The Clinical and Laboratory Standards Institute (CLSI) has revised several breakpoints since 2010 for bacteria that grow aerobically. In 2019, these revisions include changes to the ciprofloxacin and levofloxacin breakpoints for the Enterobacteriaceae and Pseudomonas aeruginosa, daptomycin breakpoints for Enterococcus spp., and ceftaroline breakpoints for Staphylococcus aureus.
KEYWORDS: CLSI, FDA, antimicrobial susceptibility testing, breakpoints
ABSTRACT
The Clinical and Laboratory Standards Institute (CLSI) has revised several breakpoints since 2010 for bacteria that grow aerobically. In 2019, these revisions include changes to the ciprofloxacin and levofloxacin breakpoints for the Enterobacteriaceae and Pseudomonas aeruginosa, daptomycin breakpoints for Enterococcus spp., and ceftaroline breakpoints for Staphylococcus aureus. Implementation of the revisions is a challenge for all laboratories, as not all systems have FDA clearance for the revised (current) breakpoints, compounded by the need for laboratories to perform validation studies and to make updates to laboratory information system/electronic medical record builds in the setting of limited information technology infrastructure. This minireview describes the breakpoint revisions in the M100 supplement since 2010 and strategies for the laboratory on how to best adopt these in clinical testing.
THE STORY BEHIND BREAKPOINT REVISIONS
Antimicrobial susceptibility testing (AST) is essential for effective management of many types of infectious diseases. Perhaps the most critical step in AST involves the interpretation of results. This interpretation occurs via the assignment of clinical breakpoints, which divide AST results, be they MIC or disk diffusion zone of growth inhibition values, into categories that correlate with the probability of clinical outcomes. Worldwide, this work of establishing breakpoints and interpretive categories is conducted by three organizations: the U.S. Food and Drug Administration Center for Drug Evaluation and Research (CDER, which is a U.S.-centric organization), and two international standards development organizations (SDOs): the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Several national committees, including the U.S. Committee on Antimicrobial Susceptibility Testing (USCAST), are affiliated with, and report to, EUCAST.
Well-known interpretive categories applied to AST zone diameter and MIC values include susceptible (S), which is a category that indicates there is a high probability of a favorable treatment outcome, and resistant (R), which indicates there is a low probability of a favorable treatment outcome. With some exceptions (e.g., urine-specific breakpoints), these categories are based on serum-achievable concentrations of the antimicrobial. In rare cases, a nonsusceptible (NS) category is applied when there are sufficient data to define the S category but not the R, i.e., generally, for new antimicrobial agents with very low resistance rates, such as the newer lipoglycopeptides. Finally, all three SDOs have one or more categories intended to accommodate ambiguity in AST data interpretation, be it due to testing variability or the possibility that a higher drug exposure (via dosing and/or infections confined to an anatomical location where the drug concentrates, such as the urine) could accommodate a higher “S” MIC breakpoint. Traditionally, this concept was addressed by the intermediate (I) category, which is how CDER continues to approach this category. CLSI additionally applies a susceptible dose-dependent (SDD) category, which is only used if there is a possibility of higher drug exposure through dosing. EUCAST redefined I to mean "increased exposure" and introduced the “area of technical uncertainty” (ATU) category, to account for testing variability, in 2019. Regardless of how categories are defined, extensive studies are performed to establish breakpoints and interpretive categories during the development of a new antimicrobial agent. However, with time, signals may arise that suggest the original breakpoints and categories no longer meet clinical needs, in which case, an investigation is performed by SDOs to determine if breakpoint revision is in order. This minireview focuses specifically on the changes made by the CLSI to interpretive categories and clinical breakpoints since 2010 and how these might be addressed by clinical laboratories that use CLSI standards. Collectively, these are referred to as “breakpoints” in this minireview, although in some cases, interpretive category changes accompanied breakpoint changes (i.e., to add a new interpretive category, such as SDD for cefepime, daptomycin, and ceftaroline).
Since 2010, the Clinical and Laboratory Standards Institute (CLSI) has revised several breakpoints for bacteria that grow aerobically. Several new revisions occurred this year, with publication of CLSI M100, 29th edition, January 2019 (Table 1). When deciding to revise an existing breakpoint, the CLSI follows criteria outlined in the M23 guideline, which are summarized in Table 2. Members of the CLSI Antimicrobial Susceptibility Testing Subcommittee or other interested parties (e.g., physicians, researchers, industry, and public health officials) submit data to suggest a breakpoint revision is needed, generally, when new data suggest the previous breakpoints no longer accurately predict treatment efficacy and revisions are warranted to address significant patient safety or public health gaps with the previous breakpoints. While historically the data that supported these breakpoint changes were not well publicized, the CLSI has started the process of publishing rationale documents for these changes, which are available free of charge on the CLSI website. There is no question that breakpoint decisions can be driven more by expert opinion than objective evidence when there is a dearth of data, but the CLSI process considers the commentary of members, advisors, and observers (i.e., the public) for these revisions. As such, breakpoints and interpretive categories published in the M100 standard represent the most up-to-date consensus position for each antimicrobial agent.
TABLE 1.
Summary of CLSI breakpoint revisions since 2010 for bacteria that grow aerobically
| Antimicrobial | Year first published by CLSI | Original MIC breakpoints (µg/ml)a
|
Year revised by CLSI | Current MIC breakpoints (µg/ml) |
Current CLSI breakpoint recognized by FDA?b | Priority for update by laboratoryc | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |||||
| Enterobacteriaceae | ||||||||||
| Aztreonam | Pre-1987 | ≤8 | 16 | ≥32 | 2010 | ≤4 | 8 | ≥16 | Yes | 1 |
| Cefazolin | ||||||||||
| Systemic | Pre-1987 | ≤8 | 16 | ≥32 | 2010 | ≤1 | 2 | ≥4 | No | 2 |
| 2011 | ≤2 | 4 | ≥8 | |||||||
| Urine | NAd | ≤8 | 16 | ≥32 | 2016 | ≤16 | ≥32 | No | 2 | |
| Surrogate for oral cephalosporins | NA | 2014 | ≤16 | ≥32 | 2 | |||||
| Cefepime | 1994 | ≤8 | 16 | ≥32 | 2014 | ≤2 | 4–8 (SDDe) | ≥16 | Yes (but calls SDD an “I”) | 1 |
| Cefotaxime | Pre-1987 | ≤8 | 16–32 | ≥64 | 2010 | ≤1 | 2 | ≥4 | Yes | 1 |
| Ceftriaxone | ||||||||||
| Ceftizoxime | ||||||||||
| Ceftazidime | Pre-1987 | ≤8 | 16 | ≥32 | 2010 | ≤4 | 8 | ≥16 | Yes | 1 |
| Ertapenem | 2003 | ≤2 | 4 | ≥8 | 2010 | ≤0.25 | 0.5 | ≥1 | Yes | 1 |
| 2012 | ≤0.5 | 1 | ≥2 | |||||||
| Imipenem | Pre-1987 | ≤4 | 8 | ≥16 | 2010 | ≤1 | 2 | ≥4 | Yes | 1 |
| Meropenem | 1998 | |||||||||
| Ciprofloxacin | Pre-1987 | ≤1 | 2 | ≥4 | 2012 | ≤0.06 | 0.12–0.5 | ≥1 | Yes (only S. typhi) | 1 |
| Levofloxacin | 1997 | ≤2 | 4 | ≥8 | 2013 | ≤0.12 | 0.25–1 | ≥2 | No | 1 |
| Ofloxacin (Salmonella only) | 1990 | ≤2 | 4 | ≥8 | 2013 | ≤0.12 | 0.25–1 | ≥2 | N | 3 |
| Ciprofloxacin | Pre-1987 | ≤1 | 2 | ≥4 | 2019 | ≤0.25 | 0.5 | 1 | No | 2 |
| Levofloxacin (other Enterobacteriaceae) | 1997 | ≤2 | 4 | ≥8 | ≤0. 5 | 1 | ≥2 | |||
| Pseudomonas aeruginosa | ||||||||||
| Colistin | 1979 | ≤2 | 4 | ≥8 | 2017 | ≤2 | ≥4 | No | 3 | |
| Imipenem | Pre-1987 | ≤4 | 8 | ≥16 | 2012 | ≤2 | 4 | ≥8 | Yes | 1 |
| Meropenem | 1998 | |||||||||
| Piperacillin | Pre-1987 | ≤64 | ≥128 | 2012 | ≤16 | 32–64 | ≥128 | Yes | 3 | |
| Ticarcillin | Pre-1987 | |||||||||
| Piperacillin-tazobactam | 1993 | ≤64/4 | ≥128/4 | 2012 | ≤16/4 | 32/4–64/4 | ≥128/4 | Yes | 1 | |
| Ticarcillin-clavulanatef | 1987 | 3 | ||||||||
| Ciprofloxacin | Pre-1987 | ≤1 | 2 | ≥4 | 2019 | ≤0.5 | 1 | ≥2 | No | 2 |
| Levofloxacin | 1997 | ≤2 | 4 | ≥8 | ≤1 | 2 | ≥4 | |||
| Acinetobacter spp. | ||||||||||
| Imipenem | Pre-1987 | ≤4 | 8 | ≥16 | 2014 | ≤2 | 4 | ≥8 | Yes (imipenem only) | 1 |
| Meropenem | 1998 | |||||||||
| Staphylococcus aureus | ||||||||||
| Ceftaroline | 2013 | ≤1 | 2 | ≥8 | 2019 | ≤1 | 2–4 (SDD) | ≥8 | No | 3 |
| Enterococcus spp. | ||||||||||
| Daptomycin | 2005 | ≤4 | 2019 | ≤1 | 2–4 (SDD) | ≥8g | No | 2 | ||
S, susceptible; I, intermediate; R, resistant.
