Abstract
Objectives
To examine the role of the hippocampus in stress regulation in older adults with amnestic mild cognitive impairment (aMCI).
Methods
This study combined resting-state functional MRI, structural MRI, self-reported chronic stress exposure, and an electrocardiography-based acute stress protocol to compare aMCI group (n = 17) to their cognitively healthy counterparts (HC, n = 22).
Results
For the entire sample, there was a positive correlation between chronic stress exposure and acute stress regulation. The aMCI group showed significantly smaller volumes in the right hippocampus than HC. The two groups did not differ in chronic stress exposure or acute stress regulation. In the HC group, the left hippocampal connectivity with inferior parietal lobe was significantly correlated with both the chronic stress and acute stress. In the aMCI group, the left hippocampal connectivity with both the right insula and the left precentral gyrus was significantly correlated to chronic stress exposure and acute stress regulation. Additionally, the left hippocampal connectivity with right insula significantly mediated the relationship between chronic stress exposure and acute stress regulation in aMCI group.
Conclusions
Extra hippocampal networks may be recruited as compensation to attend the maintenance of relatively normal stress regulation in aMCI by alleviating the detrimental effects of chronic stress exposure on acute stress regulation.
Keywords: Hippocampus, Mild cognitive impairment, Resting-state functional connectivity, Acute stress regulation, Chronic stress exposure
Abbreviations: aMCI, amnestic Mild Cognitive Impairment; HC, healthy control; AD, Alzheimer's Disease; HPA, Hypothalamic Pituitary Adrenal; ANS, Autonomic Nervous System; HF-HRV, high frequency heart rate variability; PSS, Perceived stress scale; MOCA, Montreal Cognitive Assessment; RAVLT, Rey's Auditory Verbal Learning Test; GDS, Geriatric Depression Scale; GLM, General Linear Model; FC, functional connectivity; LHIP, left hippocampus; RHIP, right hippocampus; LIPL, left inferior parietal lobe; LPG, left precentral gyrus; Rinsula, right insula
1. Introduction
Acute stress adaptation refers to an individual's flexible response when facing acute physiological and environmental challenges (McEwen, 2013). Cumulative exposure to chronic stress can endanger an individual's acute stress adaptation, and have a long-term adverse effect on brain structure and function (McEwen and Gianaros, 2011). The hippocampus is essential in stress regulation, as it is an important top-down regulator of the Hypothalamic Pituitary Adrenal (HPA) axis where it plays a crucial role in the stress adaptation process (Jacobson and Sapolsky, 1991; Almeida et al., 2010), including the regulation of the autonomic nervous system (ANS) (Khookhor and Umegaki, 2013). It is well known that the hippocampus is enriched with receptors for cortisol, which makes the hippocampal function especially susceptible to stress (Kim and Yoon, 1998; Knapman et al., 2012). Previous studies have found that deficits in hippocampal function and structure are associated with short- or long-term after-effect from chronic or acute stress (Astur et al., 2006; Bremner et al., 2003; Carrion et al., 2010; Murakami et al., 2005).
Compared with young adults, stress regulation can be more problematic in aging people, especially those with age-associated neurodegenerative disorders. Hyperactivity of the HPA axis, resulting in increased production of the stress hormone cortisol, has been consistently reported in patients with Alzheimer's disease (AD) related dementia (Popp et al., 2015). Increased hippocampal degeneration, associated with greater cognitive impairment, has been shown to correlate with elevated self-reported chronic stress in older adults (Zimmerman et al., 2016). Furthermore, stress dysregulation, indexed by hyperactivity of the HPA axis, can accelerate the cognitive decline and dementia severity for those at risk for AD. Amnestic mild cognitive impairment (aMCI) is an intermediate stage between normal cognitive aging and AD dementia where afflicted individuals exhibit memory deficits but intact functional abilities, and is predictive of progression to AD (Ries et al., 2008). Some research indicates stress dysregulation in aMCI, indexed by HPA axis hyperactivity, to be of similar magnitude to that in AD dementia, indicating that stress dysregulation can precede the dementia disease stage. Additionally, aMCI individuals with greater stress dysregulation show more rapid declines in memory and global cognition as well as increasing dementia severity over time, compared to aMCI individuals with ‘normal’ stress reactivity (Popp et al., 2015). However, the literature on cardiovascular reactivity/recovery-indexed stress regulation in aMCI has been contradictory, with some studies suggesting dysfunction while others indicating maintenance of normal regulation (Collins et al., 2012; Zulli et al., 2005; Nicolini et al., 2014). It leads to question the relationship between central and peripheral physiological systems in stress regulation if we aim at obtaining a clear picture of stress regulation in aMCI.
