In vitro pharmacokinetic/pharmacodynamic data of liposomal amphotericin B (L-AMB) were compared with animal data from neutropenic and nonneutropenic models of azole-susceptible and azole-resistant invasive aspergillosis. L-AMB was equally effective.
KEYWORDS: Aspergillus fumigatus, azole resistance, dose optimization, liposomal amphotericin B, neutropenia
ABSTRACT
In vitro pharmacokinetic/pharmacodynamic data of liposomal amphotericin B (L-AMB) were compared with animal data from neutropenic and nonneutropenic models of azole-susceptible and azole-resistant invasive aspergillosis. L-AMB was equally effective. The in vitro fCmax (maximum concentration of free drug)/MIC ratio associated with 50% of maximal activity was 0.31 (0.29 to 0.33), similar to that in neutropenic but not nonneutropenic mice (0.11 [0.06 to 0.20]). Simulation analysis indicated that standard L-AMB doses (1 to 3 mg/kg) are adequate for nonneutropenic patients, but higher doses (7.5 to 10 mg/kg) may be required for neutropenic patients for Aspergillus fumigatus isolates with MICs of 0.5 to 1 mg/liter.
INTRODUCTION
The pharmacodynamics (PD) of liposomal amphotericin B (L-AMB) remain relatively poorly understood because of complex pharmacokinetics (PK) that impede the in-depth comprehension of its exposure-response relationship (1). Although high L-AMB doses up to 15 mg/kg have been used (2), in the absence of a clinical dose-response relationship a dose of 3 mg/kg is generally recommended for the treatment of invasive aspergillosis (IA) (1), with an end-of-treatment favorable response of ∼40% for probable/proven cases (3). However, neutropenia may affect the clinical response to L-AMB therapy (4). We therefore studied L-AMB PD in an in vitro PK/PD model using previously published data of experimental aspergillosis in neutropenic and nonneutropenic animal models and optimized L-AMB therapy simulating human serum concentration-time profiles against azole-susceptible and azole-resistant Aspergillus fumigatus isolates in neutropenic and nonneutropenic patients.
Two clinical A. fumigatus isolates, a wild-type strain (AZN8196) and an azole-resistant strain harboring the TR34/L98H cyp51A mutation (V52-35) (5), with voriconazole/AMB CLSI MICs of 0.125/0.25 and 2/0.25 mg/liter, respectively, were studied (6). The MIC of L-AMB was 0.125 mg/liter for both isolates (6). A previously optimized two-compartment dialysis/diffusion closed PK/PD model was used (7, 8). L-AMB was injected in both compartments of the model every 24 h, while the external compartment was covered with aluminum foil to minimize light exposure and placed on a heated (37°C) magnetic stirrer. Drug levels were determined using a microbiological agar diffusion assay as previously described (8). For the preparation of calibration standard samples, stock solution of L-AMB was diluted 1:1 with methanol, heated for 10 min at 65°C to disrupt the liposomes, and then diluted further in RPMI medium (serial 2-fold dilutions) to obtain final concentrations of 8 to 0.015 mg/liter AMB (9). Internal-compartment (IC) samples were treated similarly. Plates were incubated for 24 h, and the diameter of the partial-growth (80%) inhibition zone (fine growth was ignored) was measured because this endpoint gave the largest analytical sensitivity. Because of nonlinearity between inhibition zones and L-AMB concentrations of >1 mg/liter, IC samples with expected drug levels of >1 mg/liter were first diluted so that the measured concentration would be in the linearity range of the assay. L-AMB was used for the drug assay to quantify all forms of AMB released from L-AMB in the IC (e.g., on contact with fungi, natural release during incubation, preexisting AMB in the vial of clinical formulation). However, inhibition zones of pure AMB using a 100% inhibition endpoint were the same as those of L-AMB using an 80% inhibition endpoint, although lower concentrations were detected in the L-AMB bioassay. The area under the galactomannan index (GI)-time curve (AUCGI) was determined as a surrogate marker of fungal growth as previously described (10). All experiments were carried out in duplicate and were independently performed on two different days.
