Pseudomonas aeruginosa is an opportunistic, nosocomial bacterial pathogen that forms persistent infections due to the formation of protective communities, known as biofilms. Once the biofilm is formed, the bacteria embedded within it are recalcitrant to antimicrobial treatment and host immune defenses.
KEYWORDS: Pseudomonas aeruginosa, Psl, biofilm, exopolysaccharide, glycoside hydrolase, therapeutic, wound infection
ABSTRACT
Pseudomonas aeruginosa is an opportunistic, nosocomial bacterial pathogen that forms persistent infections due to the formation of protective communities, known as biofilms. Once the biofilm is formed, the bacteria embedded within it are recalcitrant to antimicrobial treatment and host immune defenses. Moreover, the presence of biofilms in wounds is correlated with chronic infection and delayed healing. The current standard of care for chronic wound infections typically involves physical disruption of the biofilm via debridement and subsequent antimicrobial treatment. The glycoside hydrolases PelAh and PslGh have been demonstrated in vitro to disrupt biofilm integrity through degradation of the key biofilm matrix exopolysaccharides Pel and Psl, respectively. Herein, we demonstrate that PslGh hydrolase therapy is a promising strategy for controlling P. aeruginosa wound infections. Hydrolase treatment of P. aeruginosa biofilms resulted in increased antibiotic efficacy and penetration into the biofilm. PslGh treatment of P. aeruginosa biofilms also improved innate immune activity leading to greater complement deposition, neutrophil phagocytosis, and neutrophil reactive oxygen species production. Furthermore, when P. aeruginosa-infected wounds were treated with a combination of PslGh and tobramycin, we observed an additive effect leading to greater bacterial clearance than treatments of tobramycin or PslGh alone. This study demonstrates that PelAh and PslGh have promising therapeutic potential and that PslGh may aid in the treatment of P. aeruginosa wound infections.
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen, which can cause devastating infections in immunocompromised individuals. This bacterium is one of the most common causes of nosocomial infections and is frequently isolated from acute and chronic wound infections, respiratory infections, and the surface of medical devices (1–3). Furthermore, P. aeruginosa frequently exhibits recalcitrance toward conventional antimicrobials, due to its ability to form protective communities, known as biofilms (2, 4). Once encased within the biofilm matrix, P. aeruginosa becomes more tolerant to environmental stress, host immune factors, and antimicrobial treatment (5). Thus, it is unsurprising that biofilm development during infection results in chronicity and increased hospitalization costs.
The incidence of chronic wound infections is increasing worldwide as antimicrobial resistance and diseases associated with chronic wound risk, such as obesity and diabetes, increase in prevalence (6, 7). While the origin of chronic wounds can vary, recurrent infection and subsequent host inflammation delay healing (8). Although the number of chronic wound cases continues to rise, wound treatment strategies are becoming increasingly effective (9). The improvement in treatment outcomes can largely be attributed to better molecular diagnostic techniques, which allow for identification of the wound microbiota followed by targeted antimicrobial therapy. Currently, P. aeruginosa infections are treated with a combination of β-lactams, fluoroquinolones, and aminoglycosides (10). However, the incidence of infection with multidrug-resistant P. aeruginosa strains continues to increase (11). As P. aeruginosa is one of the most common organisms isolated from chronic wound infections (12), it has become increasingly important to identify targeted therapies that will enhance the effectiveness of traditional antibiotics. The connection between biofilms, chronic infection, and drug resistance is well established across bacterial species and disease states, making the biofilm a promising target for combating chronic infection and reducing the emergence of resistance. To date, various studies have determined that biofilm disruption in the wound, either mechanically via debridement or chemically via drug treatment, results in a limited window of increased antibiotic susceptibility (13–15). Therefore, in this study we sought to identify new methods for disrupting the biofilm in order to improve antibiotic efficacy.
P. aeruginosa biofilm formation is a complex process that involves the production and secretion of a variety of components, including proteins, extracellular DNA (eDNA), and exopolysaccharides (EPS). Pel and Psl are EPS that have critical roles in the structure and initiation of biofilm development, as they promote surface attachment and cell-cell adhesion (16, 17). Furthermore, Pel and Psl have direct roles in enhancing antibiotic tolerance and host immune evasion by either binding or blocking immune components and antimicrobial drugs before they reach cells within the biofilm (5, 18–20). Many P. aeruginosa strains, including PAO1, are genetically capable of producing both Pel and Psl but predominantly produce one EPS at a time. While the relative amount of each EPS produced varies by strain, Pel and Psl often serve similar functions in the biofilm (21). Therefore, Pel and Psl represent appealing antibiofilm targets for novel therapeutics.
The pel and psl synthesis operons have been well defined and both encode proteins PelA and PslG, respectively, that contain a glycoside hydrolase domain (22, 23). PelA is a multidomain protein whose deacetylation activity is required for Pel production (24). The role of the PelA and PslG hydrolytic activity remains unclear. We have previously reported that soluble recombinant forms of PelAh and PslGh are capable of hydrolyzing their respective EPS leading to disruption of established in vitro-grown biofilms (22, 25). Additionally, we determined that these enzymes increase P. aeruginosa susceptibility to colistin and HL-60 neutrophil phagocytosis (22). In the current study, we sought to determine how these enzymes improve antibiotic activity and host clearance. Herein, we provide further evidence that combined PelAh and PslGh treatment enhances antibiotic efficacy against biofilms and that this results from improved antibiotic penetration into the biofilm. PslGh treatment of P. aeruginosa also ameliorates host innate immunity by improving complement deposition, neutrophil phagocytosis, and neutrophil reactive oxygen species (ROS) production. However, PelAh treatment of P. aeruginosa did not appear to affect innate immunity. Furthermore, using a murine punch biopsy model, we demonstrate that PslGh potentiates antibiotic killing in the wound leading to reduced bacterial burden compared with wounds treated with only tobramycin.
RESULTS
PelAh and PslGh treatment increases P. aeruginosa biofilm susceptibility to antibiotic killing.
The biofilm exopolysaccharides Psl and Pel provide protection from colistin and tobramycin, which are commonly used to treat P. aeruginosa infections (18, 19, 26). Prior studies suggest that ionic interactions between EPS-associated matrix components and antibiotics results in their sequestration, preventing the compounds from reaching the bacteria within the biofilm (18, 19). Therefore, we hypothesized that disrupting the integrity of the biofilm matrix with PelAh and PslGh would increase P. aeruginosa antibiotic susceptibility. As we have previously reported, PelAh or PslGh treatment of static cultures which overproduced Pel or Psl led to increased colistin sensitivity (22). In this study, we sought to determine if hydrolase treatment improves killing activity with a variety of antibiotics, in a strain producing native levels of the exopolysaccharides. Additionally, this assay allows for the exclusion of planktonic cells, which provides a more specific measurement of the hydrolase effect on the biofilm.
