Caspofungin has a liver-dependent metabolism. Reduction of the dose is recommended based on Child-Pugh (C-P) score.
KEYWORDS: Child-Pugh score, caspofungin, critically ill, pharmacokinetics
ABSTRACT
Caspofungin has a liver-dependent metabolism. Reduction of the dose is recommended based on Child-Pugh (C-P) score. In critically ill patients, drug pharmacokinetics (PK) may be altered. The aim of this study was to investigate the prevalence of abnormal liver function tests, increased C-P scores, their effects on caspofungin PK, and whether pharmacokinetic-pharmacodynamic (PK/PD) targets were attained in patients with suspected candidiasis. Intensive care unit patients receiving caspofungin were prospectively included. PK parameters were determined on days 2, 5, and 10, and their correlations to the individual liver function tests and the C-P score were analyzed. Forty-six patients were included with C-P class A (n = 5), B (n = 40), and C (n = 1). On day 5 (steady state), the median and interquartile range for area under the curve from 0 to 24 h (AUC0–24), clearance (CL), and central volume of distribution (V1) were 57.8 (51.6 to 69.8) mg·h/liter, 0.88 (0.78 to 1.04) liters/h, and 11.9 (9.6 to 13.1) liters, respectively. The C-P score did not correlate with AUC0–24 (r = 0.03; P = 0.84), CL (r = −0.07; P = 0.68), or V1 (r = 0.19; P = 0.26), but there was a bilirubin-driven negative correlation with the elimination rate constant (r = −0.46; P = 0.004). Hypoalbuminemia correlated with low AUC0–24 (r = 0.45; P = 0.005) and was associated with higher clearance (r = −0.31; P = 0.062) and somewhat higher V1 (r = −0.15; P = 0.37), resulting in a negative correlation with the elimination rate constant (r = −0.34; P = 0.042). For Candida strains with minimal inhibitory concentrations of ≥0.064 μg/ml, PK/PD targets were not attained in all patients. The caspofungin dose should not be reduced in critically ill patients in the absence of cirrhosis, and we advise against the use of the C-P score in patients with trauma- or sepsis-induced liver injury.
INTRODUCTION
Invasive candidiasis has a major impact on morbidity and mortality in intensive care patients (1) and, thus, is of importance to initiate adequate antifungal treatment when there is a relevant clinical suspicion (2–5). The echinocandin caspofungin has been shown to be effective for treatment of invasive candidiasis (6) and is currently recommended as a first-line therapy (7, 8). Caspofungin is partially cleared by the liver (9), and an increase in AUC has been observed in patients with chronic liver insufficiency (10). Therefore, a reduction in caspofungin maintenance dose has been recommended in patients with moderate liver insufficiency, as estimated by Child-Pugh (C-P) class B (11–13).
Critically ill patients often have pathophysiological changes that may influence antifungal pharmacokinetics (PK) (14). Thus, PK results from patient cohorts other than the critically ill cannot readily be extrapolated to this patient population, especially not to critically ill patients with liver dysfunction. Data on antifungal PK in these patients is limited. However, a recent study showed that caspofungin PK in critically ill patients were similar to the results from other studies on noncritically ill patients and healthy subjects (15). This was observed despite a hepatic dysfunction classified as C-P class B in the critically ill patients. Consequentially, based on these results it was argued that a dose reduction of caspofungin in critically ill patients leads to suboptimal drug levels. In that study, it was reported that the C-P score mainly was driven by low albumin levels, and the effects of individual parameters of the C-P score were not described. Taking the hepatic metabolism of caspofungin into consideration, it is of interest to study the caspofungin PK in patients with abnormal liver function tests and increased C-P score with special reference to the individual score components bilirubin, international normalized ratio (INR), and albumin. Thus, the aim of the present study was to investigate the prevalence of abnormal liver function tests in critically ill patients with suspected fungal infection and the effects of C-P score, bilirubin, INR, and albumin on caspofungin PK. A secondary aim was to study to what extent suggested target concentrations of caspofungin were attained in this specific study population. Ultimately, the intention was to provide better guidance for caspofungin dosage in critically ill patients with abnormal liver function tests.
RESULTS
Forty-six patients were enrolled. Demographics and other characteristics are shown in Table 1. Sequential organ failure assessment (SOFA) score, C-P score, vital organ support, and liver and renal function test on treatment days 2 and 5 are shown in Table 2. The large majority of patients were formally classified as C-P class B. On day 2, only two patients demonstrated normal liver function test and one patient was classified as C-P class C. None of the patients had a diagnosis of chronic liver disease on admission. PK sampling on day 2 was obtained from all 46 patients. Samples on days 5 and 10 were obtained from 37 and 21 patients, respectively. Caspofungin was discontinued before day 10 due to there being no need for further antifungal therapy (n = 4), switch to alternative antifungals (n = 4), death (n = 6), or transportation to other units (n = 11). Median duration of caspofungin therapy was 7 days (range, 2 to 21 days).
TABLE 1.
