CF-301 (exebacase) is a recombinantly produced bacteriophage-derived lysin (cell wall hydrolase) and is the first agent of this class to enter clinical development in the United States for treating bacteremia including endocarditis due to Staphylococcus aureus. Whereas rapid bactericidal activity is the hallmark in vitro and in vivo response to CF-301 at exposures higher than the MIC, prolonged antimicrobial activity, mediated by cell wall damage, is predicted at concentrations less than the MIC.
KEYWORDS: CF-301, Staphylococcus aureus, exebacase, lysin, postantibiotic effect
ABSTRACT
CF-301 (exebacase) is a recombinantly produced bacteriophage-derived lysin (cell wall hydrolase) and is the first agent of this class to enter clinical development in the United States for treating bacteremia including endocarditis due to Staphylococcus aureus. Whereas rapid bactericidal activity is the hallmark in vitro and in vivo response to CF-301 at exposures higher than the MIC, prolonged antimicrobial activity, mediated by cell wall damage, is predicted at concentrations less than the MIC. In the current study, a series of in vitro pharmacodynamic parameters, including the postantibiotic effect (PAE), postantibiotic sub-MIC effect (PA-SME), and sub-MIC effect (SME), were studied to determine how short-duration and sub-MIC CF-301 exposures affect the growth of surviving staphylococci and extend its antimicrobial activity. Mean PAE, PA-SME, and SME values up to 4.8, 9.3, and 9.8 h, respectively, were observed against 14 staphylococcal strains tested in human serum; growth delays were extended by 6 h in the presence of daptomycin. Exposures to CF-301 at sub-MIC levels as low as 0.001× to 0.01× MIC (∼1 to 10 ng/ml) resulted in aberrant cell wall ultrastructure, increased membrane permeability, dissipation of membrane potential, and inhibition of virulence phenotypes, including agglutination and biofilm formation. A mouse thigh infection model designed to study the PAE was used to confirm our findings and demonstrate in vivo growth delays of ≥19.3 h. Our findings suggest that at CF-301 concentrations less than the MIC during therapeutic use, sustained reductions in bacterial fitness and virulence may substantially enhance efficacy.
INTRODUCTION
Staphylococcus aureus is a leading cause of bacteremia, with an annual incidence in the United States of 38.2 to 45.7 per 100,000 person-years and a 30-day all-cause mortality of up to 20% (1). S. aureus bacteremia (SAB) is additionally associated with infective endocarditis (IE) and metastatic infections such as septic arthritis, pulmonary emboli, and osteomyelitis and can lead to sepsis and septic shock (2). Methicillin-resistant S. aureus (MRSA) continues to be associated with significant morbidity and mortality, requiring the need for novel alternatives to conventional antibiotics for the treatment of bacteremia.
A promising new therapeutic approach to killing antibiotic-resistant bacterial pathogens is based on recombinantly produced bacteriophage-derived lysins (cell wall peptidoglycan hydrolases) (3, 4). Purified lysins can be applied to target bacteria to elicit rapid peptidoglycan hydrolysis, osmotic lysis, and bacterial cell death (5). The rapid killing of pathogenic bacteria upon direct contact, as opposed to hours with conventional antibiotics, serves as the basis for advancing lysins as potentially powerful new therapeutic agents. CF-301 is a novel, recombinantly produced antistaphylococcal lysin and is the first member of the lysin class to enter phase 2 of clinical development in the United States for the treatment of S. aureus bacteremia including infective endocarditis (IE), used in addition to conventional antibiotics (i.e., daptomycin, vancomycin, and semisynthetic penicillins) (6).
CF-301 was first identified as an antistaphylococcal lysin encoded within a prophage of the Streptococcus suis genome (7). It has a domain arrangement characteristic of many bacteriophage lysins (4), defined by a catalytic N-terminal domain linked to a cell wall-binding C-terminal domain. The N-terminal domain is homologous to the cysteine-histidine-dependent amidohydrolase/peptidase (CHAP) family (8), common among lysins and other bacterial cell wall-modifying enzymes. The C-terminal domain belongs to the SH3b family of cell wall peptidoglycan-binding proteins (9). Recently, d-alanyl-l-glycyl endopeptidase activity has been confirmed for CF-301 (10).
In support of the clinical development of CF-301, a range of potent in vitro antimicrobial activities have been defined, including the following hallmark features: (i) targeted and rapid bacteriolytic effect against a broad range of S. aureus isolates, (ii) a potent ability to eradicate biofilms, (iii) a low propensity for resistance, (iv) suppression of antibiotic resistance, and (v) synergy with conventional antibiotics (11, 12). Recent in vitro studies also demonstrate that MRSA isolates surviving short (and sub-MIC) exposures to CF-301 undergo phenotypically stable increases in oxacillin susceptibility, consistent with resensitization (13, 14) and likely mediated by cell wall perturbations (15). The potent in vivo efficacy of CF-301 has also been reported using multiple different animal infection models and includes demonstrations of antimicrobial activity at very low (possibly sub-MIC) concentrations when tested in addition to conventional antistaphylococcal antibiotics (12, 16–18). In the rabbit infective endocarditis (IE) model, for example, a single bolus dose of CF-301 at a concentration as low as 0.09 mg/kg administered in addition to 4 daily doses of daptomycin (DAP; 4 mg/kg, twice a day [BID]) resulted in significantly reduced MRSA densities of >6 log10 CFU/g within heart valve vegetations, kidney, and spleen compared to 2 log10 to 3 log10 CFU/g for DAP alone after 5 days (16). These observations were also replicated in the rat IE model for CF-301 in addition to daptomycin and in the rabbit IE model for CF-301 in addition to vancomycin (16) and help to underpin the intended clinical use of CF-301 as a single dose in addition to antibiotics in the ongoing phase 2 clinical study.
Although a potent bactericidal effect defines exposures to CF-301 greater than the MIC, antibacterial effects are expected at levels lower than the MIC. We predicted that peptidoglycan hydrolysis, mediated by sub-MIC CF-301, would result in significant cell envelope damage and concomitant growth defects. To test this, we examined in vitro pharmacodynamic (PD) parameters, including the postantibiotic effect (PAE), postantibiotic sub-MIC effect (PA-SME), and sub-MIC effect (SME), which enable an understanding of the impact of short-duration and/or sub-MIC exposures on bacterial growth (19–22). These exposures are of particular interest considering the initial intended therapeutic use of CF-301 is via the administration of a single intravenous dose over a 2-h infusion in addition to standard-of-care antistaphylococcal antibiotics (17). By definition, the PAE is a suppressed phase of bacterial growth that persists after the initial exposure to an antimicrobial agent (often at supra-MIC levels) until normal bacterial growth resumes after the removal of the antibacterial agent. The PA-SME is suppressed growth during exposure to sub-MICs in the PAE phase; the PA-SME, thus, represents the time interval that includes PAE plus the additional time during which growth is suppressed by sub-MICs. Since subinhibitory concentrations may exist after dosing in therapeutic settings, the PA-SME reflects the in vivo situation more closely than the PAE. In contrast to the PA-SME, the SME measures the impact of subinhibitory levels on the growth of bacteria which have not been previously exposed to antibiotics. The effects of sub-MIC exposures in the SME format are often associated with a distinct range of physiological and morphological effects, in addition to growth defects (23, 24).
In the present study, the ability of CF-301 to suppress bacterial growth in vitro in PAE, PA-SME, and SME formats was examined using 100% human serum to better inform the intended therapeutic use of CF-301 as an intravenously administered treatment for S. aureus bloodstream infections (6). CF-301 potentiates up to 100-fold higher levels of antimicrobial activity in human blood matrices (and that of other animals, including horses) than in artificial media such as cation-adjusted Mueller-Hinton broth (caMHB) (16). For this reason, horse serum is now incorporated into the standard antimicrobial susceptibility testing (AST) medium approved by the Clinical and Laboratory Standards Institute (CLSI) (25) for determining CF-301 MICs.
This work provides evidence of a significant postexposure and sub-MIC inhibition of growth, mediated through damage to the cell envelope that furthermore impairs the expression of key virulence phenotypes, including agglutination and biofilm formation. Overall, this study provides new insights into the antimicrobial mechanisms of CF-301 affecting efficacy, beyond the general understanding of bacteriolysis, and provides important mechanistic insights which may underpin the previously reported observations (18) of the efficacy of exebacase at sub-MIC exposures when used in addition to conventional antibiotics in animal exposure target attainment models.
RESULTS
PAE, PA-SME, and SME of CF-301 on S. aureus in vitro growth.
The PAE, PA-SME, and SME values determined for CF-301 against each of 14 staphylococcal strains tested in 100% human serum are shown in Table 1. The mean PAE was 4.8 h, with a range of 3.0 to 8.7 h. Mean PA-SME and SME values of 5.4 to 9.3 h and 1.3 to 9.8 h, respectively, were observed with increasing sub-MIC amounts of CF-301 from 0.05× to 0.5× MIC. To confirm these results, assays were also performed in both 100% human plasma and the CF-301 AST medium (caMHB-HSD); both conditions yielded similar PAE, PA-SME, and SME values to those determined in serum (data not shown and see Table S1 in the supplemental material). These findings indicate a persistent inhibition of staphylococcal growth after CF-301 is either no longer present or is at less than the MIC.
TABLE 1.
