This study aimed to characterize the fecal colonization and sharing of Klebsiella pneumoniae strains between companion animals and humans living in close contact. Fecal samples were collected from 50 healthy participants (24 humans, 18 dogs, and 8 cats) belonging to 18 households.
KEYWORDS: Klebsiella pneumoniae, animal-human sharing, clonal relatedness, companion animals, dog, humans
ABSTRACT
This study aimed to characterize the fecal colonization and sharing of Klebsiella pneumoniae strains between companion animals and humans living in close contact. Fecal samples were collected from 50 healthy participants (24 humans, 18 dogs, and 8 cats) belonging to 18 households. Samples were plated onto MacConkey agar (MCK) plates with and without cefotaxime or meropenem supplementation. Up to five K. pneumoniae colonies per participant were compared by pulsed-field gel electrophoresis (PFGE) after XbaI restriction. K. pneumoniae strains with unique pulse types from each participant were characterized for antimicrobial susceptibility, virulence genes, and multilocus sequence type (MLST). Fecal K. pneumoniae pulse types were compared to those of clinical K. pneumoniae strains from animal and human patients with urinary tract infections (n = 104). K. pneumoniae colonization was detected in nonsupplemented MCK in around 38% of dogs (n = 7) and humans (n = 9). K. pneumoniae strains isolated from dogs belonged to sequence type 17 (ST17), ST188, ST252, ST281, ST423, ST1093, ST1241, ST3398, and ST3399. None of the K. pneumoniae strains were multidrug resistant or hypervirulent. Two households included multiple colonized participants. Notably, two colonized dogs within household 15 (H15) shared a strain each (ST252 and ST1241) with one coliving human. One dog from H16 shared one PFGE-undistinguishable K. pneumoniae ST17 strain with two humans from different households; however, the antimicrobial susceptibility phenotypes of these three strains differed. Two main virulence genotypes were detected, namely fimH-1 mrkD ycfM entB kfu and fimH-1 mrkD ycfM entB kpn. These results highlight the potential role of dogs as a reservoir of K. pneumoniae to humans and vice versa. Furthermore, to our best knowledge, this is the first report of healthy humans and dogs sharing K. pneumoniae strains that were undistinguishable by PFGE/MLST.
INTRODUCTION
Klebsiella pneumoniae is an important nosocomial agent that is known to spread easily (1, 2). K. pneumoniae can also cause community-onset infections in companion animals and humans and is the second most common Enterobacteriaceae species causing urinary tract infections (UTI) in humans (3–5).
Extended spectrum beta-lactamase (ESBL) and carbapenemase-producing Enterobacteriaceae are frequently multidrug resistant, which leads to important therapeutic limitations (5, 6). ESBL/carbapenemase-producing K. pneumoniae strains are frequently reported worldwide, and their dissemination is of great importance (4, 6).
Companion animals may become infected with K. pneumoniae high-risk clonal lineages, such as ST15, which is frequently a CTX-M-15 producer (4, 7–9). However, little is known about the role of healthy dogs and cats as reservoirs of such clonal lineages.
Gut colonization by K. pneumoniae is strongly linked to subsequent extraintestinal infections in hospitalized human patients (1). Moreover, Enterobacteriaceae species that cause UTI, such as K. pneumoniae, are frequently part of the host gut microbiota (1). The gut of companion animals may be colonized by high-risk clonal lineages of Escherichia coli (10) and Enterococcus faecium (11), thus potentially acting as a reservoir to humans. In humans, most studies on K. pneumoniae fecal colonization are focused on hospitalized and/or infected patients, and therefore less information is available regarding healthy humans (1, 2). Studies on the population structure of K. pneumoniae strains colonizing healthy dogs and cats are also lacking.
The transmission of pathogenic bacteria from companion animals to humans has been a growing matter of concern (12). The close contact between companion animals and humans in modern society leads to greater chances of interspecies transmission of bacteria (12), including through fecal contamination. Previous studies focused on E. coli transmission dynamics have reported that the index strains from humans or dogs with UTI are extensively shared with other human and dog household members (13–14). Sharing of E. coli between healthy humans and dogs living in close contact has also been described (15–18). To our knowledge, studies on animal-human sharing have not been conducted regarding K. pneumoniae. However, this information is crucial to a better understanding of the epidemiology of this important pathogen.
In the current study, several households composed of healthy companion animals (dogs and cats) and humans living in close contact were enrolled to evaluate the frequency of K. pneumoniae colonization and animal-human sharing. Additionally, this study aimed to characterize the population structure, antimicrobial resistance, and virulence of fecal K. pneumoniae to aid the understanding of the role of healthy companion animals as reservoirs to humans and vice versa. A special focus regarding UTI was given by comparing the fecal K. pneumoniae strains with a previously characterized collection of clinical K. pneumoniae isolates from humans, dogs, and cats with UTI (9).
MATERIALS AND METHODS
Study population.
The study population included households with at least one human and one companion animal (dog or cat) living in close contact. Prior to inclusion, all human participants were informed of the main goals of the study and were asked to sign a consent form. Participants were considered “healthy” and were included in the study if no bacterial infection nor antimicrobial use was reported in the previous month. To ensure that inclusion was anonymous, households, humans, and animals were coded with the numbered letters H, Hu, and A, respectively.
