Abstract
Depression is a debilitating mental disease, characterized by persistent low mood and anhedonia. Stress represents a major environmental risk factor for depression; the complex interaction of stress with genetic factors results in different individual vulnerability or resilience to the disorder. Dysfunctions of the glutamate system have a primary role in depression. Clinical neuroimaging studies have consistently reported alterations in volume and connectivity of cortico-limbic areas, where glutamate neurons and synapses predominate. This is confirmed by preclinical studies in rodents, showing that repeated stress induces morphological and functional maladaptive changes in the same brain regions altered in humans. Confirming the key role of glutamatergic transmission in depression, compelling evidence has shown that the non-competitive NMDA receptor antagonist, ketamine, induces, at sub-anesthetic dose, rapid and sustained antidepressant response in both humans and rodents.
We show here that the Chronic Mild Stress model of depression induces, only in stress-vulnerable rats, depressed-like anhedonic behavior, together with impairment of glutamate/GABA presynaptic release, BDNF mRNA trafficking in dendrites and dendritic morphology in hippocampus. Moreover, we show that a single administration of ketamine restores, in 24 h, normal behavior and most of the cellular/molecular maladaptive changes in vulnerable rats. Interestingly, ketamine treatment did not restore BDNF mRNA levels reduced by chronic stress but rescued dendritic trafficking of BDNF mRNA.
The present results are consistent with a mechanism of ketamine involving rapid restoration of synaptic homeostasis, through re-equilibration of glutamate/GABA release and dendritic BDNF for synaptic translation and reversal of synaptic and circuitry impairment.
Keywords: Chronic stress, Ketamine, Stress vulnerability, Glutamate release, BDNF, Antidepressant
Highlights
-
•
Chronic mild stress (CMS) induces anhedonic behavior and maladaptive changes in the hippocampus (HPC) of vulnerable rats.
-
•
CMS reduces basal and evoked release of glutamate in the HPC of vulnerable rats.
-
•
SCMS reduces evoked release of GABA in the HPC of vulnerable rats.
-
•
CMS reduces expression of BDNF mRNA and trafficking along dendrites in the HPC of vulnerable rats.
-
•
CMS reduces length of apical dendrites in CA3 pyramidal neurons of vulnerable rats.
-
•
Ketamine injection (10 mg/kg) restores in 24h anhedonic behavior and most maladaptive changes, except BDNF expression.
-
•
The present results suggest that the antidepressant mechanism of ketamine involves restoration of synaptic homeostasis.
1. Introduction
Major depressive disorder (MDD) is a debilitating mental disease, characterized by persistent low mood and anhedonia (loss of interest in pleasurable activities) (American Psychiatric Association, 2013). The etiopathogenesis of MDD is thought to involve a complex interaction of genetic and environmental factors, among which behavioral stress is a major one (Jaffee and Price, 2007; Uher, 2014). Indeed, especially chronic stress exposure may induce maladaptive alterations in vulnerable subjects, leading to increased risk of developing neuropsychiatric disorders (Han and Nestler, 2017; McEwen, 2016; Popoli et al., 2012).
Dysfunctions of the glutamate system have been shown to have a primary role in MDD (Duman et al., 2016; Murrough et al., 2017; Musazzi et al., 2013; Sanacora et al., 2012). Consistently, neuroimaging studies have reported volume and connectivity alterations in cortico-limbic areas, including hippocampus (HPC), prefrontal cortex (PFC) and amygdala, where glutamate neurons/synapses predominate. Preclinical studies with stress-based animal models of depression have showed atrophy/remodeling of dendrites in the same brain regions altered in humans, suggesting that stress-induced maladaptive changes have a primary role in the chain of events leading to the development of MDD (Autry and Monteggia, 2012; Krishnan and Nestler, 2008; McEwen, 2016; Popoli et al., 2012; Sanacora et al., 2012).
Further supporting the key role of glutamatergic transmission in MDD, the non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist ketamine (KET) has been consistently reported to induce at low sub-anesthetic dose rapid (within hours) and sustained (up to several days) antidepressant response in both clinical and preclinical studies (Berman et al., 2000; Kadriu et al., 2019; Zanos and Gould, 2018; Zarate et al., 2006).