Demographic and other characteristics of included patientsc
| Parameter | Value(s) |
|---|---|
| Demographics | |
| No. male/no. female (%) | 35/11 (76/24) |
| Age [yr; median (range)] | 64 (19–89) |
| Weight [kg; median (range)] | 80 (50–114) |
| No. with weight of ≤80 kg | 23 |
| No. with weight of >80 kg | 23 |
| BMI [kg/m²; median (range)] | 25.1 (16.0-43.5) |
| ICU, n | |
| Surgical and medical | 24 |
| Cardiothoracic | 19 |
| Burn | 3 |
| Primary diagnosis at admission to ICU, n | |
| Cardiac arrest | 3 |
| Cardiothoracic surgery | 8 |
| Pulmonary disease | 8 |
| Aortic surgery | 5 |
| Abdominal surgery or pancreatitis | 4 |
| Kidney transplantation | 1 |
| Hematological malignancies | 3 |
| Burn injury | 4 |
| Primary infection | 10 |
| APACHE II score, n (range) | 19 (3–40) |
| Indication for treatment,a n (%) | |
| Candidemia | 3 (7) |
| Abdominal candidiasis | 5 (11) |
| Empirical | 37 (80) |
| Prophylaxis | 1 (2) |
| Species, nb | |
| C. albicans | 27 |
| C. glabrata | 1 |
| C. parapsilosis | 3 |
| Yeast; not specified | 1 |
| Mixed | 8 |
| C. albicans | 5 |
| C. glabrata | 4 |
| C. parapsilosis | 4 |
| C. tropicalis | 1 |
| C. lusitaniae | 1 |
| C. guilliermondii | 1 |
Indication as stated in the medical record.
Invasive isolates were C. albicans (5), C. glabrata (1), and C. parapsilosis (1).
Six patients had negative cultures.
TABLE 2.
Child-Pugh score, SOFA score, vital organ support, and parameters of hepatic and renal functiona
| Parameter | Value(s) on day: |
|
|---|---|---|
| 2 (n = 46) | 5 (n = 37) | |
| Child-Pugh, n (%) | ||
| Score 5 | 2 (4) | 2 (5) |
| Score 6 | 3 (7) | 0 |
| Score 7 | 25 (54) | 24 (65) |
| Score 8 | 3 (7) | 6 (16) |
| Score 9 | 12 (26) | 5 (14) |
| Score 10 | 1 (2) | 0 |
| Class A | 5 (11) | 2 (5) |
| Class B | 40 (87) | 35 (95) |
| Class C | 1 (2) | 0 |
| SOFA score, median (range) | 9 (2–21) | 7 (2–17) |
| Vital organ support, n (%) | ||
| CRRT | 21 (45) | 15 (40) |
| Ventilator | 41 (89) | 30 (81) |
| ECMO | 9 (20) | 9 (20) |
| Parameters of hepatic and renal function, median (range) |
||
| P-bilirubin, μmol/liter | 24 (4–286) | 18 (4–296) |
| P-albumin, g/liter | 18.5 (11–44) | 20 (12–39) |
| P-INR | 1.2 (0.9–1.8) | 1.2 (1.0–1.7) |
| P-ALT, μkat/liter | 0.64 (0.15–10.5) | 0.86 (0.15–8.0) |
| P-ALP, μkat/liter | 1.7 (0.59–8.6) | 1.8 (0.9–8.0) |
| P-creatinine, μmol/liter | 97 (29–550) | 96 (36–289) |
| GFR, ml/min/1.73m² | 84b (23–116) | 89c (25–117) |
Values are given in absolute numbers (percent) or median (range). SOFA score, sequential organ failure assessment score; CRRT, continuous renal replacement therapy mainly performed as continuous venovenous hemodialysis; ECMO, extracorporeal membrane oxygenation; P, plasma; INR, international normalized ratio; ALT, alanine aminotransferase; ALP, alkaline phosphatase; μkat/liter, microkatal/liter; GFR, glomerular filtration rate as estimated by the Modification of Diet in Renal Disease Study equation.
Twenty-five patients (patients with CRRT excluded).
Twenty-two patients (patients with CRRT excluded).
All patients were given a caspofungin loading dose of 70 mg. Of the 23 patients with a body weight of >80 kg, 12 continued with 70 mg, whereas 11 patients received a dose of 50 mg. All patients with body weight of <80 kg received 50 mg. In no case was there a dose reduction to 35 mg. Each individual patient received the same maintenance dose during the entire study. No dose adjustment of caspofungin was made for patients receiving treatment with extracorporeal membrane oxygenation (ECMO).
Caspofungin pharmacokinetics.
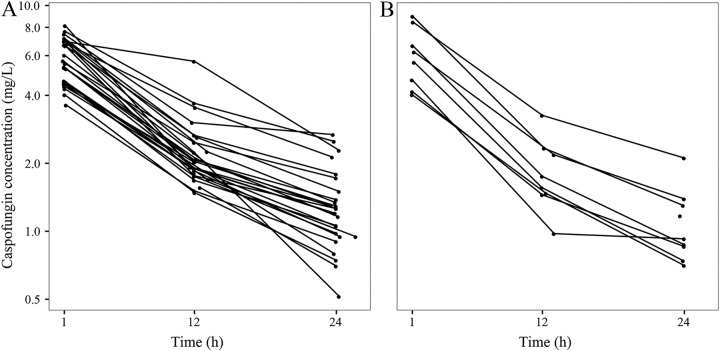
Plasma concentration-time curves on day 5 are shown in Fig. 1. PK parameters estimated by using all data and specific values for days 2, 5, and 10 are presented in Table 3. For the 21 patients completing PK sampling up to day 10, the specific values for each separate day are listed in Table S1 in the supplemental material. Changes over time were limited. In the group of patients with a body weight of >80 kg, no difference in AUC from 0 to 24 h (AUC0–24) was registered in the two subgroups receiving maintenance doses of 50 mg and 70 mg (Fig. 1).