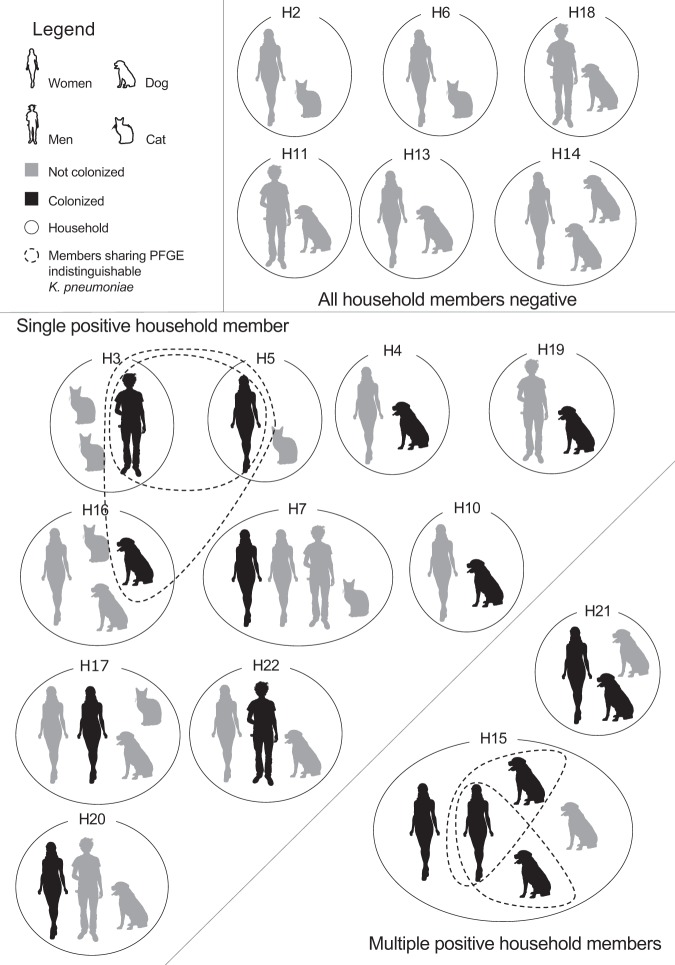
A total of 50 participants (24 humans, 18 dogs and 8 cats) living in 18 households were enrolled in 2016. Therefore, the household composition varied in the number of humans and companion animals (Fig. 1). Human participants had lived in the same household as the included companion animals for at least 6 months, except for one cat that had been recently adopted to H16 (Fig. 1).
FIG 1.
Fecal colonization and sharing of K. pneumoniae among household members.
All human participants were more than 18 years of age, and 70.8% (n = 17/24) were women.
Companion animal ages ranged from 2 months to 17 years, and 57.7% (n = 15/26) were females. Thirty-three percent (n = 8/24) of humans and 19.2% (n = 5/26) of companion animals had undergone antimicrobial treatment within the previous year. All cats lived exclusively indoors except one. All dogs had access to the outdoors; 83.3% (n = 15/18) lived indoors with the owners, while 16.7% (n = 3/18) stayed in private yards.
Sample collection and bacteriological methods.
Ethical approval for this study was obtained from the Comissão de Ética e Bem-Estar Animal (CEBEA) from the Faculty of Veterinary Medicine of the University of Lisbon. All fecal samples were collected using noninvasive methods after informed, written consent was obtained. Enrolled humans collected their own fecal samples and the fecal samples from the coliving companion animals into sterile containers. Immediately after collection, fecal samples were stored at 4°C until processing.
One gram of homogenized fecal sample was added to 10 ml of sterile 0.85% NaCl (Merck, Germany) solution and mixed thoroughly. Ten microliters of fecal suspension were plated onto MacConkey agar plates (Scharlau, Spain), with or without 1.5 μg/ml of cefotaxime (Sigma-Aldrich, USA) or meropenem (Sigma-Aldrich, USA) supplementation. To improve detection of low numbers of K. pneumoniae, 1 g of feces was added to 5 ml of sterile buffered peptone water (Biokar Diagnostics, France), vortexed, and incubated at 36 ± 1°C for 18 h. A negative quality control consisting of buffered peptone water alone was also incubated. Following incubation, 1 μl of buffered peptone water fecal suspension was plated onto the MacConkey agar plates described above. MacConkey agar plates were incubated at 36 ± 1°C for 18 h, followed by inspection for K. pneumoniae suspected colonies.
To guide presumptive K. pneumoniae identification, suspected colonies obtained from MacConkey agar plates were streaked onto UriSelect agar plates (Bio-Rad, USA). Up to five K. pneumoniae suspected colonies per participant were isolated and stored in 20% glycerol (Sigma-Aldrich, USA) brain heart infusion broth (Biokar Diagnostics, France) at −20°C until processing. Total DNA was extracted by the boiling method and K. pneumoniae species confirmed by PCR as previously described (19, 20).
All fecal samples had a high number of CFU of Enterobacteriaceae after direct plating onto MacConkey agar plates, thus confirming sample viability.
K. pneumoniae population structure analysis.
All K. pneumoniae isolates were compared by pulsed-field gel electrophoresis (PFGE) after 3 h XbaI (New England Biolabs, USA) restriction. Restriction fragments were separated on a CHEF-DR II apparatus (Bio-Rad, USA) using a 1% agarose gel (agarose pulse-field grade; NZYtech–Genes and Enzymes, Portugal) and previously described electrophoresis conditions (5 to 20 s for 4 h followed by 25 to 50 s for 18 h at 14°C, 6 V/cm2) (21).
K. pneumoniae strains with unique pulse types from each animal or human were further typed by MLST according to the published consensus MLST scheme (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
Collection of clinical UTI K. pneumoniae isolates.
The fecal K. pneumoniae PFGE restriction patterns from this study were compared with those of a previously typed collection of clinical K. pneumoniae strains isolated from dogs and cats (n = 27) and from humans (n = 77 [19 hospital patients and 58 community patients]) with UTI (9).
Susceptibility testing.
K. pneumoniae strains with unique pulse types from each animal and human were tested for antimicrobial resistance to amoxicillin/clavulanate 30 μg, cefoxitin 30 μg, cefotaxime 30 μg, meropenem 10 μg, ciprofloxacin 5 μg, gentamicin 10 μg, amikacin 30 μg, nitrofurantoin 300 μg, chloramphenicol 30 μg, tetracycline 30 μg, and trimethoprim-sulfamethoxazole 25 μg (Oxoid, Hampshire, UK). Antimicrobial susceptibility was conducted by disk diffusion according to CLSI guidelines (22).
Antimicrobial resistance determinants.
K. pneumoniae isolates that were not susceptible to at least one of the tested beta-lactams, except ampicillin, were tested by PCR for the presence of blaTEM, blaSHV, blaOXA, blaCTX-M-type, blaCIT-type, blaDHA-type, blaMOX-type, blaACT-type, blaFOX-type, and blaMIR-type beta-lactamase genes using previously described primers (23–25). K. pneumoniae strains with unique pulse types were tested for the presence of efflux pump (oqxAB) (26) and outer membrane protein (ompK35 and ompK36) (27) coding genes.
Virulence genes.
