Nineteen Proteus mirabilis isolates producing the carbapenemase OXA-23 were recovered over a 2-year period in 19 French hospitalized patients, of whom 12 had community onset infections. The isolates exhibited a slightly reduced susceptibility to carbapenems.
KEYWORDS: carbapenemase, OXA-23, Proteus mirabilis, spread, clonality
ABSTRACT
Nineteen Proteus mirabilis isolates producing the carbapenemase OXA-23 were recovered over a 2-year period in 19 French hospitalized patients, of whom 12 had community onset infections. The isolates exhibited a slightly reduced susceptibility to carbapenems. Whole-genome analysis revealed that all 19 isolates formed a cluster compared to 149 other P. mirabilis isolates. Because of its susceptibility to carbapenems, this clone may be misidentified as a penicillinase producer while it constitutes a reservoir of the OXA-23-encoding gene in the community.
INTRODUCTION
The emergence of carbapenem resistance in the Enterobacteriaceae is mainly linked to horizontal diffusion of carbapenemases belonging to Ambler classes A, B, and D (1). While the OXA-48-like enzymes are the most prevalent carbapenemases in several European countries, including France, carbapenem-hydrolyzing enzymes of OXA-23 and OXA-58 types are generally confined to Acinetobacter species (2). The blaOXA-23 and blaOXA-58 genes have occasionally been reported in Enterobacteriaceae species, especially in Proteus mirabilis. The spread of an OXA-23-producing clone of P. mirabilis was first revealed during a survey conducted between 1996 and 1999 in France (3). Later, the blaOXA-23 gene was detected sporadically in Escherichia coli in Singapore and India and, more recently, in P. mirabilis from Finland (4–6). Besides, P. mirabilis strains with a chromosomally integrated or plasmid-borne blaOXA-58 gene were characterized in Belgium and in Germany, respectively (7, 8).
Here, we report on the regional spread of a blaOXA-23-positive P. mirabilis clone. Between November 2016 and May 2018, 19 isolates of P. mirabilis were found to exhibit an unusual penicillinase-like resistance phenotype (see below). The isolates were recovered from 19 patients hospitalized in ten different wards at the University Hospital of Besançon, France (Table 1). None of these patients had travelled abroad, and three had received amoxicillin or the amoxicillin-clavulanate combination within the 2 months before the isolation of P. mirabilis strain. Twelve patients were detected positive within the first 2 days following their admission, among whom seven had no history of hospitalization within the preceding 6 months (Table 1). An epidemiologic link between the cases could not be found. Together, these elements strongly suggested an acquisition of the P. mirabilis isolates within the community. Their antimicrobial susceptibility was determined by the disk diffusion method on Mueller-Hinton agar (Bio-Rad, Marnes-la-Coquette, France) and interpreted according to current EUCAST guidelines (http://www.eucast.org). All of the isolates were resistant to amoxicillin and ticarcillin, with no recovery of their susceptibility with the addition of clavulanate. They also displayed decreased susceptibility to piperacillin-tazobactam but remained susceptible to expanded-spectrum cephalosporins (cefotaxime, ceftazidime, and cefepime). All isolates appeared to be susceptible to ertapenem but with inhibition zone diameters around the disk close to the breakpoint (i.e., 25 to 26 mm). This unusual antibiotic resistance profile evoked production of a class D β-lactamase expressing a poor carbapenem-hydrolyzing activity. All of the isolates were thus screened by PCR for the Ambler class D carbapenemase-encoding genes blaOXA-48-like, blaOXA-23-like, blaOXA-24-like, and blaOXA-58-like (9, 10), and finally turned out to harbor blaOXA-23. We tested the ability of ChromID Carba Smart medium (bioMérieux, La Balme-les-Grottes, France) to grow the P. mirabilis isolates carrying blaOXA-23 by plating ∼107 CFU of five representative isolates (PmOXA23-1 to PmOXA23-5). However, none of them could develop on that selective chromogenic medium. Of note, enterobacteria that produce other class D carbapenemases, such as OXA-244, are also not detected by this method (11). The transferability of blaOXA-23, using E. coli and Acinetobacter baumannii as recipient strains, was tested as described previously (12). No transconjugants were obtained despite several attempts under different conditions (not shown), suggesting a chromosomal location for the resistance determinant. In order to identify other antibiotic resistance genes, the total DNA of each P. mirabilis strain was fully sequenced on an Illumina NextSeq platform. The DNA libraries for whole-genome sequencing were prepared using the Nextera XT kit with a 2 × 150-bp paired-end approach (BioProject accession number PRJNA490489). De novo assembly of the contigs was performed with SPAdes v3.11 (13), while the resistance genes were identified by using BLAT software with the ResFinder database (http://cge.cbs.dtu.dk/services/ResFinder, accessed 22 November 2018) (14). The mean size of the P. mirabilis genomes was 3.99 Mb, with a G+C content of 38.8%. We found that, in addition to blaOXA-23, all isolates harbored genes conferring resistance to aminoglycosides [aadA1, aph(3″)-Ib, and aph(6)-Id], sulfamides (sul2, except in isolate PmOXA23-9), trimethoprim (drfA), chloramphenicol (cat), and tetracyclines [tet(J)] (Table 1). Most isolates also possessed the phenicol resistance gene floR and the aminoglycoside resistance genes aac(3)-IIa and aph(3′)-Ia. Finally, isolates PmOXA23-3 and PmOXA23-5 contained the genes aac(3)-IVa and aph(4)-Ia and the lincosamide nucleotidyltransferase-encoding gene lnu(G) (Table 1).