The in vitro PK/PD model was evaluated using the previously published in vivo results of nonneutropenic and neutropenic murine models of disseminated aspergillosis (11, 12), where mice were infected with the two A. fumigatus strains used in the present study and treated intravenously with seven 4-fold-increasing L-AMB doses of 0.004 to 16 mg/kg once daily for 14 days. Based on previous PK studies in neutropenic CD-1 mice, intravenous 10 and 3 mg/kg L-AMB resulted in peak serum total concentrations (tCmaxs) of 47.1 and 15.7 mg/liter, respectively, indicating a 1:5 dose:tCmax ratio for this dose range (9). The same ratio was used for nonneutropenic mice because neutropenia does not affect L-AMB PK (13). Thus, 4-fold-increasing L-AMB tCmaxs of 0.02 to 80 mg/liter were targeted in the in vitro PK/PD model simulating the biphasic time-concentration profile observed in mouse serum (9, 14). The in vitro exposure-response relationship (percentage growth inhibition versus fCmax [maximum concentration of free drug]/MIC) after 72 h of incubation was compared with the in vivo exposure-response curve (percentage survival versus fCmax/MIC) after 14 days of treatment using the extra sum-of-squares F test. Survival data were normalized to span from 0% survival in drug-free controls to 100%. The in vivo fCmax was calculated on the basis of the unbound fraction (fu) of AMB in human serum (15). Total AMB in serum of L-AMB-treated subjects was largely liposome associated in steady state, and the percentage of protein binding (PB) of nonliposomal AMB was concentration dependent, following the equation PB = 95.3% − (99.5% − 95.3%) × (1 − e0.11 · total AMB) with a maximum fu the limit of AMB water solubility of ∼0.8 mg/liter (15). Data were analyzed using nonlinear regression analysis based on the sigmoidal Emax (maximum effect) model with variable slope described previously (7, 8) with GraphPad Prism 7.0 (GraphPad Software, San Diego, CA). Exposure indices associated with 20% (EI20), 50% (EI50), and 80% (EI80) of maximal activity were also estimated.
To bridge the in vitro data with human PK, Monte Carlo simulation analysis was performed using the Normal random number generator function of Excel spreadsheet (MS Office 2007) for 5.000 patients treated with the standard intravenous dosage of 3 mg/kg of L-AMB once daily. In particular, this dosage resulted in a steady-state mean ± standard deviation (SD) tCmax in human plasma of 21.87 ± 12.47 mg/liter, which corresponds to an fCmax of 0.19 ± 0.11 mg/liter based on concentration-dependent fu of L-AMB in human serum estimated as described above (15, 16). The probability of target attainment (PTA) was calculated for isolates with AMB MICs of 0.03 to 8 mg/liter. In order to find which are the most clinically relevant EIs, the cumulative fractional responses (CFRs) were estimated for a previously published AMB MIC distribution of clinical A. fumigatus isolates, with an MIC range of 0.03 to 8 mg/liter and 70% of isolates having MICs of 0.5 to 1 mg/liter (17), and for the standard L-AMB dose of 3 mg/kg previously used for the primary therapy of probable/proven IA in a randomized trial (3). The PTA using the best EI was then calculated for A. fumigatus isolates, with CLSI AMB MICs ranging from 0.008 to 8 mg/liter and for L-AMB doses of 1, 3, 5, 7.5, and 10 mg/kg with steady-state tCmax ± SD (fu; fCmax ± SD) of ∼10.94 ± 6.24 (1.7%; 0.19 ± 0.11), 21.87 ± 12.47 (0.86%; 0.19 ± 0.11), ∼43.74 ± 24.94 (0.53%; 0.23 ± 13, assuming close to linear PK for this dose range), 115.1 ± 104.9 (0.5%; 0.56 ± 0.52), and 164.7 ± 119.7 (0.5%; 0.82 ± 0.60) mg/liter, respectively (2, 18). To account for adverse events (nephrotoxicity and hypokalemia) that may cause discontinuation of L-AMB therapy and worsen clinical outcome, adjusted PTA (adjPTA) and CFR (adjCFR) were calculated as PTA − PTA × % patients with adverse events and CFR − CFR × % patients with adverse events, respectively, where the percentage of adverse events was ∼2%, 15%, 20%, 25%, and 30% for 1, 3, 5, 7.5, and 10 mg/kg, respectively (2, 4, 19). In addition, the percentage of patients with fCmax higher than the plasma solubility of AMB (∼0.8 mg/liter) (15), indicating unnecessary exposure, was calculated for each L-AMB dose.