Recognized by the FDA on the STIC website (see text).
Prioritization is based on the authors’ opinion and should be discussed at the institutional level with physicians, pharmacy, antibiotic stewardship teams, and hospital leadership. Refer to the text and supplemental table for more details.
NA, not available.
SDD, susceptible dose dependent.
Ticarcillin-clavulanate is no longer available globally; although present on some commercial cAST panels, this antimicrobial need not be reported.
The enterococcal breakpoints have been further revised by the CLSI, in January 2019; the subcommittee approved a revised breakpoint for E. faecium of ≤4 µg/ml, SDD, and ≤8 µg/ml, R, with no susceptible category.
TABLE 2.
CLSI M23 Criteria used by CLSI to determine if a breakpoint warrants reevaluation for possible revision
| Criterion | Example of recent revisions |
|---|---|
| Recognition of a new resistance mechanism(s) | Carbapenems for Enterobacteriaceae |
| New PK/PD data indicate an existing breakpoint is too high/low | Fluoroquinolones for Enterobacteriaceae and P. aeruginosa |
| Recognition that the antimicrobial dosage regimens used in widespread clinical practice differ substantially from the dosage regimens that were used to establish previous breakpoints | Cefazolin for Enterobacteriaceae |
| Introduction of new formulations of the antimicrobial agent, which result in different PK characteristics | Ceftaroline for S. aureus |
| New data emerge to demonstrate the previous breakpoints were not optimal for common uses of an antimicrobial agent | Penicillin for Streptococcus pneumoniae (infections other than meningitis) |
| New data demonstrate poor prediction of clinical response using previous breakpoints | Daptomycin for Enterococcus spp.; Piperacillin-tazobactam for P. aeruginosa |
| A specific public health need is identified that is not addressed by previous breakpoints | Colistin for P. aeruginosa and Acinetobacter spp.; carbapenems for Enterobacteriaceae; aztreonam and cephalosporins for Enterobacteriaceae |
| Significant rates of discordance are documented between MIC and disk diffusion test results when testing recent clinical isolates | Ceftaroline for S. aureus (initial reason for investigation of breakpoint) |
| Changes are made to CLSI-approved reference methods that affect the initial breakpoints | No recent breakpoint revisions were due to changes in CLSI reference methods |
| Revised breakpoints to simplify testing and eliminate need for additional tests to detect specific resistance mechanisms | Cephalosporins for Enterobacteriaceae (ESBLs) |
| Differences exist between breakpoints established by CLSI and those of other regulatory organizations responsible for determining breakpoints (e.g., EUCAST) | Fluoroquinolones for Enterobacteriaceae and P. aeruginosa |
The CLSI officially refers to the breakpoints that have recently been revised as “current breakpoints.” Consequently, the term “current breakpoints” will be used in the remainder of this review. Clinical laboratories have struggled to adopt the current breakpoints in a timely manner, due to several factors, which include both regulatory and laboratory-level challenges. Recent surveys performed in California illustrate some of these challenges. In 2015, more than one-third of laboratories were using obsolete Enterobacteriaceae carbapenem breakpoints (1). Interviews conducted by the Los Angeles County Department of Public Health with hospital laboratories in their jurisdiction that utilized obsolete breakpoints in 2017 demonstrated that more than three-quarters (27/34; 79%) incorrectly assumed the commercial AST system (cASTs) used by their laboratory applied current breakpoints because it was a Food and Drug Administration (FDA)-cleared system. When further queried, 17/34 (50%) indicated they did not know how to change breakpoints on their cASTs, and 10/34 (29%) indicated they lacked the resources necessary to perform a validation study that would be required to allow use of current breakpoints on their cASTs if the cASTs was not FDA-cleared with the current breakpoints (2).
This minireview discusses the CLSI breakpoint revision process, CLSI clinical breakpoint revisions since 2010 and reasoning behind these revisions, status of FDA recognition of these breakpoints, and clearance of the current breakpoints on cASTs. In addition, strategies that laboratories may use to prioritize and adopt the revised (current) CLSI breakpoints are presented. For details on how the CLSI sets breakpoints for new antimicrobial agents and performs review of existing breakpoints, it is suggested the reader review the CLSI M23 guideline. M23 describes the data required for establishing a breakpoint, as well as the signals that suggest a breakpoint is in need of revision. For new agents, the sponsor (pharmaceutical company) presents a completed data packet to the FDA and then to the CLSI, but tentative breakpoints may be established by the CLSI prior to the antimicrobial agent’s FDA approval (e.g., as was done for cefiderocol in 2019). The terminologies associated with breakpoint development and revision in the United States and used in this review are summarized in Table 3.
TABLE 3.