The K. pneumoniae strains that were tested for antimicrobial susceptibility were also screened by PCR for the presence of the following virulence genes: type 1 (fimH-1), type 3 (mrkD), and FimH-like (kpn) fimbriae adhesins (28, 29), outer membrane lipoprotein (ycfM) (28), catecholate siderophore receptor (iroN) (30), enterobactin (entB) (29), aerobactin (iutA) (29), iron transporter with phosphotransferase function (kfu) (29), yersiniabactin high-pathogenicity island (YHPI; irp-1, irp-2, fyuA, and ybtS) (29, 31, 32), serum resistance-associated outer membrane lipoprotein (traT) (31), regulator of mucoid phenotype A (rmpA) (29), and allantoin metabolism-associated gene (allS) (29).
Data analysis.
K. pneumoniae PFGE patterns were compared using BioNumerics software version 6.6, (bioMérieux, France) and the Dice/unweighted pair group method with arithmetic mean (UMPGA) clustering method with a tolerance of 1.5% and a clustering cutoff of 80%. Previously proposed criteria for bacterial strain typing were used (33).
Fisher’s exact test was used for comparisons between groups, with an alpha value of 0.05, using SAS statistical software package for Windows version 9.3 (SAS Institute, Inc., Cary, NC, USA).
K. pneumoniae STs from this study were compared with all known STs from the Institut Pasteur K. pneumoniae database (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html) through eBURSTV3 analysis (http://eburst.mlst.net/). K. pneumoniae STs were assigned to the same group if they shared identical alleles at 6 out of 7 loci with at least one other ST.
RESULTS
K. pneumoniae colonization was detected in 16 participants (7 dogs and 9 humans) from 12 households (Fig. 1). All samples were negative for K. pneumoniae growth in MacConkey agar plates with cefotaxime or meropenem supplementation.
The fecal colonization by K. pneumoniae was equally high in dogs (38.9%, n = 7/18) and in humans (37.5%, n = 9/24). However, K. pneumoniae colonization was not detected in cats despite the use of preenrichment prior to plating.
The majority of positive households (83.3%, n = 10/12) had a single colonized participant, and therefore within-household sharing of K. pneumoniae was absent in these cases (Fig. 1). The two households with multiple colonized participants included colonized humans and dogs (Fig. 1).
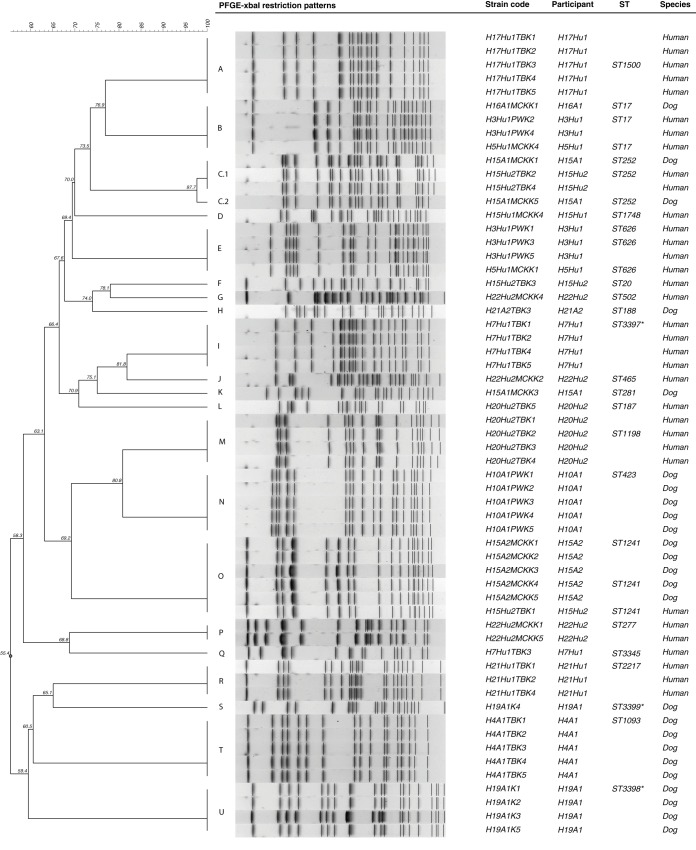
A total of 59 K. pneumoniae isolates were obtained from the 16 positive participants and the number of isolates per participant ranged from one to five. The PFGE analysis revealed a total of 22 different restriction patterns (A to U) (Fig. 2). In samples from which two to five K. pneumoniae colonies were isolated, 61.5% (n = 8/13) of the participants were colonized by two or more different K. pneumoniae strains according to the PFGE results (Fig. 2, Table 1). The PFGE analysis of the K. pneumoniae isolates from the participants living in household H21 showed that the human and dog were colonized by unrelated K. pneumoniae strains (pulse types R and H, respectively) (Fig. 2). In household H15, which was composed of two colonized humans and two colonized dogs, it is interesting to notice that while both humans were colonized by unrelated K. pneumoniae strains, the human H15Hu2 shared one K. pneumoniae strain undistinguishable by PFGE with dog H15A1 (pulse type C.1) and another with dog H15A2 (pulse type O) (Fig. 2). In both dogs from household H15, the colonizing K. pneumoniae strain was detected without preenrichment, while in the human H15Hu2 preenrichment was needed.
FIG 2.
PFGE analysis of commensal K. pneumoniae from humans and animals living in close contact. An asterisk (*) indicates a new ST described in this study (ST3397, ST3398, and ST3399).
Two K. pneumoniae pulse types, namely E and B, were shared between humans living in distinct households (Fig. 1 and 2). K. pneumoniae pulse types E and B were shared by one human from household H3 and another from household H5. Furthermore, K. pneumoniae pulse type B was also shared by dog H16A1 from household H16 (Fig. 1 and 2). The colonized human participants from households H3 and H5 and the human living in close contact (H16Hu1) with the colonized dog H16A1 share the same workplace and thus are epidemiologically related. Notably, the colonized dog H16A1 does not visit the workplace of human H16Hu1.
Overall, within-household human-animal sharing of K. pneumoniae strains occurred in 5.5% (n = 1/18) of all included households and in 8.3% (n = 1/12) of positive households. Considering the positive participants, there were a total of 20 human-animal pairs where potential within-household sharing was possible. Based on PFGE results, 10% (n = 2/20) of these human-animal potential pairs shared undistinguishable K. pneumoniae strains. Although several households included multiple human participants, none of the colonized humans shared K. pneumoniae strains with the coliving humans (n = 5 households) (Fig. 1). The same was true for animals living with colonized dogs (n = 3 households) (Fig. 1).