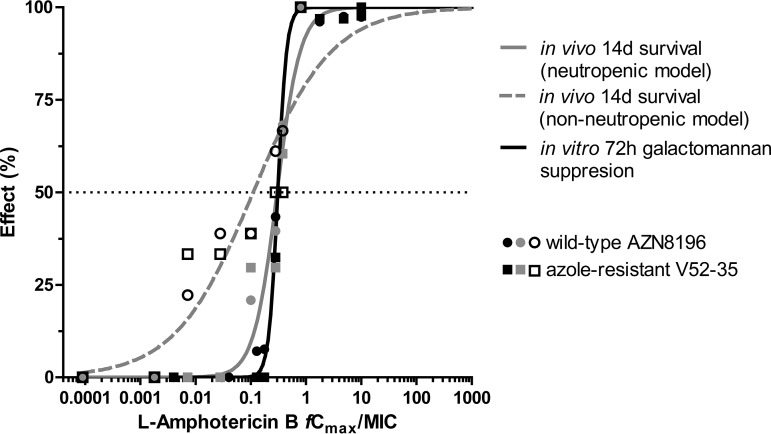
A biphasic time-concentration profile of L-AMB was simulated in the in vitro model attaining the target Cmaxs and half-life at α phase (t1/2,α, ∼2 h) but longer t1/2,β (>24 h). After 72 h of incubation, galactomannan production was completely suppressed in both isolates by L-AMB regimens of fCmaxs ≥0.32 mg/liter. The in vitro PK/PD relationship for the two A. fumigatus isolates followed a sigmoidal pattern (R2 = 0.99), with mean (95% confidence interval [CI]) EI20, EI50, and EI80 of 0.24 (0.22 to 0.26), 0.31 (0.29 to 0.33), and 0.40 (0.34 to 0.46) fCmax/MIC and 2.5 (2.2 to 2.8), 3.6 (3.3 to 4), and 5.3 (4.1 to 6.7) fAUC0-24/MIC, respectively. The in vitro exposure-galactomannan suppression relationship was similar to the in vivo exposure-survival relationship of the neutropenic animal model (R2 ≥ 0.96) (Fig. 1). The in vitro EI50 and slope (95% CI) were 0.31 (0.29 to 0.33) and 5.4 (3.03 to 7.80), similar to the in vivo EC50 and slope of 0.33 (0.25 to 0.36) and 2.06 (1.11 to 3.09), respectively (extra sum-of-squares F test, P = 0.77). No differences were found in the exposure-effect relationship between the two A. fumigatus isolates. The in vivo exposure-survival relationship in the nonneutropenic animal model followed a sigmoidal pattern (R2 = 0.85), with means (95% CI) of EI20, EI50, EI80, and slope of 0.011 (0.003 to 0.038), 0.11 (0.06 to 0.20), 1.06 (0.35 to 3.24), and 0.61 (0.35 to 0.88), respectively (Fig. 1).
FIG 1.
In vitro and in vivo PK/PD relationship based on galactomannan suppression and survival rate of experimental nonneutropenic (12) and neutropenic (11) murine models of aspergillosis. In vitro PK/PD relationship was similar to in vivo PK/PD relationship in neutropenic animal model.