Terminology used in this minireview regarding breakpoints
| Term | Definition |
|---|---|
| Current breakpoint | Breakpoints revised and published in the current CLSI M100 Standard (i.e., M100S 29th edition at the time of this writing) |
| Obsolete breakpoint | Breakpoints published in prior editions (i.e., as of this writing, M100S 28th edition and prior) |
| FDA-recognized breakpoint | Breakpoints listed on the FDA STIC website; www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm575163.htm |
| Off-label breakpoint | Breakpoints used on a cASTs that are different than those FDA cleared on the cASTs |
| cASTs | Commercial antimicrobial susceptibility test system. In the United States, these include manual (disk and gradient diffusion) and automated devices. Manufacturers must use FDA CDER-recognized breakpoints |
| M100 | CLSI standard that lists CLSI breakpoints, quality control ranges, antimicrobial agents recommended for testing and reporting and some additional information related to testing procedures |
| M23 | CLSI guidance document that outlines the process and data required for approval of new breakpoints and revised breakpoints |
| STIC | Susceptibility test interpretive criteria; language used by the FDA for “breakpoint” |
| CDER | Center for Drug Evaluation and Research; branch of the FDA that regulates antimicrobial agents and breakpoints in the United States |
| CDRH | Center for Devices and Radiological Health; branch of the FDA that regulates medical devices in the United States, including cASTs |
CLSI VERSUS FDA BREAKPOINTS AND COMMERCIAL AST SYSTEMS
For years, AST in the United States has been complicated by the fact that two primary organizations set and revise breakpoints: the CLSI and the CDER branch of the FDA (3, 4). CLSI breakpoints are published in M100 and FDA breakpoints were previously published in each antimicrobial agent’s “prescribing information,” or drug label. This changed in December 2017 when the FDA established the Susceptibility Test Interpretive Criteria (STIC) website as a result of a provision in the 21st Century Cures Act (3, 4). Today, the FDA breakpoints are only listed on the STIC website.
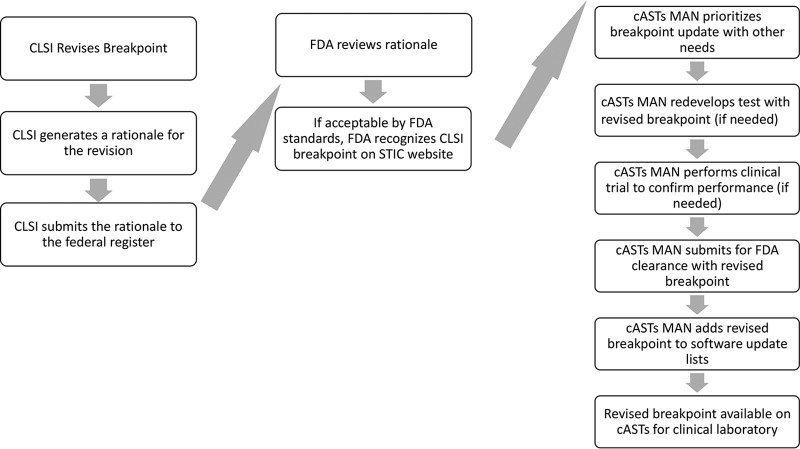
A second outcome of the 21st Century Cures Act is the new ability of the CDER to officially recognize CLSI breakpoints, including those recently revised by the CLSI. For this to occur, the CLSI generates a rationale document summarizing the data used by the CLSI to justify the breakpoint, as outlined in Fig. 1 These rationale documents are available online (https://clsi.org/meetings/ast/rationale-documents/) and is submitted to the Federal Register and reviewed by the CDER; provided that the data and rationale meet CDER requirements, the CDER publishes the CLSI breakpoints on the STIC website as the official FDA breakpoints. The timelines for these steps are not yet well established, but it is likely at least a year will pass between CLSI publication of a revised breakpoint in M100 and its recognition by the FDA on the STIC website.
FIG 1.
Process for revised breakpoint implementation on cASTs; roles of the CLSI, FDA, and cASTs manufacturers. cASTs, commercial antimicrobial susceptibility test system; cASTs MAN, commercial antimicrobial susceptibility test system manufacturer.
Manufacturers of cASTs must use FDA breakpoints; therefore, they cannot adopt CLSI breakpoints until they are recognized by the FDA on the STIC website (Fig. 1). However, once the FDA recognizes the CLSI breakpoint, cASTs manufacturers may submit test performance data to the Center for Devices and Radiological Health (CDRH) branch of the FDA to obtain clearance of their cASTs with the current breakpoints. Historically, it has taken several years (ranging from 1 to 9 or more years) for manufacturers to update their cASTs with current breakpoints. Because cASTs are labeled by the FDA as class II devices, CDRH, unfortunately, does not have a mechanism to mandate that manufacturers revise the breakpoints on their cASTs sooner, or at all. In contrast, class III devices have a stricter postmarket review process which might allow routine requirement of breakpoint revision. However, class III devices require a much more stringent data set and review process, which could result in significant delays for new antimicrobial clearance on these systems. It is clear that changes to this process and more coordination between the cASTs manufacturers and the CDRH are needed. The CDRH requirements for clearance of existing cASTs with current breakpoints involve demonstration that results are accurate and reproducible, and these requirements are designed so as not to be overly burdensome for the manufacturer. Manufacturers must recognize their responsibility to their customers and the patients they serve and revise breakpoints within a timely manner.
While waiting for the FDA to recognize current CLSI breakpoints and for manufacturers to incorporate these into their cASTs, laboratories have the option to adopt current CLSI breakpoints following internal laboratory validation of the cASTs’ performance with the current breakpoints. To do this, the cASTs panel must contain antimicrobial concentrations that encompass the current breakpoints; however, these are not always available. Validation of breakpoints in this manner is considered “off-label use” and is a significant challenge to many laboratories, requiring extensive time, resources, and expertise. Nonetheless, to ensure patient safety and favorable outcomes for infections, laboratories should endeavor to adopt breakpoint revisions as soon as possible. As such, laboratories will need to prioritize breakpoints for implementation; suggestions for how to prioritize breakpoint revisions are described below. Many strategies include the use of manual testing and manual interpretation of MIC values and zone diameters, such as by gradient diffusion or disk diffusion, as an interim measure. However, laboratories should be cognizant that some level of validation of these tests with current breakpoints is prudent, as the analytical performance characteristics of these tests may not be the same as it was with obsolete breakpoints, due to less tight correlation with the reference broth microdilution method. Generally, the CLSI evaluates the performance of disk diffusion through the process of revising disk breakpoints, and so it is anticipated these tests will perform well with current breakpoints.
PRIORITIZING THE ADOPTION OF CURRENT CLSI BREAKPOINTS IN THE CLINICAL LABORATORY
The effort involved in implementing current breakpoints on a cASTs that is not yet FDA cleared for these breakpoints in the clinical laboratory may be substantial. It is important to understand when use of an obsolete breakpoint is likely to result in poor patient outcomes and/or impact therapy choices at an institutional level and prioritize implementation of current breakpoints accordingly. It should be reinforced that all CLSI breakpoints are defined by consensus, in which not only CLSI-appointed voting members and appointed advisors, but also reviewers (anyone wishing to participate in CLSI open meetings), are encouraged to provide feedback and commentary. As such, the publication of these truly defines the best practices for AST; however, they may apply to differing degrees to different patient populations. Herein, we assign priority 1 (highest), 2, or 3 (lowest) for a laboratory’s consideration as follows and as summarized in Table 1. This priority ranking is the authors’ opinion, based on the availability of literature to support the breakpoint change, time since the breakpoint was revised by the CLSI/FDA, and practicality. (For example, are there automated systems on the market that can accommodate the breakpoint?) It should be emphasized that the decision on how to address each breakpoint is an institutional decision, and the value to discussions with all vested parties cannot be overemphasized.
Priority 1: all laboratories to implement now.
Priority 2: laboratories to implement following determination of the institutional need; generally, breakpoints not yet recognized by the FDA fall into this category.
Priority 3: laboratories may not need to implement, dependent on institutional need.
Table 4 lists CLSI breakpoints revised since 2010 that have been recognized by the FDA CDER but not all are available on all cASTs. This table will continually evolve, and so laboratories are encouraged to check in with their cASTs manufacturer representative for the most-up-to-date information.