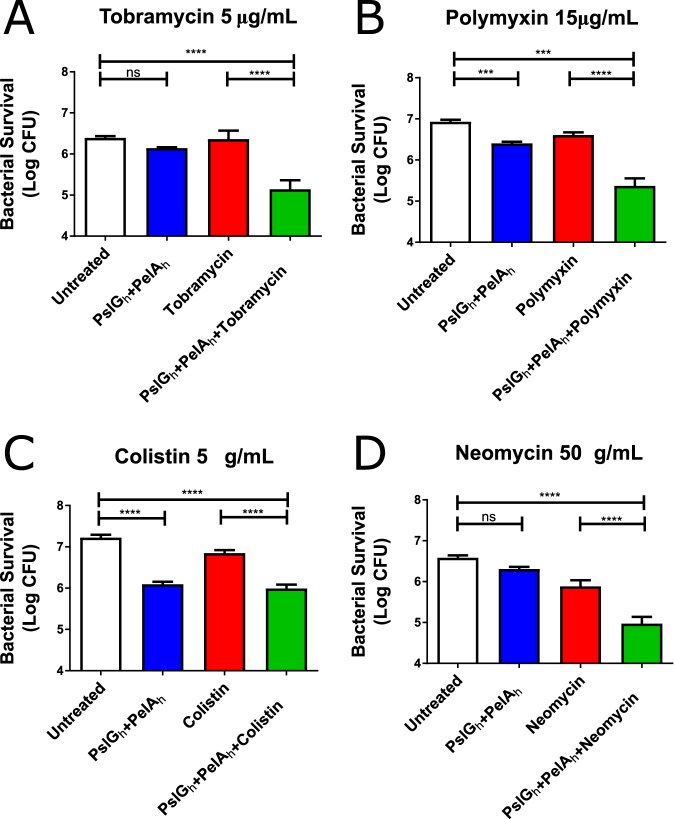
PAO1 biofilms were grown on polystyrene pegs immersed in liquid medium and treated with an antibiotic with or without combined PelAh and PslGh treatment. Following the combined treatment with PelAh and PslGh, P. aeruginosa was significantly more susceptible to killing by tobramycin, polymyxin, colistin, and neomycin (Fig. 1A to D). While PelAh was included in our biofilm treatments, PAO1 produces a predominantly Psl matrix (21). Therefore, the increased antibiotic efficacy observed here is likely due to the activity of PslGh rather than PelAh. Additionally, it is worth noting that hydrolase treatment without antibiotics reduced bacterial numbers. As the recombinant hydrolases do not exhibit bactericidal activity (22), the decrease in CFUs is likely due to biofilm disruption and cell removal during the washing steps.
FIG 1.
Combined PelAh and PslGh treatment of PAO1 biofilms improves antibiotic efficacy. PAO1 biofilms were treated as indicated followed by antibiotic treatment with (A) tobramycin, (B) polymyxin, (C) colistin, and (D) neomycin. Bacterial survival was determined by CFU quantification. Bars indicate mean ± SEM. Statistical significance was determined by one-way ANOVA followed by Bonferroni’s post hoc tests. ***, P < 0.001; ****, P < 0.0001.
PslGh treatment improves antibiotic penetration into the biofilm.
We next sought to investigate how hydrolase treatment improves antibiotic susceptibility. A previous study demonstrated that penetration of tobramycin into the P. aeruginosa biofilm is impaired (19). Considering PAO1 predominantly produces Psl (21), we measured the effect of PslGh hydrolase treatment on tobramycin biofilm penetration. Mature PAO1 biofilms were stained with Syto9 (green) and then treated with Cy5-labeled tobramycin (purple) with or without PslGh pretreatment. The intensity of Cy5 signal was measured across the surface of microcolony biofilm structures in order to determine how well tobramycin could penetrate into the biofilm. As previously demonstrated (19), we observed tobramycin localized to the periphery of the biofilm structure when untreated (Fig. 2A). However, when the biofilm was treated with PslGh for 5 minutes prior to antibiotic exposure for 30 minutes, tobramycin was located throughout the structure with less antibiotic at the periphery (Fig. 2B). This finding suggests that PslGh treatment improves antibiotic penetration into the biofilm, which likely contributes to hydrolase-mediated antibiotic potentiation.
FIG 2.
PslGh treatment improves tobramycin penetration into the biofilm. Four-day-old biofilms grown under flow conditions were untreated (A) or treated with 100 nM PslGh for 5 min (B) followed by application of Syto9- and Cy5-labeled tobramycin for 30 min. Biofilm structures were imaged with confocal microscopy (n > 9). The intensity of the tobramycin fluorescence signal (purple line) and bacterial population (green line) was measured across the surface of biofilm microcolonies to determine the location of tobramycin (bottom).
Combined PelAh and PslGh treatment leads to increased complement protein C3 deposition.
The complement system, which consists of a collection of circulating proteins in the serum and tissues, is an important component of the innate immune response. Complement proteins provide the host with a number of protective functions to enhance pathogen recognition and phagocytosis (27, 28). Some complement factors, such as C3b, function as opsonins that bind to the surface of bacterial cells. Furthermore, complement factors can initiate a direct killing process by recruiting additional factors to the bacterial cell surface leading to cell lysis (29, 30). Psl inhibits the deposition of the complement components C3, C5, and C7 resulting in a significant fitness advantage over Psl-deficient strains during infection (20). We hypothesized that hydrolase-mediated degradation of Psl from P. aeruginosa cells would improve complement deposition. Following hydrolase treatment, PAO1 was exposed to pooled human serum and bound C3 was quantified. For each strain tested, C3 binding values were normalized to the average of the same strain when untreated. We observed that hydrolase treatment significantly improves complement deposition onto PAO1 (Fig. 3). As predicted, hydrolase treatment of PAO1 cells lacking both Psl and Pel did not alter C3 deposition, indicating the observed effect on PAO1 is due to degradation of the EPS.
FIG 3.

PelAh plus PslGh treatment improves C3 surface deposition on PAO1 and CF127. Bacterial cultures untreated (white bars) or treated (blue bars) with PelA and PslGh were exposed to pooled human serum. C3 bound to the surface of bacterial cells was then measured using flow cytometry. Bars indicate mean ± SEM. Statistical significance was determined by Student’s t test (*, P < 0.05; **, P < 0.01).
