.
RESULTS
During 2016 and 2017, isavuconazole susceptibility was determined for 429 mold isolates and 1,069 yeast isolates from 1,325 patients. The MICs for isavuconazole and comparators (voriconazole, itraconazole, posaconazole, and amphotericin B for molds and voriconazole, fluconazole, amphotericin B, anidulafungin, and micafungin for yeasts) were evaluated separately for 2016 and 2017, but as no difference was seen for the 2 years (modal MICs and MIC50s within ±1 dilution step [data not shown]), data were pooled and are presented together.
Molds.
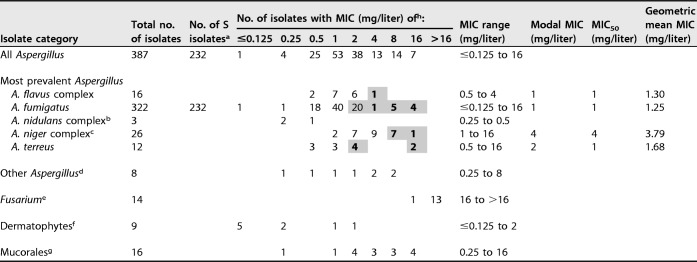
Aspergillus accounted for 90.2% (n = 387) of the 429 mold isolates (Table 1). Adopting the EUCAST ECOFFs available for the five most prevalent Aspergillus complex isolates, 24 (6.4%) were non-wild type, and among the three species for which clinical breakpoints have been established, 36/335 (10.7%) isolates were classified as resistant (R). In detail, 30/322 (9.3%) A. fumigatus isolates (from 28 patients) were classified as resistant, and 10/322 (3.1%) from 9 patients were classified as non-wild type. Three (15%) A. fumigatus isolates with isavuconazole MICs of 2 mg/liter (classified as resistant but within the wild-type range) were cross-resistant to itraconazole (MIC ≥16 mg/liter). Two of these isolates harbored Cyp51A alterations and were also cross-resistant to either posaconazole and voriconazole (G54A; MICs of ≥4 mg/liter and 2 mg/liter, respectively) or posaconazole only (M220K; MIC of ≥4 mg/liter). All 10 A. fumigatus isolates with MICs of >2 mg/liter (and, thus, both resistant and non-wild type) were nonsusceptible to itraconazole, posaconazole, and voriconazole. Eight of these isolates (80%) harbored Cyp51A alterations (TR34/L98H [n = 3], Trip343/L98H [n = 1], TR120/F46Y/M172V/E427K [n = 1], G432S [n = 1], and G448S [n = 2]). Thus, overall, 13/322 (4%) of the A. fumigatus isolates were classified as isavuconazole resistant and cross-resistant to other mold-active azoles, and 10/13 (76.9%) harbored target gene alterations, including 4/13 (30.8%) whose alterations were due to an environmental resistance mechanism.
TABLE 1.
MICs and geometric mean MICs of isavuconazole against the 429 mold isolates
aNumber of isolates sensitive to itraconazole, posaconazole, and voriconazole determined by azole agar screening.
bOne isolate each of the A. nidulans complex, A. quadrilineatus, and A. spinulosporus.
cTwenty-two isolates of the A. niger complex, three isolates of A. tubingensis, and one of A. luchuensis.
dThree A. calidoustus isolates and one isolate each of A. fischeri, A. giganteus, A. persii, A. similis, and A. turcosus.
eTwo F. dimerum, two F. proliferatum, seven F. solani sensu stricto, and three F. solani complex isolates.
fTwo M. canis, four T. rubrum, one T. interdigitale, and two T. mentagrophytes complex isolates.
gTwo M. circinelloides isolates, three R. pusillus isolates, four R. microsporus isolates, and one isolate each of C. muscae, L. corymbifera, L. ramosa, Lichtheimia species, R. oryzae, Syncephalastrum racemosum, and Mucorales species.
hResistant Aspergillus isolates are shaded, and non-wild-type isolates are shown in boldface type.
Six A. terreus (50%) isolates from five patients were classified as isavuconazole resistant and non-wild type, including 4/4 isolates with MICs of 2 mg/liter, the wild-type CYP51A target gene, and susceptibility to the other three azoles. The remaining two isolates (17%), with an isavuconazole MIC of >2 mg/liter, harbored an M217I alteration (corresponding to the M220I alteration in A. fumigatus).
Finally, among other molds, low MICs (≤0.25 mg/liter) were observed against Microsporum canis, Trichophyton rubrum, Trichophyton interdigitale, Circinella muscae, and Saprochaete capitata, and high MICs (≥16 mg/liter) were observed against Scedosporium apiospermum, Fusarium spp. (including F. dimerum, F. proliferatum, and F. solani complex isolates), and 4/16 Mucorales isolates (Mucor circinelloides [n = 3] and Rhizopus oryzae). However, numbers were low.
The in vitro activity of isavuconazole against A. flavus, A. fumigatus, A. nidulans, and A. terreus isolates on a milligram-per-liter basis was comparable to those of voriconazole and amphotericin B and slightly lower than those of itraconazole and posaconazole (Table 2). For A. niger isolates, the isavuconazole modal MIC and MIC50 were two 2-fold dilutions higher than those for voriconazole and itraconazole and four 2-fold dilutions higher than those for posaconazole and amphotericin B. As isavuconazole and the comparators are all regarded as valid options for A. fumigatus infections, the proportions of isolates with MICs above the ECOFF against A. fumigatus were compared for each compound as an indicator of relative coverage (Table 2). The proportions of A. flavus and A. terreus isolates with MICs above the A. fumigatus ECOFF were lower for isavuconazole than for amphotericin B and voriconazole, whereas in contrast, the proportion of A. niger isolates less susceptible than A. fumigatus was highest for isavuconazole (Table 2).
TABLE 2.
