Abstract
Bovine mastitis (BM) presents a high incidence, being Staphylococcus aureus one of the major causative agents. Antibiotics comprise the most common therapeutic approach, but due to their indiscriminate use, high rates of increasingly resistant bacterial species have been markedly pointed out. Particularly, S. aureus possesses a pronounced ability to form biofilms, and therefore, are of pivotal interest due to its alarming pathogenicity. The present study investigates the antibacterial properties of Eucalyptus globulus methanol: water extracts, alone and in combination with Juglans regia, against S. aureus isolates from BM. All isolates and reference strain proved to be good biofilm producers after 24 h of bacterial growth. Individually, the studied plant extracts (PE) lead to a considerable biofilm cells reduction, but their combination revealed to be the most effective strategy. When tested in combination, both extracts led to a 3 and 5 log reduction for S. aureus ATCC 25923 and S. aureus 1, respectively. Based on these findings, both PE seem to be promissory antimicrobial agents for upcoming use on dairy industry contaminations, BM and even S. aureus-triggered food poisoning. Further studies are needed to understand which of the compounds present in the extracts are responsible for the observed effects, including their corresponding modes of action.
Keywords: Microbiology
1. Introduction
Staphylococcus aureus is among the most common etiological agents of BM in dairy cattle. Its zoonotic ability is an important public health concern, being even given a pivotal attention by dairy industries due to their ability of being the causative agent of foodborne illness [1, 2]. Some authors showed that antibiotic-resistant S. aureus, associated with chronic mastitis, were detected in milk processing lines [1, 3].
Antimicrobials are the first-line approach to treat infectious disorders. Particularly in the case of BM, antibiotics are the most commonly used, despite their low efficacy rates when administered intramammarly [4, 5, 6, 7, 8], and consequent risk of rising pathogens’ resistance and hampering mastitis management [9]. In addition, the biofilm formation ability evidenced by some species, hinders the control of this pathology. Several authors have shown a strong biofilm formation capacity by several mastitis pathogens, such as S. aureus, coagulase negative Staphylococcus and Escherichia coli [10, 11, 12]. This ability is considered a protective mechanism [6, 13]; in fact, sessile cells confer greater protection, with microorganisms grown in this mode being 10 to 10.000 times more resistant to antimicrobials than non-embedded bacteria [6, 14, 15, 16]. More specifically, the production of slime and biofilms represents a serious problem, namely in the dairy chain, since bacteria incorporated in its own matrix, can survive the usual sanitation and cleaning process [2]. In this sense, and taking into account that the presence of biofilms in the dairy industry must be avoided due to the risk of milk contamination, food poisoning and even occurrence/recurrence of BM [17], the discovery of effective antimicrobial agents for control dairy industry pathogens is of paramount importance, namely those targeting microbial biofilms.
The antimicrobial potential of PE rich in phenolic compounds, among other bioactive phytochemicals, has been increasingly recognized [18]. In fact, there are several studies highlighting the antimicrobial activity of Eucalyptus globulus Labill. and Juglans regia L. against a wide range of potentially pathogenic microbes [19, 20, 21, 22, 23]. However, its effect on S. aureus biofilm cells from mastitis remains largely unstudied. Thus, based on the latest advances, the antibacterial activity of methanol: water extracts obtained from E. globulus (family Myrtaceae) and J. regia (family Juglandaceae) were individually and jointly investigated against one of the major pathogens in the dairy industry, S. aureus.
2. Material and methods
2.1. Plant material
Two different plants were used in this work: leaves of E. globulus Labill. (leaves) from commercial origin (Garray - Soria, Spain) and wild samples of J. regia L. harvested in Trás-os-Montes, Bragança, North-Eastern Portugal. Plant scientific nomenclature according to The Plant List (2013), version 1.1 (2013).
2.2. Standards and reagents
Analytical grade purity methanol was provided by Pronalab (Lisbon, Portugal). Tryptic Soy Broth (TSB) and Tryptic Soy Agar (TSA) were provided, respectively, by Liofilchem (Roseto degli Abruzzi, Italy) and Merck (Darmstadt, Germany), and prepared according to the manufacturer's instructions. Water was treated in a Milli-Q water purification system (TGI Pure Water Systems, Greenville, SC, USA).