FIG 1.
Caspofungin plasma concentration-time curves on day 5 in patients receiving the 50-mg maintenance dose (A) and the 70-mg maintenance dose (B).
TABLE 3.
PK parameters estimated by using all data or specific data
| Parametera | PK values [median (range)] for: |
|||
|---|---|---|---|---|
| All data (n = 46) | Day 2 (n = 46) | Day 5 (n = 37) | Day 10 (n = 21) | |
| Cmax (mg/liter) | 5.1 (1.1–8.8) | 5.6 (3.6–9.0) | 5.3 (3.6–9.4) | |
| Cmin (mg/liter) | 1.1 (0.5–2.3) | 1.2 (0.5–2.7) | 1.1 (0.5–2.6) | |
| AUC0-24 (mg·h/liter) | 59.6 (32.7–105) | 61.1 (32.8–108) | 57.8 (44.0–113) | 58.4 (37.2–105) |
| CL (liters/h) | 0.87 (0.47–1.70) | 0.84 (0.46–1.78) | 0.88 (0.44–1.58) | 0.90 (0.47–1.88) |
| V1 (liters) | 11.2 (6.7–26.4) | 11.2 (6.7–26.4) | 11.9 (7.5–20.1) | 11.2 (7.7–19.4) |
| V2 (liters) | 6.9 (1.6–22) | 6.9 (2.6–13) | 5.3 (1.5–7.1) | 5.5 (3.3–6.6) |
| k10 (/h) | 0.074 (0.047–0.11) | 0.072 (0.048–0.11) | 0.079 (0.054–0.12) | 0.077 (0.061–0.12) |
Cmax, maximal concentration; Cmin, minimal concentration; AUC0–24, area under the concentration-time curve from 0 to 24 h; CL, clearance; V1, central volume of distribution; V2, peripheral volume of distribution; k10, elimination rate constant from V1 at equilibrium between V1 and V2. Shrinkage (%): all data, CL of 11.2, V1 of 9.3, and V2 of 33.7; day 2, CL of 2.6, V1 of 4.6, and V2 of 54.0; day 5, CL of 17.6, V1 of 28.9, and V2 of 63; day 10, CL of 11.5, V1 of 42.1, and V2 of 76.7.
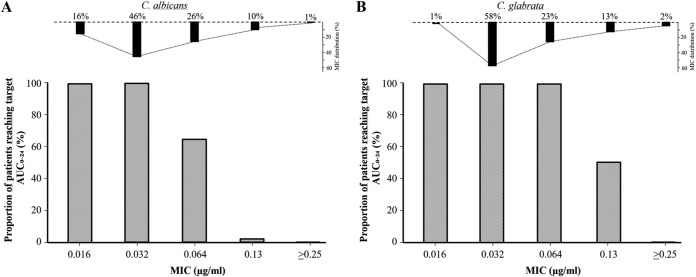
The correlations between the pharmacokinetic parameters and C-P score, the individual components of the C-P score, and SOFA score are shown in Table 4. The C-P score did not correlate with AUC0–24, clearance (CL), and central volume of distribution (V1), but there was a negative correlation with the elimination rate constant (k10) (Fig. 2). Bilirubin was the most important parameter in this negative correlation. Furthermore, low albumin levels correlated with low AUC0–24 (Fig. 3) and were associated with higher clearance and somewhat higher V1, resulting in a significantly negative correlation with the elimination rate constant (Table 4). No significant correlations were seen between PK parameters and the plasma liver enzymes alanine aminotransferase and alkaline phosphatase or the glomerular filtration rate (GFR) (Table S2). The 21 patients with continuous renal replacement therapy (CRRT) did not differ with respect to PK parameters compared with the group not receiving CRRT (Table S3A), and there were no significant differences detected in PK parameters in the nine patients receiving ECMO compared with patients not receiving ECMO (Table S3B). The proportions of patients on day 5 reaching target AUC for Candida albicans and C. glabrata with different MICs are demonstrated in Fig. 4. Target AUC was not reached in all patients for strains with MICs of ≥0.064 μg/ml.
TABLE 4.
Correlation coefficients between PK parameters and Child-Pugh score, plasma bilirubin, albumin, INR, and SOFA score on day 5a (n = 37)
| Parameter | AUC0-24 | CL | V1 | k10 |
|---|---|---|---|---|
| C-P score | 0.03 | −0.07 | 0.19 | −0.50** |
| Bilirubin | 0.00 | 0.02 | 0.22 | −0.46** |
| Albumin | 0.45** | −0.31 | −0.15 | −0.34* |
| INR | −0.08 | 0.00 | −0.03 | −0.04 |
| SOFA score | 0.03 | −0.05 | 0.20 | −0.47** |
C-P, Child-Pugh; INR, international normalized ratio; SOFA, sequential organ failure assessment; AUC0–24, area under the concentration-time curve from 0 to 24 h; V1, central volume of distribution; V2, peripheral volume of distribution; k10, the elimination rate constant from V1 at equilibrium between V1 and V2. *, P < 0.05. **, P < 0.01.