Modal MICs/MIC50s and MIC ranges for isavuconazole and comparators for the 429 mold isolatesh
| Isolate category | Total no. of isolates | Isavuconazole |
Voriconazole |
Itraconazole |
Posaconazole |
Amphotericin B |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Modal MIC/MIC50 (range) or MIC range (mg/liter) | % of isolates with MIC > 2 mg/liter | Modal MIC/MIC50 (range) or MIC range (mg/liter) | % of isolates with MIC > 1 mg/liter | Modal MIC/MIC50 (range) or MIC range (mg/liter) | % of isolates with MIC > 1 mg/liter | Modal MIC/MIC50(range) or MIC range (mg/liter) | % of isolates with MIC > 0.25 mg/liter | Modal MIC/MIC50 (range) or MIC range (mg/liter) | % of isolates with MIC > 1 mg/liter | ||
| All Aspergillus | 387 | 1/1 (≤0.125 to 16) | 8.9 | 1/1 (0.25 to 16) | 8.5 | 0.25/0.5 (≤0.125 to >16) | 9.8 | 0.125/0.125 (≤0.03 to >4) | 8.80 | 0.5/0.5 (0.03 to >4) | 3.1 |
| Most prevalent Aspergillus | |||||||||||
| A. flavus complex | 16 | 1/1 (0.5 to 4) | 6.3 | 1/1 (0.5 to 2) | 12.5 | 0.25/0.25 (≤0.125 to 0.5) | 0 | 0.125/0.125 (0.06 to 0.25) | 0 | 1/1 (0.5 to 4) | 18.8 |
| A. fumigatus | 322a | 1/1 (≤0.125 to 16) | 3.1 | 0.5/0.5 (0.25 to 16) | 4.0 | 0.25/0.25 (≤0.125 to >16) | 6.8 | 0.125/0.125 (≤0.03 to >4) | 6.2 | 0.5/0.5 (0.125 to 1) | 0 |
| A. nidulans complexb | 3 | 0.25 to 0.5 | 0/3 | 0.25 to 0.5 | 0/3 | ≤0.125 to 0.5 | 0/3 | 0.06 to 0.5 | 1/3 | 0.25 to 1 | 0/3 |
| A. niger complexc | 26 | 4/4 (1 to 16) | 65.4 | 1/1 (0.5 to 4) | 42.3 | 1/1 (0.25 to >16) | 38.5 | 0.25/0.25 (≤0.03 to 0.5) | 23.1 | 0.25/0.25 (0.03 to 0.5) | 0 |
| A. terreus | 12 | 2/1 (0.5 to 16) | 16.7 | 0.5/1 (0.5 to 8) | 25 | ≤0.125/0.25 (≤0.125 to >16) | 16.7 | 0.06/0.06 (≤0.03 to 0.5) | 16.7 | 1/1 (1 to 4) | 50 |
| Other Aspergillusd | 8 | 0.25 to 8 | 50 | 0.25 to 8 | 50 | ≤0.125 to >16 | 50 | 0.06 to >4 | 62.5 | 0.125 to >4 | 25 |
| Fusariume | 14 | (16 to >16) | 100 | (4 to >16) | 100 | (4 to >16) | 100 | >4 | 100 | 0.5 to >4 | 38.5 |
| Dermatophytesf | 9 | ≤0.125 to 2 | 0/9 | ≤0.125 to 1 | 0/9 | ≤0.125 to >16 | 3/9 | ≤0.03 to 0.5 | 2/9 | 0.25 to 1 | 0/8 |
| Mucoralesg | 16 | 0.25 to 16 | 62.5 | 8 to >16 | 100 | ≤0.125 to >16 | 60 | ≤0.03 to >4 | 68.8 | 0.03 to 2 | 6.3 |
A total of 232/322 A. fumigatus isolates were determined to be sensitive to itraconazole, posaconazole, and voriconazole by azole agar screening.
One isolate each of the A. nidulans complex, A. quadrilineatus, and A. spinulosporus.
Twenty-two A. niger complex, three A. tubingensis, and one A. luchuensis isolate.
Three A. calidoustus isolates and one isolate each of A. fischeri, A. giganteus, A. persii, A. similis, and A. turcosus.
Two F. dimerum, two F. proliferatum, seven F. solani sensu stricto, and three F. solani complex isolates.
Two M. canis, four T. rubrum, one T. interdigitale, and two T. mentagrophytes complex isolates.
Two M. circinelloides isolates, three R. pusillus isolates, four R. microsporus isolates, and one isolate each of C. muscae, L. corymbifera, L. ramosa, Lichtheimia species, R. oryzae, S. racemosum, and Mucorales species.
The proportions of isolates with MICs above the A. fumigatus EUCAST ECOFF are illustrated.
Yeast.
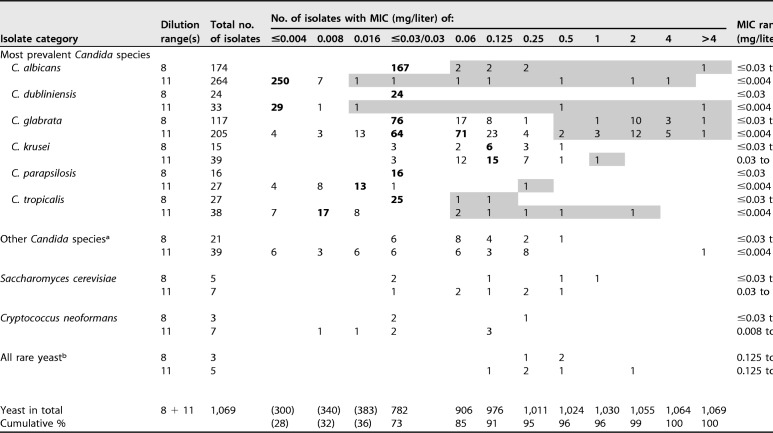
Candida accounted for 97.2% (n = 1039) of the 1,069 yeast isolates (Table 3). Other species were Saccharomyces cerevisiae (12; 1.1%), Cryptococcus neoformans (10; 0.9%), and rare yeast (8; 0.7%). Overall, 91% of MICs fell at ≤0.125 mg/liter, and 95% were ≤0.25 mg/liter.