TABLE 4.
cASTs with FDA clearance for current CLSI breakpointsa
| Organism group | Antimicrobial agent | BD phoenix | Beckman coulter MicroScan | bioMérieux Vitek 2 | Thermo Fisher Sensititre |
|---|---|---|---|---|---|
| Enterobacteriaceae | Cefepime | Y | N | Y | Y |
| Cefotaxime | N | Y | Y | Y | |
| Ceftriaxone | Y | Y | Y | Y | |
| Ceftazidime | N | N | N | N | |
| Ertapenem | Y | Y | Y | Y | |
| Imipenem | Y | Y | Y | Y | |
| Meropenem | Y | N | N | Y | |
| Enterobacteriaceae (Salmonella) | Ciprofloxacin | S. typhi | S. typhi; S. enteritidis | ||
| Pseudomonas aeruginosa | Imipenem | Y | Y | Y | Y |
| Meropenem | Y | Y | N | Y | |
| Piperacillin-tazobactam | Y | N | N | Y | |
| Acinetobacter spp. | Imipenem | Y | Y | Y | Y |
Includes agents for which FDA and CLSI MIC breakpoints are the same. Y, yes breakpoints current with CLSI/FDA breakpoints; N, no breakpoints, not current with CLSI/FDA breakpoints. Contact manufacturer for updated information on those breakpoints listed as not yet current here.
Table 5, in contrast, lists compounds for which the breakpoints currently differ between the CLSI and the FDA CDER, which have been addressed since 2010 by either organization. It should be noted that there are over 100 exceptions to the CLSI tables at present on the FDA CDER STIC website, primarily for nonfermenting Gram-negative bacilli. For many of these, the clinical data required to set an FDA breakpoint were unavailable at the time the antimicrobial was introduced. These will be addressed based on public health need and the availability of data to support, or suggest a revision for, current CLSI breakpoints. It should be noted as well that there are rare instances where an FDA breakpoint exists with no CLSI breakpoint, such as for tigecycline or cefditoren. In general, these breakpoints were set by the CDER at the time of the drug’s first approval.
TABLE 5.
Agents for which current CLSI breakpoints are not recognized by the FDAa
| Organism group | Antimicrobial agent |
|---|---|
| Enterobacteriaceae | Cefazolin |
| Ciprofloxacin | |
| Levofloxacin | |
| Enterobacteriaceae (Salmonella) | Levofloxacin |
| Pseudomonas aeruginosa | Cefepimeb |
| Ceftazidimeb | |
| Ciprofloxacin | |
| Levofloxacin | |
| Acinetobacter spp. | Meropenem |
| S. aureus | Ceftaroline |
| Enterococcus spp. | Daptomycin |
Manufacturers of cASTs must use FDA breakpoints.
FDA updated the cefepime and ceftazidime P. aeruginosa breakpoints in 2012, whereas the CLSI did not. The current FDA breakpoint does not include an intermediate category, which makes clearance of cASTs challenging due to the higher rates of very major errors (VMEs), major errors (MEs), and results without any minor errors (mE).
PRIORITY 1 BREAKPOINTS
Enterobacteriaceae: carbapenem breakpoints.
All laboratories should adopt the current carbapenem breakpoints now! Carbapenem-resistant Enterobacteriaceae (CRE) have been designated an urgent public health threat by the Centers for Disease Prevention and Control (CDC), and the use of current breakpoints is imperative for both patient treatment and infection control. Carbapenems are a mainstay therapy for infections caused by Enterobacteriaceae that are not susceptible to extended-spectrum cephalosporins due to extended-spectrum β-lactamase (ESBL), chromosomal AmpC, or other resistance mechanisms. Carbapenem usage may be soon amplified by the results of the MERINO trial, which documented a significant treatment advantage for meropenem over piperacillin-tazobactam for treatment of ESBL-producing Escherichia coli and Klebsiella pneumoniae bloodstream isolates (5). For carbapenem therapy, significant differences in 30-day mortality for patients have been observed based on carbapenem MIC, with one study showing a 38.9% 30-day mortality if the carbapenem MIC was 2 to 8 µg/ml, as opposed to 5.6% if the isolate carbapenem MIC was ≤1 µg/ml (6). Furthermore, the application of obsolete carbapenem breakpoints to a collection of carbapenemase-producing Enterobacteriaceae was shown to result in 19% being interpreted as susceptible to meropenem (1). Many laboratories continue to use the modified Hodge test (MHT) and obsolete breakpoints (1). This practice is inferior to the use of current breakpoints, as the MHT is no longer recommended as a reliable phenotypic test for carbapenemase production (7), yielding significant uncertainty regarding the isolate’s true susceptibility to the carbapenems.
The use of current carbapenem breakpoints is also imperative to public health initiatives. Computer modeling suggested ongoing use of obsolete breakpoints alone was responsible for a 3% to 5% annual increase in the prevalence of CRE, due to missed opportunities for infection control interventions (8). Laboratories may find supplementation of current carbapenem breakpoints with a carbapenemase test (such as the modified carbapenem inactivation method, Carba-NP, or molecular testing) a useful practice for infection control purposes, but testing to identify the carbapenem resistance mechanism does not supplant the need to adopt current breakpoints, as not all carbapenem resistance is due to carbapenemase and no carbapenemase test detects all carbapenemases (1).
Enterobacteriaceae: aztreonam, ceftriaxone, cefotaxime, ceftazidime, ceftizoxime, and cefepime breakpoints.
Current extended-spectrum cephalosporin and aztreonam breakpoints should be adopted by all laboratories that have not yet done so. The CLSI first began discussions to revise the aztreonam, ceftriaxone, cefotaxime, ceftazidime, and ceftizoxime breakpoints for the Enterobacteriaceae in 1994, when an increasing extended-spectrum β-lactamase (ESBL) prevalence among the Enterobacteriaceae led to the recognition that the breakpoints were too high to predict clinical outcomes. The CLSI introduced the ESBL test to the M100 as an interim measure to address this public health threat (3) and subsequently made revisions to the breakpoints in 2005 based on data described elsewhere (9). ESBL screening and confirmatory testing were found unnecessary when applying the revised (current) breakpoints. It took the CLSI and FDA CDER 5 years to reach alignment on the processes for how to implement the revision, and the current breakpoints were published in 2010 (3).
Cefepime breakpoints were not adjusted until 2014, as the pharmacokinetic/pharmacodynamic PK/PD data reviewed in 2010 supported the now-obsolete breakpoints. However, a review of the breakpoints in 2013 with new PK/PD and clinical outcome data supported a revision. One consideration that made establishing a revised breakpoint for cefepime challenging was the number of FDA-approved cefepime doses, each of which predicted a different susceptible breakpoint. As such, the CLSI introduced the SDD designation.
Widespread adoption of the current breakpoints has been painstakingly slow. A major hurdle is that not all cASTs manufacturers have obtained FDA clearance with current breakpoints, in particular, for ceftazidime (Table 4). In addition, many laboratories have been reluctant to adopt these changes, due either to the belief that clinical outcomes are best predicted by ESBL presence or absence or to infection control concerns. It should be emphasized that the change to current breakpoints does not preclude the use of ESBL testing for infection control or patient care purposes. Importantly, most laboratories that employ cASTs or the CLSI ESBL confirmatory test only report ESBLs in Escherichia coli, Klebsiella species, and Proteus species; however, other species of Enterobacteriaceae may harbor ESBLs. This testing gap may serve as a reservoir for silent transmission. The use of current breakpoints is the best method by which to predict the probability of therapeutic response for these species of Enterobacteriaceae, as it allows detection of MICs that would predict a high likelihood of treatment failure (9).