FIG 2.
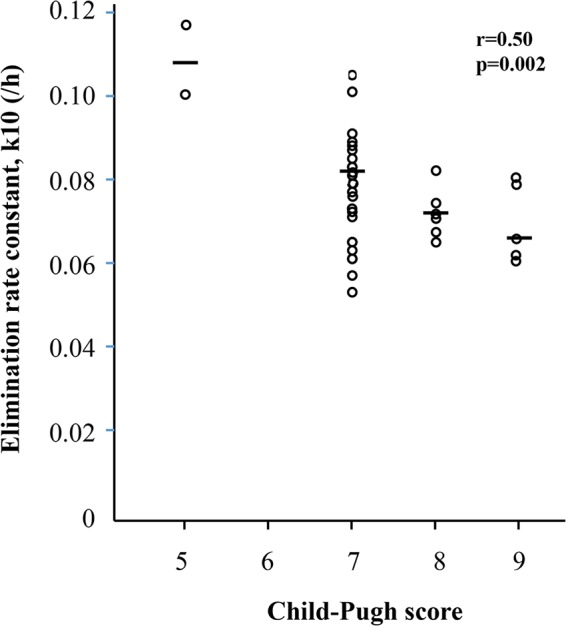
Elimination rate constants, k10, on day 5 at different C-P levels (n = 37). Horizontal bars demonstrate median values.
FIG 3.
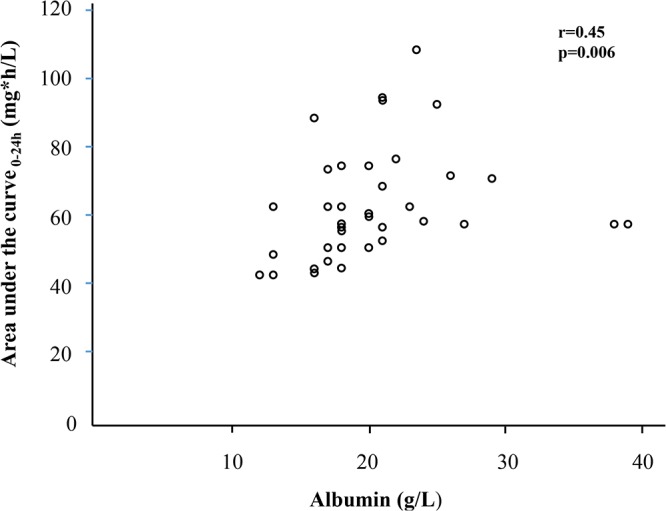
Correlation plot between AUC0–24 and albumin levels on day 5 (n = 37). The two patients with low AUC0–24 despite the highest albumin levels were the two classified as C-P class A with a score of 5 and associated with high elimination rates, as demonstrated in Fig. 4. Correlation analysis restricted to patients with C-P class B resulted in an r value of 0.51 (P = 0.002; n = 35).
FIG 4.
Proportion of patients on day 5 reaching target concentrations for Candida albicans (A) and C. glabrata (B) with different MICs. The distribution of the MIC values for C. albicans and C. glabrata are included at the top. Similar results were found on days 2 and 10. AUC/MIC ratios for C. albicans and C. glabrata of 865 and 450, respectively, were used in the calculations and refer to Andes et al. (32). The MIC distributions were obtained from Pfaller et al. (21).
DISCUSSION
The results of the present study demonstrate that the majority of critically ill patients who are candidates for antifungal treatment due to a suspected invasive candida infection have abnormal liver function tests, suggesting liver dysfunction expressed as C-P score. Although no patient suffered from chronic liver disease, all but two demonstrated abnormal results on day 2 and were formally classified as C-P class A (n = 5), B (n = 40), or C (n = 1). The high prevalence of abnormal liver function tests in critically ill patients at risk of invasive candida infection warrants a better understanding of the optimal caspofungin dosage in this group of patients.
In the current study, no correlation was observed between C-P score and the PK parameters AUC0–24, CL, and V1. Thus, the caspofungin dose should not be reduced in critically ill patients with abnormal liver function tests in the absence of chronic liver disease. This conclusion is further supported by the absence of caspofungin accumulation in the patients studied over the period of 10 days. The discrepancy from the study by Mistry et al., where caspofungin exposure was increased in patients with chronic liver disease, classified as C-P class B (10), emphasizes that data obtained in these patients cannot be extrapolated to patients with trauma- or sepsis-induced liver injury and vice versa.