Overall, 27 K. pneumoniae strains were typed by MLST and characterized for antimicrobial resistance and for the presence of virulence genes.
A total of 21 STs, corresponding to the different PFGE pulse types, were detected, thus revealing high K. pneumoniae population diversity in colonized dogs and humans (Table 1). Three novel K. pneumoniae STs were described, namely ST3397 to ST3399. In eBURSTv3 analysis, the novel ST3398 and ST3399 strains isolated from a dog were found to be singletons. The K. pneumoniae ST3397 strain isolated from a human was a double-locus variant from ST65.
TABLE 1.
Characterization of fecal K. pneumoniae strains
| Household | Household member | Strain identifier | Pulse type | Sequence type | Clonal complexb | Antimicrobial resistancec | Virulence genesd |
|---|---|---|---|---|---|---|---|
| H3 | Human Hu1 | H3Hu1PWK1 | E | ST626 | None | fimH-1, mrkD, ycfM, entB, kfu, allS | |
| H3 | Human Hu1 | H3Hu1PWK2 | B | ST17 | CC11 | None | fimH-1, mrkD, ycfM, entB, kpn |
| H4 | Dog A1 | H4A1TBK1 | T | ST1093 | CC11 | (AK), NIT | fimH-1, mrkD, ycfM, entB, kfu |
| H5 | Human Hu1 | H5Hu1MCKK1 | E | ST626 | (NIT) | fimH-1, mrkD, ycfM, entB, kfu, allS | |
| H5 | Human Hu1 | H5Hu1MCKK4 | B | ST17 | CC11 | (CTX), (CN), (AK) | fimH-1, mrkD, ycfM, entB, kpn |
| H7 | Human Hu1 | H7Hu1TBK1 | I | ST3397a | CC11 | None | fimH-1, mrkD, ycfM, entB, kfu, allS |
| H7 | Human Hu1 | H7Hu1TBK3 | Q | ST3345 | CC11 | NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H10 | Dog A1 | H10A1PWK1 | N | ST423 | CC11 | NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H15 | Dog A1 | H15A1MCKK1 | C.1 | ST252 | CC11 | NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H15 | Dog A1 | H15A1MCKK3 | K | ST281 | CC505 | NIT | fimH-1, mrkD, ycfM, entB, kpn, YHPI |
| H15 | Dog A1 | H15A1MCKK5 | C.2 | ST252 | CC11 | (CN), (AK), NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H15 | Dog A2 | H15A2MCKK1 | O | ST1241 | NIT | fimH-1, mrkD, ycfM, entB, kfu | |
| H15 | Human Hu1 | H15Hu1MCKK4 | D | ST1748 | Singleton | NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H15 | Human Hu2 | H15Hu2TBK1 | O | ST1241 | NIT | fimH-1, mrkD, ycfM, entB, kfu | |
| H15 | Human Hu2 | H15Hu2TBK2 | C.1 | ST252 | CC11 | NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H15 | Human Hu2 | H15Hu2TBK3 | F | ST20 | CC11 | CN, (AK), NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H16 | Dog A1 | H16A1MCKK1 | B | ST17 | CC11 | (CN), (AK) | fimH-1, mrkD, ycfM, entB, kpn |
| H17 | Human Hu1 | H17Hu1TBK3 | A | ST1500 | CC11 | NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H19 | Dog A1 | H19A1K1 | U | ST3398a | Singleton | (CN), NIT | fimH-1, mrkD, ycfM, entB, kfu |
| H19 | Dog A1 | H19A1K4 | S | ST3399a | Singleton | (CN), NIT | fimH-1, mrkD, ycfM, entB, kfu |
| H20 | Human Hu2 | H20Hu2TBK2 | M | ST1198 | CC11 | C, NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H20 | Human Hu2 | H20Hu2TBK5 | L | ST187 | CC187 | (CTX), NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H21 | Dog A2 | H21A2TBK3 | H | ST188 | Singleton | (CN) | fimH-1, mrkD, ycfM, entB, kfu |
| H21 | Human Hu1 | H21Hu1TBK1 | R | ST2217 | Singleton | (CN), NIT | fimH-1, mrkD, ycfM, entB, kfu |
| H22 | Human Hu2 | H22Hu2MCKK1 | P | ST277 | CC11 | (CN), NIT | fimH-1, mrkD, ycfM, entB, kpn |
| H22 | Human Hu2 | H22Hu2MCKK2 | J | ST465 | CC11 | TE | fimH-1, mrkD, ycfM, entB, kpn |
| H22 | Human Hu2 | H22Hu2MCKK4 | G | ST502 | CC502 | None | fimH-1, mrkD, ycfM, entB, kpn |
ST3397, ST3398, and ST3399 are new STs described in this study.
Clonal complexes (CC) were assigned based on the predicted founder ST based on a population snapshot by eBURST analysis of all K. pneumoniae sequence types known until 31 August 2018.
Intermediate resistance is indicated in parentheses. AK, amikacin; C, chloramphenicol; CN, gentamicin; CTX, cefotaxime; NIT, nitrofurantoin; TE, tetracycline.
fimH-1, type 1 adhesin; mrkD, type 3 adhesin; kpn, FimH-like adhesin; ycfM, outer membrane lipoprotein; entB, enterobactin; kfu, iron transporter with phosphotransferase function; YHPI, yersiniabactin high-pathogenicity island; allS, allantoin metabolism-associated gene.
Most K. pneumoniae strains were susceptible to the tested antimicrobials. The only exception was the antimicrobial nitrofurantoin, against which 70.4% (n = 19/27) of strains were not susceptible. Furthermore, several strains were intermediately resistant against gentamicin and/or amikacin (Table 1). The two K. pneumoniae strains with intermediate resistance to cefotaxime were negative for all of the beta-lactamase genes tested except blaSHV.