Salmonella spp.: fluoroquinolone breakpoints.
Over the course of the past several years, the CLSI has updated the fluoroquinolone breakpoints for Salmonella spp. several times (Table 1), as has been described elsewhere (10–12). Treatment of nontyphoidal Salmonella (NTS) infections limited to the gut usually consists of fluid and electrolyte replacement; antimicrobial treatment of NTS diarrhea is not required and may in fact prolong the carrier state. Routine AST is not necessary for isolates recovered from stool cultures; however, certain patient populations (e.g., infants and those immunocompromised) may be considered for antimicrobial therapy, in which case, AST would be warranted. Isolates recovered from patients with disseminated disease, indicated by isolation of Salmonella spp. from specimens other than stool, should be subjected to AST. Enteric fever, caused by Salmonella typhi and Salmonella paratyphi, is always managed with antimicrobial therapy, and AST should be performed on these isolates.
When AST is performed for Salmonella spp., the CLSI recommends testing a fluoroquinolone and interpretation of results with Salmonella-specific MIC breakpoints for ciprofloxacin, levofloxacin, and ofloxacin. Salmonella-specific disk diffusion breakpoints for ciprofloxacin are available, but none have been set for levofloxacin or ofloxacin. Because U.S. laboratories are likely to encounter Salmonella spp. sporadically, AST can be conducted on a per-request basis, using manual methods such as ciprofloxacin disk diffusion or ciprofloxacin/levofloxacin gradient diffusion, which perform well (10, 11). In the past, nalidixic acid was used as a surrogate for fluoroquinolone resistance in Salmonella. However, Salmonella isolates with some fluoroquinolone resistance mechanisms (such as the plasmid-mediated quinolone resistance [PMQR] gene) may test susceptible to nalidixic acid but resistant to ciprofloxacin; importantly, these resistance mechanisms are increasing (12). Clinical data demonstrating the success of fluoroquinolone therapy for extraintestinal salmonellosis are directly linked to MICs of ≤0.06 µg/ml for ciprofloxacin and ≤0.12 µg/ml for levofloxacin (12), and these agents are used by most physicians when treating this disease.
Pseudomonas aeruginosa and Acinetobacter spp.: carbapenem breakpoints.
Multidrug resistance among the nonfermenting Gram-negative bacteria is a significant concern for many institutions. Carbapenems are often used as primary therapeutic choices for infections due to isolates in this organism group, and the probability of outcomes are best reflected by the current breakpoints, which are recognized by the FDA. All cASTs in the United States, except for Vitek 2 for meropenem and P. aeruginosa, have obtained FDA clearance for the current breakpoints. If laboratories are Vitek 2 users, they should contact the manufacturer to learn when the breakpoints will be updated. Meropenem breakpoints for Acinetobacter spp. have not been updated by any systems, as the FDA has yet to recognize Acinetobacter spp. meropenem breakpoints. The CLSI rationale document for meropenem breakpoints for Acinetobacter spp. is under review by the FDA at the time of this writing, and the FDA has indicated this is a priority for the agency.
Pseudomonas aeruginosa: piperacillin-tazobactam breakpoints.
The obsolete piperacillin-tazobactam breakpoint is a poor predictor of clinical response for P. aeruginosa infections, which was recognized by the CLSI in 2005. A warning comment was added to the M100 in 2006 while breakpoints were under evaluation regarding the need for high-dose therapy for serious infections, the likelihood of clinical failure associated with monotherapy for susceptible isolates, and the need to administer a second antimicrobial agent (fluoroquinolone or aminoglycoside) with in vitro activity against the isolate. A study performed in 2008 confirmed these warnings, evaluating 34 patients with bacteremia caused by P. aeruginosa isolates with MICs of 32 to 64 µg/ml (susceptible by obsolete breakpoints, but intermediate by current breakpoints). This study documented an 85.7% mortality for patients treated with piperacillin-tazobactam versus 22.2% if treated with another antimicrobial (13). The above-mentioned warnings were removed from CLSI M100 when breakpoints were updated in 2012, and a comment was added to indicate the need for a dose of 3 g every 6 h (q6h) for susceptible isolates. Some cASTs have not updated piperacillin-tazobactam breakpoints for P. aeruginosa (Table 4), and it is unclear if laboratories that are using the obsolete breakpoints are adding the former CLSI-recommended comments to patient reports. The risk of problematic reporting is highest in institutions that do not have dedicated staff (e.g., infectious diseases or pharmacy) that are knowledgeable about piperacillin-tazobactam dosing in the context of P. aeruginosa MICs. Continued use of the obsolete breakpoint is a significant patient safety concern.
In contrast, ticarcillin-clavulanate is no longer available globally, and so laboratories can cease reporting this agent and there is no need to update breakpoints.
PRIORITY 2 BREAKPOINTS
Enterobacteriaceae: cefazolin breakpoints.
In 2010, the CLSI updated the cefazolin breakpoint for the Enterobacteriaceae, based on target attainment for the FDA-approved dose of cefazolin (1 g q8h); this breakpoint was recognized by the FDA. In 2011, the CLSI revised the cefazolin breakpoint a second time, primarily due to the recognition that the dose of cefazolin used most often clinically (2 g q8h) predicted a higher susceptible breakpoint than the FDA-approved dose; however, the FDA has not recognized this current CLSI breakpoint (Table 1). In 2014, the CLSI further approved testing cefazolin as a surrogate for the oral cephalosporins cefaclor, cefdinir, cefpodoxime, cefprozil, cefuroxime, cephalexin, and loracarbef for treatment of uncomplicated urinary tract infections caused by E. coli, K. pneumoniae or Proteus mirabilis (14). Breakpoints for cefazolin were expanded to include systemic use of intravenous (i.v.) and intramuscular cefazolin for uncomplicated urinary tract infections (UTIs) in 2016. The reason the urine-specific cefazolin susceptible breakpoint is ≤16 µg/ml and the systemic breakpoint is ≤ 2 µg/ml is because cefazolin concentrates in the urine, allowing a much higher probability of treatment success for isolates with MICs of 4, 8, and 16 µg/ml at that anatomical site compared to that in blood (14).
No cASTs has obtained FDA clearance for either current FDA or CLSI systemic cefazolin breakpoints, and many do not have concentrations of antimicrobial low enough for laboratories to apply these breakpoints (Table 2). The stated reason manufacturers have not attempted clearance for their cASTs with the current CLSI breakpoints has to do with the fact that both the FDA and CLSI susceptible breakpoints (≤1 µg/ml and ≤2 µg/ml, respectively) bisect the wild-type MIC mode values for E. coli and Klebsiella spp., which are 1 µg/ml and 2 µg/ml, respectively. As such, a higher than normal error rate is seen when testing to compare a cASTs to a reference method, as wild-type isolates may yield MICs that are intermittently susceptible and resistant by either method, due to the inherent 2-fold (± 1-fold) dilution variability of MIC testing. The FDA has not yet recognized the urine breakpoint, and as such, cASTs may not submit data to the FDA for clearance of their devices with this breakpoint.