Many neuroimaging studies have reported patients with AD show both disrupted structural integrity of the hippocampus (Ries et al., 2008; Sohn et al., 2014) and disruptions in the networks of the ANS that result in stress dysregulation (Collins et al., 2012; Femminella et al., 2014). However, compensatory functional networks involving the hippocampus and parietal and frontal regions have been revealed to support goal-oriented behaviors, and potentially stress regulation, in individuals with aMCI (Sohn et al., 2014; Filippi et al., 2018; Cha et al., 2013). Given the prevalence of stress dysregulation in AD and the detrimental effects that chronic stress can have on the brain, understanding the neural mechanisms of stress is critical for promoting biologically successful aging, especially in individuals at high risk of AD. However, it is still unclear whether the relationship between chronic and acute stress in aMCI can be explained by the hippocampus, especially the hippocampus involved networks.
The objective of the present study was to examine the relationship between the hippocampus and stress regulation in older adults with aMCI. We hypothesized that due to hippocampal atrophy and disrupted functional connectivity in neurodegeneration, the acute stress adaptation (indexed by the cardiovascular reactivity and recovery to acute cognitive stress) would be vulnerable to the effects of chronic stress exposure (indexed by perceived chronic stress) in the aMCI group relative to cognitively healthy ager controls (HC).
2. Methods
2.1. Participants
The data from thirty-nine participants (17 aMCI, 22 HC) was used in this study. The aMCI group were recruited from university-affiliated memory clinics using the clinical diagnosis of “mild cognitive impairment due to Alzheimer's disease” set forth by Albert et al. (2011). They had deficits in memory based on an intensive neuropsychological battery but retained intact basic activities of daily living and absence of dementia per the NINCDS-ADRDA criteria (McKhann et al., 2011). Age, sex, and education-matched HC participants without self-reported history of aMCI or dementia were recruited from the community.
2.2. Design and assessments
The study consisted of two sessions: Session 1, neuropsychological and psychosocial-behavioral interviews; Session 2, acute stress protocol. In Session 1, the neuropsychological battery included a global cognition measure (Montreal Cognitive Assessment, MOCA), a verbal memory and learning measure (Rey's Auditory Verbal Learning Test, RAVLT), a measure of perceived chronic stress exposure (the 10-item Perceived Stress Scale, PSS), and a measure of depressive systems designed for the geriatric population (Geriatric Depression Scale, GDS), as well as measures to collect background demographic information. In Session 2, the protocol included a baseline ECG recording (10 min), an ECG with computerized cognitive tasks being administered (20 min), and recovery ECG recording (10 min, after 30 min relaxation). The computerized tasks included a Stroop task and Dual 1-back task, which have been widely used to elicit cognitive/perceived stress due to their cognitive demands (Moses et al., 2007), (Castaldo et al., 2015). Each of the tasks lasted 10 min and the order was inter-balanced across participants. Immediately after the baseline ECG recording, individuals took part in the baseline fMRI scans.
2.3. Chronic stress exposure
Chronic stress exposure was measured using the Perceived Stress Scale (PSS) (Cohen et al., 1983). The PSS consists of 10 questions, in which participants rate the severity of perceived stress over the past month using a five-point scale from “Never” (0) to “Very Often” (4). A mean score across 10 items was used in the current study with higher scores indicating more severe exposure to chronic stress. The Cronbach's alpha of the items in the current study was 0.91. The PSS has been validated in older adults and can reliably represent the perceived experience of stress in this group (Ezzati et al., 2014).
2.4. ECG data acquisition and analysis
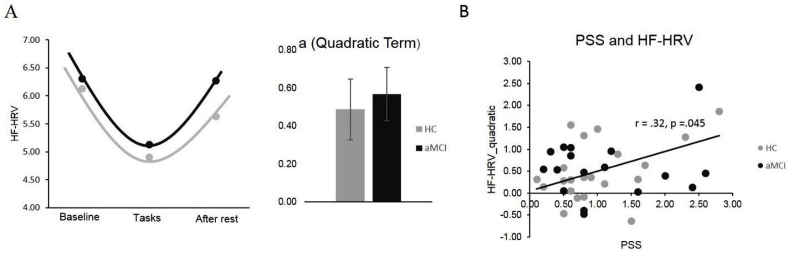
Acute stress adaptation was measured using the high frequency domain (0.15–0.40 Hz) of participants' heart rate variability (HF-HRV), which was recorded using 3-lead ECG (Mindware, https://www.mindwaretech.com/) in Session 2. HF-HRV is a sensitive biomarker of acute stress as it is representative of parasympathetic influences, which are the only cardiovascular indices capable of producing rapid, millisecond, changes in the heart's beat to beat timing (Thayer et al., 2012). The function of parasympathetic nervous system, both reactivity and recovery, reflects an individual's adaptation to stress. The ECG data was processed in HRV software (Mindware) as described previously (Berntson et al., 1997; Lin et al., 2017a). The first and last minute of each recording were excluded to avoid potential noise and intervals between R peaks were preprocessed to remove ectopic beats and artifacts. HF-HRV values were derived over 20-s intervals, as analyzing multiple short epochs to examine a dynamic signal is beneficial in psychophysiological studies in which variability changes over time (Berntson et al., 1997; Lin et al., 2016). HF-HRV data was expressed in absolute units and then natural log transformed for normalization. The mean HF-HRV was obtained by averaging the HF-HRV data in baseline, stress tasks, and recovery period, respectively. We examined the pattern of the acute stress response (baseline, mean reactivity across two tasks, and recovery) with repeated measure ANOVA for the entire sample. Different models of HF-HRV are functions used to describe and quantify the pattern of HF-HRV changes over time. Given the typical HF-HRV decrease following a cognitively stressful test (Vazan et al., 2017) the quadratic model has been found to describe brain-regulated HF-HRV acute stress response effectively (Casement et al., 2018; Lin et al., 2017b), and we found it to be superior to other models (see “Results”). This model reflects the U-shape of the HF-HRV response: high at baseline, lower during the task, and rebounded after rest. The quadratic term ‘a’ represents ‘curvature’ of HF-HRV function and how fast HF-HRV raises or drops, which is representative of stress adaptation; the linear term ‘b’ represents the minimum HF-HRV can reach, and the constant ‘c’ represents the initial HF-HRV level. We extracted the quadratic term ‘a’, dubbed HF-HRV_quadratic, for the following analysis.