While PAO1 predominantly produces Psl, there are common clinical P. aeruginosa variants that produce Pel or high levels of Pel and Psl (21). Although no interaction between Pel and complement has been reported, we investigated the effect of hydrolase treatment on two clinical strains, namely, PA14 and CF127, which produce only Pel or high basal levels of both Pel and Psl, respectively. Hydrolase treatment of PA14 did not appear to have any significant impact on C3 deposition, while we observed significantly greater C3 deposition onto CF127 when treated with PelAh and PslGh (Fig. 3). Although the temperature conditions tested here are similar to the human body, PA14 transcription of the pel synthesis operon is low at 37°C (31). Thus, PelAh treatment of PA14 may not improve C3 deposition because Pel was not produced under the conditions tested. Therefore, we conclude that hydrolase-aided complement deposition under these conditions may be limited specifically to PslGh removing surface-associated Psl (Fig. 3).
PelAh and PslGh potentiate the neutrophil response to P. aeruginosa.
Neutrophils are crucial for the control and clearance of P. aeruginosa infections (32), and they utilize a variety of methods, including phagocytosis and the release of antimicrobials, to remove bacteria from the host. In order to elucidate how hydrolase treatment promotes neutrophil killing of P. aeruginosa, we assessed the effect of hydrolase treatment on phagocytosis and ROS production, an important group of antimicrobial compounds. We observed a significant increase in uptake of PAO1 by human neutrophils following combined treatment with PelAh and PslGh (Fig. 4A). Treatment of PAO1 and CF127 with the hydrolases prior to neutrophil exposure also results in 2- to 3-fold greater neutrophil ROS production (Fig. 4B). As expected, treatment of PAO1ΔpelΔpsl did not have an effect on neutrophil phagocytosis or ROS production, indicating the response to PAO1 and CF127 is specific to EPS hydrolysis. Increased production of ROS suggests increased bacterial recognition, phagocytosis, and antimicrobial activity (29). One pathway that leads to neutrophil phagocytosis and subsequent ROS production is when the neutrophil complement receptor binds to complement components bound to an antigen (29, 33). Therefore, elevated ROS production in response to treated Psl-producing strains is likely a result of increased complement deposition.
FIG 4.
Combined PelAh and PslGh treatment enhances neutrophil function against P. aeruginosa. (A) Combined PelAh and PslGh treatment increased neutrophil phagocytosis. (B) PelAh plus PslGh treatment promoted neutrophil ROS production during infection. Bars indicate mean ± SEM. Statistical significance was determined by Student’s t test. (*, P < 0.05; **, P < 0.01).
As previously demonstrated, differences in the structure of lipopolysaccharides (LPS) produced by PA14 result in increased sensitivity to a variety of factors, relative to PAO1 (34). Due to increased serum sensitivity, we were unable to determine the effect of hydrolase treatment on the phagocytosis of PA14 and CF127, but we were able to measure the ROS response. Similar to our complement deposition studies, hydrolase treatment of PA14 did not affect neutrophil ROS production (Fig. 4B), which could again be the result of low levels of Pel production under the conditions tested. Thus, the activity observed here for CF127 and PAO1 is likely specific to PslGh and the Psl EPS produced by these strains. Collectively, our results suggest that PslGh-mediated removal of Psl improves bacterial recognition and killing.
PelAh and PslGh are nontoxic to host cells and do not impede the host immune response.
Prior to using these hydrolases in vivo, we sought to evaluate the safety of introducing these enzymes into the host environment. We have previously reported no negative effects following exposure of IMR-90 lung fibroblasts to PelAh and PslGh (22). To expand on this study, we next evaluated whether the enzymes have any toxic effect on primary human cells. Red blood cells (RBCs) were isolated from healthy human blood and treated with PelAh and PslGh, either alone or in combination. We observed no significant loss of intact RBCs, suggesting no cell death was occurring at the concentration of protein used (Fig. 5A). To assess any impact on neutrophil function, human neutrophils were exposed to PelAh and PslGh, either alone or in combination for 1 hour with or without the ROS-inducing stimulant phorbol myristate acetate. The presence of either hydrolase did not impede the ability of neutrophils to produce ROS, and the enzymes themselves did not stimulate an ROS response in neutrophils. These data further indicate that the hydrolases have no negative effects on host cells (Fig. 5B). The lack of neutrophil response when incubated with PelAh and PslGh also suggests that the purified hydrolases lack significant amounts of any contaminating agonist (e.g., LPS, flagellin, and peptidoglycan).
FIG 5.
PelAh and PslGh are not toxic to host cells and do not impede wound healing. (A) Primary human red blood cell viability is unaffected by PelAh or PslGh treatment. (B) Human neutrophil activity is unaffected by PelAh and PslGh treatment. (C, D, and E) Treatment of porcine burn wounds with up to 1 mg/ml of PslGh had no negative effects on wound healing (D) and did not exacerbate necrosis or inflammation (D, E). Arrows indicate the epithelial tongue, and asterisks indicate large populations of neutrophils (E). Bars indicate mean ± SEM. Statistical significance was determined using two-way ANOVA followed by Bonferroni’s post hoc tests. ns, not significant.
Since we observed no cytotoxicity or impairment of immune cell function in vitro, we next used a porcine burn wound model to examine the effects of PslGh in vivo. The porcine wound closely resembles a human wound, due to anatomical similarity and a comparable reepithelization process (35, 36). These factors make the porcine wound model ideal for identifying any negative host response to the hydrolases. As PslGh activity appears primarily responsible for improving the immune response, we limited our wound studies to treatment with this enzyme alone. Treatment of burn wounds with PslGh had no significant effect on wound size, tissue necrosis, or inflammation (Fig. 5C to E). Collectively, these results suggest that PslGh treatment has no negative effects for the host.
Loss of Psl and Pel production attenuates P. aeruginosa wound infection.
Prior studies have demonstrated that Pel and Psl protect P. aeruginosa from antimicrobial treatment and host clearance, leading to more severe infection (18, 19, 26, 37). However, it remains unclear if EPS production contributes to virulence in vivo. Here, we utilized a murine punch biopsy wound infection model, which allowed us to monitor the progression of infection in real time. Biopsy wounds were infected with either PAO1-lux or PAO1ΔpelΔpsl-lux. Forty-eight and 72 hours postinfection (hpi), the bacterial burden was nearly 1 log lower in PAO1ΔpelΔpsl-infected wounds than in PAO1-infected wounds (Fig. 6). However, at 96 hpi, there was no longer a significant difference in bacterial burden between PAO1 and PAO1ΔpelΔpsl (Fig. 6B). These results suggest that the loss of EPS impacts initial colonization, but this may not necessarily affect the long-term outcome of an infection.