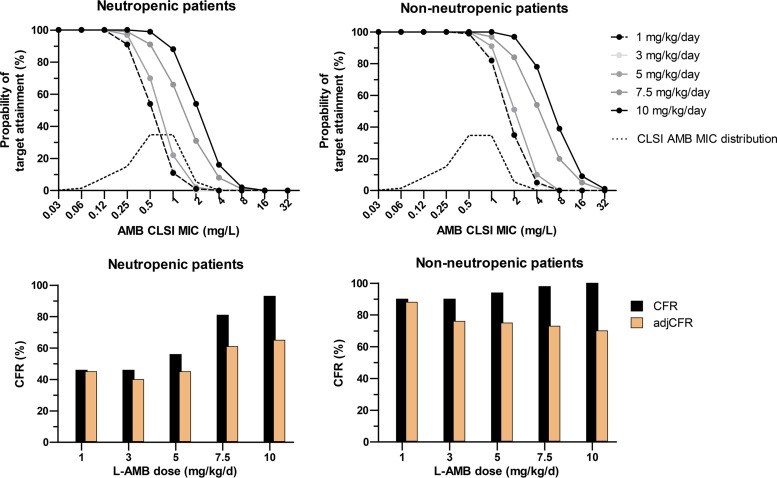
Based on Monte Carlo simulation analysis, the CFRs and adjCFRs for EI80, EI50, and EI20 were 34%, 46%, and 59% and 29%, 39%, and 50%, respectively, using the in vitro PK/PD targets that correlated with the PK/PD targets in neutropenic mice. Thus, the CFR and adjCFR for EI50 were better correlated with the 40% favorable response at the end of treatment (2 weeks) of (mostly neutropenic) patients with proven/probable cases of IA treated with 3 mg/kg of L-AMB in a large randomized clinical trial (3). The proportions of neutropenic and nonneutropenic patients attaining the corresponding EI50 targets of 0.31 and 0.11 fCmax/MIC, respectively, are shown in Fig. 2. High PTAs (>80%) were found for 1 and 3 mg/kg for isolates with MICs up to 0.25 mg/liter in neutropenic patients and 1 mg/liter in nonneutropenic patients (Fig. 2). The PTAs increased slightly (<16%) with the 5-mg/kg dose for both groups of patients. In neutropenic patients, high CFRs (>81%) were found for isolates with MICs of 0.5 and 1 mg/liter, with 7.5 and 10 mg/kg, respectively. However, because of 20% and 30% adverse events with those doses, respectively, the adjCFR reached only 60%, whereas 20% to 40% of patients will be exposed to doses that cannot increase the fu of AMB because of the water-solubility limit of 0.8 mg/liter (15). In nonneutropenic patients, the CFR was high (90% to 100%) for all L-AMB doses, including 1 mg/kg, for which the highest adjCFR (88%) was found compared with those at higher doses (70% to 76%).
FIG 2.
Probabilities of target attainment of liposomal amphotericin B (L-AMB) monotherapy for A. fumigatus isolates with different CLSI AMB MICs and different L-AMB doses in simulated neutropenic and nonneutropenic patients (top). Cumulated fractional response (CFR) adjusted based on adverse events (adjCFR) in simulated neutropenic and nonneutropenic patients (bottom).
Although the in vitro model was validated using animal data from a model of disseminated aspergillosis rather than a model of pulmonary aspergillosis, which is the most frequent clinical entity, previous studies have shown that the efficacy of L-AMB is similar in these two models, reaching maximal efficacy at ≥10 mg/kg daily (20, 21). The in vitro exposure-response relationship was very similar to the in vivo exposure-response relationship in neutropenic mice (Fig. 2), validating the link between in vitro L-AMB concentrations measured with the bioassay and the in vivo free concentration-dependent fu of AMB calculated based on the total (mainly liposomal in steady state) AMB in serum. However, more complex PK phenomena (dose-dependent tissue distribution, concentration-dependent tissue binding, time-dependent tissue accumulation, equilibrium among the different AMB forms in interstitial fluid) cannot be excluded.