Two main virulence genotypes were detected, namely fimH-1 mrkD ycfM entB kfu and fimH-1 mrkD ycfM entB kpn (Table 1). The allS gene was only detected in K. pneumoniae strains from humans belonging to ST626 and to novel ST3397. The yersiniabactin high-pathogenicity island was present in one strain from a dog belonging to ST281 (Table 1). All K. pneumoniae isolates were negative for rpmA, iutA, iroN, and traT genes and positive for ompK35 and ompK36. Moreover, only one strain lacked oqxAB genes according to PCR.
The K. pneumoniae strains from the participants sharing identical pulse type/STs also shared the same virulence genotype. However, the antimicrobial resistance phenotype was not always similar (pulse types E and B) (Table 1).
The K. pneumoniae strains shared between the H15 human/dog pairs belonged to ST252 (pulse type C.1) and ST1241 (pulse type O) and also had an identical antimicrobial resistance phenotype and an identical virulence genotype (Table 1). Both household H15 human participants reported that they allowed all the household dogs (n = 3) to lick their faces and sleep in their beds. No difference in dog-dog or dog-human behavior was noted regarding the three dogs living together. Interestingly, the dog H15A1 was colonized by two variants (pulse type C.1 and C.2) of the same K. pneumoniae pulse type (Fig. 1, Table 1). The K. pneumoniae strains (pulse type B) shared between the two humans and one dog from different households all belonged to ST17; however, these strains presented different antimicrobial resistance phenotypes (Table 1).
Some of the fecal K. pneumoniae strains, mostly from humans, showed a ≥80% similarity to strains from humans with UTI (Fig. 3). Of note, dog H10A1 was colonized with a K. pneumoniae strain that was closely related (92.3% similarity) to a clinical ST423 CTX-M-15-producing K. pneumoniae strain (PC25/15B) from a human (Fig. 3). The dog and human from household H10 did not have prior clinical history of UTI, were not under antimicrobial treatment in the last year, and had no contact with the hospital environment.
FIG 3.
Clusters of K. pneumoniae isolated from clinical UTI and from fecal samples of human and companion animal origin. UTI-C, community patient with UTI; UTI-H, hospitalized patient with UTI.
DISCUSSION
To our best knowledge, this is the first report of the fecal colonization and sharing of K. pneumoniae clonal lineages between healthy humans and dogs living in close contact.
The high K. pneumoniae population diversity detected in this study is in line with that in previous reports (7, 8, 34). There are several K. pneumoniae STs disseminated worldwide, including in Portugal, that are considered to be high-risk clonal lineages or are recognized as important international outbreak clones (35, 36). Considering that the gut is a reservoir of pathogenic Enterobacteriaceae (1), it is interesting to notice that only the high-risk ST17 and the international outbreak ST20 K. pneumoniae clonal lineages were detected in this study. The K. pneumoniae high-risk clonal lineages are frequently ESBL and carbapenemase producers (36). The fact that most of the fecal K. pneumoniae strains from this study were susceptible to beta-lactams may explain the low frequency of high-risk clonal lineages detected.
K. pneumoniae ST15, which is a high-risk clonal lineage, seems to predominate among clinical CTX-M-15-producing strains from companion animals from several countries (4, 7–9). In a previous study conducted in dogs and cats with UTI from Portugal, most uropathogenic K. pneumoniae isolates also belonged to ST15 (9). The absence of colonized dogs with K. pneumoniae ST15 was therefore a surprise.
Several studies have reported the colonization and sharing of E. coli strains between companion animals and humans (13–18). To our best knowledge, data on K. pneumoniae is still lacking. The use of PFGE over whole-genome sequencing (WGS) could be considered a limitation of the current study, since the latter is more discriminatory and is necessary to definitely ascertain the similarity of bacterial strains. However, previous studies on K. pneumoniae outbreaks have found that WGS-based phylogeny is consistent with the PFGE and MLST data combined, especially in strains differing in less than 3 bands (37, 38). Therefore, the detection of dogs and a human living in the same household (H15) colonized by K. pneumoniae strains belonging to the same ST, with undistinguishable PFGE restriction patterns, with an identical antimicrobial resistance phenotype and an identical virulence genotype is strongly suggestive of human-dog K. pneumoniae sharing.
Household H15 is also remarkable because although 2 humans and 2 dogs were colonized by K. pneumoniae, within-household sharing was only detected between human-dog pairs. The absence of human-human or dog-dog sharing in this study is likely related to the low number of households with multiple colonized humans or dogs. The K. pneumoniae ST252 clonal lineage shared between the human H15Hu2 and dog H15A1 has been previously detected in fecal samples from hospitalized patients and long-term-care facility residents from Portugal (39, 40). This could suggest that colonization had human origin. However, the higher K. pneumoniae ST252 and ST1241 fecal burden detected in these dogs could point to dog-to-human transmission. Allowing the dog to lick the face has been suggested as a risk factor for dog-human E. coli sharing (18). However, in household H15 this was not a determining factor. A common source of K. pneumoniae acquisition should also be hypothesized, but since the three dogs had a common living environment and behaviors, it would be likely that the three dogs would be colonized by the same strains. K. pneumoniae is known to spread easily (1); therefore, additional studies are necessary to clarify its routes of human-dog dissemination.
The two humans and one dog living in different households that were colonized by PFGE-undistinguishable K. pneumoniae ST17 strains were epidemiologically related, and therefore the K. pneumoniae transmission could have occurred through direct (human-human) or indirect (human-dog) contact. However, it should be noted that the antimicrobial resistance phenotypes of these K. pneumoniae ST17 strains differed. Thus, since the K. pneumoniae ST17 clonal lineage is disseminated worldwide (36), the circulation of this strain in the community and the colonization of these participants through unrelated sources is the likely explanation. For instance, retail meat has been pointed to as a potential source of uropathogenic K. pneumoniae to humans (41). The detection of a high-risk K. pneumoniae clonal lineage colonizing a healthy dog highlights its possible role as a reservoir. Furthermore, other K. pneumoniae clonal lineages that were detected in dogs, namely ST188, ST252, ST281, ST423, and ST1093, have also been previously implicated in human infections (39, 41–44).