PAE, PA-SME, and SME of CF-301 against 14 S. aureus strains in 100% human seruma
| Strain (description)b | CF-301 MIC (μg/ml) | PAE (h)c | Effect (h) at MIC of:d |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5× |
0.25× |
0.1× |
0.05× |
|||||||
| PA-SME | SME | PA-SME | SME | PA-SME | SME | PA-SME | SME | |||
| NRS 123 (MRSA) | 1 | 3.3 ± 0.6 | 5.6 ± 0.6 | 6.0 ± 0 | 4.6 ± 0.6 | 5.0 ± 0 | 4.6 ± 0.6 | 2.6 ± 1.5 | 3.6 ± 1.5 | 1.0 ± 0 |
| JMI 227 (MRSA) | 0.5 | 3.0 ± 0 | 11.7 ± 0.6 | 9.3 ± 0.6 | 10.0 ± 0 | 5.0 ± 0 | 5.3 ± 0.6 | 4.3 ± 0.6 | 3.6 ± 1.5 | 2.7 ± 0.6 |
| JMI 22056 (MRSA) | 1 | 4.0 ± 0 | 5.3 ± 0.6 | 6.3 ± 0.6 | 4.6 ± 0.6 | 3.6 ± 1.15 | 4.6 ± 0.6 | 2.6 ± 0.6 | 3.0 ± 0 | 0.7 ± 0.6 |
| ATCC 43300 (MRSA) | 1 | 4.7 ± 0.6 | 10.0 ± 0 | 9.3 ± 0.6 | 9.6 ± 0.6 | 7.0 ± 0 | 8.6 ± 0.6 | 3.6 ± 0.6 | 7.0 ± 0 | 2.0 ± 0 |
| NRS 671 (MRSA) | 0.5 | 6.0 ± 0 | 9.6 ± 1.15 | 7.6 ± 0.6 | 7.6 ± 0.6 | 6.0 ± 0 | 5.0 ± 1 | 3.3 ± 0.6 | 4.6 ± 0.6 | 1.0 ± 0 |
| CAIRD 426 (MRSA) | 0.5 | 3.7 ± 0.6 | 5.3 ± 0.6 | 6.0 ± 0 | 5.0 ± 0 | 4.6 ± 0.6 | 4.0 ± 0 | 3.6 ± 1.5 | 3.0 ± 0 | 0 |
| ATCC 25923 (MSSA) | 1 | 5.7 ± 0.6 | 9.0 ± 0 | 12.0 ± 0 | 8.6 ± 0.6 | 10.3 ± 0.6 | 8.3 ± 0.6 | 4.6 ± 0.6 | 8.0 ± 0 | 2.3 ± 0.6 |
| ATCC 29213 (MSSA) | 1 | 8.7 ± 0.6 | 12.0 ± 0 | 12.0 ± 0 | 11.6 ± 0.6 | 5.6 ± 0.6 | 10.6 ± 0.6 | 3.0 ± 0 | 8.3 ± 0.6 | 1.6 ± 0.6 |
| NRS 121 (LRSA) | 0.5 | 4.0 ± 0 | 11.7 ± 0.6 | 12.3 ± 0.6 | 7.6 ± 0.6 | 5.3 ± 0.6 | 7.0 ± 0 | 3.6 ± 1.5 | 6.0 ± 0 | 1.0 ± 0 |
| NRS 271 (LRSA) | 0.5 | 4.0 ± 0 | 9.3 ± 0.6 | 12.0 ± 0 | 7.3 ± 0.6 | 7.3 ± 0.6 | 5.0 ± 1 | 4.6 ± 0.6 | 3.0 ± 0 | 0.7 ± 0.6 |
| AB 2145 (DRSA) | 1 | 5.3 ± 0.6 | 12.0 ± 0 | 11.6 ± 0.6 | 9.0 ± 0 | 8.3 ± 0.7 | 6.0 ± 0 | 5.0 ± 0 | 5.6 ± 0.6 | 0.7 ± 0.6 |
| CFS 954 (DRSA) | 2 | 3.6 ± 0.6 | 8.3 ± 0.6 | 11.6 ± 0.6 | 7.6 ± 0.6 | 11.0 ± 0 | 6.6 ± 1.15 | 4.0 ± 0 | 4.3 ± 0.6 | 3.3 ± 0.6 |
| VRS1 (VRSA) | 0.5 | 7.6 ± 1.15 | 9.0 ± 0 | 9.0 ± 0 | 9.0 ± 0 | 6.3 ± 1.5 | 9.3 ± 0.6 | 3.6 ± 0.6 | 9.3 ± 0.6 | 0 |
| VRS2 (VRSA) | 0.25 | 4.6 ± 1.15 | 11.7 ± 0.6 | 11.6 ± 0.6 | 8.6 ± 0.6 | 10.3 ± 0.6 | 6.0 ± 0 | 4.6 ± 0.6 | 6.3 ± 0.6 | 1.6 ± 0.6 |
| Mean | 4.8 ± 1.7 | 9.3 ± 2.4 | 9.8 ± 2.4 | 7.9 ± 2.0 | 6.8 ± 2.3 | 6.5 ± 2.0 | 3.8 ± 1.0 | 5.4 ± 2.2 | 1.3 ± 1.0 | |
Mean values of 3 experiments are indicated (± standard deviations).
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; LRSA, linezolid-resistant S. aureus; DRSA, daptomycin-resistant S. aureus; VRSA, vancomycin-resistant S. aureus.
To induce the PAE, all strains were exposed to 4× MIC of CF-301 except LRSA, which was exposed to 2× MIC.
CF-301 concentrations (MIC values) inducing the SME in both the PA-SME and SME experimental formats are indicated.
Daptomycin (DAP) was tested as a comparator agent in both 100% human serum and caMHB (supplemented with Ca2+). In serum, DAP demonstrated a mean PAE of 2.5 h and mean PA-SME and SME values from 2.3 to 3.1 h and 0.31 to 1.7 h, respectively, with increasing sub-MIC amounts from 0.05× to 0.25× (Table S1). The effects observed for DAP in caMHB (plus Ca2+) were similar to that in serum. The antistaphylococcal effects of DAP reported here are also similar to those reported in the literature (26). Overall, our findings are consistent with a superior postexposure effect for CF-301 compared to DAP.
PA-SME of CF-301 in addition to DAP.
In view of the initial intended therapeutic use of CF-301 in addition to standard-of-care (SOC) antistaphylococcal antibiotics including DAP (6), CF-301 and DAP were tested together in staggered approaches using the PA-SME format in 100% human serum. Under one condition, the PAE was induced with CF-301 for 1 h and, after lysin removal, the SME was induced using DAP over a range of concentrations from 0.01× to 0.25× MIC. Mean PA-SME values of 6 to 11.6 h were observed over the sub-MIC DAP range, with a significant synergistic effect of 2.7 to 6.3 h (Table 2). Under a second condition, the PAE was induced with DAP alone, and after removal of the antibiotic, the SME was induced with CF-301 using 0.05× to 0.5× MIC levels. Mean PA-SME values of 5.3 to 13.3 h were observed, with a synergistic effect of 3.6 to 6.6 h. Regardless of the PA-SME format (CF-301 to induce PAE, DAP to induce SME, and vice versa), there was a strong and persistent inhibition of growth. All attempts to directly combine CF-301 and DAP together at once, each at single agent concentrations of ≥0.125× MIC, resulted in culture sterilization, thus precluding a combined PAE approach and necessitating the staggered PA-SME method.
TABLE 2.
Induction of the PA-SME using CF-301 in addition to DAP for MRSA strain NRS 123
| Condition | PAE induction (×MIC) |
SME phase (×MIC) |
Mean effects (h)a
|
Synergy effect (h)b | ||||
|---|---|---|---|---|---|---|---|---|
| CF-301 | DAP | CF-301 | DAP | CF-301 | DAP | CF-301+DAP | ||
| PAE with CF-301/SME with DAP | 4 | No | No | 0.25 | 3.3 ± 0.6 | 2.0 ± 1.0 | 11.6 ± 0.6 | 6.3 |
| 0.1 | 1 | 8.3 ± 0.6 | 4.3 | |||||
| 0.05 | 1 | 8.0 ± 0 | 4.3 | |||||
| 0.01 | 0 | 6.0 ± 0 | 2.7 | |||||
| PAE with DAP/SME with CF-301 | No | 1.5 | 0.5 | No | 6.0 ± 0 | 0.7 ± 0.6 | 13.3 ± 0.6 | 6.6 |
| 0.25 | 5.0 ± 0 | 11.3 ± 0.6 | 5.6 | |||||
| 0.1 | 2.6 ± 1.5 | 7.0 ± 1.0 | 3.7 | |||||
| 0.05 | 1.0 ± 0 | 5.3 ± 0.6 | 3.6 | |||||
Values (in hours) are shown for CF-301 and DAP as single agents (in PAE and SME formats, separately) and for CF-301+DAP in dual agent combinations (in PA-SME formats).
The synergy effect (in hours) indicates the added benefit of using each agent in combination (in the PA-SME format) over the sum of each agent tested alone (in the PAE and SME formats).
Sub-MIC CF-301 alters growth kinetics and doubling time.
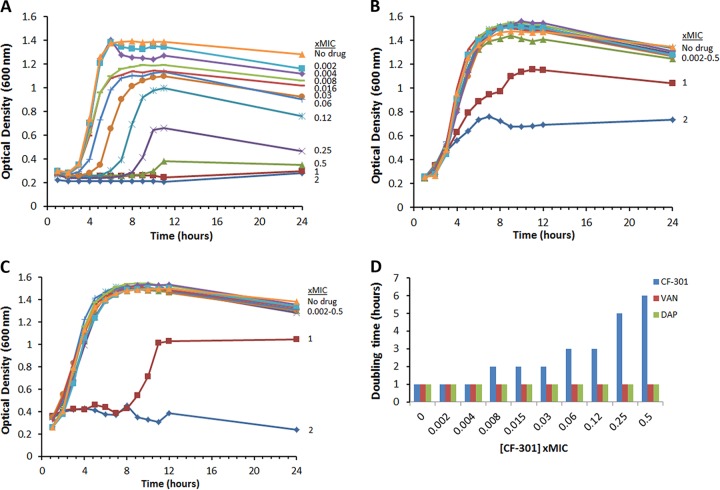
The impact of sub-MIC CF-301 on staphylococcal growth kinetics was compared to that of DAP and vancomycin (VAN) using two growth curve formats in caMHB medium supplemented with 50% human serum (caMHB-50% HuS). This medium was chosen, in lieu of 100% serum, to facilitate the accurate measurement of optical density in a 96-well plate format; CF-301 MIC values determined in caMHB-50% HuS are standardly equivalent to those determined in 100% HuS. In our study, exposures ranging from 0.008× to 0.5× MIC (8 to 500 ng/ml) CF-301 resulted in diminished exponential growth and an increased lag phase (Fig. 1A), whereas sub-MIC exposures to either DAP or VAN had little impact on growth (Fig. 1B and C). In shaking flasks, exposures to CF-301 over a 0.008× to 0.5× MIC range resulted in 1 to 5 h increases in doubling times compared to that in unexposed cultures (Fig. 1D). Sub-MIC exposures to either DAP or VAN had no impact on doubling times. The growth observed for DAP and VAN at 1× and/or 2× MIC levels (though not observed for CF-301) is attributed to the well-aerated growth conditions, with agitation, compared with the static growth conditions of the MIC assay. Overall, these findings are consistent with a greater sensitivity to sub-MIC CF-301 than to antibiotics for exposures in serum.
