CASE
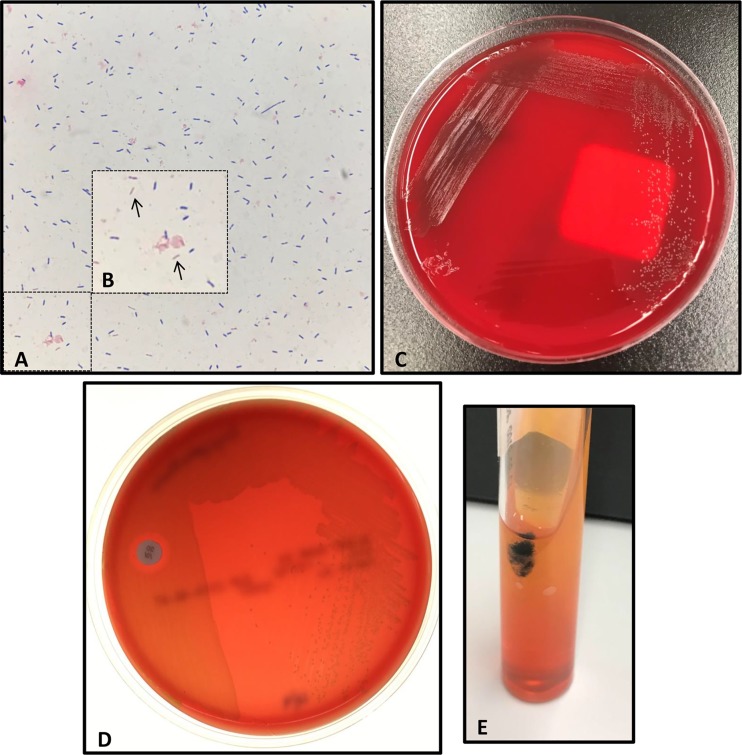
A 59-year-old male with a past medical history of rheumatoid arthritis for which he was on high-dose prednisone (20 mg daily) for more than 3 years, chronic obstructive pulmonary disease, chronic lower back pain, lower extremity neuropathy, gout, and hypertension presented to the emergency department (ED) with a 2-day history of increasing shortness of breath and subjective fever. The patient had a family history significant for heart disease but denied any personal history of coronary artery or valvular heart disease. The patient also noted an ulceration on his right great toe with drainage for 1 to 2 months. Upon arrival in the ED, the patient was tachycardic (heart rate, 100 to 149 bpm) and febrile to 39.4°C with normal respiration (blood oxygen saturation [SpO2], 94%). Initial laboratory studies were significant for elevated white blood cells at 18,600 cells/mm3 (reference, 3,800 to 9,000), 91.4% neutrophils, lactate at 5.9 mmol/liter (reference, 0.5 to 2.2), and N-terminal pro-b-type natriuretic peptide (NT-pro-BNP) of 151 (reference, 52 to 125 pg/ml). On physical examination, a 1-cm ulcer was noted on his great right toe that was associated with erythema but from which no sinus tract or significant purulence was noted. Chest, abdomen, and pelvis computed tomography with contrast revealed minor bronchiolar densities in the right upper lobe, likely representing early pulmonary bronchiolitis. Two sets of blood cultures were obtained, and the patient was started on intravenous (i.v.) levofloxacin and i.v. metronidazole. Twelve hours into incubation, the aerobic and anaerobic bottles (VersaTrek, TREK Diagnostic Systems, Cleveland, OH) of both sets of blood cultures signaled positive, and short Gram-positive bacilli with rounded ends were observed on Gram stain (Fig. 1A and B), prompting initiation of empirical treatment with i.v. vancomycin, cefepime, and metronidazole. Within 1 day, pinpoint, alpha-hemolytic colonies grew on 5% sheep blood and chocolate agars incubated at 37°C in 5% CO2 and brucella blood agars incubated anaerobically (Fig. 1C). The isolate was identified as Erysipelothrix rhusiopathiae with scores of 2.26 and 2.14 using the research use only (RUO) BDAL v7 database of the Bruker biotyper matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Billerica, MA), indicating a high-confidence species-level identification (scores, >2.0). Additional biochemical testing demonstrated that the isolate was catalase negative, resistant to vancomycin (30 µg; BD, Sparks, MD), and produced hydrogen sulfide when incubated on triple sugar iron (TSI) Agar (Remel, Lenexa, KS), consistent with Erysipelothrix rhusiopathiae (Fig. 1D and E).