The colonization of humans and dogs by K. pneumoniae was equally high (∼38%), and 10% of the potential within-household human-animal pairs shared K. pneumoniae strains undistinguishable by PFGE. Since this study relied on standard culture procedures, K. pneumoniae colonization and sharing could be underestimated due to the overgrowth of other Enterobacteriaceae species. Nevertheless, a previous study from India has reported that only 26% of the healthy dogs were colonized by K. pneumoniae (45). Considering that 61.5% of participants were colonized by multiple strains, it can be speculated that the PFGE typing of a higher number of colonies per sample could be advantageous in future studies to detect additional sharing pairs. The nasopharynx is also a K. pneumoniae colonization site (2). In retrospect, we find that the study of nasopharyngeal colonization could have undisclosed further epidemiological links between colonized dogs and humans. The absence of colonized cats in this study may be related to the number of cats tested, since infections caused by K. pneumoniae have been previously reported in cats (9).
The frequency of K. pneumoniae virulence genes agrees with previously published data (28, 29, 34). The absence or low frequency of virulence genes associated with higher K. pneumoniae invasiveness (34) is a positive outcome from this study. The first hypervirulent K. pneumoniae ST23 isolate detected in Portugal was only recently described in a human patient (46). Therefore, the absence of hypervirulent K. pneumoniae clonal lineages in this study was expected.
According to the annual report of the European Antimicrobial Resistance Surveillance Network, Portugal is among the countries with a higher frequency of resistance to third-generation cephalosporins, carbapenems, and fluoroquinolones in invasive K. pneumoniae strains (6). Additionally, a high frequency of fecal colonization by ESBL/AmpC-producing K. pneumoniae has been reported in long-term-care facility residents from Portugal (40). The high susceptibility and lack of multidrug-resistant K. pneumoniae isolates in the present study is, therefore, considered a positive finding. Nevertheless, these results may be a consequence of the study design due to the limited sample size and because it relied on healthy humans and animals without reported infections or antimicrobial use in the prior month.
Another interesting finding from this study was the detection of one healthy dog (H10A1) colonized by a K. pneumoniae ST423 strain that was 92.3% similar by PFGE to one strain isolated from a human with UTI. The use of WGS is warranted to fully disclose the relatedness of these strains; nevertheless, this finding should not be neglected. K. pneumoniae can cause other important infections, such as pneumonia (1, 34). For this reason, future comparative studies should include K. pneumoniae strains from other clinical origins to further understand the role of dogs as reservoirs of pathogenic K. pneumoniae. In fact, the K. pneumoniae clonal lineages detected in dogs from this study have been previously isolated from several types of human infections besides UTI (39, 42–44).
To conclude, this study presents novel epidemiological data regarding K. pneumoniae colonization and suggests that healthy humans and dogs may share similar K. pneumoniae clonal lineages. The role of dogs as reservoirs of K. pneumoniae clonal lineages previously described in human infections is noteworthy, even though those strains were neither multidrug resistant nor hypervirulent. Some questions remain to be answered regarding the routes of transmission and persistence of K. pneumoniae colonization over time in coliving humans and dogs. Future studies using longitudinal designs should be conducted to clarify these issues regarding infected and healthy companion animals. Meanwhile, good hygiene practices and proper fecal disposable should be advised to dog caretakers to minimize the chances of direct or indirect K. pneumoniae interspecies transmission.
ACKNOWLEDGMENTS
We thank the team of curators of the Institut Pasteur MLST system (Paris, France) for importing novel alleles, profiles, and isolates at http://bigsdb.web.pasteur.fr.
We thank all the participants for accepting to be enrolled and for providing the samples and epidemiological data as requested.
We acknowledge the PetRisk Consortium and all its members: Constança Pomba, Adriana Belas, Cátia Marques, Juliana Menezes, Luís Telo Gama, and Rodolfo Leal (Faculdade de Medicina Veterinária, Universidade de Lisboa, Portugal); Gonçalo da Graça Pereira (Centro para o conhecimento animal, Portugal); Stefan Schwarz and Claudia Feudi (Friedrich Loeffler Institute of Farm Animal Genetics, Freie Universität Berlin, Germany); Scott Weese, Joyce Rousseau, and Rebecca Flancman (Ontario Veterinary College, University of Guelph, Canada); Anette Loeffler and Sian Frosini (Royal Veterinary College, United Kingdom); and Vincent Perreten (Institute of Veterinary Bacteriology, University of Bern, Switzerland).
This work was funded by FEDER funds through the Programa Operacional Factores de Competitividade–COMPETE and by national funds through the FCT–Fundação para a Ciência e a Tecnologia (project UID/CVT/00276/2019) and by the Joint Programming Initiative on Antimicrobial Resistance Project JPIAMR/0002/2016 (PETRisk Consortium). A.B. and C.M. hold Ph.D. grants SFRH/BD/113142/2015 and SFRH/BD/77886/2011, respectively.
We have no financial or personal relationships that could inappropriately influence or bias the content of the paper.