FIG 1.
The growth of MRSA strain NRS 123 (MW2) over 24 h in the presence and absence of CF-301 (A), DAP (B), or VAN (C). The growth medium in each case was supplemented with 50% human serum and MIC values are based on MICs of 1, 4, and 1 μg/ml for CF-301, DAP, and VAN, respectively. (D) Doubling times (in hours) were determined using a spectrophotometric-based method.
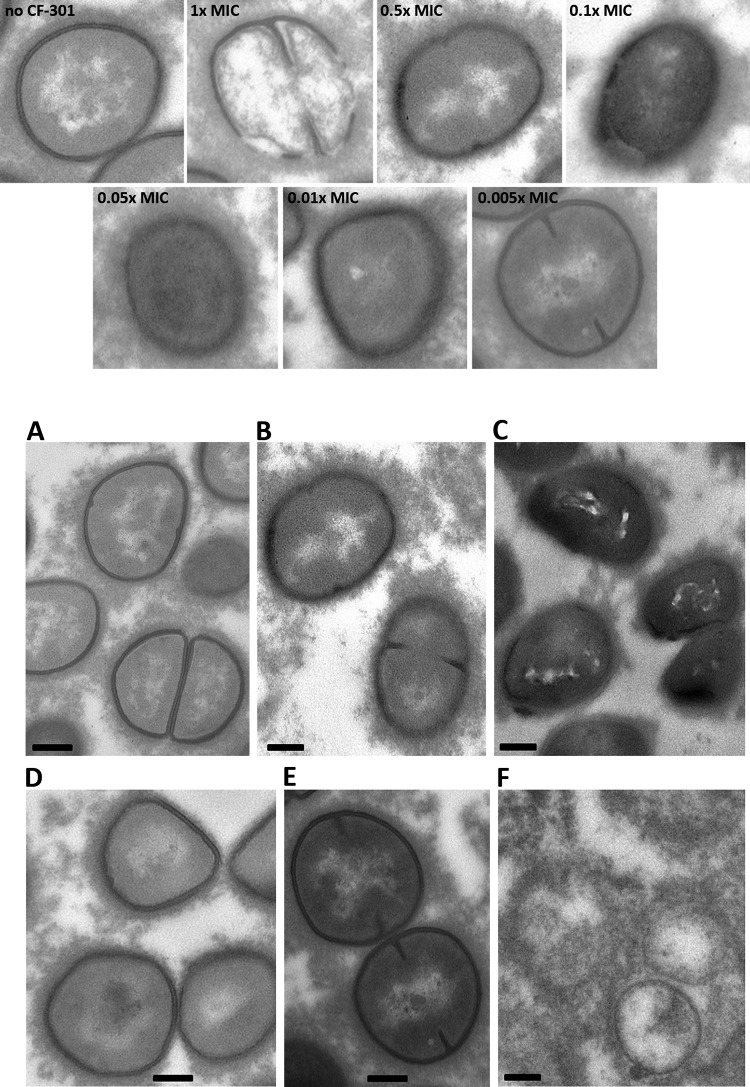
Sub-MIC CF-301 alters cell envelope structure.
The inhibitory effect of sub-MIC CF-301 on growth may be attributed to the time required for recovery from nonlethal cell wall damage. We used transmission electron microscopy (TEM) to observe cell wall ultrastructure immediately after 1-h sub-MIC exposures to CF-301 in serum. Without CF-301 treatment, staphylococci had clearly defined cell walls of uniform thickness, as expected for “healthy” bacteria (Fig. 2A). In contrast, treatments with CF-301 at 0.1× and 0.05× MIC levels (100 and 50 ng/ml) resulted in poorly defined and highly diffuse cell walls, with various levels of thickness and regions of potential destabilization (Fig. 2B and C). We observed diminished effects at 0.01× and no effect at 0.005× MIC (Fig. 2D and E). The impact of sub-MIC exposures on cell wall ultrastructure is distinct from the overt lysis observed at and above the MIC (Fig. 2F) and is in agreement with our findings of diminished growth rates for exposed bacteria.
FIG 2.
Exposures to sub-MIC CF-301 over 1 h in 100% human serum result in gross changes in S. aureus cell wall ultrastructure. Treatments were are follows: (A) buffer control; (B) 0.1× MIC CF-301; (C) 0.05× MIC CF-301; (D) 0.01× MIC CF-301; (E) 0.005× MIC; and (F) 1× MIC. Magnification, ×13,000. Scale bars, 200 nm. Indicated MIC values are based on an MIC of 1 μg/ml.
Sub-MIC CF-301 disrupts cell membrane integrity.
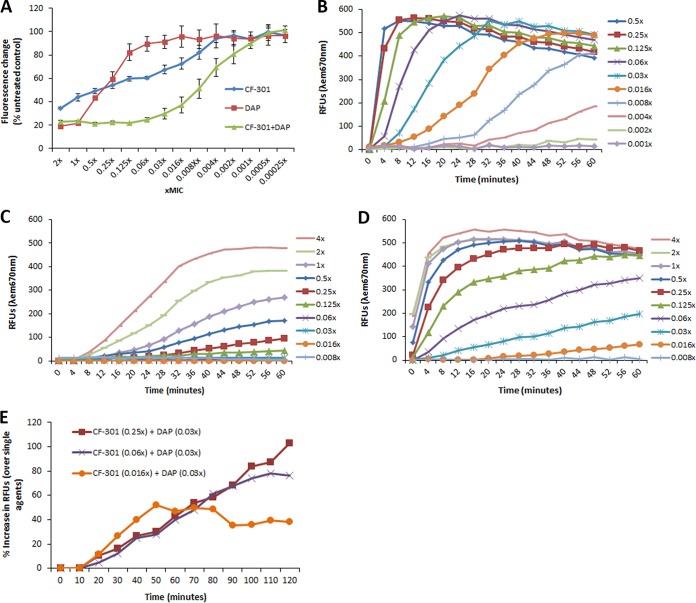
To determine the impact of sub-MIC CF-301-mediated cell envelope damage, we examined cytoplasmic membrane integrity using a propidium iodide exclusion assay previously used to examine DAP-treated staphylococci (27). Dose-dependent increases in membrane permeability were observed for treatments with CF-301 as low as 0.008× MIC (8 ng/ml) (Fig. 3A). We also tested the antibiotic DAP as a comparator agent, which is known to target the cytoplasmic membrane and which resulted in increased permeability only at levels ≥0.125× MIC (Fig. 3A). Combinations of CF-301 plus DAP resulted in pronounced synergistic effects on permeability at concentrations of each agent as low as 0.002× MIC.
FIG 3.
Disruption of S. aureus membrane integrity by sub-MIC CF-301. (A) The ratio of SYTO 9 fluorescence (all bacteria) to propidium iodide fluorescence (bacteria with damaged cytoplasmic membranes) was determined after 1 h of treatments with CF-301 and compared to that for untreated samples to calculate the percent control. Lower values indicate more membrane permeability. (B) Changes in membrane potential after treatment with CF-301, expressed as relative fluorescence units (RFUs). (C) Effect of DAP treatment on membrane potential. (D) Effect of nisin treatment on membrane potential. (E) Changes in membrane potential after treatment with CF-301 in addition to DAP. The percent increase in RFUs at each time point is shown for the dual treatment over the sum of each agent tested alone.
Consistent with our hypothesis that nonlethal damage at sub-MIC levels is responsible for growth delays and postexposure effects, we observed no impact on bacterial viability over a range of sub-MIC CF-301 concentrations (from 0.125× to 0.002× MIC), demonstrating increased permeability (see Fig. S1). The use of HEPES (a low-ionic-strength buffer) in these assays, rather than human serum (associated with high background levels of fluorescence here), was ruled out as the cause of permeabilization, largely because untreated controls lacking CF-301 and/or DAP in HEPES yielded green/red fluorescence ratios consistent with ratios previously reported (28) for nonpermeabilized healthy populations (data not shown). While HEPES might have impacted signal intensity in the presence of CF-301, the rapid onset of permeabilization in sub-MIC CF-301-treated samples matches the timing of cell envelope destabilization observed by TEM in Fig. 2. Overall, our findings remain consistent with decreased membrane integrity after treatment with a wide range of sub-MIC CF-301 levels.
Sub-MIC CF-301 dissipates membrane potential.
The addition of CF-301 over 0.125 to 0.5× MIC levels resulted in full membrane depolarization within 10 min of initiating treatment, as determined by the 3,3-dipropylthiacarbocyanine iodide [DiSC3(5)] release assay (27, 29, 30). A more gradual dissipation was observed for CF-301 levels as low as 0.004× and 0.008× MIC, with maximal depolarization observed by 60 min for the 0.008× MIC treatment (Fig. 3B). In contrast, DAP alone demonstrated only partial depolarization at sub-MIC levels, with activity detected only for the 0.5× and 0.25× sub-MIC treatments (Fig. 3C). In the assay buffer alone (5 mM HEPES, 5 mM glucose, and 50 μg/ml CaCl2), there was no DiSC3(5) release over the entire time course; thus, the depolarization was induced by the CF-301 and DAP treatments. As with the membrane permeabilization study described above, the possibility that the assay buffer used here affected the CF-301 and/DAP cannot be discounted; however, the very rapid onset of depolarization is consistent with the timing of cell envelope destabilization observed by TEM in Fig. 2
The pore-forming toxin, nisin, was additionally used a positive control because of its ability to rapidly dissipate transmembrane electrostatic potential (31). In Fig. 3D, nisin treatments resulted in full depolarization within 60 min at sub-MIC levels as low as 0.125× MIC (1 μg/ml). More gradual dissipation was observed at nisin levels down to 0.03× MIC (0.24 μg/ml). In a direct comparison with CF-301 at, for example, 0.06× MIC levels (0.06 μg/ml for CF-301 and 0.48 μg/ml for nisin), CF-301 activity exceeded that of nisin by >100%; significantly, CF-301 demonstrated activity in excess of 300% of nisin levels within 10 min. In contrast, treatment with DAP at 0.06× MIC (0.03 μg/ml) resulted in activities <5% of nisin over 2 h.