Different hypotheses on the mechanisms underlying KET antidepressant action have been proposed (for recent reviews, see (Kadriu et al., 2019; Zanos and Gould, 2018)). Importantly, the behavioral effects of KET have been suggested to be related to rapid activation of neurotrophic signaling and synaptic translation of Brain-Derived Neurotrophic Factor (BDNF) (Kavalali and Monteggia, 2015, 2012; Monteggia and Kavalali, 2013). BDNF is the main neurotrophin in the adult brain, where it is involved in the regulation of synapse formation, function and plasticity (Chao, 2003; Waterhouse and Xu, 2009). Disinhibition of glutamate transmission by KET and the subsequent increase in glutamate release activate ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, thus leading to the release of BDNF (Abdallah et al., 2015; Duman, 2014; Duman et al., 2016). Recent studies reported that local BDNF protein synthesis at synapses plays a critical role in the regulation of local spines and dendrites morphology (Baj et al., 2011; Kellner et al., 2014; Song et al., 2017). Transcription of BDNF is regulated by at least nine different promoters, giving rise to different splice variant transcripts (Aid et al., 2007), which were proposed to bear a spatial code for local translation of BDNF (Baj et al., 2011). Indeed, splice variants containing transcripts from exon 2 (BDNF-2) and 6 (BDNF-6) were shown to be trafficked to distal dendrites, and suggested to play a key role in regulating local dendritic morphology (Aid et al., 2007; Baj et al., 2011; Chiaruttini et al., 2008; Kellner et al., 2014). Interestingly, both chronic treatment with antidepressants and physical exercise have been shown to increase trafficking of BDNF transcripts at distal dendrites (Baj et al., 2012). However, at present, it is not known whether trafficking of BDNF mRNA is regulated by stress or KET treatment.
In the present work, we show that in the Chronic Mild Stress (CMS) model of depression anhedonic behavior, and the impairment of: (1) glutamate presynaptic release; (2) BDNF mRNA dendritic trafficking and (3) dendritic morphology in HPC, are restricted to stress-vulnerable animals. Moreover, we show that a single administration of sub-anesthetic KET is able to restore behavior and these cellular/molecular alterations in vulnerable rats.
2. Material and methods
2.1. Animals
Experiments were performed in accordance with the European Community Council Directive 2010/63/UE and approved by the Italian legislation on animal experimentation (Decreto Legislativo 26/2014, authorization N 308/2015-PR).
A total of 144 male Sprague-Dawley 150–175 g rats (Charles River, Calco, Italy) were housed two per cage at 20–22 °C, 12 h light/dark cycle (light on 7:00 a.m. off 7:00 p.m.), water and food ad libitum, except when required for CMS.
2.2. Chronic mild stress
96 rats were exposed once or twice daily to random, mild and unpredictable stressors for five weeks (Strekalova et al., 2011; Willner, 2017) (Fig. 1a). CMS included: up to 12 h of food or water deprivation, overcrowding, social isolation, cage soiled with water in sawdust, 45° cage tilting, light-on overnight, light/dark cycle reversal, 5 min of forced swim.
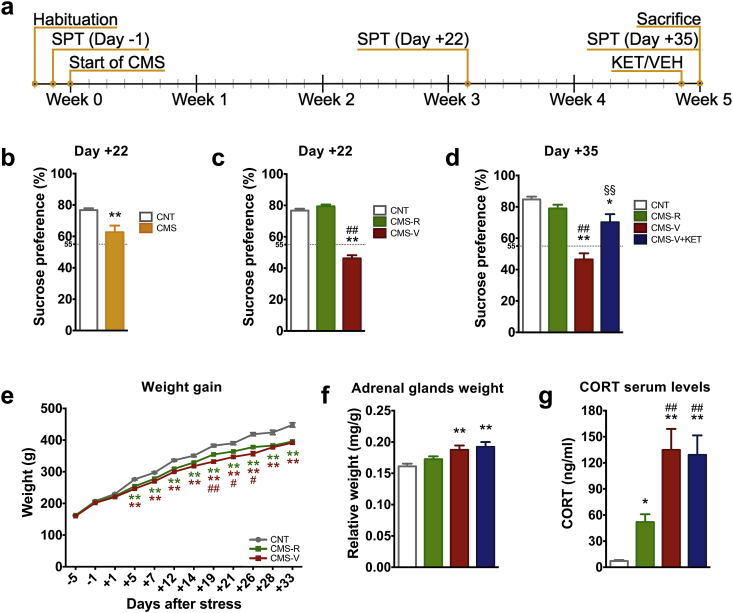
Fig. 1.