2.3. Preparation of methanol:water extracts
Methanol: water extracts were obtained by extracting each sample (1 g) with 30 mL of methanol: water (80:20, v/v) at 25 °C and 150 rpm for 1 h, and then filtering through Whatman No. 4 paper. The final residue was again extracted with an additional 30 mL portion of the methanol:water mixture. The combined extracts were evaporated at 35 °C under reduced pressure (rotary evaporator Büchi R-210, Flawil, Switzerland) and then lyophilized (FreeZone 4.5, Labconco, Kansas City, MO, USA).
The lyophilized methanol:water extract was re-dissolved in water, performing stock solutions of 50 mg/mL, from which several dilutions were made.
2.4. Determination of adherence/biofilm formation
Seven S. aureus were selected to this work: reference strain (S. aureus ATCC 25923) and six BM isolates, kindly provided by the Portuguese lab “Segalab - Laboratório de Sanidade Animal e Segurança Alimentar SA”. Biofilms were grown in 96-well plates, containing 200 μL of each one of the selected S. aureus (1 × 106 cells/mL), suspended in TSB. Each culture plate included a negative control consisting of four wells containing TSB without bacterial inoculum. Plates were incubated over 2 h and 24 h at 37 °C and 120 rpm. After these incubation periods, planktonic cells were carefully removed, and adhered/biofilm cells were twice washed with NaCl 0.9 % (w/v). Then, cells were fixed for 15 min at ambient temperature by adding 200 μL of methanol. After discarding methanol and air drying adhered/biofilm cells, 200 μL of 1 % (v/v) Crystal Violet (CV) was added to each well for 5 min. CV stain was removed and adherent/biofilm cells air-dried overnight. Then, 200 μL of acetic acid 33 % (v/v) was added, and plates examined for the presence/absence of adhered/biofilms cells. For that, optical density (OD) was measured at 570 nm and un-inoculated wells stained as blank controls.
According to the critical OD value (ODc), that is defined as three standard deviations above the mean OD of the negative control, the biofilm production was scored as: non-biofilm producers [(-), OD ≤ ODc], weak biofilm producers [(+), ODc < OD ≤ 2 × ODc], moderate biofilm producers [(++), 2 × ODc < OD ≤ 4 × ODc], or strong biofilm producers [(+++), OD > 4 × ODc] [24].
2.5. Antibacterial susceptibility to Eucalyptus globulus Labill. extract
2.5.1. Evaluation on adhered bacteria
Two concentrations of E. globulus extract (3.125 and 6.25 mg/mL) were used to assess its in vitro effect against biofilm synthesis (bacterial adherence). For that, E. globulus was added after distribution of the bacterial inoculum into 96-well plates, with final bacteria concentration of 1×106 cells/mL. The plates were then incubated for 24 h at 37 °C and 120 rpm, and biofilm formation was determined by colony forming units (CFU) assay. Each clinical isolate was tested at least three times, in three different occasions.
2.5.2. Evaluation on biofilms
The antibacterial activity of E. globulus methanol: water extract in preformed biofilms was determined by examining its effect on viable bacteria, within the biofilm matrix and the total biofilm biomass. Bacterial biofilms were allowed to grow in 96-well plates over 24 h at 37 °C and 120 rpm. Then, two different concentrations of plant extract (3.125 and 6.25 mg/mL) were added to the wells and the effect on total survival of viable bacteria evaluated through incubation for 24 h at 37 °C and 120 rpm. Finally, surviving bacteria were determined by CFU assay. For that, the wells were thoroughly scraped and resuspended in 1 mL of 0.9% NaCl. Viable cells were determined by performing 10-fold serial dilutions in saline solution and plating in TSA. Colonies were counted after 24 h incubation at 37 °C.
2.6. Antibacterial susceptibility to Juglans regia, individually and combined with Eucalyptus globulus extract
The antibacterial activity of J. regia extract in preformed biofilms was tested alone and in combination with E. globulus. For that, two different S. aureus strains were used, namely S. aureus ATCC 25923 and S. aureus 1.
Strains were grown in 96-well plates over 24 h at 37 °C and 120 rpm. Then, 16-fold minimum inhibitory concentration (MIC) of J. regia extract (12.5 and 25 mg/mL for S. aureus ATCC 25923 and S. aureus 1, respectively), alone and in combination with 16-fold MIC of E. globulus (3.125 mg/mL), were added to the wells and incubated over 24 h at 37 °C and 120 rpm. The surviving viable bacteria were obtained by CFU assay.