TABLE 3.
MICs and geometric means of isavuconazole against the 1,069 yeast isolatesc
aFifteen C. lusitaniae isolates; 11 C. guilliermondii isolates; 6 C. orthopsilosis isolates; 5 C. kefyr isolates; 4 isolates of C. fermentati, C. inconspicua, and C. pelliculosa; 2 C. norvegensis isolates; and 1 isolate each of C. auris, C. bovina, C. duobushaemulonii, C. famata, C. metapsilosis, C. nivariensis, C. palmioleophila, C. pararugosa, and C. utilis.
bTwo Geotrichum candidum and Rhodotorula mucilaginosa isolates and one isolate each of Arxula adeninivorans, Exophiala dermatitidis, Geotrichum silvicola, and Magnusiomyces capitatus.
Modal MICs are highlighted in boldface type for species with ≥10 isolates, and presumably non-wild-type MIC ranges are shaded for Candida isolates with MICs >2 dilution steps above the modal MIC (≥2 dilution steps when the MIC distribution was clearly truncated with the modal MIC at the lowest dilution tested [0.004 mg/liter]). The numbers and percentages in parentheses represent underestimates of the true numbers and proportions due to the truncation of the tested drug concentration ranges.
The vast majority of the MICs against the most susceptible Candida species fell at or below the lowest concentrations tested (Table 3), i.e., for C. albicans, 417/438 (95.2%) overall and 250/264 (94.7%) for the extended concentration range, specifically, and for C. dubliniensis, 53/57 (93.0%) and 29/33 (87.9%), respectively. Consequently, modal MIC and MIC50 values were ≤0.004 mg/liter. For the other common Candida species, the modal MIC/MIC50 values (milligrams per liter) were as follows: 0.008/0.008 for C. tropicalis, 0.016/0.016 for C. parapsilosis, 0.06/0.06 for C. glabrata, and 0.125/0.125 for C. krusei. In all cases, the modal MIC and MIC50 values set using the 11-dilution range were supported by the 8-dilution range adopted in the first part of the study period, suggesting a robust performance of EUCAST isavuconazole testing during the study period.
For 65/979 (6.6%) isolates from these six most prevalent Candida species (isolated from 60 patients), an isavuconazole MIC >2 dilution steps above the modal MIC for a given species (≥2 dilutions if the modal MIC fell at the lowest concentration tested [0.004 mg/liter]) could be documented, suggesting a non-wild-type phenotype (indicated with gray shading in Table 3). In detail, this was the case for 14/438 (3.2%) C. albicans, 3/57 (5.3%) C. dubliniensis, 38/322 (11.8%) C. glabrata, 1/54 (1.9%) C. krusei, 1/43 (2.3%) C. parapsilosis, and 8/65 (12.3%) C. tropicalis isolates. When fluconazole and voriconazole susceptibilities were investigated for these isolates, 96.1% were nonsusceptible/non-wild type to fluconazole, 86.2% were nonsusceptible/non-wild type to voriconazole, and all were nonsusceptible/non-wild type to at least one of the two (data not shown). For C. glabrata, 13/38 (34.2%) of the isavuconazole non-WT isolates presented fluconazole MICs of >16 mg/liter, rendering classification as intermediate (I) or R impossible. One of these isolates was voriconazole susceptible (S), whereas the remaining 12 were classified as voriconazole non-WT.
The rare Candida species were characterized by isavuconazole MICs spanning the entire ≤0.004- to >4-mg/liter concentration range, suggesting differential susceptibilities of the involved species. The most resistant species was C. duobushaemulonii (n = 1), with an MIC of >4 mg/liter, whereas the single C. auris isolate, originating from a Norwegian patient, as well as isolates belonging to C. bovina, C. famata, C. kefyr, C. metapsilosis, C. nivariensis, C. palmioleophila, C. pararugosa, C. pelliculosa, and C. utilis had isavuconazole MICs of ≤0.06 mg/liter. Similarly, the MICs for rare yeast were diverse, with Magnusiomyces capitatus being the isolate with the highest isavuconazole MIC of 2 mg/liter.
On a milligram-per-liter basis, the in vitro activity of isavuconazole was more similar to that of voriconazole against the yeast isolates than to that of fluconazole and similar to that of the echinocandins, except for C. parapsilosis, Cryptococcus, C. glabrata, and C. krusei (Table 4).
TABLE 4.