The decision to adopt cefazolin breakpoints for systemic use, treatment of uncomplicated urinary tract infections, and/or as a surrogate test for oral cephalosporins should be discussed with antimicrobial stewardship teams, physicians, and pharmacies at individual institutions. Regardless of the path, all options would require the laboratory to apply the breakpoint off-label on their cASTs (for the urine breakpoints) or through the use of alternative test methods (for the systemic breakpoint, as not all of the cASTs have dilutions low enough to permit testing with the CLSI and/or FDA systemic breakpoints). While some institutions use cefazolin as a deescalation agent for bloodstream infections caused by susceptible isolates of E. coli and Klebsiella spp., many hospitals have relegated the use of cefazolin to a presurgical prophylaxis agent. If this is the case, laboratories need not develop a strategy to adopt current cefazolin systemic breakpoints, as most presurgical use is for Gram-positive coverage. If, however, it is determined cefazolin is used as a deescalation option by clinicians, laboratories should develop an algorithm, either by modifying the breakpoints on their existing cASTs if possible or through use of an alternative test system if requested. An example algorithm is in Fig. S1 in the supplemental material.
Not all hospitals use oral cephalosporins or cefazolin to treat uncomplicated urinary tract infections. Indeed, these agents are only recommended for treatment of uncomplicated cystitis by the Infectious Diseases Society of America (IDSA) when other agents (nitrofurantoin, trimethoprim-sulfamethoxazole, and fosfomycin) cannot be used. The IDSA further warns that beta-lactams generally have lower efficacy and more side effects than the first-line agents (15). Nonetheless, there are circumstances when cephalosporins may be considered for uncomplicated cystitis, and the laboratory should determine if these exist in routine practice at their institution. An example may be those institutions that manage care for a large number of elderly patients, for whom nitrofurantoin may be counterindicated due to diminished renal function, trimethoprim-sulfamethoxazole resistance rates are >20%, and fluoroquinolone resistance is high.
No cASTs has obtained FDA clearance for cefazolin as a surrogate test for the oral cephalosporins, although this could theoretically be done by demonstrating analytical performance as a surrogate for oral cephalosporin MICs, by FDA breakpoints. To do this, the manufacturer would have to demonstrate the correlation of cefazolin MICs with those of various oral cephalosporins on their system. Given the number of oral cephalosporins in use, this is a large endeavor for all manufacturers and unlikely to be a priority. In contrast, FDA clearance of urine cefazolin breakpoints on cASTs is not possible, as the FDA has not recognized this breakpoint. Nonetheless, all cASTs should be able to accommodate the urine breakpoints, should laboratories choose to implement them. Many laboratories have found the reporting of cefazolin results as a surrogate for the oral cephalosporins to be challenging, and some have chosen to report this on patient reports as “oral cephalosporins” as opposed to “cefazolin,” akin to what is done for Staphylococcus aureus and cefoxitin, where results are reported for oxacillin rather than the surrogate agent, cefoxitin. An alternative approach has been to report the cefazolin MIC without interpretation but with a comment explaining the interpretation (e.g., ≤16 µg/ml is susceptible) when the infection is an uncomplicated urinary tract infection.
Enterobacteriaceae: fluoroquinolone breakpoints.
In 2018, the CLSI reviewed data compiled and used by USCAST and EUCAST to revise the ciprofloxacin and levofloxacin breakpoints for Enterobacteriaceae, other than Salmonella spp. These data demonstrated, for critically ill patients, the probability of target attainment for ciprofloxacin and levofloxacin was low for isolates with MICs of >0.25 µg/ml for ciprofloxacin and >0.5 µg/ml for levofloxacin. These data are described in detail elsewhere (S. Butler-Wu, in preparation).
The current CLSI ciprofloxacin and levofloxacin breakpoints have not been recognized by the FDA and as such are not FDA cleared on cASTs (Table 4). Off-label implementation of the breakpoints can, however, be done by some systems (Table 4). Reporting isolates with ciprofloxacin MICs of ≤1 µg/ml or levofloxacin MICs of ≤2 µg/ml (i.e., susceptible by the obsolete breakpoints) is not an acceptable practice, as these isolates may be susceptible, intermediate, or resistant with the current breakpoints. Because the majority of Enterobacteriaceae have MICs of ≤1 µg/ml to ciprofloxacin or MICs of ≤2 µg/ml to levofloxacin, testing all isolates that meet these criteria by a manual method (gradient diffusion or disk diffusion) is not likely to be feasible. Data from the SENTRY collection reviewed by the CLSI during breakpoint deliberations demonstrated that 81% of U.S. isolates of Enterobacteriaceae had a ciprofloxacin MIC of ≤1 µg/ml and 82.3% of isolates had a levofloxacin MIC of ≤2 µg/ml in 2011 to 2012 (S. Butler-Wu, in preparation).
One alternative is to only report fluoroquinolone MICs when specifically requested by the treating physician for select specimen types and when requested to test isolates with MICs of ≤1 µg/ml to ciprofloxacin or ≤2 µg/ml to levofloxacin by an alternative methodology. By doing so, the laboratory could focus testing only for those cases where a fluoroquinolone is being considered for therapy. For example, fluoroquinolone usage is being deemphasized by the FDA and IDSA for treatment of uncomplicated urinary tract infections, and some institutions have followed suit due to risk of collateral damage (e.g., Clostridioides difficile) and adverse drug effects (16) associated with these antimicrobials. As such, not reporting a fluoroquinolone for urine isolates (the majority of isolates tested by the laboratory) may be a viable option, provided institutional leadership is in agreement. One Canadian study demonstrated that the restriction of fluoroquinolone susceptibility result reporting on the laboratory report was associated with a significant decrease in fluoroquinolone usage and resistance in P. aeruginosa (17). However, laboratories are cautioned that some institutions utilize fluoroquinolones as prophylaxis agents for critical patients (e.g., for patients with hematological malignancies and prolonged neutropenia), and the knowledge of fluoroquinolone susceptibility for isolates recovered from these patients while on prophylaxis is likely to be desired on a routine basis.
Pseudomonas aeruginosa: fluoroquinolone breakpoints.
Similar to that for the Enterobacteriaceae, the CLSI updated the fluoroquinolone breakpoints for P. aeruginosa in 2019 (S. Butler-Wu, in preparation). Also similar to the situation with the Enterobacteriaceae, the P. aeruginosa breakpoints have not been recognized by the FDA and are not available on cASTs. The breakpoints were lowered by a single dilution (i.e., susceptible breakpoint of ≤0.5 versus ≤1 µg/ml and ≤1 versus ≤2 µg/ml for ciprofloxacin and levofloxacin, respectively). This change is predicted to impact only 10% of isolates, and most systems can accommodate the breakpoint revision, if validated off-label (Table 4).
Enterococcus spp.: daptomycin breakpoints.
Daptomycin breakpoints were updated in 2019 by the CLSI, for the enterococci, in response to overwhelming literature that demonstrated poor treatment outcomes for patients infected with vancomycin-resistant Enterococcus (predominantly Enterococcus faecium) if the MIC was >1 µg/ml and standard doses of daptomycin (6 mg/kg/day) were used. The current breakpoint for daptomycin includes a new susceptible dose-dependent breakpoint of 2 to 4 µg/ml and a new resistant breakpoint of ≥8 µg/ml. Obsolete breakpoints include only a susceptible breakpoint of ≤4 µg/ml. The susceptible dose-dependent category is intended for serious infections (e.g., endocarditis) caused by enterococci, where doses of 10 to 12 mg/kg/day have been shown to be more effective than the standard dose (18, 19). These elevated doses of daptomycin are not, however, FDA approved.