Synergistic effect of CF-301 and DAP on dissipation of membrane potential.
The analysis of membrane potential was extended to include combinations of sub-MIC CF-301 and DAP. Representative findings are shown in Fig. 3E, in which 0.25×, 0.06×, and 0.016× MIC levels of CF-301 (0.25, 0.06, and 0.016 μg/ml, respectively) were combined with 0.03× MIC DAP (0.015 μg/ml) to provide 102%, 76%, and 38% increases in relative fluorescence unit (RFU) values, respectively, over the sum of single agent values determined in Fig. 3B (CF-301) and Fig. 3C (DAP).
Sub-MIC CF-301 inhibits S. aureus agglutination.
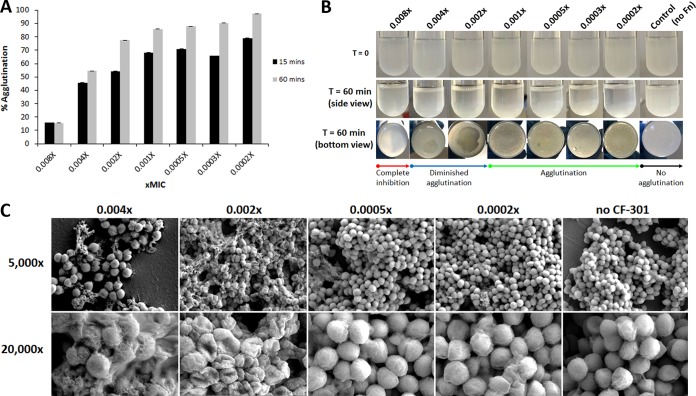
Based on the observed disruption of cell envelope structure and function by sub-MIC CF-301, we hypothesized that such treatments would inhibit the activity of surface-bound staphylococcal virulence proteins and provide an antivirulence function. Among other activities, S. aureus expresses a unique array of fibrinogen-binding proteins on its surface to promote agglutination (clumping) in blood and facilitate infections, such as endocarditis, involving clustered bacteria in association with host matrix proteins (32). We used two complementary assays to examine the impact of 1-h exposures to sub-MIC CF-301 on in vitro expression of the clumping phenotype. In one assay, performed in the presence of recombinant human fibronectin, the sub-MIC treatments resulted in near complete inhibition of agglutination (percent agglutination, ≤14% of the pretreatment control) at levels down to 0.008× MIC and partial agglutination at 0.004× (54% agglutination) (Fig. 4A). Visible signs of altered agglutination were apparent for treatments down to at least 0.002× MIC (Fig. 4B). At lower concentrations, the settling of large clumps was more uniform and resulted in agglutination levels similar to that in pretreatment controls. These findings support our hypothesis that sub-MIC CF-301 treatments can exert antivirulence functions.
FIG 4.
Sub-MIC CF-301 inhibits agglutination of S. aureus. (A) Quantitative measure of agglutination after 1-h exposures to sub-MIC CF-301 (MIC values shown) and addition of 50 μg/ml fibrinogen for 15 or 60 min. (B) Visualization of tube agglutination over 60 min; control, no fibrinogen (Fn) added. (C) Scanning electron micrographs of agglutination in human plasma after 1-h exposures to sub-MIC CF-301 (MIC values shown); control, no CF-301 added. Scale bars, 5 μm (×5,000 magnification images) and 1 μm (×20,000 magnification images).
In a second assay, the impact of sub-MIC treatments on agglutination was examined in the presence of human plasma by using scanning electron microscopy (SEM). Treatments as low as 0.004× MIC CF-301 resulted in small diffuse clumps of bacteria that were distinct from the larger accumulations apparent at lower concentrations and in the absence of CF-301 (Fig. 4C). Individual bacteria observed at levels ≥0.004× MIC had rough damaged surfaces (Fig. 4C and Fig. S1), while bacteria in the 0.002× treatment had a “deflated” nonspherical appearance. In contrast, bacteria treated at levels of ≤0.0005× MIC had a smooth appearance and were similar to that observed without exposure to CF-301. These findings agree with our hypothesis regarding the antivirulence properties of CF-301 at sub-MIC levels.
CF-301 inhibits S. aureus biofilm formation.
We next examined the impact of sub-MIC CF-301 on biofilm formation. Biofilms are central to the pathogenesis of S. aureus and require the coordinated activity of cell wall-anchored proteins (33). As with clumping, we hypothesized that the cell wall hydrolytic activity of sub-MIC CF-301 would inhibit biofilm formation. To examine this, we determined the PAE and SME on staphylococcal biofilm formation after 1-h exposures to CF-301 concentrations ranging from 4× to 0.05× MIC. The CF-301 antibiofilm PAE resulting from exposures over a range of 1× to 4× MIC was observed to be 8 to 20 h (Table 3). The SME resulting from exposures of 0.1× to 0.5× MIC was 3 to 7 h. These findings support our hypothesis regarding the antivirulence properties of sub-MIC CF-301.
TABLE 3.
CF-301 PAE and SME for CF-301 on biofilm formation by S. aureus strain ATCC BAA-42
The indicated MICs were used to induce the effect over a 1-h incubation at 37°C. Values are based on an MIC of 1 μg/ml.
T, time (in hours) required for the OD600 of solubilized biofilm biomass from CF-301-exposed cultures to reach 75% of OD600 for the untreated control.
CF-301 exhibits an extended in vivo PA-SME.
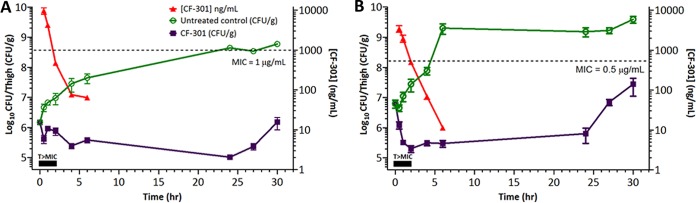
The neutropenic mouse thigh model was performed as described (34) to confirm our in vitro CF-301 PA-SME findings. This model tests for staphylococcal regrowth inhibition at CF-301 levels less than the MIC and is considered to primarily provide a description of the sub-MIC effect that is further influenced by in vivo infection conditions (35). Here, a 50% effective concentration (EC50) dose of CF-301 was used in BALB/c mice (12), providing a 2 h time above MIC (T>MIC) and sub-MIC exposures for up to 4 h (Fig. 5). The resulting in vivo PA-SMEs for MRSA strains CAIRD 426 and NRS 123 (MW2) were 19.3 h and >23.5 h, respectively. These effects were significantly longer than the 3 to 5.3 h and 3.6 to 5.6 h in vitro PA-SME values determined using CAIRD 426 and NRS 123, respectively, in Table 1.
FIG 5.
In vivo antistaphylococcal PAE of CF-301 in the neutropenic mouse thigh infection model using MRSA strains MW2 (A) and CAIRD 426 (B). Dashed lines indicate strain-specific MIC levels. CF-301 serum concentrations are indicated by the red lines. Tissue burdens (CFU/g) for the untreated controls and the CF-301 treated animals are shown in green and purple, respectively. Black rectangles indicate the interval over which CF-301 serum levels exceed the MIC. Mean values are indicated ± standard deviations.
DISCUSSION
The potent concentration-dependent in vitro bactericidal activity of CF-301 at and greater than the MIC is well described and serves an important basis of its therapeutic use (4, 5, 12). Heretofore, however, there has been no description of antimicrobial activity for CF-301 exposures at levels less than the MIC. The postexposure or sub-MIC effect is of particular importance for CF-301, considering both the intended therapeutic use of CF-301 as a single intravenously administered dose, used in addition to conventional antistaphylococcal antibiotics for the treatment of S. aureus bacteremia including endocarditis (6), and the associated expectation that lysin levels will become less than the MIC during treatment. To elucidate postexposure and sub-MIC effects of CF-301 and to better understand the duration and potency of antimicrobial activity, we examined three in vitro PD parameters, including the PAE, PA-SME, and SME.
Prolonged PAE, PA-SME, and SME effects for CF-301 were observed in human serum, as well as in plasma and the CF-301 AST medium, caMHB-HSD. Assays were performed in the presence of biologically relevant human blood matrices to account for both the intention to use CF-301 as a systemic treatment (36) and for the notable capacity of CF-301 to synergize with specific human blood components that are not present in reference media (i.e., caMHB) (37). The CF-301 PAE, PA-SME, and SME values were notably longer than values both determined here for DAP and reported for both DAP and VAN in the literature (26, 38). Overall, the prolonged postexposure and sub-MIC effects of CF-301 on staphylococcal growth support a persistent antimicrobial activity after the lysin is no longer present or concentrations are less than the MIC. These findings extend the general understanding of CF-301 antimicrobial activity beyond the potent immediate bacteriolytic effect at and higher than the MIC.
Significant inhibitions of growth in PAE, PA-SME, and SME formats were previously attributed to drug-induced nonlethal damage and the time required for recovery from such damage (22, 35). We predict that nonlethal damage to the cell envelope, mediated by the peptidoglycan hydrolytic activity of CF-301, contributes to an extended lag phase and reduced bacterial doubling time. Whereas an alternative explanation for growth delays could be the time required for outgrowth of CF-301-resistant bacterial subpopulations (including slow-growing persistent forms), we previously reported an inability to select for such variants in serial passage resistance studies (11). Furthermore, in the current study, we observed no resistant subpopulations or small colony variants after treatments. Our hypothesis holds that limited or sub-MIC exposures to CF-301 result in physical and functional changes in the cell envelope that account for growth delays.
The exact nature of potentially nonlethal damage to the cell envelope caused by limited or sub-MIC CF-301 exposures was examined. Consistent with the peptidoglycan hydrolytic activity of CF-301, short exposures to sub-MIC levels destabilized the cell wall. Furthermore, while the sub-MIC activity of CF-301 did not result in killing, we observed dose-dependent increases in membrane permeability and (gradual to rapid) dissipation of membrane potential. The pattern of rapid membrane depolarization observed over a range of CF-301 concentrations was similar to that of the membrane pore-forming toxin, nisin, and distinct from the gradual dissipation observed with DAP concentrations as low as 0.125× MIC. Although CF-301 does not directly act on the bacterial cell membrane, as do nisin and DAP, the observed effects on cell membrane permeability and electrostatic potential are likely the result of osmotic stress induced by the peptidoglycan hydrolytic activity of CF-301 (and the observed destabilization of the cell envelope) at very low concentrations. It is known that localized cell wall hydrolysis can result in the extrusion of the inner membrane and the formation of pores or blebs, as well as the uncoupling of cell wall synthesis and hydrolysis, growth arrest, and changes in cell wall thickness (39, 40). Damage to the cell wall and dysregulation of synthesis/hydrolysis can furthermore result in membrane depolarization (41). The exact mechanism by which sub-MIC CF-301 activity alters inner membrane function remains to be determined.