FIG 6.
Loss of Psl and Pel production attenuates P. aeruginosa wound infection. (A) Representative IVIS images of luminescent signal in PAO1-lux- and PAO1ΔpelΔpsl-lux-infected wounds 2 days postinfection. (B) Wound bacterial burden was quantified by measuring the emitted light from P. aeruginosa-infected mouse wounds. Bars indicate mean ± SEM. At least five mice were included in each treatment group, and statistical significance was determined using Student’s t test. (*, P < 0.05; **, P < 0.01).
Topical wound treatment with PslGh improves bacterial clearance and tobramycin efficacy.
Currently, the most effective method for treating chronic wound infections involves regular debridement followed by antimicrobial treatment (9, 38). The process of debridement removes dead tissue and bacteria, and the physical disruption of biofilm integrity leads to a short period of increased antimicrobial susceptibility (14). Therefore, we hypothesized that disruption of a wound infection with hydrolases would improve P. aeruginosa susceptibility to topical antibiotic treatment. While tobramycin is predominantly administered orally, there is evidence that suggests topical application of tobramycin combats infection and helps reduce the risk of developing antibiotic resistance (38–40). We tested multiple concentrations of the antibiotic on PAO1-lux-infected wounds. A total of 30 μg/kg and 3 μg/kg of tobramycin drastically reduced bacterial wound burden, while 0.3 μg/kg of tobramycin was sufficient to lower bacterial burden without fully eliminating the luciferase signal from the wound (see Fig. S1 in the supplemental material). Therefore, we chose to use 0.3 μg/kg for our combined treatment experiments to avoid masking a detectable effect from hydrolase treatment.
To determine if PslGh potentiates antibiotic activity, PAO1-lux-infected wounds were treated with tobramycin and PslGh, either alone or in combination, as indicated (Fig. 7). Since Psl appears particularly important at the early stages of infection and biofilm development (Fig. 6) (16, 20), we chose to treat wounds with PslGh at the time of inoculation. Using an in vivo imaging system (IVIS) and CFU quantification, at 72 hpi, we found the bacterial burden was significantly reduced in wounds treated with PslGh and tobramycin compared with that of wounds treated with only tobramycin (Fig. 7). Although we observed improved innate immune activity following treatment with PslGh (Fig. 3 and 4), under the conditions tested, the impact of PslGh activity alone was not sufficient to improve bacterial clearance in vivo (Fig. 7). While innate immunity may contribute to clearance in the PslGh- and tobramycin-treated wounds, it is likely that the additive killing effect we observed here is due to the potentiation of antibiotic activity. Combined, these results suggest that PslGh treatment effectively reduces wound bacterial burden when combined with antibiotic therapy.
FIG 7.
PslGh enhances the effectiveness of tobramycin during wound treatment. (A) Representative IVIS images of luminescent signal in PAO1-lux-infected wounds 72 hpi following daily treatments with PslGh and/or tobramycin as indicated. (B) The wound bacterial burden was quantified at the indicated time points by measuring light emission. (C) The 72 hpi bacterial burden was quantified by homogenizing wound biopsy specimens and quantifying tissue CFUs. Bars indicate mean ± SEM. At least seven mice were included in each treatment group, and statistical significance was determined using two-way (B) and one-way ANOVA (C) followed by Bonferroni’s post hoc tests. (*, P < 0.05; **, P < 0.01; ns, no significance).
DISCUSSION
As chronic P. aeruginosa wound infection and antibiotic resistance become more prevalent, it is crucial that new methods are developed to improve treatment. Disruption of the biofilm through physical or chemical means improves antibiotic susceptibility in chronically infected wounds (13–15). There is growing evidence to suggest that glycoside hydrolases can be utilized to disrupt P. aeruginosa biofilms (22). Similarly, it has been demonstrated that treatment with various other glycoside hydrolases improves antibiotic efficacy against Aspergillus fumigatus, Staphylococcus epidermidis, and Escherichia coli biofilms (41, 42). Collectively, these studies suggest glycoside hydrolases may represent a new class of therapy, which could be used in treating biofilm infections in a wide range of microbial species. In the current study, we sought to gain further insight into the efficacy of PelAh and PslGh therapy. We demonstrate that PelAh and PslGh treatment enhances antibiotic efficacy against biofilms and that PslG improves antibiotic penetration into the biofilm (Fig. 1 and 2). While PelAh does not appear to impact innate immune activity, PslGh treatment improves complement deposition and neutrophil killing activity against Psl-producing P. aeruginosa strains (Fig. 3 and 4). Collectively, these functions likely contribute to improving bacterial clearance. While our investigation was limited to neutrophils, many immune cells, such as monocytes and dendritic cells, also utilize complement factors during phagocytosis (27). It is possible that enhanced complement deposition following glycoside hydrolase treatment could broadly improve the antimicrobial activity of other immune cells; however, additional studies will be necessary to determine the extent of these effects.
We further demonstrate that PslGh treatment potentiates tobramycin antimicrobial activity leading to reduced bacterial burden in infected wounds (Fig. 7). Although we do not observe complete bacterial clearance from the wound 72 hpi, we expect that bacterial load would continue to decrease over time as the wound heals. Future studies that look specifically at the progression of infection and wound healing during treatment will be necessary to determine the optimal timing and dosing conditions for glycoside hydrolase treatments.
We demonstrated that treatment of PAO1 biofilms in vitro with PelAh and PslGh improves the efficacy of tobramycin, polymyxin, colistin, and neomycin leading to nearly 1 log greater bacterial killing than antibiotic treatment alone (Fig. 1A to D). Tobramycin and colistin are commonly used to treat P. aeruginosa infection, and the concentrations used here are similar to levels predicted to be in the wound fluid following intravenous antibiotic administration (43). These antibiotics also utilize different mechanisms to kill bacteria, suggesting hydrolase antibiotic potentiation is not specific to a single antibiotic mode of action. We propose that the additive effect between PslGh and antibiotics may be due to improved penetration of the antibiotic into the biofilm. Tseng et al. determined that Psl inhibits tobramycin penetration into the biofilm and proposed that antibiotic sequestration to the periphery of the biofilm could protect metabolically active cells within the biofilm interior (19). In agreement with this hypothesis, we observe that PslGh treatment increases tobramycin penetration into PAO1 biofilm structures (Fig. 2).