FIG 1.
Phenotypic and biochemical features of Erysipelothrix rhusiopathiae. (A) Gram stain of positive blood culture broth demonstrating Gram-positive bacilli. (B) Inset of image (A) highlighting Gram-positive bacilli appearing at times Gram negative (arrows). (C) Small, alpha-hemolytic colonies of E. rhusiopathiae growing on 5% sheep blood agar after 3 days of incubation. (D) Growth of E. rhusiopathiae isolate abuts 30-µg vancomycin disk demonstrating vancomycin resistance. (E) Hydrogen sulfide production is noted by formation of black precipitate when a triple sugar iron (TSI) Agar slant is inoculated with E. rhusiopathiae.
Following report of the E. rhusiopathiae result, the patient’s antimicrobial therapy was switched to i.v. penicillin G and a transesophageal echocardiogram (TEE) was performed. A 0.9- by 0.5-cm vegetation was seen on the posterior mitral leaflet consistent with endocarditis. No significant valve destruction was found, precluding surgical intervention, but mild mitral regurgitation was noted. The open wound on the patient’s right great toe was thought to be the source of infection, as the patient reported frequently walking barefoot at home and has pet cats and dogs. Drainage fluid from the ulcer was submitted for aerobic culture. Gram stain of the primary specimen demonstrated no inflammatory cells or organisms seen, and the culture grew rare mixed Gram-positive organisms. X-ray and magnetic resonance imaging of the right foot revealed soft tissue swelling and no evidence of osteomyelitis. The patient remained clinically asymptomatic and was discharged with 6 weeks of i.v. penicillin G. At 4 weeks postdischarge, the patient was readmitted with low-grade fevers and left upper extremity swelling consistent with deep vein thrombosis secondary to the peripherally inserted central catheter line. Posttreatment blood cultures remained negative, and follow-up TEE findings were unchanged compared with those of previous studies. The patient completed the remaining week of antimicrobial therapy with i.v. ceftriaxone and recovered with no further complications.
DISCUSSION
Erysipelothrix rhusiopathiae is a facultative anaerobic non-spore-forming Gram-positive bacillus. First isolated by Koch in 1880, E. rhusiopathiae was identified as the causative agent of swine erysipelas and later recovered from a human patient with localized infection in 1909 (1). E. rhusiopathiae is widely distributed but is most frequently associated with pigs and can also be found in the digestive tracts of various animals, including fish, sheep, and cattle, as well as dogs and cats. Two additional species in the genus, namely Erysipelothrix tonsillarum and Erysipelothrix inopinata, have been recovered from the tonsils of healthy pigs and cattle and the environment. Although a rare cause of human infection, E. rhusiopathiae enters the environment when shed in the urine or feces of infected animals and are transmitted to humans from direct contact through cuts, abrasions, and injuries.
E. rhusiopathiae is largely a zoonotic pathogen that can cause disease in a variety of animals. In pigs, E. rhusiopathiae can cause an acute infection characterized by septicemia and diffuse erythema resulting in sudden death. Subacute and chronic infections presenting as cutaneous lesions or local arthritis and endocarditis can also occur. In other animals, including sheep, cattle, and birds, E. rhusiopathiae can cause skin lesions and arthritis and can result in death (2).