In the present study, bilirubin was the component with the largest impact on the C-P score and the parameter mainly responsible for the negative correlation between C-P score and the elimination rate constant, k10. Furthermore, hypoalbuminemia correlated with low AUC0–24 and negatively to the elimination rate constant (Table 4). Thus, despite increasing the C-P score, hypoalbuminemia did not contribute to the negative correlation between the C-P score and elimination rate constant. In this aspect, hypoalbuminemia actually exerted an effect opposite that of hyperbilirubinemia. This is not surprising, since albumin is affected not only by liver dysfunction but also by severe trauma and infection, common conditions in the intensive care unit (ICU). It may be speculated that hypoalbuminemia resulting in an increased free fraction of caspofungin exposes the drug to a more effective elimination. This mechanism may be an important factor to take into account in caspofungin dosage. Our results on AUC0–24, CL, V1, and the question of dose adjustment are in agreement with the study by Muilwijk et al., but in their study C-P score was mainly driven by albumin, and hypoalbuminemia did not influence the PK parameters (15). However, taking into consideration the high caspofungin protein binding of 97% at normal plasma protein levels (9) and the increased number of patients in the current study, our findings are not unexpected. Our results are also in agreement with those from the study by Nguyen et al. in which higher albumin levels were associated with higher trough concentrations of caspofungin (16). In a prior study on critically ill patients treated with the highly protein-bound echinocandin anidulafungin, no correlation was observed between exposure and plasma protein concentration (17). The authors in that study speculated that the lack of correlation was due to influences of factors with counteracting effects on the exposure. As shown in the current study, hyperbilirubinemia and hypoalbuminemia may represent two such counteracting factors.
There was a relatively large interindividual variation in the AUC0–24 and the other PK parameters, which is in agreement with previous studies on caspofungin pharmacokinetics in ICU patients (14, 15, 18). The AUC0-24 values in our patients compared with those observed in these studies and healthy subjects are shown in Table S4 in the supplemental material. Our AUC0-24 values were lower than those reported in healthy subjects (9), which is in agreement with the DALI study (14) and the recent study by van der Elst et al. (18) with 7 and 20 patients, respectively, but in contrast to the study by Muilwijk et al. with 21 patients (15). The discrepancy with the latter study may be explained by the wide variation seen in all studies and possibly because our patients had a higher clearance due to higher weight and less pronounced renal dysfunction. Although caspofungin clearance was not associated with glomerular filtration rate in the current study, a slight increase in caspofungin exposure has been reported in patients with renal dysfunction (19). In contrast to voriconazole (20), the limited data in the present study do not indicate that caspofungin dosing needs adjustment in patients with ECMO.
The current results show that the majority of patients attain caspofungin target exposures for most C. albicans and C. glabrata strains (Fig. 4). For strains with MICs of ≥0.064 μg/ml, this seems more uncertain. Candida spp., such as C. lusitaniae, C. krusei, and C. parapsilosis, have higher MICs than C. albicans and C. glabrata (21, 22), suggesting additional uncertainty on whether target concentrations will be achieved. The number of patients in the current study not reaching target concentration is higher than that of patients assessed as treatment failures in the clinical trials (6, 23). It may be speculated that patients with low albumin and a shift from the bound to free form of caspofungin benefit more than expected from current PK/pharmacodynamics (PD) data, a theory that also highlights the need of free drug measurements. Besides a large variation in the murine data, it must be emphasized that human PK/PD data may differ. However, in the case of fluconazole there has been good agreement between human and murine data (24, 25). As previously proposed for anidulafungin (17) and caspofungin (18), current data suggest that therapeutic drug monitoring (TDM) is relevant in critically ill patients, at least in candidiasis caused by strains with higher MICs. Higher daily doses of up to 200 mg of caspofungin have been studied and are reported as well tolerated (26, 27). On the other hand, there are concerns related to higher doses of caspofungin. In vitro data indicate that the fungicidal effect of caspofungin is hampered at higher concentrations, the so-called paradoxical effect (PE) (28–30). A study by Rueda et al., however, showed no influence of PE on clinical outcome (31). Whether PE has any influence on clinical outcome and, if so, at what caspofungin levels remains to be elucidated. TDM has a crucial role in understanding the clinical role of PE.
In addition to being considerably larger than previous studies, the strength of the present study is its separate analyses of individual components of the C-P score, demonstrating counteracting effects of bilirubin and albumin. Thus, by expanding the knowledge of liver function and how PK parameters are affected in the critically ill, we can make more robust decisions on dosage of caspofungin. The prospective design, enrollment of consecutive ICU patients, and relation of findings to target values represent additional advantages. Nevertheless, there are several limitations. First, PK data were not obtained using frequent sampling but rather from three time points per study day, which has previously been used in other PK studies on caspofungin (14). Second, correlation analysis would have been a more robust statistical method if the C-P score had been more evenly distributed among C-P classes. This implies that our results are mainly applicable to critically ill patients with C-P classes A and B. However, the patients were included consecutively and, hence, the distribution reflects the extent of liver dysfunction in an ICU population with suspected or proven invasive fungal infection. Another weakness is the fact that the criteria for target attainment are based on murine data, since human data are lacking (32). This emphasizes the need for human PK/PD studies to establish optimal caspofungin exposure. Lastly, as discussed above, data were based on total plasma concentrations of caspofungin. To fully understand the role of albumin in caspofungin PK/PD, methods for measuring the free fraction of caspofungin are of paramount importance.