In vivo, AMB is present as liposome-associated, protein-bound, and free drug, and it is believed that liposome-associated AMB serves as a pool of the other forms of AMB but can also exert antifungal activity by delivering AMB directly to fungal cell membranes (1). The biologically active drug is the free drug together with the AMB released on L-AMB contact with fungi, and this might have been the AMB concentration that was measured with bioassay and exerted its antifungal activity in the in vitro model. This may explain the 2-fold-lower MICs of L-AMB compared with MICs of AMB of the isolates in the present study. However, because of spatial and diffusion restrictions in vivo, the free fu of AMB that diffuses from blood and is released from tissue L-AMB to interstitial fluid is probably exerting the most direct antifungal activity (15). L-AMB in tissues may serve as delivery system that sequesters AMB in interstitial fluid rather than directly into cell membrane of fungi. Indeed immunohistochemistry staining showed that L-AMB was found locally at the sites of infection in the brain but not in close proximity to fungal hyphae (22). In addition, peak L-AMB concentrations in tissue are usually only 10% of serum concentrations in mouse lung and kidney after i.p. treatment, although accumulation does occur, with tissue trough levels being twice the serum trough levels after 5 days of i.v. treatment (9, 14). Tissue binding of L-AMB in mouse lung, kidney, and liver was estimated to be 1% to 10% (14). In human lungs, autopsy studies showed similar levels (16 h after the last dose on day 10) (23) compared with previously reported serum trough concentrations with the standard dose (16). However, in those studies, tissue homogenates were used where blood and tissue compartments were mixed. Although the bioavailable AMB concentration in tissues (i.e., fu and liposome-associated AMB that reach the fungus in interstitial space) is not known, the low PK/PD index found in the present study and the low response rates found in clinical trials and animal models with the standard dose cannot be explained by the high total or liposome-associated AMB concentration in blood or tissues. Therefore, free AMB is pharmacodynamically more important than total L-AMB concentration, particularly in deep-tissue fungal infections like invasive aspergillosis.
The in vitro PK/PD index for L-AMB is higher than the corresponding index for conventional AMB (C-AMB) previously found in the same model (0.31 versus 0.15) (24), indicating that higher L-AMB than C-AMB concentrations are required for the same effect. A similar observation was found in an in vitro static model of human alveoli and in animal models, indicating that some of the active compound is locked in the liposome, rendering it inert (25, 26). Interestingly, the PK/PD index of L-AMB determined in the present study (tCmax/MIC, 31, taking into account the ∼1% fu) was similar to the PK/PD index determined in the static model (tCmax/MIC, 31.04) in the alveolar compartment where conidia were inoculated and drug diffused into it from the endovascular compartment via the endothelial/alveolar cell lines. This further supports our hypothesis that the in vitro model assesses the PD of bioactive diffusible AMB. C-AMB reaches higher free-drug AMB concentrations than L-AMB on a milligram-per-kilogram basis, but its dose-limited nephrotoxicity does not allow for increasing C-AMB doses >1 mg/kg, whereas up to 15 mg/kg of L-AMB has been used with significant toxicity (>20%), observed at doses of >5 mg/kg (2).
L-AMB was equally active against azole-resistant isolates, which align with results in animal models (11) and clinical recommendations for treating azole-resistant aspergillosis (27). Monte Carlo analysis in the present study showed that 1- and 3-mg/kg doses provide similar PTAs. With the caveat of nonlinear L-AMB PK, despite the 2-fold-higher tCmax attained with 3- versus 1-mg/kg doses (22 versus 11 mg/liter, respectively), the unbound AMB value was similar (∼0.19 mg/liter) because of the different protein binding (99.14% versus 98.3%, respectively). Indeed, in one of the first clinical trials of L-AMB against IA, the 1-mg/kg L-AMB dose was equally effective to a 4-mg/kg L-AMB dose (clinical response, 64% versus 48%; radiological response, 58 versus 54%, respectively) (4). In that study, the low-dose arm had shorter neutrophil recovery time (14 versus 24 days, respectively) and lower renal toxicity (1% versus 11%, respectively), which might influence clinical outcome. Based on the Monte Carlo analysis of the present study using the nonneutropenic PK/PD target, the adjCFR was higher for the 1-mg/kg dose than for other doses. In neutropenic patients, higher doses up to 10 mg/kg could attain the PD target for almost all wild-type isolates.