Because of the intended therapeutic use of CF-301 as an adjunctive therapy, in addition to antistaphylococcal antibiotics (6), we also incorporated DAP directly into the in vitro PD studies with CF-301. We previously demonstrated potent in vitro and in vivo synergistic antimicrobial activities for CF-301 and DAP, likely driven by complementary activities on peptidoglycan and cell membrane targets, respectively (12). In accordance with these findings, a synergistic effect on growth was observed here using sub-MIC combinations of CF-301 and DAP. The synergy can most likely be attributed to a significant dose-dependent sub-MIC effect observed for CF-301 in addition to DAP on both membrane permeability and membrane depolarization. These findings are supportive of a persistent antimicrobial activity for CF-301 in addition to DAP during treatment.
Beyond the inhibitory effect on growth, exposures to sub-MIC CF-301 suppressed the expression of key staphylococcal virulence phenotypes, including biofilm formation and agglutination. We observed extended PAEs and SMEs on both biofilm formation and agglutination. This suppression of virulence phenotypes was most likely mediated by interference with the surface expression/activity of staphylococcal virulence proteins. Damage to the cell envelope resulting from nonlethal peptidoglycan hydrolysis may therefore serve antivirulence functions and may additionally impact other traits required for S. aureus pathogenesis, including IgG binding by protein A and exotoxin activities.
Translation of our in vitro findings was confirmed in a complementary study using a methodology to induce the in vivo PA-SME in a thigh infection of neutropenic mice (22, 34). In this model, plasma CF-301 levels remained higher than the MIC for 1 to 2 h and were less than the MIC for an additional 4 h (reaching as low as 10 ng/ml). The resulting PA-SME resulted in growth delays of 19.3 and >23.5 h for the two strains analyzed. This observed in vivo effect was superior to the in vitro effect of up to 9.3 h and is consistent with the understanding that in vivo PAEs tend to be longer than in vitro PAEs (42). Regrowth was clearly delayed by up to 17 h after CF-301 levels became lower than the MIC, which was probably due to postantibiotic subinhibitory concentrations (i.e., the PA-SME). Based on our in vitro findings, including sub-MIC effects on biofilm formation and agglutination, we hypothesize that the extended in vivo PAEs are mediated through the diminished capacity of staphylococci to adhere to, persist, and grow in tissues. This inhibition of growth, observed in the context of neutropenic animals, is expected to be more pronounced in nonneutropenic animals in which staphylococci may be more susceptible to immune surveillance (i.e., the postleukocyte enhancement effect [43]). Overall, these observations may have important therapeutic implications for improving clinical outcomes in invasive MRSA infections.
The PAE observations presented here may have clinical relevance in that these findings may underpin important observations whereby a single dose of CF-301 used in addition to multiple-day dosing with an antistaphylococcal antibiotic has consistently demonstrated multi-log-fold-greater reductions in bacterial burden compared to that with the antibiotic alone in murine bacteremia and both rat and rabbit infective endocarditis (IE) models (12, 16, 18). In the rabbit IE model, for example, a single bolus dose of CF-301 at a concentration as low as 0.09 mg/kg administered in addition to 4 daily doses of DAP (4 mg/kg, BID) resulted in significantly reduced MRSA densities of >∼6 log10 CFU/g within all relevant target tissues of infected animals compared to 2 log10 to 3 log10 CFU/g for DAP alone after 5 days (16). Based on the pronounced in vivo PAEs of 19.3 and >23.5 observed in the current study (and the in vitro postexposure effects of CF-301 alone and in addition to DAP), we consider the PAE to be a potentially important contributing factor to the efficacy observed in other animal studies (12, 16, 18).
Importantly, the PAE observations in the current study and the synergy observed in animal models (12, 16, 18) were informative to the decision to study CF-301 in a phase 2 study in which a single intravenous (i.v.) dose of CF-301 is administered in addition to conventional antibiotics to patients with S. aureus bacteremia including endocarditis, with the goal of improving clinical responder rates compared to that with antibiotics alone. We have completed a substantial body of work which translates the in vitro PAE and in vivo animal efficacy to pharmacodynamically optimized human dosing for phase 2 by leveraging animal target attainment data and population pharmacokinetics (PK) from animal studies and the phase 1 clinical study (36). Dose-fractionation studies using the murine neutropenic thigh infection model demonstrated that the area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC/MIC ratio) is the PK/PD index most predictive of efficacy, as measured by bacterial burden reduction. AUC/MIC ratios of 0.1 to 0.2 were associated with maximal efficacy of CF-301 when administered in addition to antibiotics (e.g., daptomycin, vancomycin or oxacillin [18]), consistent with the in vivo efficacy of CF-301 at sub-MICs. These animal target attainment studies served to inform target efficacious exposure and dose selection for the ongoing phase 2 study, and PK/PD studies from phase 2 will be used to further assess the need for pharmacodynamic optimization. Data from the phase 2 study will also be used, with data from supportive animal models, to explore additional CF-301 dosing paradigms as part of the developmental life cycle of CF-301.
Overall, the data from our current study demonstrate that the antimicrobial effect of CF-301 extends beyond the well-described rapid bactericidal activity. Additional postexposure and sub-MIC effects have the potential to limit bacterial growth and impair the activity of important virulence factors associated with the cell envelope. Such bacteriostatic and antivirulence functions suggest a more far-reaching and nuanced antimicrobial effect for CF-301 and, potentially, lysins in general. Importantly, there is no evidence that the beneficial effects of CF-301 at sub-MIC levels are counterbalanced by the selection for resistant derivatives (11, 12), as can be the case with small molecule antibiotics (44). Overall, our findings highlight the remarkable potential of this lysin (and possibly the lysin class) to serve as an adjunctive therapeutic strategy used in addition to conventional antibiotics to treat life-threatening bacterial infections, particularly those which result in substantial morbidity and mortality despite treatment with conventional antibiotics. Further studies to elucidate the mechanism(s) of postexposure and sub-MIC effects reported here, including the nature of cell envelope damage, the potential role for induction of the cell wall stress stimulon (31–34), and the specific impact on cell wall anchored and secreted virulence factors, will be pursued.
MATERIALS AND METHODS
Antimicrobial agents, bacterial strains, and growth media.
CF-301 (>99% pure) was prepared by ContraFect Corporation (Yonkers, NY). Daptomycin (DAP) and nisin were obtained from Sigma-Aldrich (St. Louis, MO). Clinical isolates and laboratory strains were obtained from JMI laboratories (JMI), BEI Resources (NRS), American Type Culture Collection (ATCC), and the ContraFect (CFS) in-house collection. The S. aureus strains used in this study are described in Table 1 and were stored frozen at −80°C. Frozen isolates were revived on BBL Trypticase soy agar plates with 5% sheep blood (TSAB; Becton, Dickinson & Company [BD]) and incubated overnight at 37°C in ambient air. Additional growth media included sterile-filtered human serum from pooled human male AB plasma (Sigma-Aldrich), plasma from humans (Sigma-Aldrich), tryptic soy broth (TSB) from Hardy Diagnostics (VWR International), TSB with 0.2% d-glucose (TSBg), BBL cation-adjusted Mueller-Hinton II broth (caMHB; BD), and caMHB supplemented with horse serum to a final concentration of 25% and dithiothreitol (DTT) to a final concentration of 0.5 mM (caMHB-HSD). The human serum and horse serum used in these studies were sterile filtered and not delipidated; in the case of both the human serum (either undiluted or in combination with caMHB) and horse serum (diluted in caMHB), there was no cloudiness or precipitation observed. The caMHB-HSD is the standard AST medium for CF-301, approved for use by the CLSI (25), and enables MIC results identical to that determined in both 100% human serum and caMHB supplemented with 50% human serum (45). The d-glucose and DTT were obtained from Sigma-Aldrich.
MIC determinations.
MICs were determined by broth microdilution (BMD) according to Clinical and Laboratory Standards Institute methodology (CLSI; M07-A11) (46) with some exceptions. The MIC values for CF-301 (or the indicated antibiotic) for studies using 100% human serum (or plasma) or caMHB supplemented with 50% human serum (caMHB-50% HuS) were determined in those same culture conditions; in the case of DAP, the caMHB was also supplemented with Ca2+ to 50 μg/ml. For studies using either caMHB-HSD (i.e., the CF-301 AST medium) or caMHB supplemented with Ca2+ to 50 μg/ml (i.e., the DAP AST medium), we again used medium-specific MIC values. Thus, the concentrations of CF-301, DAP, and VAN used for each indicated treatment were based on MIC values specific for the medium used in that experiment (i.e., serum, plasma, caMHB-HSD, and caMHB-50% HuS).
Determination of in vitro PAE.
The analysis of 14 S. aureus strains was performed as described (47) with slight modifications. Bacterial cultures were adjusted to a 0.5 McFarland standard, diluted 1:100 in the indicated medium, and grown to a density of 105 to 106 CFU/ml (in logarithmic phase) at 37°C with agitation at 225 rpm. With the exception of linezolid-resistant strains, each culture was exposed to CF-301 at 4× MIC for 1 h at 37°C with agitation at 200 rpm. The linezolid-resistant strains were exposed to CF-301 at 2× MIC. The 4× and 2× values was chosen based on range-finding studies to identify the highest CF-301 concentration to enable an ∼3 log10 CFU/ml drop in viability within 1 h. After exposure, CF-301 was removed by 1:1,000 dilution into freshly prepared medium and then further incubated at 37°C with agitation at 200 rpm for 24 h. To confirm the removal of CF-301 at the dilution step, control cultures were initially included whereby the bacteria (for dilution) were first suspended in 25 mg/ml activated charcoal (Sigma-Aldrich) to inactivate CF-301 as described (11); this did not alter the results compared to those with dilution without suspension in charcoal (data not shown); thus, the use of charcoal was not continued. For each PAE test culture, bacterial concentrations were determined by quantitative plating just before and immediately after dilution; growth was then followed by quantitative plating at 1-h intervals for 9 h and at 24 h. To prevent carryover effects of free drug, culture samples removed for quantitative plating were first suspended in a 25-mg/ml activated charcoal suspension to inactivate residual CF-301 as above. The PAE was defined as T – C, where T is the time required for viability counts of an antibiotic-exposed culture to increase by 1 log10 compared to counts immediately after removal of CF-301 and C is the corresponding time for growth control not exposed to CF-301.