There are a wide range of wound management strategies utilized in the clinical setting. Currently, the most effective treatment strategy for chronic wound infections involves periodic wound debridement and subsequent administration of topical, oral, and/or intravenous (i.v.) antimicrobials (14). As next-generation sequencing diagnostic testing becomes routine, specialized treatments are becoming more common for effectively treating these infections (9, 38). Selecting appropriate antimicrobials that specifically target the organisms present greatly reduces the risk of developing antibiotic resistance and reduces the length of hospitalization required for treatment (10). Targeted wound treatments have already had a noticeable impact on reducing health care costs (9), which emphasizes the importance of developing therapeutics that specifically target common wound pathogens, such as P. aeruginosa. Here, we demonstrate that Psl functions as an important virulence factor in the wound environment. At 48 and 72 hpi, the bacterial burden of PAO1-infected wounds was nearly 1 log greater than PAO1ΔpslΔpel-infected wounds. (Fig. 6A and B). Interestingly, the difference in wound burden between strains becomes less significant 96 hpi (Fig. 6B), which indicates EPS production is particularly important during the early stages of infection. Pel and Psl have important roles in surface attachment and initiating biofilm formation (16, 17, 44), so it is possible that the loss of EPS primarily impedes the initial establishment of a P. aeruginosa infection. While further studies will be required to determine the optimal timing for targeting EPS, our data suggest that impeding Psl formation with hydrolases or other therapeutics can attenuate P. aeruginosa infection.
While it is now well established that disrupting the EPS matrix improves antibiotic efficacy, a recent study suggests biofilm disruption may also promote bacterial dissemination (15). Fleming and Rumbaugh observed increased rates of septicemia when treating P. aeruginosa-infected wounds with the hydrolases α-amylase and cellulase (15). However, when paired with an antibiotic, the hydrolases reduced bacterial wound burden. Although we did not directly evaluate bacterial dissemination, we did not observe changes in clinical signs associated with sepsis following PslGh treatment. One possibility for these observed differences could be the specificity of PslGh for Psl (22). Cellulase and α-amylase have a broad range of targets and could affect other factors in the host environment. Additionally, Fleming and Rumbaugh report that smaller wound sizes reduced the risk of septicemia (15). They did not observe septicemia in mice with a single 0.5-cm wound, but nearly 60% of the mice died with a single 1.0-cm wound. In our study, the mice were administered two 0.6-cm wounds, and we observed no difference in mouse survival rate following PslGh treatment. It remains unclear how wound size impacts dissemination rates, but it is possible that the size of an individual wound is important for determining the systemic spread of an infection. A third factor that may contribute to bacterial dissemination could be the concentration of hydrolase administered. Our study used a lower hydrolase dose, and it is possible that high concentrations of hydrolase cause rapid dissociation of the biofilm leading to dissemination. Additional studies that evaluate these risk factors will be crucial for determining the safest and most effective way of utilizing biofilm-disrupting agents therapeutically.
In conclusion, PelAh and PslGh appear to be promising new therapeutics for treating P. aeruginosa biofilm infections. We demonstrate here that PelAh and PslGh improves antibiotic efficacy and determined that PslGh potentiates antimicrobial activity during wound infection. We anticipate biofilm disruption will become a necessary aspect of managing P. aeruginosa infections and PslGh hydrolase therapy may be an effective method to address this challenge.
MATERIALS AND METHODS
Ethics statement.
All animal procedures performed for this study were preapproved by The Ohio State University Institutional Animal Care and Use Committee (IACUC) under the protocol numbers 2017A00000028 (pig) and 2017A00000033 (mouse). All blood was obtained from healthy human adults. Informed written consent was obtained from all donors, and The Ohio State University Institutional Review Board (IRB) protocol number 2015H0162 approved all procedures.
Bacterial growth and media.
In all experiments, excluding antibiotic susceptibility assays, P. aeruginosa strains were grown overnight rotating at 37°C in lysogeny broth (LB) without sodium chloride (LBNS), and when appropriate, 300 μg/ml of carbenicillin was added to maintain plasmids. Log-phase cultures were used in all experiments (optical density at 600 nm [OD600], 0.5 to 0.8), which were prepared by inoculating 100 to 200 μl of overnight cultures into LBNS.
Recombinant protein production.
PslGh and PelAh were cloned into an expression plasmid as described previously (22, 25). Clearcoli cells were transformed with the expression plasmid and grown in autoinduction medium or terrific broth (TB) for PslGh and PelAh, respectively, with 50 μg/ml kanamycin. For PslGh, the autoinduction medium inoculated with the Clearcoli cells was incubated at 37°C overnight. For PelAh, bacteria were grown in TB and induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) when the cells reached an OD600 of 1.2 to 1.4. The cells were incubated postinduction overnight at 18°C with shaking at 200 rpm before being harvested by centrifugation at 5,000 × g for 30 min at 4°C. Both proteins were purified using nickel-affinity chromatography and, subsequently, buffer was exchanged into 20 mM Tris (pH 8.0), 150 mM NaCl, and 10% (vol/vol) glycerol or 20 mM Tris (pH 7.5), 150 mM NaCl, and 2% (vol/vol) glycerol for PelAh and PslGh, respectively, and stored at −20°C until required. The protein yield for PelAh and PslGh using the methods described is on average ∼80 to 100 mg/liter of medium used.
Antibiotic susceptibility.
Bacterial cells were scraped from Luria agar plates (LA) and transferred to Dulbecco’s phosphate-buffered saline (PBS) (Gibco number 14190-144) and then diluted to an OD600 of 0.26. Cells were further diluted 1:30 in tryptic soy broth (TSB). A total of 150 μl of cells was added to each well of a Calgary biofilm device (CBD) plate (Innovotech) and incubated with the lid for 10 h shaking at 37°C. The polystyrene pegs of the CBD plate were rinsed twice by transferring the peg lid to a new 96-well plate filled with 200 μl of sterile PBS and incubated for 1 min. Three pegs per condition were removed with sterile needle-nose pliers and sonicated for 10 min in a tube containing sterile PBS. CFUs were calculated by serial dilution to determine the average initial number of bacteria present on peg biofilms prior to treatment. Following peg removal, the lid and remaining pegs were inserted into a new 96-well plate containing antibiotics and/or PelAh and PslGh. Plates were treated for 4 h at 37°C in a humidified chamber. Pegs were washed once in PBS as described above and then transferred to a new plate containing 200 μl of TSB. The pegs and plate were sonicated together for 10 min, and viable remaining bacteria were quantified by serial dilution. All experiments were run in triplicate in at least three independent experiments. Statistical significance was determined by one-way ANOVA followed by Bonferroni’s post hoc tests.