Modal MICs/MIC50s and proportions of non-wild-type isolates for isavuconazole and comparators for the yeast isolatese
| Isolate category | Dilution range | Total no. of isolates | ISA |
FLC |
VRC |
AMBa
|
ANF |
MFG |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modal MIC/MIC50 (range) or range (mg/liter) | % of non-WT isolates | Modal MIC/MIC50 (range) or range (mg/liter) | % of non-WT isolates | Modal MIC/MIC50 (range) or range (mg/liter) | % of non-WT isolates | Modal MIC/MIC50 (range) or range (mg/liter) | % of non-WT isolates | Modal MIC/MIC50 (range) or range (mg/liter) | % of non-WT isolates | Modal MIC/MIC50 (range) or range (mg/liter) | % of non-WT isolates | |||
| C. albicans | 8 | 174 | ≤0.03/≤0.03 (≤0.03 to >4) | 3.2 | 0.25/0.25 (≤0.125 to >16) | 2.7 | ≤0.03/≤0.03 (≤0.03 to 2) | 2.5 | ND | 0 | ≤0.008/≤0.008 (≤0.008 to 0.03) | 0.3 | ≤0.008/≤0.008 (≤0.008 to 0.03) | 3.2 |
| 11 | 264 | ≤0.004/≤0.004 (≤0.004 to 4) | 0.25/0.25 (0.03 to >32) | ≤0.004/≤0.004 (≤0.004 to 2) | 0.25/0.25 (≤0.016 to 0.5) | ≤0.004/≤0.004 (≤0.004 to 0.125) | 0.008/0.008 (≤0.004 to 0.5) | |||||||
| C. dubliniensis | 8 | 24 | ≤0.03/≤0.03 (≤0.03) | 5.3 | ≤0.125/≤0.125 (≤0.125 to 4) | 5.3 | ≤0.03/≤0.03 (≤0.03) | 5.3 | ND | 0 | 0.016/0.016 (≤0.008 to 0.5) | 2.2 | 0.016/0.016 (≤0.008 to 1) | 6.5 |
| 11 | 33 | ≤0.004/≤0.004 (≤0.004 to >4) | 0.125/0.25 (0.06 to >32) | 0.008/0.008 (≤0.004 to >4) | 0.03/0.03 (≤0.016 to 0.25) | 0.008/0.008 (≤0.004 to 0.03) | 0.016/0.016 (≤0.004 to 0.06) | |||||||
| C. glabrata | 8 | 117 | ≤0.03/≤0.03 (≤0.03 to >4) | 11.8 | 4/4 (0.5 to >16) | 7.8d | 0.06/0.06 (≤0.03 to 4) | 7.8 | ND | 0 | 0.06/0.06 (0.016 to 1) | 1.9 | ≤0.008/≤0.008 (≤0.008 to 1) | 2.7 |
| 11 | 205 | 0.06/0.06 (≤0.004 to >4) | 4/4 (0.5 to >32) | 0.06/0.06 (0.016 to >4) | 0.25/0.25 (0.03 to 1) | 0.016/0.016 (0.008 to 1) | 0.016/0.016 (≤0.004 to 0.5) | |||||||
| C. krusei | 8 | 15 | 0.125/0.125 (≤0.03 to 0.5) | 1.9 | >16/>16 (16 to >16) | 0 | 0.25/0.25 (0.125 to 1) | 1.9 | ND | 0 | 0.06/0.06 (0.03 to 0.06) | 0 | 0.125/0.125 (0.06 to 0.25) | 0 |
| 11 | 39 | 0.125/0.125 (0.03 to 1) | 32/32 (8 to >32) | 0.25/0.25 (0.125 to 2) | 0.5/0.5 (0.5 to 1) | 0.03/0.03 (0.016 to 0.06) | 0.125/0.125 (0.06 to 0.5) | |||||||
| C. parapsilosis | 8 | 16 | ≤0.03/≤0.03 (≤0.03) | 2.3 | 1/1 (≤0.125 to 4) | 8.7 | ≤0.03/≤0.03 (≤0.03 to 0.06) | 3.3 | ND | 0 | >1/>1 (1 to >1) | 0 | (1/>1)/>1 (1 to >1) | 0 |
| 11 | 27 | 0.016/0.016 (≤0.004 to 0.25) | (0.5/1)/1 (0.5 to >32) | 0.016/0.016 (0.008 to 2) | 0.5/0.5 (0.125 to 1) | 0.5/1 (0.5 to 2) | 2/2 (0.5 to 4) | |||||||
| C. tropicalis | 8 | 27 | ≤0.03/≤0.03 (≤0.03 to 0.125) | 12.3 | 0.5/0.5 (≤0.125 to 16) | 10.8 | ≤0.03/≤0.03 (≤0.03 to 0.25) | 12.3 | ND | 0 | 0.03/0.03 (≤0.008 to 0.5) | 1.0 | 0.016/0.016 (≤0.008 to 0.5) | 1.0 |
| 11 | 38 | 0.008/0.008 (≤0.004 to 2) | 0.5/0.5 (0.125 to >32) | 0.016/0.03 (0.008 to >4) | 0.25/0.25 (0.125 to 1) | 0.016/0.016 (≤0.004 to 0.03) | 0.03/0.03 (≤0.004 to 0.06) | |||||||
| Other Candida speciesb | 8 | 21 | ≤0.03 to 0.5 | ≤0.125 to >16 | ≤0.03 to 2 | 0.016 to >1 | 0.03 to 0.5 | |||||||
| 11 | 39 | ≤0.004 to >4 | 0.06 to >32 | ≤0.004 to >4 | 0.06 to >4 | ≤0.004 to 2 | 0.016 to 0.5 | |||||||
| Saccharomyces cerevisiae | 8 | 5 | ≤0.03 to 1 | 2 to >16 | 0.06 to 0.5 | 0.06 to 0.25 | 0.125 to 0.25 | |||||||
| 11 | 7 | 0.03 to 0.5 | 2 to 16 | 0.06 to 0.5 | 0.25/0.25 (0.06 to 1) | 0.016 to 0.125 | 0.06 to 0.125 | |||||||
| Cryptococcus neoformans | 8 | 3 | ≤0.03 to 0.25 | 1 to 16 | ≤0.03 to 0.25 | >1 | >1 | |||||||
| 11 | 7 | 0.008 to 0.125 | 2 to 16 | 0.016 to 0.125 | 0.25/0.25 (0.03 to 0.5) | >4 | >4 | |||||||
| All rare yeastc | 8 | 3 | 0.125 to 0.5 | 8 to >16 | 0.06 to 2 | >1 | >1 | |||||||
| 11 | 5 | 0.125 to 2 | 16 to >32 | 0.25 to >4 | 0.06 to 1 | 0.125 to >4 | 0.03 to >4 | |||||||
Amphotericin B was tested only in a wide concentration range.