2.7. Statistical analysis
Statistical analysis was performed with Prism software package (GraphPad Software). Results were analyzed using one-way ANOVA followed by Tukey's multiple comparisons test (p < 0.05).
3. Results
One of the main goals of this study was to determine the anti-staphylococcal activity of methanol: water extracts from E. globulus and J. regia against bovine mastitis-associated Staphylococcus aureus biofilms. To achieve this goal, firstly, both bacterial ability to adhere and to form biofilms were determined, and no adherence was stated after 2 h cultivation. However, as shown in Table 1, all studied strains were good biofilm producers after 24 h incubation. Then, the antibacterial activity of E. globulus extract against several S. aureus strains was tested, either in biofilm formation or in preformed biofilms (24 h). MIC value of E. globulus extract for S. aureus strains was previously determined by broth microdilution susceptibility testing method, ranging from 0.19 to 0.39 mg/mL [23]. On the other hand, since biofilms are 10–1000 times less susceptible than planktonic cells to antimicrobials [25], the extract concentrations tested were higher than MIC value (16-fold MIC: 3.125–6.25 mg/mL).
Table 1.
Staphylococcus aureus adhesion and biofilm formation after 2 and 24 h cultivation, respectively.
| Strain | Adhesion Mean OD ± SD | Biofilm Mean OD ± SD | Biofilm formation evaluation |
|---|---|---|---|
| S. aureus 1 | 0.05 ± 0.01 | 0.69 ± 0.21 | +++ |
| S. aureus 2 | 0.07 ± 0.02 | 0.78 ± 0.20 | +++ |
| S. aureus 3 | 0.07 ± 0.00 | 0.80 ± 0.15 | +++ |
| S. aureus 4 | 0.06 ± 0.01 | 0.66 ± 0.18 | +++ |
| S. aureus 5 | 0.02 ± 0.01 | 0.49 ± 0.13 | +++ |
| S. aureus 6 | 0.00 ± 0.01 | 0.54 ± 0.13 | +++ |
| S. aureus ATCC 25923 | 0.05 ± 0.01 | 0.61 ± 0.15 | +++ |
OD, optical density; SD, Standard deviation; (+++) strong biofilm producer.
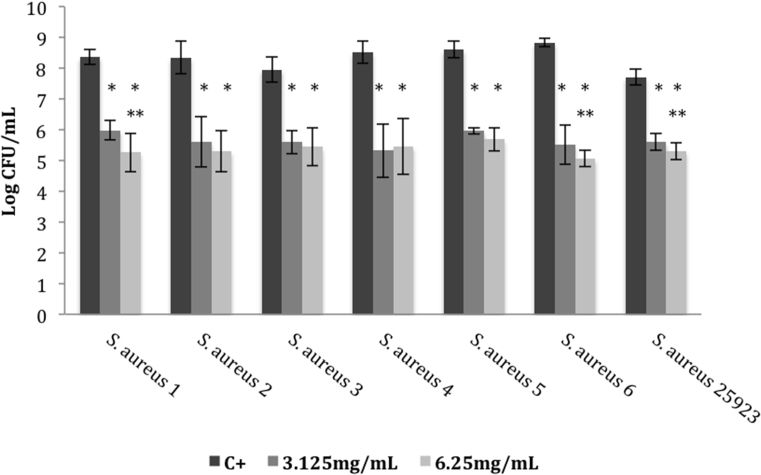
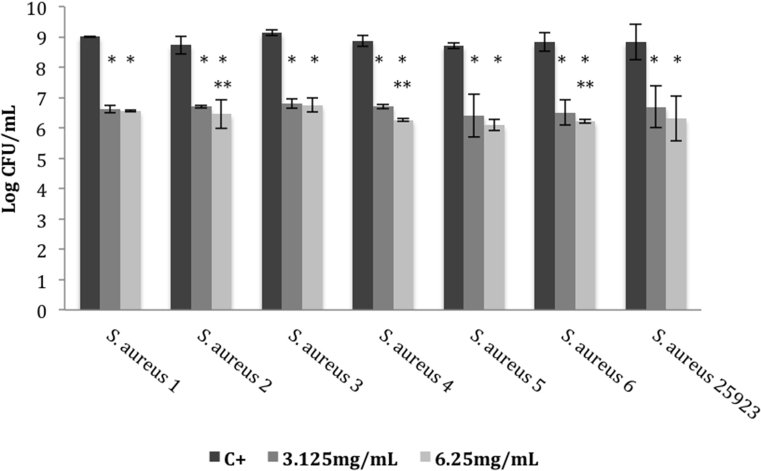
When the effect of E. globulus extract in adhered bacteria was assessed, a pronounced cell reduction (2–4 log) was stated, when compared to the positive control (p < 0.05) (Fig. 1). In addition, when comparing the two concentrations tested, 3.125 and 6.25 mg/mL, the last one revealed to be more effective against S. aureus 1, 6 and ATCC 25923 strains (p < 0.05). Moreover, the studied extract was also able to significantly inhibit preformed biofilms, leading to a CFU reduction of approximately 2–3 log, when compared to the positive control. As shown in Fig. 2, similar findings were also found for both concentrations tested (3.125 mg/mL and 6.25 mg/mL) against the studied strains, except to S. aureus 2, 4 and 6 isolates, in which the inhibitory ability was higher at 6.25 mg/mL (p < 0.05).