Tobramycin penetration of in vitro biofilms treated with PslGh.
Tobramycin penetration and continuous flow cell biofilm reactors were prepared and assembled as previously described (19, 45). Log-phase cultures, grown in LB, were diluted in 1% (vol/vol) LB to a final OD600 of 0.01. Flow cell chambers were then inoculated with these diluted cultures and incubated inverted for 1 h before initiation of flow. Biofilms, which were continuously supplied with fresh 1% (vol/vol) LB at 10 ml/h, were grown for 4 days at room temperature. A Zeiss LSM 510 confocal laser scanning microscope was used to image the biofilms, and Volocity software (Quorum Technologies) was used for compiling and quantifying images.
Four-day-old biofilms were treated with 5 μg/ml PslGh statically for 5 min followed by incubation with 2.5 μM Syto 9 and 20 μg/ml of Cy5-tobramycin for 30 min. Biofilms were then flushed for 30 min and imaged to measure antibiotic penetration. To determine the aggregate boundaries the Syto 9 (stains intact cells), the signal was analyzed and the widest z-slices were used to quantify the spatial distribution of Cy5-tobramycin in at least 9 images per condition. A graph of fluorescence intensity versus aggregate diameter was created for both Syto 9 (green) and Cy5-tobramycin (purple).
C3 deposition.
Surface-bound C3 was quantified as described previously (20). Log-phase P. aeruginosa cultures with OD600 of 0.5 were suspended in 1 ml of sterile PBS. Cultures were treated with 50 μg/ml of PslGh and 105 μg/ml of PelAh for 1 h at 37°C with nutation. Cells were washed once with PBS and suspended in 1 ml of 20% (vol/vol) pooled human serum (catalog number NHS; Complement Technology, Inc.) and incubated for 1 h at 37°C with nutation. The cells were then washed once in sterile PBS and fixed overnight in 4% (wt/vol) paraformaldehyde. C3 on the surface of the bacterial cells was measured by staining with a rabbit α-C3 antibody (Complement Technology, Inc.) followed by staining with an Alexa Fluor 647 goat α-rabbit secondary antibody (1:1,000). Bacterial cells that were not exposed to human serum served as a negative control. All strains were tested in at least three independent experiments. Statistical significance was determined by Student’s t test.
Neutrophil internalization.
Neutrophil internalization was measured using previously described methods (37). Neutrophils were isolated from healthy human donor blood by Ficoll-Hypaque separation followed by 1.5% (wt/vol) dextran sedimentation. The plasmid pMRP9 was transformed into PAO1 and PAO1ΔpslΔpel allowing for constitutive green fluorescent protein (GFP) expression. P. aeruginosa was treated for 1 h with 105 μg/ml of PelAh and 50 μg/ml PslGh at 37°C with nutation. The bacterial cultures were washed once in PBS and then opsonized in 20% (vol/vol) human serum for 5 min. The neutrophils were infected with log-phase P. aeruginosa, multiplicity of infection (MOI) of 1:50, for 30 min at 37°C with nutation. The neutrophils were washed twice in PBS by centrifugation at 2,500 × g for 2 min. Neutrophils were then fixed in 4% (wt/vol) paraformaldehyde overnight. Extracellular bacteria were stained with the α-Pseudomonas antibody (1:2,500) and goat α-rabbit Alexa Fluor 647 (1:1,000) to exclude any bacteria that were not fully taken up by the neutrophil. A BD FACSCanto II flow cytometer (BD Biosciences) and FlowJo 9.0 analysis software were used to calculate the neutrophil population that was GFP+/Alexa647−. All experiments were done in duplicate with neutrophils from at least three different donors. Statistical significance was determined by Student’s t test.
Reactive oxygen species quantification.
P. aeruginosa cultures were grown to log phase, and 0.5 OD600 was suspended in 1 ml of PBS. Cells were treated with 105 μg/ml of PelAh and 50 μg/ml PslGh for 1 h on a nutator at 37°C. Cells were washed once in PBS. Cells were then suspended in 1 ml of 20% (vol/vol) pooled human serum in Hanks’ balanced salt solution (HBSS) and incubated for 30 min on a nutator at 37°C. Cells were washed once with PBS, and neutrophils in HBSS were infected with bacteria (MOI, 1 neutrophil:50 bacteria). ROS was measured as previously described (46), and luminescence was measured every 3 min over a 1-h period in the presence a luminol reporter (100 μM). The area under the concentration-time curve (AUC) was measured to determine the relative ROS produced by neutrophils over the time course. Results were normalized to the neutrophil response of the ROS inducer phorbol myristate acetate to correct for variation between donors. Experiments were done in triplicate in three independent experiments using neutrophils from different donors. Statistical significance was determined by Student’s t test.
Cell toxicity.
Human red blood cells (RBCs) were isolated from healthy donor blood from three different individuals, and the cells were exposed to 1 mg/ml of PelAh and PslGh for 1 h. RBC viability was measured by measuring the OD700 to quantify the remaining intact cells. A total of 1% (vol/vol) Triton X-100 was used as a positive control to lyse cells. Statistical significance was determined using two-way ANOVA followed by Bonferroni’s post hoc tests.
Porcine model and wound assessment.
Porcine infections were carried out using a previously established model (47). A pig was burned along the dorsolateral spine/trunk in 5.08- by 5.08-cm squares, creating 6 full-thickness burn wounds. Each wound was individually wrapped with Tegaderm, and then the entire site was bandaged and left for 3 days. The delivery vehicle PF-127 is a solid at room temperature and a liquid at 4°C. PslGh at two concentrations, namely, 0.1 mg/ml and 1.0 mg/ml, was suspended in 20% (wt/vol) PF-127 at 4°C and added to each wound 3, 4, and 7 days postwounding. Strip biopsy specimens were taken from all wounds after 7 days and fixed in 10% (vol/vol) neutral buffered formalin prior to being processed and embedded in paraffin for histological analysis. All tissue sectioning and staining was done by the Comparative Pathology Core at The Ohio State University College of Veterinary Medicine. Formalin-fixed tissues were paraffin embedded, sectioned (5 μm), and stained with hematoxylin and eosin (H&E). The slides were scanned with an Aperio slide scanner and analyzed and measured using ImageScope Software (Leica Biosystems, Buffalo Grove, IL). Wound closure was measured by a pathologist in a blind manner and determined by measuring the distance between the leading edges of the wounds, as previously described (48). Additionally, H&E-stained tissue was used to assess necrosis and inflammatory markers for differences between vehicle-treated and PslGh-treated tissue.
Murine wound infection.