These findings are in line with those of animal experiments, where the efficacy of L-AMB was suboptimal in neutropenic mice with ∼30% 14-day survival at the standard dose of 3 mg/kg (21) and ∼60% at 10 to 15 mg/kg (28), whereas 1 to 4 mg/kg L-AMB in nonneutropenic mice resulted in 80% survival against isolates with MICs of 0.5 to 1 mg/liter (29). However, high L-AMB doses are associated with a high percentage of adverse events that usually worsen clinical outcome and can result in drug discontinuation. When this toxicity was taken into account, the adjCFR increased only slightly at high doses (up to 60% to 65%, as in animal models). Indeed, clinical trials with high doses (≥4 mg/kg) failed to show superiority over lower doses (≤3 mg/kg) (3, 4). Given the large PK variation (60% with the standard dose [16] and >76% with higher doses [2]) and the narrow therapeutic index, high L-AMB doses may be of benefit for only a subset of neutropenic patients with suboptimal concentrations (e.g., tCmax, <30, <15, and <7.5 mg/liter for isolates with MICs of 1, 0.5, and 0.25 mg/liter, respectively, based on the findings of the present study). Finally, given the water-solubility limit of AMB at 0.8 mg/liter (15), the PK/PD target of 0.31 fCmax/MIC could not be attained for isolates with MICs of ≥2 mg/liter, justifying the clinical susceptibility breakpoint for AMB and A. fumigatus. Higher L-AMB doses would not further increase free AMB because of the water-solubility limit, with 20% and 40% of patients treated with 7.5 and 10 mg/kg, respectively, attaining unnecessary drug exposures. Such exposure may be necessary for tissue compartments where L-AMB penetration is poor, e.g., in myocardium and brain (23), although animal models of central nervous system and pulmonary infections showed that high doses (>10 mg/kg) are not more effective (28, 30).
In conclusion, the results of the in vitro PK/PD model were comparable to the outcomes of L-AMB therapy in vivo in a neutropenic murine model of experimental aspergillosis and in patients with IA. Given the limited treatment options for azole-resistant IA, our results showed that L-AMB has a role in the management of azole-resistant A. fumigatus infections because its efficacy against the wild-type isolate and the isolate harboring the azole resistance mechanism was similar. Simulation analysis indicated that a lower than the standard L-AMB dose may be sufficient for nonneutropenic patients without difficult-to-treat infections when toxicity prohibits the use of the standard dose or a step-down dose-reduction approach on neutrophil recovery. However, a higher-than-standard dose may be required, particularly in patients with profound and prolong neutropenia infected with isolates with AMB MICs of 0.5 to 1 mg/liter when low serum L-AMB levels are expected. This hypothesis warrants further clinical verification.
REFERENCES
- 1.Stone NRH, Bicanic T, Salim R, Hope W. 2016. Liposomal amphotericin B (AmBisome): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 76:485–500. doi: 10.1007/s40265-016-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh TJ, Goodman JL, Pappas P, Bekersky I, Buell DN, Roden M, Barrett J, Anaissie EJ. 2001. Safety, tolerance and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob Agents Chemother 45:3487–3496. doi: 10.1128/AAC.45.12.3487-3496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Dellow E, Herbrecht R, Donnelly JP. 2011. Efficacy outcomes in a randomised trial of liposomal amphotericin B based on revised EORTC/MSG 2008 definitions of invasive mould disease. Mycoses 54:e449–e455. doi: 10.1111/j.1439-0507.2010.01947.x. [DOI] [PubMed] [Google Scholar]
- 4.Ellis M, Spence D, de Pauw B, Meunier F, Marinus A, Collette L, Sylvester R, Meis J, Boogaerts M, Selleslag D, Krcmery V, von Sinner W, MacDonald P, Doyen C, Vandercam B. 1998. An EORTC international multicenter randomized trial (EORTC number 19923) comparing two dosages of liposomal amphotericin B for treatment of invasive aspergillosis. Clin Infect Dis 27:1406–1412. doi: 10.1086/515033. [DOI] [PubMed] [Google Scholar]