Human Erysipelothrix sp. infection is rare and is usually due to exposure to infected animals, such as fish or swine. As such, individuals with occupational exposures to animals or animal products, such as veterinarians, abattoir workers, and fisherman, are at greatest risk for infection (3). Infections can manifest in one of the following three forms: (i) a localized cutaneous lesion or erysipeloid, (ii) a generalized cutaneous form, and (iii) a septicemic form which is often associated with infectious endocarditis. In the localized cutaneous form, the erysipeloid develops 2 to 4 days postexposure, and infection is typically limited to the fingers or hand. Surface swabs are not useful for isolation of E. rhusiopathiae from cutaneous lesions since the organisms reside deep in the subdermal layer. Rather, biopsy specimens are optimal for isolation of the organism. In bloodstream infections, E. rhusiopathiae can be recovered from standard blood cultures and is strongly associated with endocarditis. E. rhusiopathiae endocarditis occurs in patients with underlying heart conditions and animal exposure and can have mortality approaching 40% (1). Although as many as 90% of E. rhusiopathiae bloodstream infections are associated with endocarditis, newer reports suggest endocarditis due to systemic E. rhusiopathiae infection is rare (4). In the absence of occupational exposures, risk factors for invasive Erysipelothrix rhusiopathiae infection include immunocompromising conditions, such as chronic kidney disease, diabetes mellitus, and treatment with high-dose steroids (as was the case with this patient). In patients with E. rhusiopathiae endocarditis, excessive alcohol use, in addition to underlying heart conditions, has also been identified as a significant risk factor (1, 4).
Identification of Erysipelothrix species can present some diagnostic challenges. The organism can have an uneven Gram stain appearance and may even appear Gram negative at times, similar to this case (Fig. 1A and B). Colonies are pinpoint and alpha-hemolytic on routine medium, as shown in Fig. 1C, and the short Gram-positive rods can resemble streptococci and enterococci. Similar to enterococci, E. rhusiopathiae is also positive for pyrrolidonyl aminopeptidase (PYR) activity, which may contribute to misidentification. Erysipelothrix species can be differentiated from enterococci by the ability to produce hydrogen sulfide (H2S) on TSI Agar (Fig. 1E). Biochemical testing and Gram stain appearance may readily distinguish Erysipelothrix sp. isolates from other non-spore-forming Gram-positive bacilli, including Corynebacterium spp., Listeria monocytogenes, and Lactobacillus spp. (Table 1). Positive catalase reactions differentiate Corynebacterium spp. and Listeria spp. from Erysipelothrix spp., which are negative for most rapid biochemical tests, including catalase, oxidase, indole, methyl red, Voges-Proskauer, and hydrolysis of esculin or urea (5). Lactobacillus species are catalase-negative long, slender Gram-positive bacilli that can be vancomycin resistant and are also associated with bloodstream infection and endocarditis. However, Lactobacillus species are negative for H2S production on TSI Agar. Another unique characteristic of E. rhusiopathiae is the “bottle-brush” or “pipe cleaner” appearance of growth in a gelatin stab. Further biochemical testing is necessary for species-level differentiation of E. rhusiopathiae from E. tonsillarum, which ferments sucrose and is positive for alkaline phosphatase (5).
TABLE 1.
Biochemical characteristics differentiating Erysipelothrix rhusiopathiae from select non-spore-forming Gram-positive bacillia
| Characteristic | Erysipelothrix spp. | Lactobacillus spp. | Listeria spp. | Corynebacterium spp. |
|---|---|---|---|---|
| Gram stain | Short bacilli, rounded ends; occurs singly, short chains or long filaments | Long and slender bacilli; occurs singly or in chains | Short, regular bacilli; occurs singly or short chains | Pleomorphic coccobacilli |
| Size | ||||
| Width (μm) | 0.2–0.5 | 0.5–1 | 0.4–0.5 | 0.3–0.6 |
| Length (μm) | 0.8–2.5 | 2–10 | 0.5–2 | 1.5–8 |
| Hemolysis | α | α/None | β | None |
| Catalase | − | − | + | + |
| Esculin hydrolysis | − | + | + | −b |
| H2S on TSI | + | − | − | − |
| Motility | − | − | + | − |
| Notable intrinsic resistance | Vancomycin | Vancomycinc | Cephalosporins | None |
+, positive; −, negative.
Rare Corynebacterium species are positive for esculin hydrolysis.
Some Lactobacillus species are intrinsically vancomycin resistant, including Lactobacillus rhamnosus and Lactobacillus casei/L. paracasei, among others.