TABLE 1.
Clinical and microbiological features associated with blaOXA-23-positive P. mirabilis isolates
| Isolate | Isolate dataa |
Presence of antibiotic resistance gene |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospitalization ward | Patient gender | Age range (yrs) | Clinical sample | Date of isolation | Strain isolated within 48 hours of admission | History of hospitalization within the preceding 6 mo | aac(3)-IIa | aac(3)-IVa | aadA1 | aph(3'')-Ib | aph(3')-Ia | aph(4)-Ia | aph(6)-Id | blaOXA-23 | cat | dfrA1 | floR | lnu(G) | sul2 | tet(J) | |
| PmOXA23-1 | Geriatric medicine | F | 90–99 | Urine | November 2016 | No | No | + | + | + | + | + | + | + | + | + | |||||
| PmOXA23-17 | Surgery ward | F | 60–69 | Urine | February 2017 | No | No | + | + | + | + | + | + | + | + | + | + | + | |||
| PmOXA23-2 | Hematology | M | 50–59 | Urine | February 2017 | No | Yes | + | + | + | + | + | + | + | + | + | + | + | |||
| PmOXA23-19 | Surgery ward | M | 70–79 | Bone | January 2017 | No | Yes | + | + | + | + | + | + | + | + | + | + | ||||
| PmOXA23-3 | Medical ICU | F | 60–69 | Wound | March 2017 | Yes | Yes | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| PmOXA23-18 | Surgery ward | F | 60–69 | Abscess | March 2017 | Yes | Yes | + | + | + | + | + | + | + | + | + | + | + | |||
| PmOXA23-4 | Geriatric medicine | F | 90–99 | Bone | March 2017 | No | Yes | + | + | + | + | + | + | + | + | + | + | ||||
| PmOXA23-5 | Surgery ward | F | 90–99 | Urine | April 2017 | Yes | No | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| PmOXA23-6 | Geriatric medicine | M | 80–89 | Urine | July 2017 | Yes | Yes | + | + | + | + | + | + | + | + | + | |||||
| PmOXA23-7 | Pediatrics | F | 0–9 | Urine | August 2017 | Yes | Yes | + | + | + | + | + | + | + | + | + | + | + | |||
| PmOXA23-8 | Medical ICU | F | 50–59 | Urine | October 2017 | Yes | No | + | + | + | + | + | + | + | + | + | + | ||||
| PmOXA23-9 | Surgery ward | F | 90–99 | Joint fluid | November 2017 | Yes | No | + | + | + | + | + | + | + | + | + | |||||
| PmOXA23-10 | Cardiology | M | 80–89 | Urine | November 2017 | Yes | No | + | + | + | + | + | + | + | + | + | + | + | |||
| PmOXA23-11 | Gynecology department | F | 40–49 | Vagina | January 2018 | Yes | No | + | + | + | + | + | + | + | + | + | + | + | |||
| PmOXA23-12 | Urology | M | 70–79 | Urine | February 2018 | Yes | Yes | + | + | + | + | + | + | + | + | + | + | ||||
| PmOXA23-13 | Reproductive Biology | M | 30–39 | Sperm | February 2018 | Yes | No | + | + | + | + | + | + | + | + | + | + | + | |||
| PmOXA23-14 | Surgery ward | M | 70–79 | Bone | March 2018 | No | No | + | + | + | + | + | + | + | + | + | + | ||||
| PmOXA23-15 | Cardiology | F | 80–89 | Urine | May 2018 | No | Yes | + | + | + | + | + | + | + | + | + | + | + | |||
| PmOXA23-16 | Emergency room | F | 80–89 | Urine | May 2018 | Yes | No | + | + | + | + | + | + | + | + | + | |||||
aICU, intensive care unit; F, female; M, male.