One significant challenge for this current breakpoint is that it bisects the wild-type population of E. faecium, where the modal MIC is 2 to 4 µg/ml. As such, a single isolate may test S, SDD, or R based on MIC variability, which appears to be greater for E. faecium than other bacteria (20). This variability of results makes validation of the breakpoint a challenge, regardless of test methodology. This challenge is not as great for Enterococcus faecalis, but most E. faecalis isolates are ampicillin and vancomycin susceptible, and as such, daptomycin therapy would only be considered in special circumstances. The CLSI reviewed the E. faecium testing challenges in January 2019 and opted to further refine the E. faecium-specific breakpoints, with an SDD category of ≤4 µg/ml, a resistant category of ≥8 µg/ml, and no susceptible category. The daptomycin breakpoints for other enterococcal species (including E. faecalis) were also revised, to a susceptible category of ≤2 µg/ml, an intermediate category of 4 µg/ml, and a resistant category of ≥8 µg/ml. These breakpoints are present as a footnote in Table 1 and will be published in the M100 30th edition in January 2020. Laboratories should therefore wait until more definitive information is available before updating daptomycin breakpoints. In the interim, laboratories may consider the following steps: (i) ensure the species is identified and reported when an Enterococcus is recovered from blood culture, given treatment failures for daptomycin therapy have predominantly been documented for E. faecium infections; (ii) consider adding a comment to the laboratory report when E. faecium is isolated from blood regarding the value of an infectious diseases consult to optimize daptomycin dosing regimen, with consideration of doses of 8 to 12 mg/kg/day, as will be suggested by the CLSI in the M100 S 30th edition.
PRIORITY 3
Pseudomonas aeruginosa: colistin.
Current CLSI colistin breakpoints for P. aeruginosa exclude an intermediate category; isolates that were historically considered intermediate to colistin (MIC of 4 µg/ml) are now interpreted as resistant. Colistin testing is a significant challenge to the clinical laboratory, and the only CLSI-endorsed method is broth microdilution, which is rarely performed in clinical laboratories. The FDA has not recognized any colistin breakpoints (CLSI or otherwise), and as such, there are no FDA-cleared cASTs available for colistin in the United States. Alternative agents (e.g., ceftolozane-tazobactam) may be more efficacious than colistin and should be considered first-line agents, when susceptible, for infections due to multidrug-resistant P. aeruginosa. Should the laboratory choose to perform colistin testing, due to physician demand and/or local epidemiology, it would require a validation study using current CLSI breakpoints. It should be noted that the CLSI now indicates laboratories can extrapolate the polymyxin B MIC based on the colistin MIC (but not vice versa).
Staphylococcus aureus: ceftaroline breakpoints.
The ceftaroline breakpoint for Staphylococcus aureus was revised in 2019 to introduce an SDD interpretation for isolates with ceftaroline MICs of 2 to 4 μg/ml. This SDD category is based on a dosage of ceftaroline that is not currently FDA approved, i.e., 600 mg q8h, infused over 2 h. Because this dose of ceftaroline is not commonly used in the United States, at this point, it is not necessary for laboratories to update the breakpoints. In contrast, laboratories in South America, which see more isolates with MICs of 1 or 2 µg/ml to ceftaroline and have access to this dosing regimen, may consider adopting the revised breakpoint. Laboratories in the United States may consider informing their institutional pharmacists of this current CLSI ceftaroline breakpoint, as they may opt to use the higher doses off-label, in select instances. It is anticipated the current CLSI ceftaroline breakpoint will not be recognized by the FDA, as the dosage regimen used to establish the SDD category is not FDA approved.
GENERAL CONSIDERATIONS TO LABORATORY ADOPTION OF CURRENT BREAKPOINTS
Laboratories must work closely with members of the antimicrobial stewardship team, infection control, pharmacy, and infectious diseases and/or others on their health care teams when determining how best to approach updating breakpoints in their facility. In the vast majority of cases (Table S1), if the antimicrobial is in use at the institution, the laboratory should report AST results with current breakpoints. It cannot be overemphasized that the implementation of current breakpoints is imperative for patient safety that requires both laboratory attention and institutional support. Some large integrated health networks have implemented routine breakpoint updates as part of the laboratory quality system.
Regardless of the institution, performing validation studies to update breakpoints when cASTs manufacturers have not yet obtained FDA clearance is a time-consuming task. As such, laboratories should approach these evaluations with a clear understanding of which breakpoint updates are the highest priority for their institution. Knowledge of antimicrobial formulary and institutional treatment guidelines may save the laboratory significant time and effort, as results for agents not in use can simply be suppressed. Furthermore, the choice between FDA, CLSI, and EUCAST breakpoints should be discussed, as these may depend on the routine dosing regimens used at the institution.
An example is the Enterobacteriaceae ceftazidime breakpoint. No cASTs have obtained FDA clearance with the current breakpoints. Many facilities use ceftazidime only for treatment of Pseudomonas aeruginosa infections. If this is the case, the laboratory may consider suppressing ceftazidime results for all Enterobacteriaceae as opposed to updating their cASTs with current ceftazidime breakpoints. However, if this path is chosen, laboratories should develop mechanisms (e.g., via laboratory information system alerts) to ensure ceftazidime results are not reported with obsolete breakpoints if a physician phones the laboratory to request results for this drug or in times of antimicrobial shortages, such as for cefepime, when ceftazidime may be used with increased frequency. Such a scenario may result in an inaccurate picture of ceftazidime activity versus other expanded cephalosporins that were tested and reported with current breakpoints.
Laboratories may also consider practical approaches to implementing current breakpoints. An example is for daptomycin and Enterococcus, where the current breakpoint for resistance (≥8 µg/ml) is the same as the obsolete nonsusceptible breakpoint. As such, laboratories could report resistant results, but suppress the MIC for susceptible isolates, with a comment regarding the use of high-dose daptomycin for treatment of E. faecium infections. Because the breakpoint for resistant versus nonsusceptible remains the same, the laboratory may not need to perform validation of their system with this strategy. However, if the laboratories adopt the current breakpoint, a validation is needed, as cASTs may not yield the same categorical agreement for a susceptible breakpoint of ≤1 µg/ml as for ≤4 µg/ml. Further details of such strategies are presented in Table S1 and Fig. S1.
RESOURCES FOR VALIDATION STUDIES FOR OFF-LABEL BREAKPOINTS
Several organizations have developed work aids to assist clinical laboratories with the validation of breakpoints on cASTs, should their system not be FDA cleared for use with current CLSI breakpoints (21). Materials from the California Department of Public Health (CADPH) can be found at https://www.cdph.ca.gov/Programs/CHCQ/HAI/Pages/CA_ARLN.aspx.
Well-characterized isolates from the CDC & FDA Antibiotic Resistance Isolate Bank are suggested for the validations, and instructions for procuring them are provided with the CADPH materials and also on the CDC website (https://wwwn.cdc.gov/ARIsolateBank). Local health departments are increasing their capacities to assist clinical laboratories with AST and are likely to provide assistance as well.
IMPACT OF IMPLEMENTATION OF CURRENT BREAKPOINTS ON LOCAL CUMULATIVE ANTIBIOGRAMS
Cumulative antibiograms list the percentage of isolates of a given species susceptible (%S) to antimicrobial agents appropriate for use in treating infections caused by the species. Results generated from routine AST of clinical isolates are used to prepare the report. Since all breakpoint revisions to date have involved lowering the susceptible breakpoint (with the exception of the urine cefazolin breakpoint), the %S in the antibiogram will likely be lower when switching to the current breakpoints. In addition, reporting of specific agents on select isolates (e.g., ciprofloxacin only when requested on isolates of Enterobacteriaceae or daptomycin only on isolates of E. faecium from blood) may misrepresent the susceptibility of isolates causing infection in the facility and skew data when comparing these agents with others that are available for all isolates. It is important to convey the implementation of the current breakpoints including any selective reporting practices to those who use cumulative antibiogram reports so %S data can be evaluated appropriately.