Fig. 1.
Logarithm of number of colony forming units (CFUs) of different S. aureus strains cultured within distinct concentrations of Eucalyptus globulus methanol:water extract. Effect tested on biofilm production. Error bars represent standard deviations (SD). C+: positive control; *p < 0.05 (one-way ANOVA, Tukey's multiple comparisons test) comparatively to the positive control; **p < 0.05 comparatively to 3.125 mg/mL.
Fig. 2.
Logarithm of number of colony forming units (CFUs) of different S. aureus strains cultured within distinct concentrations of Eucalyptus globulus methanol:water extract. Effect tested on preformed biofilms. Error bars represent standard deviations (SD). C+: positive control; *p < 0.05 (one-way ANOVA, Tukey's multiple comparisons test) comparatively to the positive control; **p < 0.05 comparatively to 3.125 mg/mL.
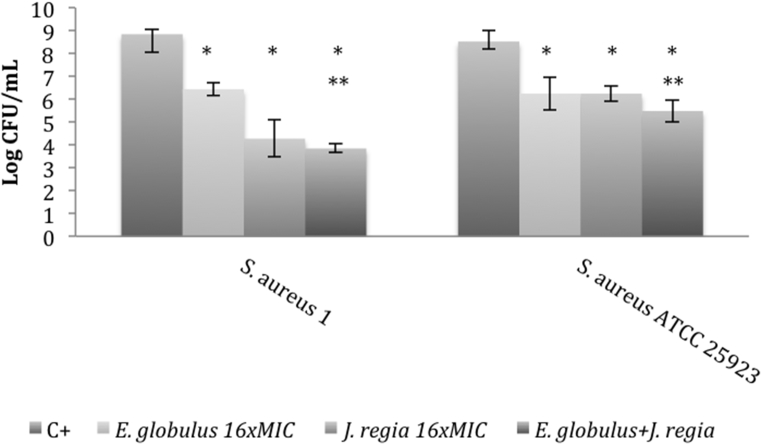
Previously, the authors verified that J. regia methanol: water extract also evidenced an interesting antibacterial activity against S. aureus species (disk diffusion assay), with MIC value ranging from 0.78-1.56 mg/mL [23]. In this sense, aiming to achieve a more prominent antimicrobial activity, J. regia was tested alone and in combination with E. globulus against S. aureus biofilm cells. For that, two strains were used, one BM clinical isolate and the reference strain, S. aureus ATCC 25923. At 16-fold MIC, J. regia extract evidenced higher antibacterial activity against the S. aureus 1 isolate than E. globulus. However, when combined with E. globulus, the extracts’ mixture revealed to be slightly more effective against the studied strains than when singly used. Nevertheless, neither additive nor synergistic effects were observed. In addition, the reference strain ATCC 25923 evidenced to be more resistant than the clinical isolate in both conditions tested (Fig. 3).
Fig. 3.
Logarithm of number of colony forming units (CFUs) of two S. aureus strains cultured within Eucalyptus globulus and Juglans regia methanol:water extracts alone, and in combination, at 16-fold MIC concentration. Effect tested on preformed biofilms. Error bars represent standard deviations (SD). C+: positive control; *p < 0.05 (one-way ANOVA, Tukey's multiple comparisons test) comparatively to the positive control; **p < 0.05 comparatively to E. globulus and J. regia alone.