Automated identification systems, including Vitek2 (bioMérieux, Durham, NC) and BD Phoenix (BD Diagnostics, Sparks, MD), as well as the API Coryne and Strep systems (bioMérieux, Durham, NC), can reliably identify E. rhusiopathiae. MALDI-TOF mass spectrometry can also identify E. rhusiopathiae. FDA-cleared databases for both Bruker biotyper CA and Vitek MS systems do not include Erysipelothrix species. However, the Bruker biotyper RUO database includes mass spectral profiles (MSP) for both E. rhusiopathiae and E. tonsillarum, while the Vitek MS KnowledgeBase RUO library only includes E. rhusiopathiae. While 16S rRNA sequencing has been used to identify Erysipelothrix species, the >99% sequence identity of the 16S rRNA gene between some Erysipelothrix species may preclude use of this method for species-level identification (2).
Erysipelothrix spp. are intrinsically resistant to vancomycin; thus, prompt and accurate identification is critical to ensure effective antibiotic therapy. Ampicillin and penicillin are the treatment of choice for both localized and systemic Erysipelothrix infections, as resistance to these agents has not been described. Other beta-lactams, including extended-spectrum cephalosporins and carbapenems, have comparable in vitro activity against Erysipelothrix isolates. In penicillin-allergic patients, erythromycin, clindamycin, and fluoroquinolones can be considered. Although susceptibility testing is usually unnecessary, testing may be warranted for penicillin-allergic patients, as resistance to clindamycin and erythromycin has been noted. Antimicrobial susceptibility testing guidelines and interpretive breakpoints for Erysipelothrix rhusiopathiae are published in the M45 document from Clinical and Laboratory Standards Institute (CLSI) (6). Treatment with aminoglycosides and sulfonamides is not recommended, as Erysipelothrix isolates are frequently resistant to these agents.
In summary, we present a case of Erysipelothrix rhusiopathiae bloodstream infection and endocarditis in a patient on chronic steroids due to rheumatoid arthritis. This isolate was identified using MALDI-TOF MS and confirmed with biochemical testing. Rapid and accurate identification of this isolate facilitated prompt initiation of appropriate antimicrobial therapy, which contributed to successful treatment and recovery of the patient.
SELF-ASSESSMENT QUESTIONS
- Which of the following exposures is associated with the highest risk of Erysipelothrix rhusiopathiae exposure and subsequent infection?
-
a.Sustaining a cut while working in an abattoir
-
b.Consuming unpasteurized dairy products
-
c.Hunting game in a wooded area
-
d.Harvesting crops on a farm
-
a.
- Erysipelothrix spp. exhibit which of the following phenotypes on biochemical tests, differentiating them from other aerobic, non-spore-forming Gram-positive bacilli?
-
a.Catalase (+), beta-hemolytic, H2S on triple sugar iron (TSI) Agar (−)
-
b.Catalase (+), motility (−), H2S on TSI Agar (+)
-
c.Catalase (−), alpha-hemolytic, H2S on TSI Agar (+)
-
d.Catalase (−), motility (+), H2S on TSI Agar (−)
-
a.
- Which of the following is the treatment of choice for Erysipelothrix rhusiopathiae?
-
a.Penicillin
-
b.Vancomycin
-
c.Clindamycin
-
d.Gentamicin
-
a.
For answers to the self-assessment questions and take-home points, see https://doi.org/10.1128/JCM.02032-18 in this issue.
REFERENCES
- 1.Gorby GL, James E, Peacock J. 1988. Erysipelothrix rhusiopathiae endocarditis: microbiologic, epidemiologic, and clinical features of an occupational disease. Rev Infect Dis 10:317–325. doi: 10.1093/clinids/10.2.317. [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, Chang BJ, Riley TV. 2010. Erysipelothrix rhusiopathiae. Vet Microbiol 140:405–417. doi: 10.1016/j.vetmic.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Reboli AC, Farrar WE. 1989. Erysipelothrix rhusiopathiae: an occupational pathogen. Clin Microbiol Rev 2:354–359. doi: 10.1128/CMR.2.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan EM, Marcelin JR, Adeel N, Lewis RJ, Enzler MJ, Tosh PK. 2017. Erysipelothrix rhusiopathiae bloodstream infection—a 22-year experience at Mayo Clinic, Minnesota. Zoonoses Public Health 64:e65–e72. doi: 10.1111/zph.12348. [DOI] [PubMed] [Google Scholar]
- 5.Wellinghausen N. 2015. Listeria and Erysipelothrix, p 462–473. In Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 11th ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2016. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. CLSI document M45-ED3. Clinical Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed] [Google Scholar]