Fifteen C. lusitaniae isolates; 11 C. guilliermondii isolates; 6 C. orthopsilosis isolates; 5 C. kefyr isolates; 4 isolates of C. fermentati, C. inconspicua, and C. pelliculosa; 2 C. norvegensis isolates; and one isolate each of C. auris, C. bovina, C. duobushaemulonii, C. famata, C. metapsilosis, C. nivariensis, C. palmioleophila, C. pararugosa, and C. utilis.
Two Geotrichum candidum and Rhodotorula mucilaginosa isolates and one isolate each of Arxula adeninivorans, Exophiala dermatitidis, Geotrichum silvicola, and Magnusiomyces capitatus.
Potentially an underestimate of the true non-wild-type proportion, as some of the C. glabrata isolates were not tested at fluconazole concentrations above 16 mg/liter.
ISA, isavuconazole; FLC, fluconazole; VRC, voriconazole; AMB, amphotericin B; ANF, anidulafungin; MFG, micafungin; ND, not done.
Correlation between susceptibilities to isavuconazole and comparators.
Finally, the correlation between isavuconazole and comparator MICs was investigated (Table 5). A significant correlation was observed between isavuconazole and voriconazole MICs for all mold and Candida species, although it was weak (R2 of <0.5 for Mucorales, C. krusei, and C. parapsilosis). Moreover, the correlation between isavuconazole and voriconazole was stronger than that between isavuconazole and any other comparator for all species except A. terreus and Mucorales species (the best correlation was observed for isavuconazole and posaconazole). A good and highly significant correlation was also observed between isavuconazole and fluconazole for C. albicans, C. glabrata, and C. tropicalis but not for C. parapsilosis.
TABLE 5.
Correlation between isavuconazole MICs and those of voriconazole, itraconazole, posaconazole, and amphotericin Ba
| Species | Voriconazole |
Itraconazole |
Posaconazole |
Fluconazole |
Amphotericin B |
No. of isolatesb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | P | R2 | P | R2 | P | R2 | P | R2 | P | ||
| A. flavus | 0.598 | 0.0004 | 0.090 | 0.2581 | 0.196 | 0.086 | ND | ND | 0.229 | 0.0608 | 16 |
| A. fumigatus | 0.766 | <0.0001 | 0.289 | <0.0001 | 0.071 | 0.0115 | ND | ND | 0.003 | 0.6276 | 89 |
| A. niger | 0.573 | <0.0001 | 0.537 | 0.0002 | 0.225 | 0.0177 | ND | ND | 0.0414 | 0.3405 | 21–24 |
| A. terreus | 0.740 | 0.0003 | 0.763 | 0.0004 | 0.807 | 0.0002 | ND | ND | 0.585 | 0.0038 | 10–12 |
| Mucorales | 0.381 | 0.0108 | 0.072 | 0.3336 | 0.691 | <0.0001 | ND | ND | 0.101 | 0.2291 | 15–16 |
| C. albicans | 0.896 | <0.0001 | ND | ND | ND | ND | 0.786 | <0.0001 | 0.0215 | 0.4564 | 9–28 |
| C. dubliniensis | 0.938 | 0.0015 | ND | ND | ND | ND | 0.857 | 0.008 | 0.675 | 0.0234 | 6–7 |
| C. glabrata | 0.862 | <0.0001 | ND | ND | ND | ND | 0.817 | <0.0001 | 0.0092 | 0.1361 | 216–244 |
| C. krusei | 0.417 | <0.0001 | ND | ND | ND | ND | 0.207 | 0.0008 | 0.0146 | 0.3983 | 51 |
| C. parapsilosis | 0.448 | 0.0002 | ND | ND | ND | ND | 0.00871 | 0.6573 | 0.0934 | 0.1289 | 25–26 |
| C. tropicalis | 0.786 | <0.0001 | ND | ND | ND | ND | 0.668 | <0.0001 | 0.0679 | 0.1306 | 33–35 |
| Other Candida | 0.514 | <0.0001 | ND | ND | ND | ND | 0.439 | <0.0001 | 0.000464 | 0.8845 | 42–48 |
The strongest correlation (highest R2 coefficient) for each species across the four comparators is highlighted in boldface type, and significant P values are underlined. ND, not done.
Number of paired values for comparison (isolates with MICs below the lowest concentration were excluded from analyses, whereas isolates with MICs above the concentration range were elevated to the nearest higher 2-fold dilution).
DISCUSSION
This study confirmed previous findings of potent in vitro activity of isavuconazole against most human-pathogenic fungi (3–5, 9, 11). The rate of acquired resistance in Aspergillus and Candida spp. was overall low and stable (4). Exceptions were resistance rates of around 10% in A. fumigatus, A. terreus, C. glabrata, and C. tropicalis. However, resistance rates may be overestimated, as fungal isolates referred to a reference laboratory may not be representative of the general population. For the two Aspergillus species, however, the resistance was in part due to a stringent susceptibility breakpoint bisecting the wild-type populations of A. fumigatus and A. terreus, leading to a misclassification of some susceptible isolates as resistant. The ECOFF for isavuconazole against A. fumigatus is 2 mg/liter, but the clinical breakpoint established was one step lower because isolates with an MIC of 2 mg/liter may represent isolates with wild-type as well as mutant target gene sequences (1, 3). Thus, in a multicenter study, MICs straddled the ECOFF, with MICs of >2 mg/liter found in 25% of isolates harboring the M220I and M220V mutations and in 72.5% of the MIC readings of isolates withTR34/L98H alterations, suggesting a slight and more prominent reduction of susceptibility. The majority of the A. fumigatus isolates classified as wild type but resistant due to an MIC of 2 mg/liter in the present study had no cross-resistance to other azoles and no target gene alterations. Moreover, one isolate was voriconazole susceptible and harbored the M220K alteration, which previously has been shown not to affect isavuconazole susceptibility (3). Finally, one strain harbored a G54A alteration and was voriconazole intermediate. Whereas isavuconazole susceptibility in strains harboring G54E, G54R, G54V, or G54W alterations appears unaffected, to our knowledge, it is not known if G54A may affect isavuconazole and voriconazole susceptibility (3). Hence, our data suggested that the majority of isolates with an MIC of 2 mg/liter will be true wild-type isolates, and the remaining minority will be characterized by slightly reduced isavuconazole susceptibility. Buil et al. found that the probability of target attainment for isolates with isavuconazole MICs of 2 mg/liter with the isavuconazole standard dose was ∼75% (64% to 92%) when the 90% exposure index (EI90) was used as the endpoint and that a trough level of ≥1.60 mg/liter (1.42 to 1.80 mg/liter) was the target (8). Another recent study reported that approximately 10% of “real-life” clinical samples contained less than 1 mg/liter and another approximately 20% contained between 1 and 2 mg/liter of isavuconazole (12). Taken together, these observations support introducing an intermediate category for A. fumigatus and A. terreus isolates with an MIC of 2 mg/liter in a setting where therapeutic drug monitoring is available to confirm sufficient exposure.