2.5. Imaging data acquisition and analysis
The baseline MRI data was collected at the Rochester Center for Brain Imaging using a 3T Siemens TrioTM scanner (Erlangen, Germany) equipped with a 32-channel receive only head and body coil transmission. The scan began with an MPRAGE scan (TR/TE = 2530/3.44 ms, T1 = 110 ms, FA = 7, matrix = 256 × 256, resolution 1 × 1 × 1 mm, slice thickness = 1 mm, 192 slices) to provide high-resolution structural images for registration during preprocessing. The resting-state fMRI (rs-fMRI) data was collected using a gradient echo-planar imaging (EPI) sequence (TR/TE = 3000 ms/30 ms, FA = 90, slice thickness = 4 mm, matrix = 64 × 64, 4 × 4 mm in-plane resolution, 30 axial slices, volumes = 100). Participants were instructed to relax and keep their eyes open during the 5 min of rs-fMRI scanning.
2.6. Imaging data preprocessing and analysis
For the structural data, voxel-based morphometry (VBM) analysis was performed to calculate hippocampal gray volume using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Briefly, the structural images were segmented into gray matter, white matter, and cerebrospinal fluid. After an initial affine registration of the gray matter map into the Montreal Neurological Institute (MNI) space, the gray matter images were nonlinearly warped using DARTEL (Ashburner, 2007), a toolbox which implements a fast diffeomorphic registration algorithm. Then, the gray matter volume within left and right hippocampus was extracted based on the Automated Anatomical Labeling (AAL) (Tzourio-Mazoyer et al., 2002) hippocampal masks.
The functional imaging data was preprocessed and analyzed using DPARSF2.3 (http://rfmri.org/DPARSF). The first 5 vol of each participant's functional data were excluded due to potential noise related to the equilibrium of the scanner and the adaptation of the participants to the scanner. The remaining 95 vol were corrected for head-motion, slice-timing, nonlinearly warped to MNI standard template, and resampled (3 mm × 3 mm x 3 mm). Then, all data was smoothed using Gaussian kernel (FWHM 4 mm). After removing the linear trend, data was band pass filtered (0.01–0.08 Hz). Nuisance covariates, including 6 head motion parameters, global mean signal, white matter signal, and cerebrospinal fluid signals, were regressed out before functional connectivity analysis (Fox et al., 2006; Kelly et al., 2008).
The left and right hippocampus were selected as seeds by using the AAL templates (Tzourio-Mazoyer et al., 2002). To generate the hippocampal functional networks, the averaged time series within left and right hippocampus were extracted to correlate with each voxel across the entire brain, separately. Multiple linear regression was used to identify the brain regions associated with both PSS and HF-HRV_quadratic within hippocampal networks, controlling for head motion. Significant brain regions (Table 1) were identified with a threshold of corrected p < .01 (uncorrected p < .005, cluster size > 12). The correction of multiple comparisons was applied within the whole brain mask and determined by Monte Carlo simulations using the AFNI AlphaSim program (Ledberg et al., 1998). Since only using smooth kernels in preprocessing is not sufficient, the effective smoothness for AlphaSim correction was estimated based on 4D residuals using DPABI_V2.3 toolbox (Yan et al., 2016). For each participant, the correlation coefficient of hippocampal functional connectivity (z-transformed and averaged within each significant region) was extracted for the following analysis.
Table 1.