SUMMARY
Up until now, the CLSI AST Subcommittee only took action to revise breakpoints reactively, in response to the submission of compelling data that previous breakpoints are no longer accurate. However, it is anticipated that the CLSI will begin to proactively review the appropriateness of all breakpoints published in the M100, following a schedule according to antimicrobial class. Many of the CLSI breakpoints were set decades ago when antimicrobial resistance was less prevalent and less complex than it is today, and a need for breakpoint reevaluation is understandable. It is imperative that clinical laboratories adopt current breakpoints as soon as possible, to ensure both optimum outcomes for the individual patients the laboratory serves and to address serious antimicrobial resistance issues which threaten public health.
Supplementary Material
ACKNOWLEDGMENTS
R.M.H. is a voting member of the CLSI AST Subcommittee. J.A.H. and A.N.A. are members of CLSI AST Working Groups. R.M.H. is employed by Accelerate Diagnostics, and has received stocks from Accelerate Diagnostics. A.N.A. has honoraria from Accelerate Diagnostics, Roche, Cepheid, and Beckman Coulter.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00203-19.
REFERENCES
- 1.Humphries RM, Hindler JA, Epson E, Horwich-Scholefield S, Miller LG, Mendez J, Martinez JB, Sinkowitz J, Sinkowtiz D, Hershey C, Marquez P, Bhaurla S, Moran M, Pandes L, Terashita D, McKinnell JA. 2018. Carbapenem-resistant Enterobacteriaceae detection practices in California: what are we missing? Clin Infect Dis 66:1061–1067. doi: 10.1093/cid/cix942. [DOI] [PubMed] [Google Scholar]
- 2.McKinnell JA, Bhaurla S, Marquez-Sung P, Pucci A, Baron M, Kamali T, Bugante J, Schwartz B, Balter S, Terashita D, Butler-Wu S, Gunzenhauser J, Hindler J, Humphries RM. 2019. Public health efforts can impact adoption of current susceptibility breakpoints, but closer attention from regulatory bodies is needed. J Clin Microbiol 57:e01488-18. doi: 10.1128/JCM.01488-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphries RM, Ferraro MJ, Hindler JA. 2018. Impact of 21st Century Cures Act on breakpoints and commercial antimicrobial susceptibility test systems: progress and pitfalls. J Clin Microbiol 56:e00139-18. doi: 10.1128/JCM.00139-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphries RM, Hindler J, Jane Ferraro M, Mathers A. 2018. Twenty-first Century Cures Act and antimicrobial susceptibility testing: clinical implications in the era of multidrug resistance. Clin Infect Dis 67:1132–1138. doi: 10.1093/cid/ciy432. [DOI] [PubMed] [Google Scholar]
- 5.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL. 2018. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 320:984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel TS, Nagel JL. 2015. Clinical outcomes of Enterobacteriaceae infections stratified by carbapenem MICs. J Clin Microbiol 53:201–205. doi: 10.1128/JCM.03057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chea N, Bulens SN, Kongphet-Tran T, Lynfield R, Shaw KM, Vagnone PS, Kainer MA, Muleta DB, Wilson L, Vaeth E, Dumyati G, Concannon C, Phipps EC, Culbreath K, Janelle SJ, Bamberg WM, Guh AY, Limbago B, Kallen AJ. 2015. Improved phenotype-based definition for identifying carbapenemase producers among carbapenem-resistant Enterobacteriaceae. Emerg Infect Dis 21:1611–1616. doi: 10.3201/eid2109.150198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartsch SM, Huang SS, Wong KF, Slayton RB, McKinnell JA, Sahm DF, Kazmierczak K, Mueller LE, Jernigan JA, Lee BY. 2016. Impact of delays between Clinical and Laboratory Standards Institute and Food and Drug Administration revisions of interpretive criteria for carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 54:2757–2762. doi: 10.1128/JCM.00635-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN, Antimicrobial susceptibility testing subcommittee of the Clinical and Laboratory Standards Institute. 2013. Background and rationale for revised clinical and laboratory standards institute interpretive criteria (Breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. Cephalosporins and Aztreonam. Clin Infect Dis 56:1301–1309. doi: 10.1093/cid/cit017. [DOI] [PubMed] [Google Scholar]
- 10.Deak E, Hindler JA, Skov R, Sjolund-Karlsson M, Sokovic A, Humphries RM. 2015. Performance of Etest and disk diffusion for detection of ciprofloxacin and levofloxacin resistance in Salmonella enterica. J Clin Microbiol 53:298–301. doi: 10.1128/JCM.02715-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deak E, Skov R, Hindler JA, Humphries RM. 2015. Evaluation of surrogate disk tests for detection of ciprofloxacin and levofloxacin resistance in clinical isolates of Salmonella enterica. J Clin Microbiol 53:3405–3410. doi: 10.1128/JCM.01393-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries RM, Fang FC, Aarestrup FM, Hindler JA. 2012. In vitro susceptibility testing of fluoroquinolone activity against Salmonella: recent changes to CLSI standards. Clin Infect Dis 55:1107–1113. doi: 10.1093/cid/cis600. [DOI] [PubMed] [Google Scholar]
- 13.Tam VH, Gamez EA, Weston JS, Gerard LN, Larocco MT, Caeiro JP, Gentry LO, Garey KW. 2008. Outcomes of bacteremia due to Pseudomonas aeruginosa with reduced susceptibility to piperacillin-tazobactam: implications on the appropriateness of the resistance breakpoint. Clin Infect Dis 46:862–867. doi: 10.1086/528712. [DOI] [PubMed] [Google Scholar]
- 14.Schuetz AN, Brasso WB, Crandon JL, Hardy DJ, Jenkins SG, Jones RN, Knapp CC, Reller LB. 2013. Cefazolin as a class representative for oral cephalosporins and uncomplicated urinary tract infections caused by indicated Enterobacteriaceae. Diagn Microbiol Infect Dis 77:381–382. doi: 10.1016/j.diagmicrobio.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 16.Tandan M, Cormican M, Vellinga A. 2018. Adverse events of fluoroquinolones vs. other antimicrobials prescribed in primary care: a systematic review and meta-analysis of randomized controlled trials. Int J Antimicrob Agents 52:529–540. doi: 10.1016/j.ijantimicag.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Langford BJ, Seah J, Chan A, Downing M, Johnstone J, Matukas LM. 2016. Antimicrobial stewardship in the microbiology laboratory: impact of selective susceptibility reporting on ciprofloxacin utilization and susceptibility of Gram-negative isolates to ciprofloxacin in a hospital setting. J Clin Microbiol 54:2343–2347. doi: 10.1128/JCM.00950-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphries RM, Pollett S, Sakoulas G. 2013. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev 26:759–780. doi: 10.1128/CMR.00030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidd JM, Abdelraouf K, Asempa TE, Humphries RM, Nicolau DP. 2018. Pharmacodynamics of daptomycin against Enterococcus faecium and Enterococcus faecalis in the murine thigh infection model. Antimicrob Agents Chemother 62:e00506-18. doi: 10.1128/AAC.00506-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campeau SA, Schuetz AN, Kohner P, Arias CA, Hemarajata P, Bard JD, Humphries RM. 2018. Variability of daptomycin MIC values for Enterococcus faecium when measured by reference broth microdilution and gradient diffusion tests. Antimicrob Agents Chemother 62:e00745-18. doi: 10.1128/AAC.00745-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorgensen JH. 2012. Putting the new CLSI cephalosporin and carbapenem breakpoint changes into practice in clinical microbiology laboratories. J Pediatric Infect Dis Soc 1:169–170. doi: 10.1093/jpids/pis047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.