(a) Experimental plan: animals were subjected to a variable sequence of mild stressors for five weeks. Sucrose preference test (SPT) was performed to evaluate anhedonic behavior. KET or vehicle (VEH) were acutely administered 24 h before sacrifice. (b) SPT of CNT and CMS rats at Day +22 of CMS. n = CNT 101; CMS 188. Unpaired t-test: **p < 0.001 vs CNT; (c) Separation of resilient and vulnerable animals applying a cut-off at 55% of sucrose preference at Day +22 of CMS. n = CNT 101; CMS-R 100; CMS-V 88. (d) SPT of CNT and CMS rats at Day +35 of CMS, 24 h after KET/VEH treatment n = CNT 59; CMS-R 53; CMS-V 27; CMS-V + KET 25. (e) Body weight gain; n = CNT 101; CMS-R 100; CMS-V 88. (f) Adrenal glands weight. n = CNT 54; CMS-R 48; CMS-V 25; CMS-V + KET 21. (g) CORT serum levels. n = CNT 30; CMS-R 27; CMS-V 15; CMS-V + KET 15. Data are shown as means ± standard error of the mean. TPHT: *p < 0.05 vs CNT; **p < 0.001 vs CNT; #p < 0.05 vs CMS-R; ##p < 0.001 vs CMS-R; §§p < 0.001 vs CMS-V.
2.3. Sucrose preference test
Two days before CMS, sucrose habituation was performed exposing animals to two bottles containing 1% sucrose solution, for 2 h. Animals were subjected to sucrose preference test (SPT) one day before CMS (Day −1), after three weeks (Day +22), and 1 h before sacrifice (Day +35) (Fig. 1a). SPT consisted in presenting rats with two bottles, one containing 0.5% sucrose and one containing tap water, for 1 h. The position of the bottles was inverted after 30min. Sucrose preference was calculated as: [sucrose solution intake (ml)/total fluid intake (ml)]x100] (Strekalova et al., 2011).
2.4. Drug treatment
24 h before sacrifice, rats were i.p. injected with racemic KET (MSD Animal Health, Milan, Italy) (10 mg/kg) or saline.
2.5. Analysis of phenotypic changes
Body weight gain was monitored twice a week. Adrenal/total body weight ratio was calculated as: total weight of left and right adrenal glands (mg)/body weight (g) (Ieraci et al., 2016). Serum corticosterone levels were measured as in (Musazzi et al., 2017).
2.6. Preparation of purified synaptosomes and neurotransmitter release experiments
Purification and superfusion of synaptic terminals (synaptosomes) were performed as previously reported (Bonanno et al., 2005; Bonifacino et al., 2016; Treccani et al., 2014). Synaptosomes were freshly prepared from homogenized HPC of 8–12 animals/group, by centrifugation on discontinuous Percoll gradients, and superfused with a standard physiological medium (140 mM NaCl, 3 mM KCl, 1.2 mM MgSO4, 1.2 mM CaCl2, 1.2 mM NaH2PO4, 5 mM NaHCO3, 10 mM glucose, 10 mM HEPES, pH 7.4). After 36 min of superfusion, released sample (representing basal neurotransmitter release) was collected for 3 min (t = 36–39). To measure depolarization-dependent neurotransmitter overflow, a 90 s exposure of synaptosomes to KCl 15 mM, followed by 6 min of collection of released sample (t = 39–45), was used. Collected fractions were analyzed for endogenous glutamate and GABA by HPLC (Bonanno et al., 2005; Bonifacino et al., 2016; Treccani et al., 2014).
2.7. qPCR
Total RNA was extracted from HPC, using Direct-zol RNA MiniPrep (Zymo Research, Freiburg, Germany), reverse-transcribed to cDNA with iScript cDNA Reverse Transcription kit (BioRad Laboratories, Segrate, Italy) and qPCR was performed with a 7900HT Fast PCR System (Applied Biosystems, Monza, Italy) (Ieraci et al., 2016). List of primers used for qPCR is reported in Supplementary Table S1.
2.8. BDNF ELISA
BDNF protein levels were measured from homogenized HPC of 13–14 animals/group, using the BDNF Emax® ImmunoAssay System (Promega, Milan, Italy).
2.9. Riboprobes preparation for in situ hybridization
Digoxigenin (DIG)-labeled riboprobes detecting total BDNF, BDNF-2 or BDNF-6 transcripts were generated from PCR templates adapted with SP6 and T7 RNA polymerase sites. Riboprobes were transcribed using a DIG RNA Labeling Kit (Thermo Scientific) (Russo et al., 2013), purified in NucAwayTM spin columns (Ambion, Monza, Italy) and quantified with Nanodrop 1000 (Thermo Scientific).