In summary, the prevalence of patients with abnormal liver function tests in ICU patients with suspected or proven fungal infection is high. C-P score did not affect the PK parameters AUC0–24, CL, and V1. Hypoalbuminemia correlated with a reduction in AUC0–24 and an increase in the elimination rate constant. The target AUC0–24/MIC values are obtained but within a narrow margin. The current data strongly argue against a reduction of the caspofungin dose in critically ill patients with hypoalbuminemia and abnormal liver function tests in the absence of chronic liver disease and against the use of C-P score in patients with trauma- or sepsis-induced liver injury. Future strategies for optimizing the dosage of caspofungin, such as TDM, in critically ill patients with hypoalbuminemia, with or without abnormal liver function, or strains with elevated MICs need to be explored.
MATERIALS AND METHODS
Study design.
This open-label, phase IV, multiple-dose, single-center observational study was performed from January 2012 to September 2014 in three ICUs at Uppsala University Hospital, Uppsala, Sweden, a tertiary-care hospital. Adult patients, 18 years or older, treated at the combined medical and surgical, cardiothoracic, or burn ICU and receiving caspofungin for suspected or proven invasive candida infection were eligible. The patients were enrolled consecutively. The protocol was approved by the local ethics committee, and written informed consent was obtained within 24 h after initiation of caspofungin treatment. Caspofungin was administered according to its summary of product characteristics (12, 13). The first day of therapy, patients were given a loading dose of 70 mg. The daily maintenance dose was 50 mg or 70 mg in patients with body weights of >80 kg. In patients with hepatic dysfunction classified as a Child-Pugh score of 7 to 9, class B, a reduced maintenance dose of 35 mg is recommended. Dose deviations and length of treatment were at the discretion of the infectious disease consultant at each ICU. Caspofungin was administered by intravenous infusion over 1 h. PK sampling was performed on days 2, 5, and 10. If treatment with caspofungin was terminated before day 10, no further sampling was performed. PK samples were taken 1, 12, and 24 h after the start of infusion (14). Blood was collected in citrate-containing tubes and immediately centrifuged for 10 min at 3,000 rpm. Plasma was transferred to polypropylene storage tubes and stored at −80°C until analysis.
Data collection.
The following data were registered for each patient: age, gender, body weight, body mass index (BMI), medical history, comedication, indication of ICU admission, acute physiology and chronic health evaluation (APACHE) II score (33) on ICU admission, CRRT, mechanical ventilation, ECMO, and SOFA score (34). The components of the C-P score, plasma albumin, bilirubin, and INR, were registered on the days of PK sampling. Due to difficulties with assessment during intensive care, liver encephalopathy and ascites were given a score of 1 when there were no clinical signs of liver-induced encephalopathy or ascites before admittance to the ICU. Alanine aminotransferase, alkaline phosphatase, and creatinine were registered on the days of PK sampling.
Analytical method.
Total plasma concentrations of caspofungin were determined using a fully validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method, with a linear range of quantification between 0.035 and 23.1 mg/liter (see Information Supplement S1 in the supplemental material for details). Routine methods at the hospital laboratory were employed for plasma bilirubin, albumin, INR, other liver function tests, and creatinine level. The equation for modification of diet in renal disease was used for calculation of the glomerular filtration rate (35).
Pharmacokinetic and statistical analyses.
Individual PK parameters were derived based on caspofungin dosing history, observed caspofungin concentrations, and a previously described population PK model for adult ICU patients (36). The model was implemented in NONMEM (37), (ICON Development Solutions, Ellicott City, MD) and applied to obtain empirical Bayes estimates (EBEs) and associated shrinkage (38). Potential time-dependent differences in PK were investigated by comparing EBEs obtained when using all data and with the EBEs derived when each day of sampling was treated separately (see Supplemental Information S2 for details). PK parameters of interest were AUC0–24, CL, V1, peripheral volume of distribution (V2), and k10, reflecting the fraction eliminated from V1 at equilibrium between V1 and V2 (Fig. 5). Primary analyses were the correlation between the C-P score and the PK parameters. Secondary analyses were the correlations between each individual components of the C-P score and the SOFA score, respectively, with the PK parameters. Caspofungin PK and liver function were assumed to be more stable on day 5; therefore, correlations were performed on the day 5 results. Spearman rank correlation was used, since C-P score, bilirubin, albumin, INR, and several of the PK parameters were not normally distributed. A sample size of 46 patients was required based on an explanatory power of ≥10%, an α-error of 0.05, and a dropout of 20% to day 5.
FIG 5.
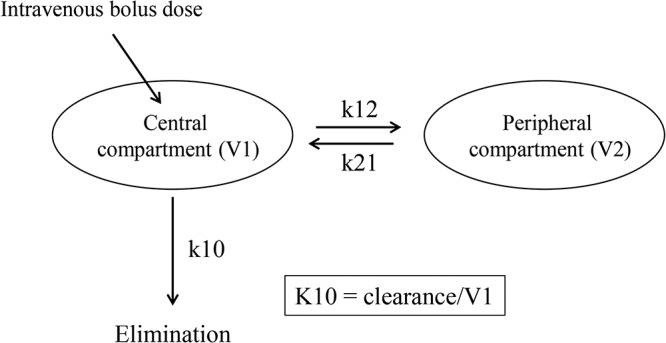
Pharmacokinetic two-compartment model. The central compartment (V1) represents blood and well-perfused organs, and the peripheral compartment (V2) represents more poorly perfused tissues. k12 and k21 are rate constants between V1 and V2, and k10 is the elimination rate constant from V1. k10 represents the fraction of caspofungin eliminated per hour from V1 at equilibrium between V1 and V2.