Determination of in vitro PA-SME.
Following PAE induction for 1 h with CF-301 (as described above), cultures samples were diluted 1:1,000 into aliquots of the medium containing four different sub-MICs of CF-301 and further incubated at 37°C with agitation at 225 rpm for 24 h. Viability was determined as described above for the in vitro PAE study. The PA-SME was defined as Tpa – C, where Tpa is the time required for cultures previously exposed to CF-301 and then exposed to different sub-MICs to increase by 1 log10 compared to counts immediately after the removal of CF-301 and C is the corresponding time for the growth control not exposed to CF-301.
Determination of in vitro SME.
The SME was induced the same way as the PA-SME, without the prior induction of the PAE. Following a 1-h growth phase (without CF-301), culture samples were diluted 1:1,000 in 100% human serum containing four different sub-MICs of CF-301 and then further incubated at 37°C with agitation at 225 rpm for up to 24 h. Viability counts were determined as described above for the in vitro PAE study. The SME was defined as Ts – C, where Ts is the time required for the cultures exposed only to sub-MICs to increase 1 log10 compared to counts immediately after dilution and C is the corresponding time for the unexposed control.
Determination of in vitro PAE and PA-SME using CF-301 in addition to DAP.
A PA-SME format was used to determine the synergistic effect of CF-301 and DAP against MRSA strain NRS 123 (MW2) in 100% human serum. Bacterial suspensions were prepared as described for the PAE study above. One set of cultures was exposed to CF-301 at 4× MIC or no drug for 1 h at 37°C with agitation at 200 rpm, diluted 1:1,000 in 100% human serum containing either no drug or different sub-MICs of DAP (including 0.25×, 0.1×, 0.05×, and 0.01× MIC), and then further incubated at 37°C with agitation at 225 rpm for 24 h. Another set of cultures was exposed to DAP at 1.5× MIC or no drug for 1 h at 37°C with agitation at 200 rpm, diluted 1:1,000 in 100% human serum containing either no drug or different sub-MICs of CF-301 (including 0.5×, 0.25×, and 0.1× MIC), and further incubated at 37°C with agitation at 225 rpm for 24 h. The 1.5× MIC of DAP was chosen (from among a range of concentrations tested), because it enabled both a 3 log10 drop in viability (without culture sterilization) and then subsequent regrowth in the absence of CF-301. Viability counts were determined as described above for the in vitro PAE study. The synergistic effect (in hours) of each combination was defined as PA-SMEa+b – (PAEa + SMEb), where PA-SMEa+b is determined using CF-301 to induce the PAE and DAP to induce the SME (and vice versa), PAEa is determined using either CF-301 or DAP to induce the PAE (with no subsequent sub-MIC exposures), and SMEb is determined using either CF-301 or DAP to induce the SME (with no previous drug exposures). The individual PAE, SME, and PA-SME values were determined as described above.
Growth kinetics.
Growth curves were performed in caMHB supplemented with human serum to a final concentration of 50% (caMHB-50% HuS) in lieu of 100% serum to enable accurate measurements of culture optical density in a 96- well microtiter plate (U-bottom, polystyrene; Falcon) format. Strain NRS 123 (MW2) was suspended at a concentration of ∼5 × 106 CFU/ml in each well of a serial 2-fold dilution series of CF-301 (ranging from 2× to 0.002× MIC) prepared in caMHB-50% HuS across the x axis of the microtiter plate. The MIC values used here were determined in caMHB-50% HuS (for CF-301 and VAN); for DAP, the caMHB was further supplemented with Ca2+ to 50 μg/ml. All MICs determined in caMHB-50% HuS were identical to those determined in 100% HuS. Culture turbidity was measured at an optical density at 600 nm (OD600) using a SpectraMax M3 multimode microplate reader (Molecular Devices) with readings every 1 min for 11 h at 24°C with agitation. An endpoint reading was also included at 24 h. Each condition was examined in quadruplicates, and controls lacking lysin were included. Mean OD600 values are reported. Doubling times (Td) were calculated in caMHB-HuS using an independent method based on OD600 values in the logarithmic phase of 25-ml cultures grown in 250-ml Erlenmeyer flasks with aeration (48).
Transmission electron microscopy.
A mid-log-phase culture of MRSA strain NRS 123 (MW2) was adjusted to an OD of 0.5 McFarland standard units and diluted 1:50 in 25 ml of 100% human serum in 250-ml Erlenmeyer flasks. CF-301 was then added over a range of concentrations (from 0.005× to 0.5× MIC) and incubated at 37°C for 1 h with agitation at 225 rpm. Treated cultures were washed twice in 1× phosphate-buffered saline (PBS), suspended for 15 min in 2× fixative solution (8% paraformaldehyde, 5% glutaraldehyde, and 0.2 M sodium cacodylate buffer, pH 7.4), pelleted by centrifuged, resuspended in 1× fixative solution, and incubated overnight at 4°C prior to analysis. Samples were postfixed in 1% osmium tetroxide, block stained with uranyl acetate, and processed according to standard procedures by The Rockefeller University Electron Microscopy Service. Samples were visualized using a Tecnai Spirit BT transmission electron microscope (FEI).
Membrane integrity.
The effect of CF-301 and/or DAP exposures on staphylococcal membrane permeability was measured using the LIVE/DEAD BacLight bacterial viability kit (Thermo Fisher Scientific) as previously described (27) with some modifications. Bacterial colonies (MRSA strain NRS 123) were suspended at an optical density of 0.5 McFarland standard units, diluted 1:50 into 50 ml of 100% human serum (in a 500-ml Erlenmeyer flask), and incubated at 37°C for 3 h with agitation at 225 rpm. Cultures were pelleted by centrifugation at 4,000 × g, washed, and resuspended in 5 mM HEPES buffer (Teknova, Hollister, CA) with 50 μg/ml CaCl2 (Sigma-Aldrich). All assays were performed in black, 96-well, polystyrene flat-bottom microtiter plates (CellStar; Greiner Bio-One). For analyses of single agent activities, 100 μl of 8× MIC concentrates (CF-301 or DAP) were serially diluted 2-fold across the x axis to enable final concentration ranges of 0.0002× to 2× MIC. For the analyses of CF-301 in addition to DAP, 16× MIC concentrates were used. Untreated control wells with no added antimicrobial agent were included for each dilution series. Next, 50 μl of bacterial inoculum was added to each well and incubated for 1 h at 24°C in the absence of light before the addition of 5 μM SYTO 9 (green fluorescent nucleic acid stain) and 30 μM propidium iodide (PI) (red fluorescent nucleic acid stain) staining solution and an additional 15-min incubation at 24°C in the absence of light. The fluorescence of SYTO 9 (excitation wavelength [λex], 485 nm; emission wavelength [λem], 530 nm) and PI (λex, 485 nm; λem, 630 nm) was measured in a SpectraMax M3 multimode microplate reader (Molecular Devices, Sunnyvale, CA). Green-fluorescing SYTO 9 enters all cells and is used for assessing total cell count. Red-fluorescing PI only enters cells with damaged cytoplasmic membranes and is used here to assess increases in bacterial permeability. The ratio of SYTO 9 fluorescence to PI fluorescence (FSYTO 9/FPI) was determined for each treatment and the buffer control. Percentage values for each treatment compared to that of the untreated control are reported. Each analysis was performed in triplicates, and means (± standard deviations) are reported. The effect of each treatment with CF-301 and/or DAP on bacterial cell viability was determined just prior to the addition of LIVE/DEAD stain by quantitative plating on TSAB plates.
Membrane potential (single agent).
The effect of CF-301 or DAP exposures on staphylococcal membrane potential was measured via spectrofluorimetry using a cationic membrane-permeable fluorescent dye, 3,3-dipropylthiacarbocyanine iodide [DiSC3(5)], which is often used as a potentiometric probe (29). The compound accumulates in the membranes of polarized cells, quenching the overall fluorescence; upon depolarization, the bound dye is released into the aqueous phase, resulting in dequenching and fluorescence emission. Bacterial colonies (MRSA strain NRS 123) were suspended at an optical density of 0.5 McFarland standard units, diluted 1:50 into 50 ml of 100% human serum (in a 500-ml Erlenmeyer flask), and incubated at 37°C for 3 h with agitation at 225 rpm. After 3 h, the culture was pelleted by centrifugation at 4,000 × g for 20 min, washed, and adjusted to an OD600 of 0.2 in assay buffer (5 mM HEPES, 5 mM glucose, and 50 μg/ml CaCl2) containing 2 μM DiSC3(5) (Sigma-Aldrich). The cell suspension was incubated at 24°C, in the absence of light, until DiSC3(5) uptake was maximal, as indicated by a stable reduction in fluorescence. Once the uptake of the dye was at the maximum, 100 mM KCl was added to the cell suspension for 15 min. For each antimicrobial agent tested, 100 μl of 4× to 8× MIC was serially diluted (in assay buffer) 2-fold across the x axes of black, 96-well, polystyrene flat-bottom microtiter plates (CellStar; Greiner Bio-One) to ultimately achieve the final concentration ranges. Nisin (30) and assay buffer alone were used as positive and negative controls, respectively. Next, 50 μl of the bacterial suspension [loaded with DiSC3(5)] was added to each well and fluorescence (λex, 622 nm; λem, 670 nm) was measured for at least 1 h at 24°C in a SpectraMax M3 multimode microplate reader (Molecular Devices, Sunnyvale, CA). Fluorescence values are reported as relative fluorescence units (RFUs). For the comparison of membrane dissipating activities of CF-301 (and DAP) to nisin, RFUs were first determined for each agent, tested at 0.06× MIC, for 2 h. Relative fluorescence at each time point was determined according to the following equation: (RFUCF-301 (or DAP)/RFUnisin) × 100.