- 5.Mavridou E, Bruggemann RJM, Melchers WJG, Verweij PE, Mouton JW. 2010. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother 54:4758–4764. doi: 10.1128/AAC.00606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard—2nd ed. CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 7.Siopi M, Mavridou E, Mouton JW, Verweij PE, Zerva L, Meletiadis J. 2014. Susceptibility breakpoints and target values for therapeutic drug monitoring of voriconazole and Aspergillus fumigatus in an in vitro pharmacokinetic/pharmacodynamic model. J Antimicrob Chemother 69:1611–1619. doi: 10.1093/jac/dku023. [DOI] [PubMed] [Google Scholar]
- 8.Elefanti A, Mouton JW, Verweij PE, Zerva L, Meletiadis J. 2014. Susceptibility breakpoints for amphotericin B and Aspergillus species in an in vitro pharmacokinetic-pharmacodynamic model simulating free-drug concentrations in human serum. Antimicrob Agents Chemother 58:2356–2362. doi: 10.1128/AAC.02661-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang T, Olson JA, Proffitt RT, Adler-Moore JP. 2010. Differences in tissue drug concentrations following intravenous versus intraperitoneal treatment with amphotericin B deoxycholate or liposomal amphotericin B. Med Mycol 48:430–435. doi: 10.3109/13693780903208249. [DOI] [PubMed] [Google Scholar]
- 10.Al-Saigh R, Elefanti A, Velegraki A, Zerva L, Meletiadis J. 2012. In vitro pharmacokinetic/pharmacodynamic modeling of voriconazole activity against Aspergillus species in a new in vitro dynamic model. Antimicrob Agents Chemother 56:5321–5327. doi: 10.1128/AAC.00549-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seyedmousavi S, Mouton JW, Melchers WJG, Verweij PE. 2017. In vivo efficacy of liposomal amphotericin B against wild-type and azole-resistant Aspergillus fumigatus isolates in two different immunosuppression models of invasive aspergillosis. Antimicrob Agents Chemother 61:e02479-16. doi: 10.1128/AAC.02479-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seyedmousavi S, Melchers WJG, Mouton JW, Verweij PE. 2013. Pharmacodynamics and dose-response relationships of liposomal amphotericin B against different azole-resistant Aspergillus fumigatus isolates in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother 57:1866–1871. doi: 10.1128/AAC.02226-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Etten EW, Otte-Lambillion M, van Vianen W, Kate MT, Bakker-Woudenberg AJ. 1995. Biodistribution of liposomal amphotericin B (AmBisome) and amphotericin B-desoxycholate (Fungizone) in uninfected immunocompetent mice and leucopenic mice infected with Candida albicans. J Antimicrob Chemother 35:509–519. doi: 10.1093/jac/35.4.509. [DOI] [PubMed] [Google Scholar]
- 14.Andes D, Safdar N, Marchillo K, Conklin R. 2006. Pharmacokinetic-pharmacodynamic comparison of amphotericin B (AMB) and two lipid-associated AMB preparations, liposomal AMB and AMB lipid complex, in murine candidiasis models. Antimicrob Agents Chemother 50:674–684. doi: 10.1128/AAC.50.2.674-684.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother 46:834–840. doi: 10.1128/AAC.46.3.834-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groll AH, Silling G, Young C, Schwerdtfeger R, Ostermann H, Heinz WJ, Gerss J, Kolve H, Lanvers-Kaminsky C, Vieira Pinheiro JP, Gammelin S, Cornely OA, Wuerthwein G. 2010. Randomized comparison of safety and pharmacokinetics of caspofungin, liposomal amphotericin B, and the combination of both in allogeneic hematopoietic stem cell recipients. Antimicrob Agents Chemother 54:4143–4149. doi: 10.1128/AAC.00425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinel-Ingroff A, Cuenca-Estrella M, Fothergill A, Fuller J, Ghannoum M, Johnson E, Pelaez T, Pfaller MA, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob Agents Chemother 55:5150–5154. doi: 10.1128/AAC.00686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hope WW, Goodwin J, Felton TW, Ellis M, Stevens DA. 2012. Population pharmacokinetics of conventional and intermittent dosing of liposomal amphotericin B in adults: a first critical step for rational design of innovative regimens. Antimicrob Agents Chemother 56:5303–5308. doi: 10.1128/AAC.00933-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, Heussel CP, Lortholary O, Rieger C, Boehme A, Aoun M, Horst H-A, Thiebaut A, Ruhnke M, Reichert D, Vianelli N, Krause SW, Olavarria E, Herbrecht R. 2007. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis 44:1289–1297. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 20.Olson JA, George A, Constable D, Smith P, Proffitt RT, Adler-Moore JP. 2010. Liposomal amphotericin B and echinocandins as monotherapy or sequential or concomitant therapy in murine disseminated and pulmonary Aspergillus fumigatus infections. Antimicrob Agents Chemother 54:3884–3894. doi: 10.1128/AAC.01554-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barchiesi F, Santinelli A, Biscotti T, Greganti G, Giannini D, Manso E. 2016. Delay of antifungal therapy influences the outcome of invasive aspergillosis in experimental models of infection. J Antimicrob Chemother 71:2230–2233. doi: 10.1093/jac/dkw111. [DOI] [PubMed] [Google Scholar]
- 22.Clemons KV, Schwartz JA, Stevens DA. 2012. Experimental central nervous system aspergillosis therapy: efficacy, drug levels and localization, immunohistopathology, and toxicity. Antimicrob Agents Chemother 56:4439–4449. doi: 10.1128/AAC.06015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogelsinger H, Weiler S, Djanani A, Kountchev J, Bellmann-Weiler R, Wiedermann CJ, Bellmann R. 2006. Amphotericin B tissue distribution in autopsy material after treatment with liposomal amphotericin B and amphotericin B colloidal dispersion. J Antimicrob Chemother 57:1153–1160. doi: 10.1093/jac/dkl141. [DOI] [PubMed] [Google Scholar]
- 24.Siopi M, Siafakas N, Vourli S, Zerva L, Meletiadis J. 2015. Optimization of polyene-azole combination therapy against aspergillosis using an in vitro pharmacokinetic-pharmacodynamic model. Antimicrob Agents Chemother 59:3973–3983. doi: 10.1128/AAC.05035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Nakeeb Z, Petraitis V, Goodwin J, Petraitiene R, Walsh TJ, Hope WW. 2015. Pharmacodynamics of amphotericin B deoxycholate, amphotericin B lipid complex, and liposomal amphotericin B against Aspergillus fumigatus. Antimicrob Agents Chemother 59:2735–2745. doi: 10.1128/AAC.04723-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lestner JM, Howard SJ, Goodwin J, Gregson L, Majithiya J, Walsh TJ, Jensen GM, Hope WW. 2010. Pharmacokinetics and pharmacodynamics of amphotericin B deoxycholate, liposomal amphotericin B, and amphotericin B lipid complex in an in vitro model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 54:3432–3441. doi: 10.1128/AAC.01586-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Flörl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Brüggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Löffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinkó J, Skiada A, Vehreschild M, Viscoli C, Cornely OA. 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24:e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Olson JA, Schwartz JA, Hahka D, Nguyen N, Bunch T, Jensen GM, Adler-Moore JP. 2015. Toxicity and efficacy differences between liposomal amphotericin B formulations in uninfected and Aspergillus fumigatus infected mice. Med Mycol 53:107–118. doi: 10.1093/mmy/myu070. [DOI] [PubMed] [Google Scholar]
- 29.Mouton JW, Te Dorsthorst DT, Meis JF, Verweij PE. 2009. Dose-response relationships of three amphotericin B formulations in a non-neutropenic murine model of invasive aspergillosis. Med Mycol 47:802–807. doi: 10.3109/13693780802672644. [DOI] [PubMed] [Google Scholar]
- 30.Clemons KV, Espiritu M, Parmar R, Stevens DA. 2005. Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob Agents Chemother 49:4867–4875. doi: 10.1128/AAC.49.12.4867-4875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]