When the correlations between isavuconazole MICs and those for the comparators were analyzed, the strongest correlation overall was found for isavuconazole and voriconazole. Thus, a significant strong to moderate correlation was found for the four most common Aspergillus species as well as for the six most common Candida species except C. krusei and C. parapsilosis, for which the correlation was significant but weak (R2 of 0.417 to 0.448), potentially due to the lack of isolates with acquired resistance for these two species. Thus, our results extend previous findings of a correlation between the azoles and, particularly, voriconazole and isavuconazole for A. fumigatus (7, 8) and the findings that isavuconazole susceptibility was lower for Candida isolates with resistance or non-wild-type susceptibility to fluconazole and voriconazole (9). No correlation was found for isavuconazole compared to itraconazole or posaconazole for A. flavus or A. fumigatus. For A. fumigatus, this may not be surprising, as it is well acknowledged that some target gene mutations specifically affect itraconazole and posaconazole (3). For A. flavus, we assume that the absence of isolates with differential susceptibility explains the lack of correlation because MIC variation in such cases can be explained solely by test variation. Therefore, taken together, our data support that voriconazole susceptibility is a strong marker of isavuconazole susceptibility in most clinically relevant Candida and Aspergillus isolates. Of note, this suggests that the azole agar screening method (EUCAST E.Def 10.1) can be adopted for identification of A. fumigatus isolates suitable as targets for isavuconazole therapy despite the fact that an isavuconazole agar is not included in the plate design (13).
Isavuconazole is licensed as a second-line option for the treatment of Mucorales infections in adults after the VITAL study showed equal clinical efficacy compared to that for matched historical controls treated with amphotericin B (14). In that study, species-specific outcome evaluation was not performed, probably in part because one-third of the cases lacked species identification. We found consistently high MICs of ≥16 mg/liter for M. circinelloides, confirming previous findings by CLSI and EUCAST testing (15, 16). Hence, species identification is highly recommended, as clinical efficacy remains to be confirmed for this species.
Isavuconazole is not licensed for the treatment of invasive candidiasis after a recent phase 3, randomized, double-blind, multinational clinical trial failed to demonstrate noninferiority at the end of intravenous (i.v.) therapy compared to caspofungin (17). Thus, the trial supported the results of other clinical studies showing superiority of echinocandins over azoles and amphotericin B and, thus, the recommendation of echinocandins as first-line agents for candidemia and invasive candidiasis (18–21). Nevertheless, the secondary endpoints (overall response to therapy 2 weeks after the end of therapy and all-cause mortality on days 14 and 56) were similar between arms, as were safety and median time to clearance from the bloodstream. On this background and taking the potent in vitro activity and attractive safety profile compared to fluconazole and voriconazole into account, isavuconazole might serve as a valid second-line option in settings where echinocandin resistance is likely or documented, mold coverage is indicated, or oral therapy is preferred.
In summary, isavuconazole displayed broad in vitro activity against most human-pathogenic species, including dermatophytes and several uncommon species. Of note, however, we confirmed low isavuconazole in vitro activity against M. circinelloides and therefore advocate for performing species identification, also for Mucorales, whenever possible. Acquired isavuconazole resistance was infrequent, except in A. terreus, C. glabrata, and C. tropicalis, and, when present, was associated with cross-resistance to other azoles. Continued surveillance remains important.
MATERIALS AND METHODS
Isolates.
In total, 1,069 yeast and 429 mold isolates from 1,325 patients were included (1 isolate each from 1,186 patients and 2 to 7 isolates from 139 patients). The isolates were prospectively obtained from clinical samples or pure cultures received at the mycology reference laboratory at Statens Serum Institut for identification and susceptibility testing during 2016 and 2017. No ethical restraints apply to studies of routinely obtained anonymized laboratory data. Same-species isolates from the same patient were excluded from the study if sampled ≤21 days apart and identical MICs (within ±1 dilution step) were seen. The isolates derived from the entire country and the following clinical specimens: blood as part of the national surveillance program (915 specimens), airways/lung/pleura/sinus (404), other normally sterile sites (47), urine (20), skin/scalp/nail (24), cervix/vagina/urethra (13), other superficial sites (39), and other/unspecified (36). Yeast identification was done using macro- and micromorphology, supplemented by thermotolerance (incubation at 37°C and 43°C), matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker, Bremen, Germany) for Candida (22), and, when needed, internal transcribed spacer (ITS) sequencing (23). Similarly, mold identification was done by classical techniques, including thermotolerance (incubation at 50°C) for discriminating A. fumigatus sensu stricto from cryptic species, which underwent β-tubulin sequencing (24). The use of the term “complex” is acknowledged for Aspergillus species other than A. fumigatus, in the absence of detailed molecular identification, although for simplicity, it is not used throughout this work. ITS and TEF (transcription elongation factor) sequencing were adopted for other molds and Fusarium species, specifically (23, 25).
Susceptibility testing.