To date, no AUC0–24/MIC ratio target has been established in humans. In animal models, the pharmacodynamic effect of echinocandins correlated best with the AUC0–24 divided by the MIC (39). In murine neutropenic infection models, AUC0–24/MIC ratios of 865 and 450 have been associated with a 1-log kill after 24 h of C. albicans and C. glabrata infection, respectively (32). In the absence of human data, these AUC0–24/MIC ratios were employed as the PK/PD target. The MIC distributions are in accordance with Pfaller et al. (21).
Data are presented as medians and interquartile ranges, unless otherwise stated. Statistica (v. 12; Statsoft, Tulsa, OK, USA) was used in the statistical calculations.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the staff of the participating intensive care units for their contribution to this study. We thank Lisa Kurland for critical reading of the manuscript.
This work was funded by the Olinder-Nielsen Family Fund for Research in Infectious Diseases (S.K.) and R&D funds of the Uppsala County Council. E.E. has a research grant from the Stockholm County Council (ALF20160331). J.S. has received lecture fees from Pfizer, Gilead, UniMedic Pharma, and MSD and reimbursement for expert testemony from Astellas and UniMedic Pharma. M.F. has received lecture fees from Pfizer, Gilead, and Unimedic Pharma AB.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02466-18.
REFERENCES
- 1.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, EPIC Group of Investigators. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 2.Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 4.Labelle AJ, Micek ST, Roubinian N, Kollef MH. 2008. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 36:2967–2972. doi: 10.1097/CCM.0b013e31818b3477. [DOI] [PubMed] [Google Scholar]
- 5.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. 2012. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 54:1739–1746. doi: 10.1093/cid/cis305. [DOI] [PubMed] [Google Scholar]
- 6.Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Smietana J, Lupinacci R, Sable C, Kartsonis N, Perfect J. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 347:2020–2029. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 7.Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, Lass-Florl C, Calandra T, Viscoli C, Herbrecht R. 2017. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 102:433–444. doi: 10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone JA, Xu X, Winchell GA, Deutsch PJ, Pearson PG, Migoya EM, Mistry GC, Xi L, Miller A, Sandhu P, Singh R, deLuna F, Dilzer SC, Lasseter KC. 2004. Disposition of caspofungin: role of distribution in determining pharmacokinetics in plasma. Antimicrob Agents Chemother 48:815–823. doi: 10.1128/AAC.48.3.815-823.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mistry GC, Migoya E, Deutsch PJ, Winchell G, Hesney M, Li S, Bi S, Dilzer S, Lasseter KC, Stone JA. 2007. Single- and multiple-dose administration of caspofungin in patients with hepatic insufficiency: implications for safety and dosing recommendations. J Clin Pharmacol 47:951–961. doi: 10.1177/0091270007303764. [DOI] [PubMed] [Google Scholar]
- 11.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. 1973. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency. 2017. Cancidas: summary of product characteristics. European Medicines Agency, Amsterdam, the Netherlands. [Google Scholar]
- 13.Food and Drug Administration. 2018. Caspofungin acetate–advisory committee meeting background. U.S. Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 14.Sinnollareddy MG, Roberts JA, Lipman J, Akova M, Bassetti M, De Waele JJ, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Dimopoulos G. 2015. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: data from multinational Defining Antibiotic Levels in Intensive care unit (DALI) patients Study. Crit Care 19:33. doi: 10.1186/s13054-015-0758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muilwijk EW, Schouten JA, van Leeuwen HJ, van Zanten ARH, de Lange DW, Colbers A, Verweij PE, Burger DM, Pickkers P, Brüggemann RJM. 2014. Pharmacokinetics of caspofungin in ICU patients. J Antimicrob Chemother 69:3294–3299. doi: 10.1093/jac/dku313. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TH, Hoppe-Tichy T, Geiss HK, Rastall AC, Swoboda S, Schmidt J, Weigand MA. 2007. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J Antimicrob Chemother 60:100–106. doi: 10.1093/jac/dkm125. [DOI] [PubMed] [Google Scholar]
- 17.van Wanrooy MJ, Rodgers MG, Uges DR, Arends JP, Zijlstra JG, van der Werf TS, Kosterink JG, Alffenaar JW. 2014. Low but sufficient anidulafungin exposure in critically ill patients. Antimicrob Agents Chemother 58:304–308. doi: 10.1128/AAC.01607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Elst KC, Veringa A, Zijlstra JG, Beishuizen A, Klont R, Brummelhuis-Visser P, Uges DR, Touw DJ, Kosterink JG, van der Werf TS, Alffenaar JC. 2017. Low caspofungin exposure in patients in intensive care units. Antimicrob Agents Chemother 61:e01582-16. doi: 10.1128/AAC.01582-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Medicines Agency. 2005. Cancidas: EPAR–scientific discussion. European Medicines Agency, Amsterdam, the Netherlands. [Google Scholar]