Anesthetized female BALB/c mice were wounded with a 6-mm punch biopsy tool generating two identical full-thickness dorsal wounds. Mice were given buprenorphine at the time of wounding and bandaged with an occlusive dressing (Opsite Flexifix). Mice were given 24 h to recover, and then 60 μl of bacterial culture containing 107 cells of PAO1 or PAO1ΔpelΔpsl was inoculated under the bandage onto the surface of each wound. All bacterial strains contained a constitutively expressed luminescent (luciferase) marker. Mice in groups receiving PslGh treatment were also administered 400 μg of PslGh at the time of infection. Topical treatments of 60 μl doses of 400 μg PslGh and/or 0.3 mg/kg of tobramycin were administered daily to each wound as indicated. Mock-treated wounds received 60 μl of sterile PBS. One hour after treatment, the wounds were washed with 500 μl of sterile PBS, and the bandage was replaced daily. Additionally, 400 μg of PslGh was reapplied after bandage replacement to relevant mouse groups to ensure PslGh was present in the wound throughout the time course. Wound luminescence was imaged daily with an IVIS Lumina II optical imaging system to assess bacterial burden. Three to 4 days postinfection, wound biopsy specimens were collected for CFU quantification. At least five mice were included in each treatment group, and statistical significance was determined using two-way and one-way ANOVA followed by Bonferroni’s post hoc tests.
Statistical analysis.
Prism (Graphpad v7.04 software) was used for all statistical analysis. The threshold for significance was set at a P value of <0.05, and the error bars in all figures indicate the standard error of the mean (SEM). Significance in each experiment was determined using the statistical test specified in each corresponding methods section.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sarah B. Chaney for her aid and consultation with the analysis of H&E slides.
This work was supported by the NIH grants 4R33A1119116-03 (M.R.P., D.J.W., and P.L.H.), AI077628 (M.R.P. and D.J.W.), and AI134895 (M.R.P. and D.J.W.) and in part by CIHR FDN154327 (P.L.H.). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The manuscript was written and edited by M.J.P., P.B., D.J.W., M.R.P., and P.L.H. The in vitro antibiotic susceptibility and penetration tests were performed by H.S. and D.P.D.S. Complement and neutrophil assays were performed by M.J.P. and P.J.H. M.J.P. and P.J.H. were responsible the porcine wound experiments, and the mouse wound experiments were completed by M.J.P. and S.D.-N. P.B., I.L., and D.R. generated and produced the recombinant proteins used in these studies. M.J.P., P.L.H., D.J.W., P.B., D.P.D.S., and M.R.P. contributed to the conceptualization and analysis of this work. All authors approved the final version of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00234-19.
REFERENCES
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 80:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 3.Mulcahy LR, Isabella VM, Lewis K. 2013. Pseudomonas aeruginosa biofilms in disease. Microb Ecol 68:1–12. doi: 10.1007/s00248-013-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fux CA, Costerton JW, Stewart PS, Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol 13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Mah TF, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 6.Dowsett C, Bielby A, Searle R. 2014. Reconciling increasing wound care demands with available resources. J Wound Care 23:552–562. doi: 10.12968/jowc.2014.23.11.552. [DOI] [PubMed] [Google Scholar]
- 7.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. 2009. Human skin wounds: a major snowballing threat to public health and economy. Wound Repair Regen 17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolcott RD, Rhoads DD, Dowd SE. 2008. Biofilms and chronic wound inflammation. J Wound Care 17:333–341. doi: 10.12968/jowc.2008.17.8.30796. [DOI] [PubMed] [Google Scholar]
- 9.Wolcott R. 2015. Economic aspects of biofilm-based wound care in diabetic foot ulcers. J Wound Care 24:189–194. doi: 10.12968/jowc.2015.24.5.189. [DOI] [PubMed] [Google Scholar]
- 10.Bassetti M, Vena A, Croxatto A, Righi E, Guery B. 2018. How to manage Pseudomonas aeruginosa infections. Drugs Context 7:1–18. doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control. 2015. Antimicrobial resistance surveillance in Europe 2015. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net), p 1–120. European Centre for Disease Prevention and Control, Solna, Sweden. [Google Scholar]
- 12.Wolcott RD, Hanson JD, Rees EJ, Koenig LD, Phillips CD, Wolcott RA, Cox SB, White JS. 2016. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Rep and Reg 24:163–174. doi: 10.1111/wrr.12370. [DOI] [PubMed] [Google Scholar]
- 13.Fleming D, Rumbaugh K. 2017. Approaches to dispersing medical biofilms. Microorganisms 5:15. doi: 10.3390/microorganisms5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, Watters C, Stewart PS, Dowd SE. 2010. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care 19:320–328. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]
- 15.Fleming D, Rumbaugh K. 2018. The consequences of biofilm dispersal on the host. Sci Rep 8:1–7. doi: 10.1038/s41598-018-29121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ. 2006. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol 188:8213–8221. doi: 10.1128/JB.01202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooley BJ, Thatcher TW, Hashmi SM, L'Her G, Le HH, Hurwitz DA, Provenzano D, Touhami A, Gordon VD. 2013. The extracellular polysaccharide Pel makes the attachment of P. aeruginosa to surfaces symmetric and short-ranged. Soft Matter 9:3871–3876. doi: 10.1039/c3sm27638d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billings N, Millan M, Caldara M, Rusconi R, Tarasova Y, Stocker R, Ribbeck K. 2013. The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog 9:e1003526. doi: 10.1371/journal.ppat.1003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng BS, Zhang W, Harrison JJ, Quach TP, Song JL, Penterman J, Singh PK, Chopp DL, Packman AI, Parsek MR, Jisun Lee S, Penterman J, Singh PK, Chopp DL, Packman AI, Parsek MR. 2013. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ Microbiol 15:2865–2878. doi: 10.1111/1462-2920.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra M, Byrd MS, Sergeant S, Azad AK, Parsek MR, McPhail L, Schlesinger LS, Wozniak DJ. 2012. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell Microbiol 14:95–106. doi: 10.1111/j.1462-5822.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colvin KM, Irie Y, Tart CS, Urbano R, Whitney JC, Ryder C, Howell PL, Wozniak DJ, Parsek MR. 2012. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ Microbiol 14:1913–1928. doi: 10.1111/j.1462-2920.2011.