EUCAST susceptibility testing was performed according to E.Def 7.3.1 for yeast. Isavuconazole and amphotericin B MICs were determined for all 1,069 yeast isolates, voriconazole and fluconazole MICs were determined for 1,068/1,069 (99.9%) isolates, micafungin MICs were determined for 1,066/1,069 (99.7%) isolates, and anidulafungin MICs were determined for 1,064/1,069 (99.5%) of the yeast isolates. For the molds, A. fumigatus isolates were screened for azole resistance according to EUCAST E.Def 10.1 using a four-well plate containing RPMI 1640–2% glucose agar supplemented with itraconazole (4 mg/liter), voriconazole (1 mg/liter), posaconazole (0.5 mg/liter), and no antifungal (positive control) (Balis Laboratorium VOF, Boven-Leeuwen, the Netherlands). In brief, 25 μl of a conidial suspension (filtered through an 11-nm filter) at a 0.5 McFarland standard was added to each well, and the plate was incubated for 48 h at 37°C before reading. Screening of agar-positive A. fumigatus isolates and all other molds was performed according to EUCAST E.Def 9.3.1, with standard filtration (11-nm filter) of the inoculum. Isavuconazole and voriconazole susceptibilities were determined for all 429 mold isolates, posaconazole susceptibility was determined for 428/429 (99.8%) isolates, and itraconazole and amphotericin B susceptibilities were determined for 427/429 (99.5%) of the mold isolates. Stock solutions of the following antimycotics were prepared at 5,000 mg/liter in dimethyl sulfoxide (Sigma-Aldrich, Brøndby, Denmark): isavuconazole (Basilea Pharmaceutica Ltd., Basel, Switzerland), voriconazole (Pfizer, Ballerup, Denmark), itraconazole (Sigma-Aldrich), posaconazole (MSD, Ballerup, Denmark), fluconazole (Sigma), amphotericin B (Sigma), anidulafungin (Pfizer, Ballerup, Denmark), and micafungin (Astellas, Tokyo, Japan). Cell culture-treated microtiter polystyrene plates (Nunc microwell 96-well microplates, catalog no. 167008; Thermo Fisher Scientific) were used throughout. Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as controls for the yeasts, and Aspergillus flavus ATCC 204304 and Aspergillus fumigatus ATCC 204305 were used as controls for the molds. For the yeast isolates, during the study period, the concentration range was extended from 8 to 11 dilutions. CYP51A sequencing was performed for non-wild-type/resistant A. fumigatus and A. terreus isolates.
Data analysis.
Modal MICs, MIC50s, geometric mean MICs (GM-MICs), and MIC ranges were determined for individual species (n ≥ 10). EUCAST ECOFFs/breakpoints were adopted for wild-type/susceptibility classification. For species without EUCAST ECOFFs, MICs >2 dilution steps above the modal MIC were regarded as non-wild type. However, for species where the modal MIC was equal to or lower than the lowest concentration tested, MICs ≥2 dilution steps above the modal MIC were regarded as non-wild type. Pearson correlation analyses with a two-tailed P value were performed for comparisons of antifungal in vitro activities (after log2 transformation) using GraphPad Prism 7.04. Correlation coefficients (squared) (R2) of ≥0.5 with a P value <0.05 were interpreted as a significant indicator of good to strong correlation, whereas R2 values of <0.5 indicated weak correlation.
ACKNOWLEDGMENTS
We thank research technician Birgit Brandt for excellent technical assistance.
This study was supported by an unrestricted grant from Basilea. The funder had no influence on the study design or on the analysis of the results.
Outside this work, we have the following potential conflicts to declare. M.C.A. has received personal speakers’ honoraria from Astellas, Basilea, Gilead, MSD, Pfizer, T2Candida, and Novartis. She has received research grants and contract work paid to the Statens Serum Institute from Astellas, Basilea, Gilead, MSD, Novabiotics, Pfizer, T2Candida, F2G, Cidara, and Amplyx. K.M.T.A. has received travel grants from Gilead and Pfizer. K.M.J. has received meeting travel grants from Amplyx, MSD, and F2G. R.K.H. has received a research grant from Gilead and travel grants from Gilead, Astellas, MSD, and Pfizer.