Correlations between hippocampal functional connectivity and HF-HRV/PSS.
| REGION | GROUP | PEAK INTENSITY | CLUSTER SIZE (# VOXELS) | MNI COORDINATES X Y Z |
HF-HRV_QUADRATIC R (P) | PSS R (P) |
|---|---|---|---|---|---|---|
| LIPL | HC | 17.97 | 16 | −512, −33, 7 | −0.45 (p = .035) | −0.54 (p = .009) |
| RINSULA | aMCI | 67.06 | 19 | 36, −12, 15 | −0.68 (p = .003) | −0.73 (p = .001) |
| LPG | aMCI | 20.57 | 33 | −39, −6, 24 | −0.60 (p = .011) | −0.55 (p = .023) |
Note. Multiple linear regressions were used to examine the hippocampal network related to PSS and HR-HRV_quadratic. Note: LIPL, left inferior parietal lobe, RINSULA, right insula, LPG, left precentral gyrus.
2.7. Other data analysis
SPSS 22.0 was used for the rest of the data analysis. An independent t-test was applied to examine the difference in sample characteristics, PSS, HF-HRV_quadratic, hippocampal volumes between HC and aMCI groups. Generalized Linear Models (GLM) with an identity link and normal distribution were used to examine the main and interaction effect of group and PSS score on HF_HRV_quadratic (Y = β0+ β1 x PSS + β2 x Group + βinteract PSS x Group). Mediation models were estimated to test whether the hippocampal network would mediate the effect of PSS on HF-HRV_quadratic by using PROCESS macro in SPSS (Preacher and Hayes, 2008). Mediating effect was estimated based on the indirect effect (defined as how PSS influences HRV through connectivity) in the PROCESS macro; and significance test was determined based on 95% confidence interval with bootstrapping the standard error of the indirect effect.
3. Results
3.1. Sample characteristics
There was no significant difference between HC and aMCI group in age, sex, education, and GDS. The aMCI group had significantly lower RAVLT and MOCA scores compared to the HC group (see Table 2).
Table 2.
Demographics and characteristics of subjects.
| AMCI (N = 17) | HC (N = 22) | T TEST (P VALUE), DF | |
|---|---|---|---|
| AGE, MEAN (SD) | 73.9 (10.7) | 71.2 (9.61) | −0.83 (.41), 37 |
| GENDER, N (% MALE) | 8 (47.1%) | 8 (36.4%) | 0.66 (.51), 37 |
| YEARS OF EDUCATION, MEAN (SD) | 15.24 (2.88) | 15.64 (2.50) | 0.465 (.65), 37 |
| MOCA, MEAN (SD) | 24.12 (2.62) | 26 (2.69) | 2.19 (.035), 37 |
| RAVLT A7 TOTAL CORRECT | 5.82 (4.80) | 9 (2.862) | 2.42 (.023), 25 |
| GDS, MEAN(SD) | 2.71 (2.52) | 2 (2.96) | −0.79 (.44), 37 |
| PSS, MEAN (SD) | 1.11 (0.81) | 0.97 (0.66) | −0.59 (.56), 37 |
| HF-HRV_QUADRATIC, MEAN (SD) | 0.57 (0.66) | 0.49 (0.66) | −0.38 (.71), 37 |
Two sample t-test was applied to examine the difference between HC and aMCI group. Note: aMCI, amnestic mild cognitive impairment; HC, healthy control; SD, standard deviation; MOCA, Montreal Cognitive Assessment; RAVLT, Rey's Auditory Verbal Learning Test; GDS, Geriatric Depression Scale; PSS, Perceived Stress Scale. Groups differed only in RAVLT scores.
3.2. Chronic stress exposure and acute stress adaptation
There was no group difference in perceived chronic stress indexed by PSS (t = −.59, df = 37, p = .56), as both aMCI and HC perceived relatively low levels of chronic stress. To assess acute stress adaptation induced by cognitive tasks, repeated measures ANOVA was applied to examine different stages (baseline, mean reactivity across two tasks, and recovery) for the entire sample. We tested three validated models of HF-HRV trajectories in response to a task: quadratic (high at baseline, lower during task, high again at rest), linear (consistent change throughout), and cubic (increase, plateau during stress, then increase again). We found that the quadratic model was superior (F = 23.68, df = 1, p = .001) to the linear (F = 0.83, df = 1, p = .37) or cubic (F = 4.26, df = 1, p = .046) models and best exemplified our data's trajectory. Hence, we extracted the quadratic parameter from the quadratic model (termed “HF-HRV_quadratic”) to be representative of the acute stress adaptation for each individual in the following analyses (see Fig. 1A). There was no significant difference in HF-HRV_quadratic between HC and aMCI group (t = −0.38, df = 37, p = .71). Combing HC and aMCI group together, there was a significant positive correlation between PSS and HF-HRV_quadratic (r = .32, df = 39, p = .045), in which higher PSS was correlated with higher HF-HRV_quadratic (see Fig. 1B). This correlation also held up separately for HC(r = 0.46, df = 22, p = .03, 95% CI [-0.13, 0.78]) but not aMCI (r = . 17, df = 17, p = .51, 95%CI [-0.49, 0.61]). The General Linear Model showed no group effects on the correlation between PSS and HF-HRV_quadratic (Wald's χ2 = 1.32, df = 1, p = .25).