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker P, Hill PJ, Snarr BD, Alnabelseya N, Pestrak MJ, Lee MJ, Jennings LK, Tam J, Melnyk RA, Parsek MR, Sheppard DC, Wozniak DJ, Howell PL. 2016. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci Adv 2:1–10. doi: 10.1126/sciadv.1501632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin MJ, Nivens DE, Weadge JT, Howell PL. 2011. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front Microbiol 2:167. doi: 10.3389/fmicb.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colvin KM, Alnabelseya N, Baker P, Whitney JC, Howell PL, Parsek MR. 2013. PelA deacetylase activity is required for pel polysaccharide synthesis in pseudomonas aeruginosa. J Bacteriol 195:2329–2339. doi: 10.1128/JB.02150-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker P, Whitfield GB, Hill PJ, Little DJ, Pestrak MJ, Robinson H, Wozniak DJ, Howell PL. 2015. Characterization of the Pseudomonas aeruginosa glycoside hydrolase PslG reveals that its levels are critical for Psl polysaccharide biosynthesis and biofilm formation. J Biol Chem 290:28374–28387. doi: 10.1074/jbc.M115.674929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GCL, Parsek MR. 2011. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog 7:e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holers VM. 2014. Complement and its receptors: new insights into human disease. Annu Rev Immunol 32:433–459. doi: 10.1146/annurev-immunol-032713-120154. [DOI] [PubMed] [Google Scholar]
- 28.Gross GN, Rehm SR, Pierce AK. 1978. The effect of complement depletion on lung clearance of bacteria. J Clin Invest 62:373–378. doi: 10.1172/JCI109138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruger P, Saffarzadeh M, Weber ANR, Rieber N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J, Hartl D. 2015. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog 11:e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnen AFP, Henneke P. 2014. Structural biology of the membrane attack complex BT - MACPF/CDC proteins - agents of defence, attack and invasion, p. 83–116. In Anderluh G, Gilbert R (ed). MACPF/CDC proteins—agents of defense, attack, and invasion. Springer Netherlands, Dordrecht, The Netherlands. [Google Scholar]
- 31.Sakuragi Y, Kolter R. 2007. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J Bacteriol 189:5383–5386. doi: 10.1128/JB.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. 2009. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun 77:5300–5310. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Kessel KPM, Bestebroer J, van Strijp JAG. 2014. Neutrophil-mediated phagocytosis of Staphylococcus aureus. Front Immunol 5:467. doi: 10.3389/fimmu.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao Y, Murphy K, Lo RY, Khursigara CM, Lam JS. 2015. Single-nucleotide polymorphisms found in the migA and wbpX glycosyltransferase genes account for the intrinsic lipopolysaccharide defects exhibited by Pseudomonas aeruginosa PA14. J Bacteriol 197:2780–2791. doi: 10.1128/JB.00337-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordillo GM, Bernatchez SF, Diegelmann R, Di Pietro LA, Eriksson E, Hinz B, Hopf HW, Kirsner R, Liu P, Parnell LKS, Sandusky GE, Sen CK, Tomic-Canic M, Volk SW, Baird For The Wound Healing Society A. 2013. Preclinical models of wound healing: is man the model? Proceedings of the Wound Healing Society Symposium. Adv Wound Care 2:1–4. doi: 10.1089/wound.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganesh K, Sinha M, Mathew-Steiner SS, Das A, Roy S, Sen CK. 2015. Chronic wound biofilm model. Adv Wound Care 4:382–388. doi: 10.1089/wound.2014.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pestrak MJ, Chaney SB, Eggleston HC, Dellos-Nolan S, Dixit S, Mathew-Steiner SS, Roy S, Parsek MR, Sen CK, Wozniak DJ. 2018. Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLoS Pathog 14:e1006842. doi: 10.1371/journal.ppat.1006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones CE, Kennedy JP. 2012. Treatment options to manage wound biofilm. Adv Wound Care (New Rochelle) 1:120–126. doi: 10.1089/wound.2011.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu AG, Antunes MB, Palmer JN, Cohen NA. 2007. Evaluation of the in vivo efficacy of topical tobramycin against Pseudomonas sinonasal biofilms. J Antimicrob Chemother 59:1130–1134. doi: 10.1093/jac/dkm087. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert ML, Wilhelmus KR, Osato MS. 1987. Comparative bioavailability and efficacy of fortified topical tobramycin. Invest Ophthalmol Vis Sci 28:881–885. [PubMed] [Google Scholar]
- 41.Snarr BD, Baker P, Bamford NC, Sato Y, Liu H, Lehoux M, Gravelat FN, Ostapska H, Baistrocchi SR, Cerone RP, Filler EE, Parsek MR, Filler SG, Howell PL, Sheppard DC. 2017. Microbial glycoside hydrolases as antibiofilm agents with cross-kingdom activity. Proc Natl Acad Sci U S A 114:7124–7129. doi: 10.1073/pnas.1702798114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Little DJ, Pfoh R, Le Mauff F, Bamford NC, Notte C, Baker P, Guragain M, Robinson H, Pier GB, Nitz M, Deora R, Sheppard DC, Howell PL. 2018. PgaB orthologues contain a glycoside hydrolase domain that cleaves deacetylated poly-β(1, 6)-N-acetylglucosamine and can disrupt bacterial biofilms. PLoS Pathog 14:e1006998. doi: 10.1371/journal.ppat.1006998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagley DH, Mac Lowry J, Beazley RM, Gorschboth C, Ketcham AS. 1978. Antibiotic concentration in human wound fluid after intravenous administration. Ann Surg 188:202–208. doi: 10.1097/00000658-197808000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao K, Tseng BS, Beckerman B, Jin F, Gibiansky ML, Harrison JJ, Luijten E, Parsek MR, Wong G. 2013. Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature 497:388–391. doi: 10.1038/nature12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen BB, Sternberg C, Andersen JB, Palmer RJJ, Nielsen AT, Givskov M, Molin S. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol 310:20–42. doi: 10.1016/S0076-6879(99)10004-1. [DOI] [PubMed] [Google Scholar]
- 46.Engels W, Endert J, Kamps MAF, Boven PA. 1985. Role of lipopolysaccharide in opsonization and phagocytosis of Pseudomonas aeruginosa. Infect Immun 49:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy S, Elgharably H, Sinha M, Ganesh K, Chaney S, Mann E, Miller C, Khanna S, Bergdall V, Powell H, Cook C, Gordillo G, Wozniak D, Sen C. 2014. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol 233:331–343. doi: 10.1002/path.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaney S, Ganesh K, Mathew-Steiner S, Stromberg P, Roy S, Sen C, Wozniak D. 2017. Histopathological comparisons of Staphylococcus aureus and Pseudomonas aeruginosa experimental infected porcine burn. Wound Repair Regen 25:541–549. doi: 10.1111/wrr.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.