REFERENCES
- 1.Arendrup MC, Meletiadis J, Mouton JW, Guinea J, Cuenca-Estrella M, Lagrou K, Howard SJ, Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. 2016. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect 22:571.e1–571.e4. doi: 10.1016/j.cmi.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Viljoen J, Azie N, Schmitt-Hoffmann AH, Ghannoum M. 2015. A phase 2, randomized, double-blind, multicenter trial to evaluate the safety and efficacy of three dosing regimens of isavuconazole compared with fluconazole in patients with uncomplicated esophageal candidiasis. Antimicrob Agents Chemother 59:1671–1679. doi: 10.1128/AAC.04586-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard SJ, Lass-Flörl C, Cuenca-Estrella M, Gomez-Lopez A, Arendrup MC. 2013. Determination of isavuconazole susceptibility of Aspergillus and Candida species by the EUCAST method. Antimicrob Agents Chemother 57:5426–5431. doi: 10.1128/AAC.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astvad KMT, Hare RK, Arendrup MC. 2017. Evaluation of the in vitro activity of isavuconazole and comparator voriconazole against 2635 contemporary clinical Candida and Aspergillus isolates. Clin Microbiol Infect 23:882–887. doi: 10.1016/j.cmi.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Marcos-Zambrano LJ, Gómez A, Sánchez-Carrillo C, Bouza E, Muñoz P, Escribano P, Guinea J. 2018. Isavuconazole is highly active in vitro against Candida species isolates but shows trailing effect. Clin Microbiol Infect 24:1343.e1–1343.e4. doi: 10.1016/j.cmi.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Pfaller MA, Castanheira M, Messer SA, Rhomberg PR, Jones RN. 2014. Comparison of EUCAST and CLSI broth microdilution methods for the susceptibility testing of 10 systemically active antifungal agents when tested against Candida spp. Diagn Microbiol Infect Dis 79:198–204. doi: 10.1016/j.diagmicrobio.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Gregson L, Goodwin J, Johnson A, McEntee L, Moore CB, Richardson M, Hope WW, Howard SJ. 2013. In vitro susceptibility of Aspergillus fumigatus to isavuconazole: correlation with itraconazole, voriconazole, and posaconazole. Antimicrob Agents Chemother 57:5778–5780. doi: 10.1128/AAC.01141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buil JB, Brüggemann RJM, Wasmann RE, Zoll J, Meis JF, Melchers WJG, Mouton JW, Verweij PE. 2018. Isavuconazole susceptibility of clinical Aspergillus fumigatus isolates and feasibility of isavuconazole dose escalation to treat isolates with elevated MICs. J Antimicrob Chemother 73:134–142. doi: 10.1093/jac/dkx354. [DOI] [PubMed] [Google Scholar]
- 9.Castanheira M, Messer SA, Rhomberg PR, Dietrich RR, Jones RN, Pfaller MA. 2014. Isavuconazole and nine comparator antifungal susceptibility profiles for common and uncommon Candida species collected in 2012: application of new CLSI clinical breakpoints and epidemiological cutoff values. Mycopathologia 178:1–9. doi: 10.1007/s11046-014-9772-2. [DOI] [PubMed] [Google Scholar]
- 10.Astvad KMT, Johansen HK, Røder BL, Rosenvinge FS, Knudsen JD, Lemming L, Schønheyder HC, Hare RK, Kristensen L, Nielsen L, Gertsen JB, Dzajic E, Pedersen M, Østergård C, Olesen B, Søndergaard TS, Arendrup MC. 2018. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol 56:e01564-17. doi: 10.1128/JCM.01564-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guinea J, Peláez T, Recio S, Torres-Narbona M, Bouza E. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother 52:1396–1400. doi: 10.1128/AAC.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andes D, Kovanda L, Desai A, Kitt T, Zhao M, Walsh TJ. 2018. Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Antimicrob Agents Chemother 62:e00585-18. doi: 10.1128/AAC.00585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guinea J, Verweij PE, Meletiadis J, Mouton JW, Barchiesi F, Arendrup MC, Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. How to: EUCAST recommendations on the screening procedure E.Def 10.1 for the detection of azole resistance in Aspergillus fumigatus isolates using four well azole-containing agar plates. Clin Microbiol Infect, in press. [DOI] [PubMed] [Google Scholar]
- 14.Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson GR, Alangaden GJ, Brown JM, Fredricks DN, Heinz WJ, Herbrecht R, Klimko N, Klyasova G, Maertens JA, Melinkeri SR, Oren I, Pappas PG, Ráčil Z, Rahav G, Santos R, Schwartz S, Vehreschild JJ, Young J-A, Chetchotisakd P, Jaruratanasirikul S, Kanj SS, Engelhardt M, Kaufhold A, Ito M, Lee M, Sasse C, Maher RM, Zeiher B, Vehreschild MJGT, VITAL and FungiScope Mucormycosis Investigators. 2016. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 16:828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 15.Pfaller MA, Rhomberg PR, Wiederhold NP, Gibas C, Sanders C, Fan H, Mele J, Kovanda LL, Castanheira M. 2018. In vitro activity of isavuconazole against opportunistic fungal pathogens from two mycology reference laboratories. Antimicrob Agents Chemother 62:e01230-18. doi: 10.1128/AAC.01230-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arendrup MC, Jensen RH, Meletiadis J. 2015. In vitro activity of isavuconazole and comparators against clinical isolates of the Mucorales order. Antimicrob Agents Chemother 59:7735–7742. doi: 10.1128/AAC.01919-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kullberg BJ, Viscoli C, Pappas PG, Vazquez J, Ostrosky-Zeichner L, Rotstein C, Sobel JD, Herbrecht R, Rahav G, Jaruratanasirikul S, Chetchotisakd P, Van Wijngaerden E, De Waele J, Lademacher C, Engelhardt M, Kovanda LL, Croos-Dabrera R, Fredericks C, Thompson GR III.. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive candida infections: the ACTIVE trial. Clin Infect Dis, in press. [DOI] [PubMed] [Google Scholar]
- 18.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18:19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 20.Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, Wible M, Goldstein BP, Schranz J, Krause DS, Walsh TJ. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 356:2472–2482. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- 21.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, Sobel JD, Pappas PG, Kullberg BJ. 2012. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 54:1110–1122. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 22.Jensen RH, Arendrup MC. 2011. Candida palmioleophila: characterization of a previously overlooked pathogen and its unique susceptibility profile in comparison with five related species. J Clin Microbiol 49:549–556. doi: 10.1128/JCM.02071-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Bolchacova E, Voigt K, Crous PW, Miller AN, Wingfield MJ, Aime MC, An K-D, Bai F-Y, Barreto RW, Begerow D, Bergeron M-J, Blackwell M, Boekhout T, Bogale M, Boonyuen N, Burgaz AR, Buyck B, Cai L, Cai Q, Cardinali G, Chaverri P, Coppins BJ, Crespo A, Cubas P, Cummings C, Damm U, de Beer ZW, de Hoog GS, Del-Prado R, Dentinger B, Dieguez-Uribeondo J, Divakar PK, Douglas B, Duenas M, Duong TA, Eberhardt U, Edwards JE, Elshahed MS, Fliegerova K, Furtado M, Garcia MA, Ge Z-W, Griffith GW, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci U S A 109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell K, Sutton DA, Rinaldi MG, Sarver BAJ, Balajee SA, Schroers H-J, Summerbell RC, Robert VARG, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee Y-H, Kang S, Park B, Geiser DM. 2010. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol 48:3708–3718. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]