Membrane potential (dual agent).
The effect of exposures to CF-301 and DAP together on staphylococcal membrane potential was measured as described above (with modifications) using black, 96-well, polystyrene flat-bottom microtiter plates. In a checkerboard format (49), a 2-fold dilution series of CF-301 (yielding a final concentration range of 0.5× to 0.008× MIC) across the y axis was combined with a 2-fold dilution series of DAP (yielding a final concentration range of 4× to 0.004× MIC) across the x axis. A bacterial suspension [loaded with DiSC3(5)] was prepared as described above and added to each well. Fluorescence (λex, 622 nm; λem, 670 nm) was measured over 2 h at 24°C. The percent increase in RFUs for each combination over the sum of each agent tested alone (at appropriate concentrations) was calculated. Three independent experiments were conducted, and each experiment was carried out in triplicates; mean values were used in all calculations.
S. aureus agglutination assay.
The ability of S. aureus strain NRS 123 (MW2) to agglutinate in the presence of sub-MIC CF-301 was measured according to a previously described method (50). Bacteria were suspended at an optical density of 0.5 McFarland standard units, diluted 1:100 in 10 ml of TSB in a 125-ml Erlenmeyer flask, and incubated at 37°C overnight with agitation at 225 rpm. Exponential cultures were established by dilution (1:100) into 50 ml of 100% human serum and incubating at 37°C for 1 h at 225 rpm. The culture was then divided into eight 5-ml aliquots (in 50-ml blue-cap conical centrifuge tubes) and treated with CF-301 over a range from 0.008× to 0.0002× MIC (8 ng/ml to 200 pg/ml) at 37°C for 1 h at 225 rpm. Two additional untreated control cultures were included. After treatment, bacteria were collected by centrifugation at 4,000 × g for 20 min, washed twice with 1× PBS, and resuspended in 1× PBS to an OD600 of 1.5 in 16-mm by 100-mm borosilicate glass culture tubes (VWR Scientific). Agglutination was induced by adding fibrinogen (fibrinogen from human plasma; Sigma-Aldrich) to each tube, except one of the untreated controls, at a final concentration of 50 μg/ml. Agglutination was marked by the appearance of large visible bacterial settling to form a diffuse bottom layer and clearing the supernatant of up to 70% of the initial cells present. Each tube was photographed prior to the addition of fibrinogen and 60 min later. To quantitate agglutination, 0.1-ml aliquots were first removed from each treatment (and control) prior to the addition of fibrinogen, transferred to a 96-well, polystyrene, flat-bottom non-tissue culture-treated microtiter plate (VWR Scientific), and the OD600 was measured using a SpectraMax M3 multimode microplate reader (time point T = 0). Fibrinogen was then added to a final concentration of 50 μg/ml, and the OD600 was measured at 15- and 60-min intervals. The percent agglutination was calculated as follows: [(ODT=0 − ODT=15 or 60 min)/ODT=0] × 100. Three independent experiments were conducted, and each experiment was carried out in triplicates; mean values are shown (± standard deviations).
Scanning electron microscopy.
Bacterial strain NRS 123 (MW2) was prepared and treated with sub-MIC CF-301 exactly as described for the agglutination assay. After suspending treated and washed cells in PBS at an OD600 of 1.5 in a glass culture tubes, samples were mixed 1:1 with EDTA-chelated human plasma (BioreclamationIVT) and incubated for 15 min to induce agglutination as described (51). Cells were fixed for 15 min in 2× fixative solution (16% paraformaldehyde, 10% glutaraldehyde, 0.4 M Na cacodylate buffer, pH 7.4) and stored overnight at 4°C in 1× fixative solution prior to analysis.
PAE and SME on biofilm formation.
The PAE and SME generally describe the delayed regrowth of surviving bacteria following limited exposures to an antimicrobial agent; however, the terms can be extended to describe a range of other effects. Here, the PAE and SME of CF-301 on biofilm formation were examined using MRSA strain ATCC BAA-42, a prolific biofilm-forming strain often used in studies with CF-301 (11, 12). Bacterial colonies were adjusted to a 0.5 McFarland standard, diluted 1:100 in human serum, and grown to a density of 105 to 106 CFU/ml at 37°C with agitation at 225 rpm. The PAE or SME was induced by exposure of culture aliquots to a range of CF-301 concentrations from 4× to 0.05× MIC (including buffer controls) at 37°C for 1 h with agitation at 225 rpm. Each culture was then diluted 1:1,000 in 2 ml TSBg in each well of two Falcon 24-well plates (VWR) and incubated for 36 h at 30°C in ambient air. The TSBg growth medium was used to support the formation of S. aureus biofilms, which will not form in human serum (52). At time points up to 36 h after dilution in TSBg, sets of three wells were washed 3 times each with PBS, and the adherent biofilm biomass was then stained with 0.1% crystal violet solution (Harleco; Sigma-Aldrich) and solubilized in 33% glacial acetic acid. The OD600 of solubilized biofilm material was measured using a SpectraMax M3 multimode microplate reader (Molecular Devices). The effect (PAE or SME) was defined as the time required for the OD600 of solubilized biofilm material from CF-301-exposed cultures to reach 75% of the OD600 of the untreated growth control.
Mice.
Female BALB/c mice (5 to 7 weeks of age) weighing 14 to 18 g each were obtained from Jackson Laboratories (Bar Harbor, ME), maintained in accordance with American Association for Accreditation of Laboratory Animal Care criteria, and cared for in accordance with national guidelines. The animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of ContraFect Corporation.
Mouse thigh infection model.
The in vivo PA-SME was determined using the neutropenic mouse thigh infection model as described (34). Mice were rendered neutropenic (polymorphonuclear leukocyte count, <100/mm3) by intraperitoneal (i.p.) injection of cyclophosphamide (Sigma-Aldrich) at 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) prior to intramuscular injection into the posterior thigh of a 0.1-ml bacterial inoculum (concentration, 1 × 107 CFU/ml). Mice were dosed intravenously (i.v.) into the lateral tail vein with CF-301 at 10 to 15 mg/kg beginning 2 h postinfection. Dosing was based on previously determined EC50 values (12). Buffer-treated mice served as controls. Groups of 3 mice were treated with each dosing regimen. Immediately after infection (T = 0) and at eight time points thereafter (T = 0.5, 1, 2, 4, 6, 24, 27, and 30 h), mice were euthanized and each thigh was aseptically removed, weighed, and homogenized in PBS using a Precellys24 high-throughput tissue homogenizer (Bertin Corporation, Rockville, MD). Serial 10-fold dilutions of the homogenized material were plated on TSAB plates for determinations of CFU per gram of tissue. Each symbol in the figures represents the data from three mice (six thighs).
Pharmacokinetic determinations.
The single-dose PK determination was performed in mice after the i.v. administration of CF-301 to infected animals (described above). Blood samples were obtained by retro-orbital puncture and transfer into K2EDTA tubes (Becton, Dickinson and Company) from each of three mice at the following time points: 0.5, 1, 2, 4, and 6 h after dosing. The samples were then centrifuged for 10 min at 4°C at 1,300 × g. Plasma CF-301 concentrations was determined using a validated enzyme-linked immunosorbent assay (ELISA) at ContraFect.
Determination of in vivo PAE.
The duration (in hours) of the PA-SME in infected mice was determined as described (34) by the following equation: PA-SME = T – C – M, where M represents the time serum levels exceed the MIC, T is the time required for CFU in the thighs of treated mice to increase 1 log10 compared to the count at time M, and C is the time needed for CFU in the thighs of untreated controls to increase 1 log10 compared to the viable counts at time zero (T = 0 h).
Supplementary Material
ACKNOWLEDGMENTS
We thank Ricardo Ramirez, Jimmy Rotolo, Karen Sauve, and Michael Wittekind for their contribution to this work. We also thank Nadine Soplop and Kunihiro Uryo at The Rockefeller University for electron microscopy.
This work was supported by ContraFect Corporation.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02616-18.
REFERENCES
- 1.Bergin SP, Holland TL, Fowler VG Jr, Tong SYC. 2017. Bacteremia, sepsis, and infective endocarditis associated with Staphylococcus aureus. Curr Top Microbiol Immunol 409:263–296. doi: 10.1007/82_2015_5001. [DOI] [PubMed] [Google Scholar]
- 2.Hassoun A, Linden PK, Friedman B. 2017. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care 21:211. doi: 10.1186/s13054-017-1801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wittekind M, Schuch R. 2016. Cell wall hydrolases and antibiotics: exploiting synergy to create efficacious new antimicrobial treatments. Curr Opin Microbiol 33:18–24. doi: 10.1016/j.mib.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Nelson DC, Schmelcher M, Rodriguez-Rubio L, Klumpp J, Pritchard DG, Dong S, Donovan DM. 2012. Endolysins as antimicrobials. Adv Virus Res 83:299–365. doi: 10.1016/B978-0-12-394438-2.00007-4. [DOI] [PubMed] [Google Scholar]
- 5.Fischetti VA, Nelson D, Schuch R. 2006. Reinventing phage therapy: are the parts greater than the sum? Nat Biotechnol 24:1508–1511. doi: 10.1038/nbt1206-1508. [DOI] [PubMed] [Google Scholar]
- 6.ContraFect. 2017. Safety, efficacy and pharmacokinetics of CF-301 vs. placebo in addition to antibacterial therapy for treatment of S. aureus bacteremia. https://clinicaltrials.gov/ct2/show/NCT03163446.