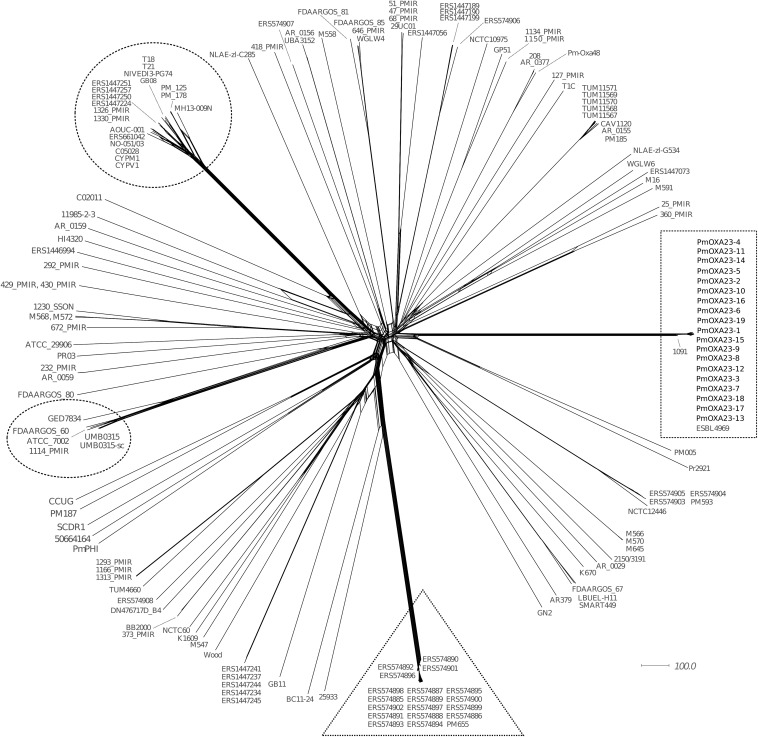
To assess the clonal relationship between the 19 P. mirabilis isolates, we compared their genomes with those of 149 P. mirabilis strains available in the NCBI database (Table S1) by whole-genome multilocus sequence typing (wgMLST; https://github.com/bvalot/pyMLST). Multilocus sequence typing (MLST) alleles were assigned with respect to 3,686 genes present in the core genome of the reference strain P. mirabilis HI4320 (15). From the 2,660 genes identified in ≥95% of the genomes, we built a distance matrix that showed the relative genomic divergence between the isolates. This revealed that our 19 isolates formed a cluster (rectangle in Fig. 1). This cluster also included the blaOXA-23-positive isolate ESBL4969, identified in Finland in 2014, and the blaOXA-58-positive isolate 1091, collected in 2015 in Belgium (6, 7). Overall, the wgMLST-based phylogeny revealed a notable diversity within the 149 P. mirabilis isolates. Another cluster of 19 P. mirabilis isolates was evidenced (triangle in Fig. 1), corresponding to CMY-2-positive isolates responsible for community-acquired infections in Ireland (16). The phylogenetic network analysis also highlighted two other genomic branches evolving from a common ancestor, which included 6 and 19 strains, respectively (represented as circles in Fig. 1).
FIG 1.
Phylogenetic network of the genomes of 168 P. mirabilis isolates. The genomic comparison included the 19 blaOXA-23 isolates collected at the University Hospital of Besançon (France) and 149 P. mirabilis isolates available in the NCBI database (Table S1). The core genome was defined as genes shared by ≥95% of the selected genomes (≥160/168 genomes). It was composed of 2,660 genes of the 3,686 genes annotated in reference strain P. mirabilis HI4320. The network was built using core genome multilocus sequence typing (cgMLST) distances with the neighborNet method in SplitTree4 (17). OXA-23- and CMY-2-positive P. mirabilis clusters are surrounded by a rectangle and a triangle, respectively. Circles represent two additional genomic branches evolving from a common ancestor.