4. Discussion
In a previous work, the authors demonstrated that among the various PE tested, E. globulus followed by J. regia were the most efficient against all S. aureus strains tested [23]. In addition, and considering that several plants are able to synthesize biomolecules (e.g. phenolic compounds) with prominent biological effects, such as antioxidant and antimicrobial effects, the chemical characterization of methanol: water E. globulus extract was also performed (HPLC-DAD/MS). Among the identified phenolic compounds (total phenolic compounds: 173 mg/g extract), the most abundant ones were gallotannins (92 mg/g), ellagic acid glycoside (21.6 mg/g), and quercetin derivatives (17.4 mg/g), being supposed that these compounds act as direct contributors to the observed antibacterial activity [23]. In fact, some of these phenolic compounds are already recognized as having a remarkable antimicrobial activity against several pathogens, namely S. aureus [26, 27]. On the other hand, regarding J. regia phenolic composition, quercetin-O-pentoside and 3-O-caffeoylquinic acid were the most plentiful phenolic compounds.
Few reports already investigated the antimicrobial activity of PE against S. aureus biofilms from cows suffering mastitis. For example, Rossi et al. (2011) showed that aquatic PE, such as Salvinia auriculata Aubl. (hexane extract) and Hydrocleys nymphoides (Humb. & Bonpl. ex Willd.) Buchenau (ethanol extract) were able to inhibit nearly to 50% S. aureus biofilm, while Diaz et al. (2010) observed a significant effect when used Senna macranthera and Baccharis dracunculifolia ethanolic extracts, Artemisia absinthium dichloromethane extract and Cymbopogon nardus ethanol/water 80% extract against S. aureus biofilms [28]. However, regarding the PE used in this study (E. globulus and J. regia methanol:water extracts), to the authors knowledge, no previous studies were carried out evaluating their effects in S. aureus biofilms. In fact, the first report investigating the bioactive effects of the studied methanol: water PE against microbial pathogens was carried out by Martins et al. (2015), but with Candida species. Therefore, it remains unstudied the effect of both PE in S. aureus biofilms. The biofilm formation ability of the several BM isolates selected was determined, and curiously all the strains were classified as strong biofilm producers. The results obtained are in accordance with those reached in other studies, demonstrating that S. aureus isolates from BM are able to produce biofilms, suggesting and even confirming the pronounced virulence of this bacterium [10, 29].
E. globulus methanol: water extract is a pool of phytochemicals, namely phenolic compounds [23, 30], with an effective bactericidal effect against S. aureus biofilm cells. The extract concentrations tested ranged from 8 to 32 times higher than MIC value, supporting the evidence that biofilms can be 10–1000 times more resistant than free floating cells [25]. Moreover, considering adhesion and consequently biofilm formation ability of S. aureus isolated from BM, it would be expected to observe a marked reduction in biofilm formation, comparatively to the controls (preformed biofilms). In fact, the obtained results showed that E. globulus extract promoted a significant and similar reduction in both S. aureus biofilm formation inhibition and preformed biofilms destruction. On the other hand, J. regia extract was tested at 16-fold MIC on S. aureus preformed biofilms and its antimicrobial activity compared with those obtained to E. globulus. Furthermore, the anti-biofilm effect of J. regia and E. globulus extracts used in combination was also determined and compared with the isolated effects, aiming to determine the existence of additive/synergistic effects. In fact, drug combination is usually implemented as strategy to obtain a greater efficacy than using antimicrobials alone. So, the combinatorial strategy has some advantages, such as helps in improving the antimicrobial efficacy once favors the occurrence of synergistic reactions and contributes to drug resistance reduction [31]. Although no synergisms were reached, a significantly higher antimicrobial efficacy was observed when both extracts were combined, reaching log reductions of 3 and 5 for S. aureus 25923 and S. aureus 1, respectively. When used alone, J. regia revealed to be equal or even more effective than E. globulus, with a log reduction of 2.28 and 4.57 for S. aureus 25923 and S. aureus 1, respectively.
Thus, the positive results presented here for E. globulus and J. regia extracts strongly emphasize their antimicrobial potential, both as effective disinfectants for cleaning and disinfection of surfaces, and for a better control of contaminating pathogens in the dairy industry, at same time that impairs the likelihood of bovine illnesses, such as BM and food-poisoning.