Fig. 1.
HF-HRV quadratic model. A) The quadratic model (ax2 + bx + c) describes the HRV trajectory during baseline, stress reactivity and recovery for HC and aMCI group, respectively. The quadratic term, a (referred to as HF-HRV_quadratic) was extracted for analysis, B) The correlation between HF-HRV_quadratic and PSS in the entire group.
3.3. Hippocampal functional connectivity associated with PSS and HF-HRV
The aMCI group had significantly smaller right hippocampus (RHIP) volume than the HC (t = 2.35, df = 36, p = .024) and a trend for, but nonsignificant smaller hippocampal volumes in the left hippocampus (LHIP, t = 1.89, df = 36, p = .067). The hippocampal volumes were not correlated with HF-HRV_quadratic (LHIP: r = −0.18, df = 36, p = .27, 95% CI [-0.47, 0.09]; RHIP: r = −0.16, df = 36, p = .34, 95% CI [-0.44, 0.15]) or PSS (LHIP: r = 0.11, df = 36, p = .51, 95% CI [-0.17, 0.39]; RHIP: r = 0.09, df = 36, p = .61, 95% CI [-0.25, 0.38]) in the entire sample.
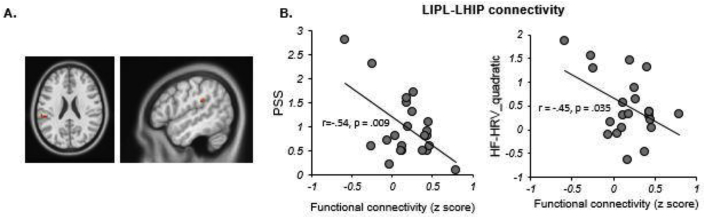
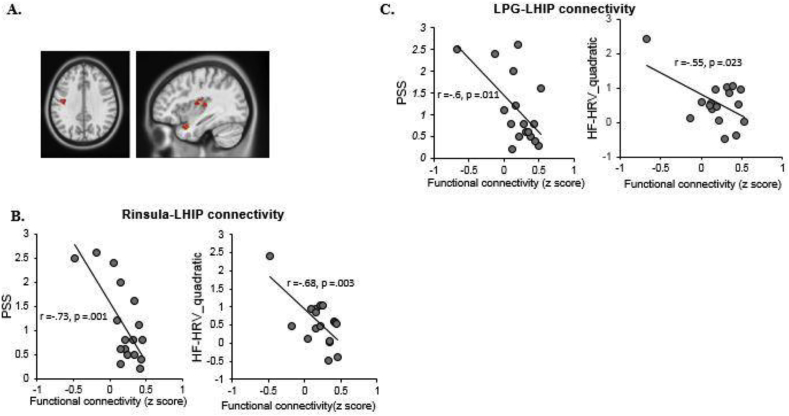
Multiple linear regressions were applied to examine the association between hippocampal functional connectivity and both PSS and the HF-HRV_quadratic across the two groups. In HC group, connectivity of LHIP with the left inferior parietal lobe (LIPL; MNI Coordinate: 51, −33, 27, cluster size = 17 voxels) was found to be negatively correlated to both PSS (r = −0.54, df = 22, p = .009, 95% CI [-0.82, 0.17]) and HF-HRV_quadratic (r = −0.45, df = 22, p = .035, 95% CI [-0.73, 0.16]) (see Fig. 2A&B). Alternatively, in the aMCI group, functional connectivity of the LHIP with the right insula (Rinsula; MNI Coordinate: 39, −12, 15, cluster size = 19) was found to be negatively correlated to both PSS (r = −0.73, df = 17, p = .001, 95% CI [-0.88,-0.31]) and the HF-HRV_quadratic (r = −0.68, df = 17, p = .003, 95% CI [-0.9,-0.05]) (see Fig. 3A&B). The aMCI group also showed functional connectivity of the LHIP with the left precentral gyrus (LPG; MNI Coordinates: 39, −6, 24, cluster size = 33 voxels) which was negatively correlated to both PSS (r = −0.60, df = 17, p = .011, 95% CI [-0.88, −0.12]) and HF-HRV_quadratic (r = −0.55, df = 17, p = .023, 95% CI [-0.85, 0.40]) (see Fig. 3A&C). We did not find any significant correlation in the right hippocampal functional connectivity. Lastly, we also compared the difference in hippocampal networks between the two groups directly and found no significant results.