- 7.Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. 2013. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:2743–2750. doi: 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Layec S, Decaris B, Leblond-Bourget N. 2008. Characterization of proteins belonging to the CHAP-related superfamily within the Firmicutes. J Mol Microbiol Biotechnol 14:31–40. doi: 10.1159/000106080. [DOI] [PubMed] [Google Scholar]
- 9.Tossavainen H, Raulinaitis V, Kauppinen L, Pentikainen U, Maaheimo H, Permi P. 2018. Structural and functional insights into lysostaphin-substrate interaction. Front Mol Biosci 5:60. doi: 10.3389/fmolb.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lood R, Molina H, Fischetti VA. 2017. Determining bacteriophage endopeptidase activity using either fluorophore-quencher labeled peptides combined with liquid chromatography-mass spectrometry (LC-MS) or Forster resonance energy transfer (FRET) assays. PLoS One 12:e0173919. doi: 10.1371/journal.pone.0173919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. 2017. Bacteriophage lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrob Agents Chemother 61:e02666-16. doi: 10.1128/AAC.02666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti VA, Huang DB, Nowinski RC, Wittekind M. 2014. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis 209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh J, Abdelhady W, Xiong YQ, Jones S, Cassino C, Bayer AS, Schuch R. 2018. Lysin CF-301 exhibits a low propensity for decreased susceptibility and prevents daptomycin (DAP) resistance in a rabbit model of S. aureus infective endocarditis (IE). Abstr Eur Congr Clin Microbiol Infect Dis, Madrid, Spain. [Google Scholar]
- 14.Oh J, Schuch R. 2017. Low propensity of resistance development in vitro in Staphylococcus aureus with lysin CF-301. Abstr ASM Microbe, New Orleans, LA. [Google Scholar]
- 15.Renzoni A, Von Dach E, Landelle C, Diene SM, Manzano C, Gonzales R, Abdelhady W, Randall CP, Bonetti EJ, Baud D, O'Neill AJ, Bayer A, Cherkaoui A, Schrenzel J, Harbarth S, Francois P. 2017. Impact of exposure of methicillin-resistant Staphylococcus aureus to polyhexanide in vitro and in vivo. Antimicrob Agents Chemother 61:e00272-17. doi: 10.1128/AAC.00272-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Indiani C, Sauve K, Raz A, Abdelhady W, Xiong YQ, Cassino C, Bayer AS, Schuch R. 2019. The antistaphylococcal lysin, CF-301, activates key host factors in human blood to potentiate methicillin-resistant Staphylococcus aureus bacteriolysis. Antimicrob Agents Chemother 63:e02291-18. doi: 10.1128/AAC.02291-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmer DB, Schmitz JE, Thandar M, Euler CW, Fischetti VA. 2017. The phage lysin PlySs2 decolonizes Streptococcus suis from murine intranasal mucosa. PLoS ONE 12:e0169180. doi: 10.1371/journal.pone.0169180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotolo J, Ramirez RA, R S, Machacek M, Khariton T, Ghahramani P, Wittekind M. 2016. PK-PD driver of efficacy for CF-301, a novel anti-staphylococcal lysin: implications for human target dose. Abstr ASM-Microbe 2016, Boston, MA. [Google Scholar]
- 19.Craig WA, Vogelman B. 1987. The postantibiotic effect. Ann Intern Med 106:900–902. doi: 10.7326/0003-4819-106-6-900. [DOI] [PubMed] [Google Scholar]
- 20.Craig WA, Gudmundsson S. 1996. Postantibiotic effect, p 296–329. InLorian V.(ed), Antibiotics in laboratory medicine. Williams and Wilkins Co., Baltimore, MD. [Google Scholar]
- 21.Lowdin E, Odenholt-Tornqvist I, Bengtsson S, Cars O. 1993. A new method to determine postantibiotic effect and effects of subinhibitory antibiotic concentrations. Antimicrob Agents Chemother 37:2200–2205. doi: 10.1128/AAC.37.10.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odenholt-Tornqvist I, Löwdin E, Cars O. 1992. Postantibiotic sub-MIC effects of vancomycin, roxithromycin, sparfloxacin, and amikacin. Antimicrob Agents Chemother 36:1852–1858. doi: 10.1128/AAC.36.9.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 24.Zhanel GG, Hoban DJ, Harding GK. 1992. Subinhibitory antimicrobial concentrations: a review of in vitro and in vivo data. Can J Infect Dis 3:193–201. doi: 10.1155/1992/793607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CLSI. 16 to 17 January 2017 AST Subcommittee Working Group meetings and plenary. AST meeting files & resources. https://clsi.org/education/microbiology/ast/ast-meeting-files-resources/.
- 26.Pankuch GA, Jacobs MR, Appelbaum PC. 2003. Postantibiotic effects of daptomycin against 14 staphylococcal and pneumococcal clinical isolates. Antimicrob Agents Chemother 47:3012–3014. doi: 10.1128/AAC.47.9.3012-3014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berti AD, Wergin JE, Girdaukas GG, Hetzel SJ, Sakoulas G, Rose WE. 2012. Altering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibiotics. Antimicrob Agents Chemother 56:5046–5053. doi: 10.1128/AAC.00502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa-Almeida R, Franco AR, Pesqueira T, Oliveira MB, Babo PS, Leonor IB, Mano JF, Reis RL, Gomes ME. 2018. The effects of platelet lysate patches on the activity of tendon-derived cells. Acta Biomater 68:29–40. doi: 10.1016/j.actbio.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Maier E, Benz R, Hancock RE. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 30.Silverman JA, Perlmutter NG, Shapiro HM. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother 47:2538–2544. doi: 10.1128/AAC.47.8.2538-2544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince A, Sandhu P, Ror P, Dash E, Sharma S, Arakha M, Jha S, Akhter Y, Saleem M. 2016. Lipid-II independent antimicrobial mechanism of nisin depends on its crowding and degree of oligomerization. Sci Rep 6:37908. doi: 10.1038/srep37908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crosby HA, Kwiecinski J, Horswill AR. 2016. Staphylococcus aureus aggregation and coagulation mechanisms, and their function in host-pathogen interactions. Adv Appl Microbiol 96:1–41. doi: 10.1016/bs.aambs.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paharik AE, Horswill AR. 2016. The staphylococcal biofilm: adhesins, regulation, and host response. Microbiol Spectr 4:VMBF-0022-2015. doi: 10.1128/microbiolspec.VMBF-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogelman B, Gudmundsson S, Turnidge J, Leggett J, Craig WA. 1988. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis 157:287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]
- 35.den Hollander JG, Fuursted K, Verbrugh HA, Mouton JW. 1998. Duration and clinical relevance of postantibiotic effect in relation to the dosing interval. Antimicrob Agents Chemother 42:749–754. doi: 10.1128/AAC.42.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassino C, Murphy G, Boyle J, Rotolo J, Wittekind M. Results of the first in human study of lysin CF-301 evaluating the safety, tolerability and pharmacokinetic profile in healthy volunteers. Abstr ECCMID 2016, Amsterdam, Netherlands. [Google Scholar]
- 37.Indiani C, Sauve K, Oh J, Ghahramani P, Raz A, Abdelhady W, Xiong YQ, Bayer AS, Cassino C, Schuch R. 2018. Lysin CF-301 (exebacase) activates latent host factors in human blood to potentiate bacteriolysis. Abstr presented at ASM Microbe, Atlanta, GA. [Google Scholar]
- 38.Dorr T, Alvarez L, Delgado F, Davis BM, Cava F, Waldor MK. 2016. A cell wall damage response mediated by a sensor kinase/response regulator pair enables beta-lactam tolerance. Proc Natl Acad Sci U S A 113:404–409. doi: 10.1073/pnas.1520333113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee TK, Huang KC. 2013. The role of hydrolases in bacterial cell-wall growth. Curr Opin Microbiol 16:760–766. doi: 10.1016/j.mib.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang KC, Mukhopadhyay R, Wen B, Gitai Z, Wingreen NS. 2008. Cell shape and cell-wall organization in Gram-negative bacteria. Proc Natl Acad Sci U S A 105:19282–19287. doi: 10.1073/pnas.0805309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashyap DR, Wang M, Liu LH, Boons GJ, Gupta D, Dziarski R. 2011. Peptidoglycan recognition proteins kill bacteria by activating protein-sensing two-component systems. Nat Med 17:676–683. doi: 10.1038/nm.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Craig WA. 1993. Post-antibiotic effects in experimental infection models: relationship to in-vitro phenomena and to treatment of infections in man. J Antimicrob Chemother 31:149–158. doi: 10.1093/jac/31.suppl_D.149. [DOI] [PubMed] [Google Scholar]
- 43.Levison ME, Levison JH. 2009. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am 23:791–815. doi: 10.1016/j.idc.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wistrand-Yuen E, Knopp M, Hjort K, Koskiniemi S, Berg OG, Andersson DI. 2018. Evolution of high-level resistance during low-level antibiotic exposure. Nat Commun 9:1599. doi: 10.1038/s41467-018-04059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh J, Sauve K, Wittekind M, Ambler J, Schuch R. 2017. Development of an antimicrobial susceptibility test (AST) for the antistaphylococcal lysin CF-301. Abstr 27th Eur Congr Clin Microbiol Infect Diss, Vienna, Austria. [Google Scholar]
- 46.CLSI. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 47.Pankuch GA, Appelbaum PC. 2006. Postantibiotic effect of ceftobiprole against 12 Gram-positive organisms. Antimicrob Agents Chemother 50:3956–3958. doi: 10.1128/AAC.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito M, Katayama Y, Hishinuma T, Iwamoto A, Aiba Y, Kuwahara-Arai K, Cui L, Matsuo M, Aritaka N, Hiramatsu K. 2014. “Slow VISA,”” a novel phenotype of vancomycin resistance, found in vitro in heterogeneous vancomycin-intermediate Staphylococcus aureus strain Mu3. Antimicrob Agents Chemother 58:5024–5035. doi: 10.1128/AAC.02470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moody J. 2010. Synergy testing: broth microdilution checkerboard and broth macrodilution methods, p 5.12.1–5.12.23. In Garcia LS. (ed), Clinical microbiology procedures handbook, vol 2 ASM Press, Washington, DC. [Google Scholar]
- 50.Walker JN, Crosby HA, Spaulding AR, Salgado-Pabon W, Malone CL, Rosenthal CB, Schlievert PM, Boyd JM, Horswill AR. 2013. The Staphylococcus aureus ArlRS two-component system is a novel regulator of agglutination and pathogenesis. PLoS Pathog 9:e1003819. doi: 10.1371/journal.ppat.1003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAdow M, Kim HK, Dedent AC, Hendrickx AP, Schneewind O, Missiakas DM. 2011. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog 7:e1002307. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abraham NM, Jefferson KK. 2010. A low molecular weight component of serum inhibits biofilm formation in Staphylococcus aureus. Microb Pathog 49:388–391. doi: 10.1016/j.micpath.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.