REFERENCES
- 1.Martin RM, Cao J, Brisse S, Passet V, Wu W, Zhao L, Malani PN, Rao K, Bachman MA. 2016. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere 1:e00261-16. doi: 10.1128/mSphere.00261-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin RM, Bachman MA. 2018. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol 8:4. doi: 10.3389/fcimb.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta K, Hooton TM, Wobbe CL, Stamm WE. 1999. The prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in young women. JAMA 11:305–308. [DOI] [PubMed] [Google Scholar]
- 4.Ewers C, Stamm I, Pfeifer Y, Wieler LH, Kopp PA, Schønning K, Prenger-Berninghoff E, Scheufen S, Stolle I, Günther S, Bethe A. 2014. Clonal spread of highly successful ST15-CTX-M-15 Klebsiella pneumoniae in companion animals and horses. J Antimicrob Chemother 69:2676–2680. doi: 10.1093/jac/dku217. [DOI] [PubMed] [Google Scholar]
- 5.Marques C, Belas A, Franco A, Aboim C, Gama LT, Pomba C. 2018. Increase in antimicrobial resistance and emergence of major international high-risk clonal lineages in dogs and cats with urinary tract infection: 16 year retrospective study. J Antimicrob Chemother 73:377–384. doi: 10.1093/jac/dkx401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. 2017. Surveillance of antimicrobial resistance in Europe 2016. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 7.Harada K, Shimizu T, Mukai Y, Kuwajima K, Sato T, Usui M, Tamura Y, Kimura Y, Miyamoto T, Tsuyuki Y, Ohki A, Kataoka Y. 2016. Phenotypic and molecular characterization of antimicrobial resistance in Klebsiella spp. isolates from companion animals in Japan: clonal dissemination of multidrug-resistant extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Front Microbiol 7:1021. doi: 10.3389/fmicb.2016.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeyama Y, Taniguchi Y, Hayashi W, Ohsaki Y, Osaka S, Koide S, Tamai K, Nagano Y, Arakawa Y, Nagano N. 2018. Prevalence of ESBL/AmpC genes and specific clones among the third-generation cephalosporin-resistant Enterobacteriaceae from canine and feline clinical specimens in Japan. Vet Microbiol 216:183–189. doi: 10.1016/j.vetmic.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Marques C, Menezes J, Belas A, Aboim C, Cavaco-Silva P, Trigueiro G, Telo Gama L, Pomba C. 2018. Klebsiella pneumoniae causing urinary tract infections in companion animals and humans: population structure, antimicrobial resistance and virulence genes. J Antimicrob Chemother doi: 10.1093/jac/dky499. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JR, Davis G, Clabots C, Johnston BD, Porter S, DebRoy C, Pomputius W, Ender PT, Cooperstock M, Slater BS, Banerjee R, Miller S, Kisiela D, Sokurenko EV, Aziz A, Price LB. 2016. Household clustering of Escherichia coli sequence type 131 clinical and fecal isolates according to whole genome sequence analysis. Open Forum Infect Dis 3:ofw129. doi: 10.1093/ofid/ofw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damborg P, Top J, Hendrickx APA, Dawson S, Willems RJL, Guardabassi L. 2009. Dogs are a reservoir of ampicillin-resistant Enterococcus faecium lineages associated with human infections. Appl Environ Microbiol 75:2360–2365. doi: 10.1128/AEM.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomba C, Rantala M, Greko C, Baptiste KE, Catry B, van Duijkeren E, Mateus A, Moreno MA, Pyörälä S, Ružauskas M, Sanders P, Teale C, Threlfall EJ, Kunsagi Z, Torren-Edo J, Jukes H, Törneke K. 2017. Public health risk of antimicrobial resistance transfer from companion animals. J Antimicrob Chemother 72:957–968. doi: 10.1093/jac/dkw481. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JR, Clabots C. 2006. Sharing of virulent Escherichia coli clones among household members of a woman with acute cystitis. Clin Infect Dis 43:e101–e108. doi: 10.1086/508541. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JR, Clabots C, Kuskowski MA. 2008. Multiple-host sharing, long-term persistence, and virulence of Escherichia coli clones from human and animal household members. J Clin Microbiol 46:4078–4082. doi: 10.1128/JCM.00980-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson JR, Owens K, Gajewski A, Clabots C. 2008. Escherichia coli colonization patterns among human household members and pets, with attention to acute urinary tract infection. J Infect Dis 197:218–224. doi: 10.1086/524844. [DOI] [PubMed] [Google Scholar]
- 16.Stenske KA, Bemis DA, Gillespie BE, D'Souza DH, Oliver SP, Draughon FA, Matteson KJ, Bartges JW. 2009. Comparison of clonal relatedness and antimicrobial susceptibility of fecal Escherichia coli from healthy dogs and their owners. Am J Vet Res 70:1108–1116. doi: 10.2460/ajvr.70.9.1108. [DOI] [PubMed] [Google Scholar]
- 17.Harada K, Okada E, Shimizu T, Kataoka Y, Sawada T, Takahashi T. 2012. Antimicrobial resistance, virulence profiles, and phylogenetic groups of fecal Escherichia coli isolates: a comparative analysis between dogs and their owners in Japan. Comp Immunol Microbiol Infect Dis 35:139–144. doi: 10.1016/j.cimid.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Naziri Z, Derakhshandeh A, Firouzi R, Motamedifar M, Shojaee Tabrizi A. 2016. DNA fingerprinting approaches to trace Escherichia coli sharing between dogs and owners. J Appl Microbiol 120:460–468. doi: 10.1111/jam.13003. [DOI] [PubMed] [Google Scholar]
- 19.Féria C, Ferreira E, Correia JD, Gonçalves J, Caniça M. 2002. Patterns and mechanisms of resistance to beta-lactams and beta-lactamase inhibitors in uropathogenic Escherichia coli isolated from dogs in Portugal. J Antimicrob Chemother 49:77–85. doi: 10.1093/jac/49.1.77. [DOI] [PubMed] [Google Scholar]
- 20.Padmavathy B, Vinoth Kumar R, Patel A, Deepika Swarnam S, Vaidehi T, Jaffar Ali BM. 2012. Rapid and sensitive detection of major uropathogens in a single-pot multiplex PCR assay. Curr Microbiol 65:44–53. doi: 10.1007/s00284-012-0126-3. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues C, Bavlovič J, Machado E, Amorim J, Peixe L, Novais Â. 2016. KPC-3-producing Klebsiella pneumoniae in Portugal linked to previously circulating non-CG258 lineages and uncommon genetic platforms (Tn4401d-IncFIA and Tn4401d-IncN). Front Microbiol 7:1000. doi: 10.3389/fmicb.2016.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing, 26th ed CLSI supplement M100S-S27 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Pérez-Pérez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guessennd N, Bremont S, Gbonon V, Kacou-NDouba A, Ekaza E, Lambert T, Dosso M, Courvalin P. 2008. Résistance aux quinolones de type qnr chez les entérobactéries productrices de bêta-lactamases à spectre élargi à Abidjan en Côte d’Ivoire. Pathol Biol (Paris) 56:439–446. doi: 10.1016/j.patbio.