Fig. 2.
The multiple linear regression analysis showed the hippocampal functional connectivity associated with both PSS and HRV in HC group. A) The LIPL-LHIP connection was correlated with PSS and HRV both. B) The scatter plots showed the significant negative correlations between functional connectivity and PSS/HRV.
Fig. 3.
The multiple linear regression analysis showed the hippocampal functional connectivity associated with both PSS and HRV in aMCI group. A) The Rinsula-LHIP and LPG-LHIP connection were correlated with PSS and HRV both. B), C) The scatter plots showed the significant negative correlations between functional connectivity and PSS/HRV.
3.4. Mediation analysis in functional connectivity and stress
Using a mediation model, Rinsula-LHIP functional connectivity significantly mediated the relationship between PSS and HF-HRV_quadratic (indirect effect = .70, SE = 0.22, Z = 3.20, p = .001, 95% CI [0.13, 1.29]) in aMCI group (see Fig. 4). There was no significant mediating effect of any hippocampal networks in HC group on acute stress. Additionally, this mediation is specific to PSS, as no mediation effect was found using other cognitive tests.
Fig. 4.
Mediation analysis. The functional connectivity of Rinsula-LHIP mediated the relationship between PSS and HF-HRV_quadratic. Note: The numbers in the figure reflect the direct effects. **p < .01; ***p < .001; ns p > .05.
4. Discussion
The present study examined a potential neural mechanism underlying the effects of chronic stress on acute stress adaptation. More perceived exposure to chronic stress (indexed by higher PSS) was significantly related to worse acute stress adaptation (indexed by higher HF-HRV_quadratic) for the entire sample. However, we did not find any significant difference between aMCI and HC in chronic stress (PSS) and acute stress adaptation (HF-HRV_quadratic). The hippocampal volume, particularly the right one, was found to be significantly smaller in individuals with aMCI compared to HC, while the left hippocampus was similar between groups. More importantly, we found that group-dependent stronger left hippocampal functional connectivity (LIPL-LHIP for HC, and Rinsula-LHIP and LPG-LHIP for aMCI) was related to less perceived chronic stress and better acute stress adaptation. Moreover, functional connectivity of Rinsula-LHIP mediated the effect of PSS on HF-HRV_quadratic. The mediation effect here indicates that HF-HRV change is dependent on the change of correlation between functional connectivity and PSS, which in turn suggests that a stronger negative correlation between connectivity and PSS predicts lower HF-HRV_quadratic and a better stress response. Overall, our findings suggest that aMCI patients may recruit extra hippocampal networks, particularly RInsula-LHIP functional connectivity, to regulate stress and alleviate the adverse effect of chronic stress exposure on acute stress responses.
There was no significant group difference in perceived chronic stress or acute stress adaptation. Abnormal HF-HRV reactivity to acute stress was observed in the middle to late stages of AD compared to healthy older adults (Femminella et al., 2014), suggesting altered acute stress regulation with more advanced neurodegeneration. For individuals with aMCI, however, there have been contradictory findings regarding the HF-HRV reactivity or recovery to acute stress (Collins et al., 2012; Zulli et al., 2005; Lin et al., 2017c). The inconsistent findings may be due to the heterogeneity in the pathophysiology in individuals with aMCI (Jacquemont et al., 2017). With respect to research into chronic stress, scores on the PSS did not differ significantly between MCI and HC in one study (Ezzati et al., 2014), which was consistent to the current study.
While the chronic and acute stress themselves were similar between our two samples, we observed distinct group patterns in the hippocampal networks involved in the chronic and acute stress regulation. For HC, the network involved posterior brain regions (IPL), while the network in aMCI was driven by anterior brain regions (insula and PG). Consistently, another study found functional connectivity of the IPL in healthy older adults that was correlated to performance in a cognitive stress task (Burianova et al., 2015). In addition, previous studies also found insula and LPG are involved in emotion regulation and compensation in MCI and AD-related neurodegeneration (Li et al., 2009; Caroli et al., 2010; Franzmeier et al., 2017). Given that anterior brain regions are known to participate in neural compensation and supporting cognitive performance in individuals at risk for AD (Reuter-Lorenz and Park, 2014), our findings suggest that similar posterior to anterior shifts may be used to compensate for other age-related brain dysfunction, such as acute stress adaptation. Therefore, we speculate that the lack of group difference in PSS and HRV may be due to the compensatory effects of the hippocampal network found in aMCI. Consistently, previous studies have shown that hippocampal functional connectivity was recruited for compensating cognitive dysfunction in age-related neurodegeneration (Wang et al., 2011). It appears that the enhanced functional connectivity strength between LPG/RInsula and LHIP in aMCI in our study may alleviate any detrimental effects of HPA axis dysregulation as well as hippocampal neurodegeneration, and compensate for any resulting inefficiencies in stress regulation. The mediating effect of the Rinsula-LHIP functional connectivity on the relationship between chronic and acute stress is especially interesting. The insular cortex, as part of the salience network, plays a critical role in stress appraisal (Menon and Uddin, 2010) and has also been shown to be a part of the regulation of HF-HRV (Thayer et al., 2012). Therefore, the LHIP-Insula connectivity may play a crucial role in understanding the relationship between chronic and acute stress. Lastly, the functional networks only exist in the less impaired side of hippocampus (left). Some emerging evidence has indicated that changes in hippocampal functional connectivity are lateralized in the brain in AD with left hippocampal functional connectivity being increased (Wang et al., 2006). Regardless, the relationship between lateralization and compensation will need further validation.