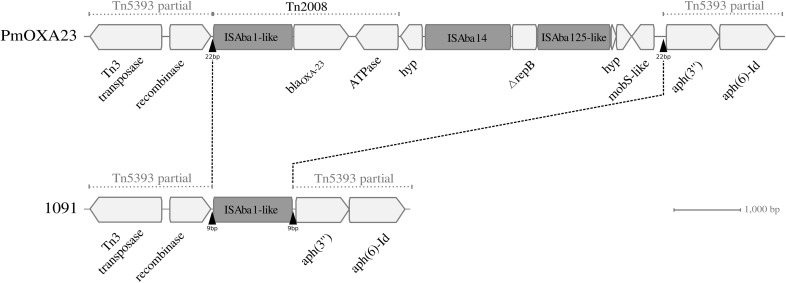
Examination of the blaOXA-23 genomic environment showed that the gene was embedded in a transposon-like structure that was itself inserted in a truncated Tn5393 transposon (Fig. 2). The structure was identical in the 19 isolates and exhibited 100% sequence identity with that of blaOXA-23-positive P. mirabilis ESBL4969 (GenBank accession number KU302354). Interestingly, only one ISAba1-like copy bounded by canonical 9-bp direct repeats (DRs) (CGCTTCATC) is inserted in Tn5393 in the clonally related OXA-58-positive strain P. mirabilis 1091 (Fig. 2). In the PmOXA-23 isolates, a 6,766-bp genetic element mapped at the same position and was bracketed by additional 13-bp DRs (TGAGCCACCTCCG), which together with the aforementioned 9-bp DRs formed a 22-bp duplication signature identical to that of isolate ESBL4969 (6). This genetic element was composed of transposon Tn2008, two other insertion sequences (ISAba14 and an ISAba125-like sequence), and a gene encoding a truncated peptide related to the RepB family of plasmid replication initiators. Despite the presence of the 22-bp target site duplication suggesting a classical insertion of a Tn2008-containing transposon-like structure, the hypothesis of a genetic recombination cannot be ruled out. The identification of the same insertion site in the P. mirabilis 1091 genome and the fact that the sequencing depth of the blaOXA-23-carrying contig was similar to that of the whole genome strongly support the notion that the element is integrated in the chromosome and not in a plasmid. Furthermore, as noted for isolate ESBL4969, the vicinity of this element is a hot spot for integration of various insertion sequences (ISAba1-like, ISAba14, ISAba125-like, ISVsa3, and IS3-like) and is subject to significant rearrangements in antibiotic resistance genes. Hence, the gene sul2 associated or not with the gene floR was absent in isolates PmOXA23-12 and PmOXA23-9, while aph(4), aac(3)-IVa, and lnu(G) were present in PmOXA23-3 and PmOXA23-5. This high degree of genetic polymorphism suggests a propensity of this region to collect genes by horizontal transfer.
FIG 2.
Schematic representation of blaOXA-23 insertion in the PmOXA23 clone. Genes are represented by light gray arrows indicating the direction of transcription. The predicted functions of genes are shown under the arrows. Gray boxes and black triangles represent insertion sequences and direct repeats, respectively. The OXA-23-carrying transposon-like structure of strain PmOXA23-13 (GenBank accession number SLUF00000000) was compared to that of an OXA-58-positive P. mirabilis 1091 isolate (accession number MCOR00000000).
In summary, the present study highlights the diffusion of an OXA-23-positive P. mirabilis clone among epidemiologically unrelated patients. Because of its very low level of resistance to carbapenems, this clone is likely to be underrecognized by medical laboratories analyzing samples from outpatients, especially if antibiotic susceptibility tests are performed with automated systems based on the broth microdilution method, which uses breakpoint concentrations of drugs only. Prevalence of the clone in the French community remains unknown and warrants more extensive investigations. The attention of microbiologists should be drawn by ertapenem-susceptible strains of P. mirabilis having inhibition zones around the ertapenem disk close to the EUCAST susceptibility breakpoint diameter (25 mm).
Accession number(s).