5. Conclusion
Overall, E. globulus and J. regia methanol: water extracts evidenced a very interesting bactericidal effect against S. aureus biofilms, with the combination of both extracts being the most effective strategy tested. Although no additive or synergistic effects were found, it should be clearly highlighted that more specific and accurate techniques need to be used in order to deepening knowledge on this field. Anyway, through this experiment, it was clearly evidenced that PE may be effectively used in the dairy industry to disinfect equipment and surfaces. However, further studies are still required, namely to elucidate the in vitro mechanisms of action. Moreover, studies focusing on the isolation of active principles and even minor phytochemicals present in E. globulus and J. regia methanol: water extracts are also of great interest in improving the efficacy and even guiding future applications of these antimicrobial agents.
Declarations
Author contribution statement
Fernanda Gomes: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Natália Martins, Isabel C.F.R. Ferreira: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mariana Henriques: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Portuguese Foundation for Science and Technology (SFRH/BPD/84488/2012) and by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UID/BIO/04469/2019 unit and BioTecNorte operation (NORTE-01-0145-FEDER-000004) funded by the European Regional Development Fund under the scope of Norte2020 - Programa Operacional Regional do Norte. N. Martins thank the Portuguese Foundation for Science and Technology (FCT-Portugal) for the Strategic project ref. UID/BIM/04293/2013 and "NORTE2020 - Northern Regional Operational Program" (NORTE-01-0145-FEDER-000012).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kümmel J., Stessl B., Gonano M., Walcher G., Bereuter O., Fricker M., Grunert T., Wagner M., Ehling-Schulz M. Staphylococcus aureus entrance into the Dairy Chain: tracking S. aureus from dairy cow to cheese. Front. Microbiol. 2016;7:1–11. doi: 10.3389/fmicb.2016.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basanisi M.G., La Bella G., Nobili G., Franconieri I., La Salandra G. Genotyping of methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk and dairy products in South Italy. Food Microbiol. 2017;62:141–146. doi: 10.1016/j.fm.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Cleto S., Matos S., Kluskens L., Vieira M.J. Characterization of contaminants from a sanitized milk processing plant. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutra L., Poutrel B. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J. Med. Microbiol. 1994;40:79–89. doi: 10.1099/00222615-40-2-79. [DOI] [PubMed] [Google Scholar]
- 5.Carter E.W., Kerr D.E. Optimization of DNA-based vaccination in cows using green fluorescent protein and protein A as a prelude to immunization against staphylococcal mastitis. J. Dairy Sci. 2003;86:1177–1186. doi: 10.3168/jds.S0022-0302(03)73701-1. [DOI] [PubMed] [Google Scholar]
- 6.Raza A., Muhammad S., Ghulam S., Atta A. Biofilm producing Staphylococcus aureus and bovine mastitis: a review. Mol. Microbiol. Res. 2013;3:1–18. [Google Scholar]
- 7.Sandgren C.H., Waller K.P., Emanuelson U. Therapeutic effects of systemic or intramammary antimicrobial treatment of bovine subclinical mastitis during lactation. Vet. J. 2008;175:108–117. doi: 10.1016/j.tvjl.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Barkema H.W., Schukken Y.H., Zadoks R.N. Invited review: the role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2010;89:1877–1895. doi: 10.3168/jds.S0022-0302(06)72256-1. [DOI] [PubMed] [Google Scholar]
- 9.Rossi C.C., Aguilar A.P., Diaz M.A.N., Ribon A.O.B. Aquatic plants as potential sources of antimicrobial compounds active against bovine mastitis pathogens. Afr. J. Biotechnol. 2011;10:8023–8030. [Google Scholar]
- 10.Darwish S.F., Asfour H.A.E. Investigation of biofilm forming ability in staphylococci causing bovine mastitis using phenotypic and genotypic assays. Sci. World J. 2013;2013 doi: 10.1155/2013/378492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melchior M.B., Vaarkamp H., Fink-Gremmels J. Biofilms: a role in recurrent mastitis infections? Vet. J. 2006;171:398–407. doi: 10.1016/j.tvjl.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes J.B.C., Zanardo L.G., Galvao N.N., Carvalho I.A., Nero L.A., Moreira M.A.S. Escherichia coli from clinical mastitis: serotypes and virulence factors. J. Vet. Diagn. Investig. 2011;23:1146–1152. doi: 10.1177/1040638711425581. [DOI] [PubMed] [Google Scholar]