There were several limitations in our study. First, the sample size was relatively small, which may give the possibility of errors, and the mediation analysis was thus carried out on a small cross-sectional dataset, abolishing the possibility of determining causal relationships. Second, the PSS measurement has been shown to be vulnerable to the effects of memory deficits or anosognosia in AD, as those with impairments are not often aware of their own deficits. Although these effects of anosognosia were less severe in aMCI (Arsenault-Lapierre et al., 2012), there remains a possibility that this phenomena may have affected ratings of perceived chronic stress in our sample, making it a limitation of this study. Future research should incorporate biomarkers such as blood or salivary cortisol samples to measure stress levels in addition to self-report measures. In addition, the current study focused on the hippocampal functional network in underlying the neural correlates of stress, as the hippocampus is a target of HPA axis dysregulation as well as regulator of ANS function. However, other brain regions, like the prefrontal cortex, also play a crucial role in stress appraisal and response, as well as in the regulation of HF-HRV (Thayer et al., 2012). Therefore, it is possible that other networks (e.g. prefrontal cortex involved central executive network) are also involved in stress regulation, which needs to be considered in future research.
In summary, our results shed a light on the brain mechanism of stress regulation in age-related neurodegeneration. Individuals with aMCI may recruit different regions in hippocampal networks to regulate acute stress that remedies the effects of chronic stress exposure. More research is needed to develop a better framework to understand the complex relationships between stress and age-associated neurodegeneration. We believe that these findings may help further elucidate the intricacies of the neurodegenerative and subsequent neural compensation found in the development of dementia.
Conflicts of interest
The authors report no conflict of interest. This work was supported by an Alzheimer's Association New Investigator grant (NIRG-14-317353) to FL.
Acknowledgments
This work was supported by an Alzheimer's Association New Investigator Award grant (NIRG-14-317353) to FL.
References
- Albert M.S. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida N.D. Quality of parental emotional care and calculated risk for coronary heart disease. Psychosom. Med. 2010;72(2):148–155. doi: 10.1097/PSY.0b013e3181c925cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault-Lapierre G. Effects of anosognosia on perceived stress and cortisol levels in Alzheimer's disease. Int. J. Alzheimer's Dis. 2012;2012 doi: 10.1155/2012/209570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Astur R.S. Hippocampus function predicts severity of post-traumatic stress disorder. Cyberpsychol. Behav. 2006;9(2):234–240. doi: 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- Berntson G.G. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bremner J.D. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatry. 2003;160(5):924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Burianova H. The relation of structural integrity and task-related functional connectivity in the aging brain. Neurobiol. Aging. 2015;36(10):2830–2837. doi: 10.1016/j.neurobiolaging.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Caroli A. Functional compensation in incipient Alzheimer's disease. Neurobiol. Aging. 2010;31(3):387–397. doi: 10.1016/j.neurobiolaging.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Carrion V.G. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. J. Pediatr. Psychol. 2010;35(5):559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casement M.D. Social stress response in adolescents with bipolar disorder. Psychoneuroendocrinology. 2018;91:159–168. doi: 10.1016/j.psyneuen.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldo R. Acute mental stress assessment via short term HRV analysis in healthy adults: a systematic review with meta-analysis. Biomed. Signal Process. Control. 2015;18:370–377. [Google Scholar]
- Cha J. Functional alteration patterns of default mode networks: comparisons of normal aging, amnestic mild cognitive impairment and Alzheimer's disease. Eur. J. Neurosci. 2013;37(12):1916–1924. doi: 10.1111/ejn.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Collins O. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol. Aging. 2012;33(10):2324–2333. doi: 10.1016/j.neurobiolaging.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Ezzati A. Validation of the Perceived Stress Scale in a community sample of older adults. Int. J. Geriatr. Psychiatry. 2014;29(6):645–652. doi: 10.1002/gps.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femminella G.D. Autonomic dysfunction in Alzheimer's disease: tools for assessment and review of the literature. J. Alzheimer's Dis. 2014;42(2):369–377. doi: 10.3233/JAD-140513. [DOI] [PubMed] [Google Scholar]
- Filippi M. Changes in functional and structural brain connectome along the Alzheimer's disease continuum. Mol. Psychiatr. 2018 doi: 10.1038/s41380-018-0067-8. https://www.nature.com/articles/s41380-018-0067-8 [DOI] [PubMed] [Google Scholar]