The DNA libraries for whole-genome sequencing in this study have been deposited in GenBank under the BioProject accession number PRJNA490489. The whole-genome shotgun project for PmOXA23-13 has been deposited in GenBank under the accession number SLUF00000000.
Supplementary Material
ACKNOWLEDGMENTS
We have no conflict of interest to declare.
This study was partially funded by the French Ministry of Health through the Santé Publique France agency.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00191-19.
REFERENCES
- 1.van Duin D, Doi Y. 2017. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans BA, Amyes SG. 2014. OXA beta-lactamases. Clin Microbiol Rev 27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnet R, Marchandin H, Chanal C, Sirot D, Labia R, De Champs C, Jumas-Bilak E, Sirot J. 2002. Chromosome-encoded class D beta-lactamase OXA-23 in Proteus mirabilis. Antimicrob Agents Chemother 46:2004–2006. doi: 10.1128/AAC.46.6.2004-2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.La MV, Jureen R, Lin RT, Teo JW. 2014. Unusual detection of an Acinetobacter class D carbapenemase gene, blaOXA-23, in a clinical Escherichia coli isolate. J Clin Microbiol 52:3822–3823. doi: 10.1128/JCM.01566-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul D, Ingti B, Bhattacharjee D, Maurya AP, Dhar D, Chakravarty A, Bhattacharjee A. 2017. An unusual occurrence of plasmid-mediated blaOXA-23 carbapenemase in clinical isolates of Escherichia coli from India. Int J Antimicrob Agents 49:642–645. doi: 10.1016/j.ijantimicag.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Osterblad M, Karah N, Halkilahti J, Sarkkinen H, Uhlin BE, Jalava J. 2016. Rare detection of the Acinetobacter class D carbapenemase blaOXA-23 gene in Proteus mirabilis. Antimicrob Agents Chemother 60:3243–3245. doi: 10.1128/AAC.03119-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girlich D, Bonnin RA, Bogaerts P, De Laveleye M, Huang DT, Dortet L, Glaser P, Glupczynski Y, Naas T. 2017. Chromosomal amplification of the blaOXA-58 carbapenemase gene in a Proteus mirabilis clinical isolate. Antimicrob Agents Chemother 61:e01697-16. doi: 10.1128/AAC.01697-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange F, Pfennigwerth N, Gerigk S, Gohlke F, Oberdorfer K, Purr I, Wohanka N, Roggenkamp A, Gatermann SG, Kaase M. 2017. Dissemination of blaOXA-58 in Proteus mirabilis isolates from Germany. J Antimicrob Chemother 72:1334–1339. doi: 10.1093/jac/dkw566. [DOI] [PubMed] [Google Scholar]
- 9.Potron A, Nordmann P, Lafeuille E, Al Maskari Z, Al Rashdi F, Poirel L. 2011. Characterization of OXA-181, a carbapenem-hydrolyzing class D beta-lactamase from Klebsiella pneumoniae. Antimicrob Agents Chemother 55:4896–4899. doi: 10.1128/AAC.00481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SG, Livermore DM. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Hoyos-Mallecot Y, Naas T, Bonnin RA, Patino R, Glaser P, Fortineau N, Dortet L. 2017. OXA-244-producing Escherichia coli isolates, a challenge for clinical microbiology laboratories. Antimicrob Agents Chemother 61:e00818-17. doi: 10.1128/AAC.00818-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potron A, Poirel L, Nordmann P. 2014. Derepressed transfer properties leading to the efficient spread of the plasmid encoding carbapenemase OXA-48. Antimicrob Agents Chemother 58:467–471. doi: 10.1128/AAC.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent WJ. 2002. BLAT—the BLAST-like alignment tool. Genome Res 12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson MM, Sebaihia M, Churcher C, Quail MA, Seshasayee AS, Luscombe NM, Abdellah Z, Arrosmith C, Atkin B, Chillingworth T, Hauser H, Jagels K, Moule S, Mungall K, Norbertczak H, Rabbinowitsch E, Walker D, Whithead S, Thomson NR, Rather PN, Parkhill J, Mobley HL. 2008. Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. J Bacteriol 190:4027–4037. doi: 10.1128/JB.01981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mac Aogain M, Rogers TR, Crowley B. 2016. Identification of emergent blaCMY-2-carrying Proteus mirabilis lineages by whole-genome sequencing. New Microbes New Infect 9:58–62. doi: 10.1016/j.nmni.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.