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Pomba C, Mendonça N, Costa M, Louro D, Baptista B, Ferreira M, Correia JD, Caniça M. 2006. Improved multiplex PCR method for the rapid detection of beta-lactamase genes in Escherichia coli of animal origin. Diagn Microbiol Infect Dis 56:103–106. doi: 10.1016/j.diagmicrobio.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Kim HB, Wang M, Park CH, Kim EC, Jacoby GA, Hooper DC. 2009. oqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Antimicrob Agents Chemother 53:3582–3584. doi: 10.1128/AAC.01574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CH, Chu C, Liu JW, Chen YS, Chiu CJ, Su LH. 2007. Collateral damage of flomoxef therapy: in vivo development of porin deficiency and acquisition of blaDHA-1 leading to ertapenem resistance in a clinical isolate of Klebsiella pneumoniae producing CTX-M-3 and SHV-5 beta-lactamases. J Antimicrob Chemother 60:410–413. doi: 10.1093/jac/dkm215. [DOI] [PubMed] [Google Scholar]
- 28.El Fertas-Aissani R, Messai Y, Alouache S, Bakour R. 2013. Virulence profiles and antibiotic susceptibility patterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol Biol (Paris) 61:209–216. doi: 10.1016/j.patbio.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Compain F, Babosan A, Brisse S, Genel N, Audo J, Ailloud F, Kassis-Chikhani N, Arlet G, Decré D. 2014. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol 52:4377–4380. doi: 10.1128/JCM.02316-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson JR, Russo TA, Tarr PI, Carlino U, Bilge SS, Vary JC Jr, Stell AL. 2000. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroN(E. coli), among Escherichia coli isolates from patients with urosepsis. Infect Immun 68:3040–3047. doi: 10.1128/IAI.68.5.3040-3047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson JR, Stell AL. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis 181:261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 32.Schubert S, Cuenca S, Fischer D, Heesemann J. 2000. High-pathogenicity island of Yersinia pestis in Enterobacteriaceae isolated from blood cultures and urine samples: prevalence and functional expression. J Infect Dis 182:1268–1271. doi: 10.1086/315831. [DOI] [PubMed] [Google Scholar]
- 33.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D, Jenney A, Connor TR, Hsu LY, Severin J, Brisse S, Cao H, Wilksch J, Gorrie C, Schultz MB, Edwards DJ, Nguyen KV, Nguyen TV, Dao TT, Mensink M, Minh VL, Nhu NT, Schultsz C, Kuntaman K, Newton PN, Moore CE, Strugnell RA, Thomson NR. 2015. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A 112:E3574–E3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manageiro V, Ferreira E, Almeida J, Barbosa S, Simões C, Antibiotic Resistance Surveillance Program in Portugal (ARSIP), Bonomo RA, Caniça M. 2015. Predominance of KPC-3 in a survey for carbapenemase-producing Enterobacteriaceae in Portugal. Antimicrob Agents Chemother 59:3588–3592. doi: 10.1128/AAC.05065-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navon-Venezia S, Kondratyeva K, Carattoli A. 2017. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev 41:252–275. doi: 10.1093/femsre/fux013. [DOI] [PubMed] [Google Scholar]
- 37.Marsh JW, Krauland MG, Nelson JS, Schlackman JL, Brooks AM, Pasculle AW, Shutt KA, Doi Y, Querry AM, Muto CA, Harrison LH. 2015. Genomic epidemiology of an endoscope-associated outbreak of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae. PLoS One 10:e0144310. doi: 10.1371/journal.pone.0144310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabirova JS, Xavier BB, Coppens J, Zarkotou O, Lammens C, Janssens L, Burggrave R, Wagner T, Goossens H, Malhotra-Kumar S. 2016. Whole-genome typing and characterization of blaVIM19-harbouring ST383 Klebsiella pneumoniae by PFGE, whole-genome mapping and WGS. J Antimicrob Chemother 71:1501–1509. doi: 10.1093/jac/dkw003. [DOI] [PubMed] [Google Scholar]
- 39.Papagiannitsis CC, Dolejska M, Izdebski R, Dobiasova H, Studentova V, Esteves FJ, Derde LP, Bonten MJ, Hrabák J, Gniadkowski M. 2015. Characterization of pKP-M1144, a novel ColE1-Like plasmid encoding IMP-8, GES-5, and BEL-1 β-lactamases, from a Klebsiella pneumoniae sequence type 252 isolate. Antimicrob Agents Chemother 59:5065–5068. doi: 10.1128/AAC.00937-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrigues C, Mendes AC, Sima F, Bavlovič J, Machado E, Novais Â, Peixe L. 2017. Long-term care facility (LTCF) residents colonized with multidrug-resistant (MDR) Klebsiella pneumoniae lineages frequently causing infections in Portuguese clinical institutions. Infect Control Hosp Epidemiol 38:1127–1130. doi: 10.1017/ice.2017.144. [DOI] [PubMed] [Google Scholar]
- 41.Davis GS, Waits K, Nordstrom L, Weaver B, Aziz M, Gauld L, Grande H, Bigler R, Horwinski J, Porter S, Stegger M, Johnson JR, Liu CM, Price LB. 2015. Intermingled Klebsiella pneumoniae populations between retail meats and human urinary tract infections. Clin Infect Dis 61:892–899. doi: 10.1093/cid/civ428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito R, Shindo Y, Kobayashi D, Ando M, Jin W, Wachino J, Yamada K, Kimura K, Yagi T, Hasegawa Y, Arakawa Y. 2015. Molecular epidemiological characteristics of Klebsiella pneumoniae associated with bacteremia among patients with pneumonia. J Clin Microbiol 53:879–886. doi: 10.1128/JCM.03067-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.da Silva HRF, Vilela MA, Almeida ACS, de Morais M. 2018. Colistin-resistant KPC-2-producing Klebsiella pneumoniae ST423 harboring an IS5-like element in the mgrB gene isolated from cerebrospinal fluid. Diagn Microbiol Infect Dis 91:184–185. doi: 10.1016/j.diagmicrobio.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Garza-Ramos U, Barrios-Camacho H, Moreno-Domínguez S, Toribio-Jiménez J, Jardón-Pineda D, Cuevas-Peña J, Sánchez-Pérez A, Duran-Bedolla J, Olguín-Rodriguez J, Román-Román A. 2018. Phenotypic and molecular characterization of Klebsiella spp. isolates causing community-acquired infections. New Microbes New Infect 23:17–27. doi: 10.1016/j.nmni.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharif NM, Sreedevi B, Chaitanya RK, Sreenivasulu D. 2017. Beta-lactamase antimicrobial resistance in Klebsiella and Enterobacter species isolated from healthy and diarrheic dogs in Andhra Pradesh, India. Vet World 10:950–954. doi: 10.14202/vetworld.2017.950-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira A, Petrucci T, Simões MJ. 2017. Klebsiella pneumoniae from K1 and hypervirulent clone ST23: first documented case in Portugal. Acta Med Port 30:496–499. doi: 10.20344/amp.7705. [DOI] [PubMed] [Google Scholar]