- Fox M.D. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems (vol 103, pg 10046, 2006) Proc. Natl. Acad. Sci. U. S. A. 2006;103(36) doi: 10.1073/pnas.0604187103. 13560-13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier N. Left frontal cortex connectivity underlies cognitive reserve in prodromal Alzheimer disease. Neurology. 2017;88(11):1054–1061. doi: 10.1212/WNL.0000000000003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jacquemont T. Amyloidosis and neurodegeneration result in distinct structural connectivity patterns in mild cognitive impairment. Neurobiol. Aging. July 2017;55:177–189. doi: 10.1016/j.neurobiolaging.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Kelly A.M.C. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Khookhor O., Umegaki H. The cholinergic stimulation of the hippocampus induced the activation of the sympathetic nervous system. Neuroendocrinol. Lett. 2013;34(1):58–61. [PubMed] [Google Scholar]
- Kim J.J., Yoon K.S. Stress: metaplastic effects in the hippocampus. Trends Neurosci. 1998;21(12):505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- Knapman A. Increased stress reactivity is associated with reduced hippocampal activity and neuronal integrity along with changes in energy metabolism. Eur. J. Neurosci. 2012;35(3):412–422. doi: 10.1111/j.1460-9568.2011.07968.x. [DOI] [PubMed] [Google Scholar]
- Ledberg A., Akerman S., Roland P.E. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8(2):113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- Li C. An fMRI stroop task study of prefrontal cortical function in normal aging, mild cognitive impairment, and Alzheimer's disease. Curr. Alzheimer Res. 2009;6(6):525–530. doi: 10.2174/156720509790147142. [DOI] [PubMed] [Google Scholar]
- Lin F. Mental fatigability and heart rate variability in mild cognitive impairment. Am. J. Geriatr. Psychiatry. 2016;24(5):374–378. doi: 10.1016/j.jagp.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. Cortical thickness is associated with altered autonomic function in cognitively impaired and non-impaired older adults. J. Physiol. Lond. 2017;595(22):6969–6978. doi: 10.1113/JP274714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. A role of the parasympathetic nervous system in cognitive training. Curr. Alzheimer Res. 2017;14(7):784–789. doi: 10.2174/1567205014666170203095128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. Cortical thickness is associated with altered autonomic function in cognitively impaired and non-impaired older adults. J. Physiol. 2017;595(22):6969–6978. doi: 10.1113/JP274714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. The brain on stress: toward an integrative approach to brain, body, and behavior. Perspect. Psychol. Sci. 2013;8(6):673–675. doi: 10.1177/1745691613506907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G.M. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses Z.B., Luecken L.J., Eason J.C. Measuring task-related changes in heart rate variability. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007;2007:644–647. doi: 10.1109/IEMBS.2007.4352372. [DOI] [PubMed] [Google Scholar]
- Murakami S. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005;53(2):129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Nicolini P. Autonomic dysfunction in mild cognitive impairment: evidence from power spectral analysis of heart rate variability in a cross-sectional case-control study. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp J. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer's type. Neurobiol. Aging. 2015;36(2):601–607. doi: 10.1016/j.neurobiolaging.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P.A., Park D.C. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol. Rev. 2014;24(3):355–370. doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M.L. Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: a review. J. Am. Geriatr. Soc. 2008;56(5):920–934. doi: 10.1111/j.1532-5415.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.S. Progressive changes in hippocampal resting-state connectivity across cognitive impairment: a cross-sectional study from normal to Alzheimer disease. Alzheimers Dis. Assoc. Disord. 2014;28(3):239–246. doi: 10.1097/WAD.0000000000000027. [DOI] [PubMed] [Google Scholar]
- Thayer J.F. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vazan R., Filcikova D., Mravec B. Effect of the Stroop test performed in supine position on the heart rate variability in both genders. Auton. Neurosci. 2017;208:156–160. doi: 10.1016/j.autneu.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Wang L. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31(2):496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wang Z. Baseline and longitudinal patterns of hippocampal connectivity in mild cognitive impairment: evidence from resting state fMRI. J. Neurol. Sci. 2011;309(1–2):79–85. doi: 10.1016/j.jns.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Yan C.G. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics. 2016;14(3):339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman M.E. Perceived stress is differentially related to hippocampal subfield volumes among older adults. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulli R. QT dispersion and heart rate variability abnormalities in Alzheimer's disease and in mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(12):2135–2139. doi: 10.1111/j.1532-5415.2005.00508.x. [DOI] [PubMed] [Google Scholar]