- 20.Spriet I, Annaert P, Meersseman P, Hermans G, Meersseman W, Verbesselt R, Willems L. 2009. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J Antimicrob Chemother 63:767–770. doi: 10.1093/jac/dkp026. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 2013. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol 51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ericsson J, Chryssanthou E, Klingspor L, Johansson AG, Ljungman P, Svensson E, Sjolin J. 2013. Candidaemia in Sweden: a nationwide prospective observational survey. Clin Microbiol Infect 19:E218–E221. doi: 10.1111/1469-0691.12111. [DOI] [PubMed] [Google Scholar]
- 23.Pappas PG, Rotstein CM, Betts RF, Nucci M, Talwar D, De Waele JJ, Vazquez JA, Dupont BF, Horn DL, Ostrosky-Zeichner L, Reboli AC, Suh B, Digumarti R, Wu C, Kovanda LL, Arnold LJ, Buell DN. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 45:883–893. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 24.Andes D, van Ogtrop M. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob Agents Chemother 43:2116–2120. doi: 10.1128/AAC.43.9.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EUCAST-AFST. 2008. EUCAST technical note on fluconazole. Clin Microbiol Infect 14:193–195. doi: 10.1111/j.1469-0691.2007.01899.x. [DOI] [PubMed] [Google Scholar]
- 26.Betts RF, Nucci M, Talwar D, Gareca M, Queiroz-Telles F, Bedimo RJ, Herbrecht R, Ruiz-Palacios G, Young JA, Baddley JW, Strohmaier KM, Tucker KA, Taylor AF, Kartsonis NA. 2009. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis 48:1676–1684. doi: 10.1086/598933. [DOI] [PubMed] [Google Scholar]
- 27.Cornely OA, Vehreschild JJ, Vehreschild MJ, Wurthwein G, Arenz D, Schwartz S, Heussel CP, Silling G, Mahne M, Franklin J, Harnischmacher U, Wilkens A, Farowski F, Karthaus M, Lehrnbecher T, Ullmann AJ, Hallek M, Groll AH. 2011. Phase II dose escalation study of caspofungin for invasive aspergillosis. Antimicrob Agents Chemother 55:5798–5803. doi: 10.1128/AAC.05134-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens DA, Espiritu M, Parmar R. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother 48:3407–3411. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. 2007. Paradoxical effect of Echinocandins across Candida species in vitro: evidence for echinocandin-specific and candida species-related differences. Antimicrob Agents Chemother 51:2257–2259. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleischhacker M, Radecke C, Schulz B, Ruhnke M. 2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur J Clin Microbiol Infect Dis 27:127–131. doi: 10.1007/s10096-007-0411-4. [DOI] [PubMed] [Google Scholar]
- 31.Rueda C, Puig-Asensio M, Guinea J, Almirante B, Cuenca-Estrella M, Zaragoza O, Padilla B, Muñoz P, Guinea J, Paño Pardo JR, García-Rodríguez J, García Cerrada C, Fortún J, Martín P, Gómez E, Ryan P, Campelo C, de los Santos Gil I, Buendía V, Gorricho BP, Alonso M, Sanz FS, Aguado JM, Merino P, González Romo F, Gorgolas M, Gadea I, Losa JE, Delgado-Iribarren A, Ramos A, Romero Y, Sánchez Romero I, Zaragoza O, Cuenca-Estrella M, Rodriguez-Baño J, Isabel Suarez A, Loza A, Aller García AI, Martín-Mazuelos E, Pérez de Pipaón MR, Garnacho J, Ortiz C, Chávez M, Maroto FL, Salavert M, Pemán J, Blanquer J, Navarro D, Camarena JJ, Zaragoza R, Abril V, Gimeno C, Hernáez S, Ezpeleta G, Bereciartua E, Hernández Almaraz JL, Montejo M, Rivas RA, Ayarza R, Planes AM, Camps IR, Almirante B, Mensa J, Almela M, Gurgui M, Sánchez-Reus F, Martinez-Montauti J, Sierra M, Horcajada JP, Sorli L, Gómez J, Gené A, Urrea M, Valerio M, Díaz-Martín A, Puchades F, Mularoni A. 2017. Evaluation of the possible influence of trailing and paradoxical effects on the clinical outcome of patients with candidemia. Clin Microbiol Infect 23:49.e1–49.e8. doi: 10.1016/j.cmi.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob Agents Chemother 54:2497–2506. doi: 10.1128/AAC.01584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. 2007. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 36.Martial LC, Bruggemann RJ, Schouten JA, van Leeuwen HJ, van Zanten AR, de Lange DW, Muilwijk EW, Verweij PE, Burger DM, Aarnoutse RE, Pickkers P, Dorlo TP. 2016. Dose Reduction of caspofungin in intensive care unit patients with Child Pugh B will result in suboptimal exposure. Clin Pharmacokinet 55:723–733. doi: 10.1007/s40262-015-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beal SL, Boeckmann AJ, Bauer RJ (ed). 2013. NONMEM 7.3.0 users guide. ICON Development Solutions, Hanover, MD. [Google Scholar]
- 38.Savic RM, Karlsson MO. 2009. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J 11:558–569. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louie A, Deziel M, Liu W, Drusano MF, Gumbo T, Drusano GL. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob Agents Chemother 49:5058–5068. doi: 10.1128/AAC.49.12.5058-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.