- 13.Silva V.O., Soares L.O., Junior A.S., Mantovani H.C., Chang Y.-F., Moreira M.A.S. Biofilm formation on biotic and abiotic surfaces in the presence of antimicrobials by Escherichia coli isolates from cases of bovine mastitis. Appl. Environ. Microbiol. 2014;80:6136–6145. doi: 10.1128/AEM.01953-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monzón M., Oteiza C., Leiva J., Lamata M., Amorena B. Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn. Microbiol. Infect. Dis. 2002;44:319–324. doi: 10.1016/s0732-8893(02)00464-9. [DOI] [PubMed] [Google Scholar]
- 15.Hall-Stoodley L., Costerton J., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 16.Davies D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 17.Melo P.D.C., Sousa C., Botelho C., Oliveira R., Nader-Filho A. NaOCl effect on biofilm produced by Staphylococcus aureus isolated from the milking environment and mastitis infected cows. Pesqui. Vet. Bras. 2014;34:109–113. [Google Scholar]
- 18.Martins N., Ferreira I.C.F.R., Barros L., Carvalho A.M., Henriques M., Silva S. Plants used in folk medicine: the potential of their hydromethanolic extracts against Candida species. Ind. Crops Prod. 2015;66:62–67. [Google Scholar]
- 19.Bachir R.G., Benali M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012;2:739–742. doi: 10.1016/S2221-1691(12)60220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abirami S., Nishanthini K., Poonkothai M. Antimicrobial activity and phytochemical sreening of the leaf extracts of Eucalyptus globulus. Int. J. Curr. Pharm. Res. 2017;9:85–89. [Google Scholar]
- 21.Ara I., Shinwari M.M.A., Rashed S.A., Bakir M.A. Evaluation of antimicrobial properties of two different extracts of Juglans regia tree bark and search for their compounds using Gas Chromatohraphy-Mass Spectrum. Int. J. Biol. 2013;5 [Google Scholar]
- 22.Zakavi F., Hagh L.G., Daraeighadikolaei A., Sheikh A.F., Daraeighadikolaei A., Shooshtari Z.L. Antibacterial effect of Juglans regia bark against oral pathologic bacteria. Int. J. Dentistry. 2013;2013 doi: 10.1155/2013/854765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes F., Martins N., Barros L., Oliveira M.B., Rodrigues M.E., Henriques M., Ferreira I. Plant phenolic extracts as an effective strategy to control Staphylococcus aureus, the dairy industry pathogen. Ind. Crops Prod. 2018;112:515–520. [Google Scholar]
- 24.Christensen G.D., Simpson W.A., Younger J.J., Baddour L.M., Barrett F.F., Melton D.M., Beachey E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert P., Allison D.G., McBain A.J. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J. Appl. Microbiol. 2002;92:98S–110S. [PubMed] [Google Scholar]
- 26.Amin M.U., Khurram M., Khattak B., Khan J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement Altern. Med. 2015;15:1–12. doi: 10.1186/s12906-015-0580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G., Wang X., Xu Y., Zhang B., Xia X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014;238:589–596. [Google Scholar]
- 28.Diaz M.A.N., Rossi C.C., Mendonça V.R., Silva D.M., De A., Ribon O.B., Aguilar A.P., Muñoz G.D. Screening of medicinal plants for antibacterial activities on Staphylococcus aureus strains isolated from bovine mastitis. Rev. Bras. Farmacogn. Brazilian J. Pharmacogn. Out. 2010;20:724–728. [Google Scholar]
- 29.Fabres-Klein M., Caizer Santos M., Contelli Klein R., Nunes de Souza G., de Oliveira Barros Ribon A. An association between milk and slime increases biofilm production by bovine Staphylococcus aureus. BMC Vet. Res. 2015;11:3. doi: 10.1186/s12917-015-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira C., Pereyra A., Patriarca A., Mazzobre M., Polak T., Abram V., Buera M., Ulrih N.P. Phenolic compounds in extracts from Eucalyptus globulus leaves and Calendula officinalis flowers. J. Nat. Prod. Resour. 2016;2(1):53–59. [Google Scholar]
- 31.Foucquier J., Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol. Res. Perspect. 2015;3 doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]