2.10. In situ hybridization
After sacrifice, brains were fixed in 4% PFA solution for 24 h and placed in 30% sucrose. Coronal slices (40 μm) were prepared and stored in cryo-protectant sectioning buffer (30% ethylene glycol, 30% glycerol and 0.05 M phosphate buffer) at −20 °C.
In situ hybridization was developed using the Vectastain® Elite ABC-Peroxidase Staining Kit (Vector Laboratories, Segrate, Italy) (La Via et al., 2013). Free-floating sections were post-fixated for 3 h in 4% PFA at RT, washed in 0.1 Tween 20 in PBS (PBST), and treated with 0.3% H2O2. The slices were then permeabilized with 2.3% sodium meta-periodate in H2O, and quickly washed in H2O. The sections were incubated in 1% sodium borohydride in 0.1 M Tris-HCl buffer pH 7.5, and washed in PBST at RT. The slices were digested with 8 μg/ml proteinase K in PBST for 20 min and washed in PBST. After digestion, the tissue slices were fixed in 4% PFA for 5 min and washed in PBST at RT. Slices were incubated at 55 °C for 90 min in pre-hybridization solution containing 20 mM Tris-HCl (pH7.5), 1 mM EDTA, 1x Denhardt's solution, 300 mM NaCl, 100 mM DTT, 0.5 mg/ml salmon sperm DNA, 0.5 mg/ml polyadenylic acid, and 50% formamide. Slices were then incubated ON at 55 °C in hybridization solution, composed of the prehybridization solution supplemented with 10% dextransulfhate and 100 ng/ml DIG-labeled riboprobes. The next day, the slices were washed in 2x saline sodium citrate, 0.1% Tween 20 (SSCT), and 50% deionized formamide at 55 °C for 30 min, for 20 min in 2x SSCT at 55 °C, and twice in 0.2 x SSCT at 60 °C for 30 min. Subsequently, the slices were detected using the peroxidase method with biotinylated donkey anti-mouse IgG antibodies and diaminobenzidine as chromogen (Vector Laboratories, Segrate, MI, Italy).
The images of in situ hybridization experiments were acquired using an LSM 510 Meta (Carl Zeiss Microscopy, Jena, Germany) microscope, and the maximal distance of hybridization signal in dendrites (maximal distance of dendritic labeling) was determined by AxionVision LE64 (Zeiss, Milano, Italy) using the function Measure Length. In particular, the hybridization signal was measured starting from the origin of dendrite from the cell body, to the point at which the in situ labeling was no longer clearly distinguishable from the background.
Dendrites of pyramidal neurons in CA1 and CA3 hippocampal regions have been analyzed; 3–4 rats/group, 2–3 slices/rat and 80–100 dendrites/slice were analyzed. All the experiments were coded and analyzed in a blinded manner.
2.11. Golgi staining and dendritic analysis
Immediately after sacrifice, left or right hemispheres were processed for Golgi staining using the Rapid Golgi Stain Kit (FD NeuroTechnologies, Inc., Columbia, MD, USA) (Nava et al., 2017). Hemispheres were coronally sliced (200 μm) on a VT1200S vibratome (Leica, Wetzlar, Germany). CA1 and CA3 areas of HPC were identified on a BX50 light microscope (Olympus, Tokyo, Japan) using newCAST software (Visiopharm, Hørsholm, Denmark). Z-stacks (1 μm per Z-step size) of 5 CA1 or CA3 pyramidal neurons with untruncated branches were acquired using a × 60 oil objective. Collapsed Z-stacks were imported in Bitplane Imaris software (version 7.7.1, Andor Technology Ltd, Belfast, Northern Ireland) and dendrites were reconstructed using the FilamentTracer function. Dendrite length and branching, and Sholl analysis were assessed (Nava et al., 2017).
2.12. Statistical analysis
For SPT (Day −1, +22 and + 35), adrenal glands weight, CORT serum levels, neurotransmitter release experiments, BDFN levels, and dendritic analyses one-way ANOVA followed by the Tukey's post-hoc test (TPHT) was used. For SPT (Day +22, considering CNT vs. CMS), unpaired two-tailed Student t-test was used. For weight gain and Sholl analysis, two-way ANOVA followed by TPHT was used. For in situ hybridization of BDNF transcripts Multilevel Covariance Analysis (MCA) was used, considering two random effects: rat and slice within rat. Dendritic trafficking was modeled on log-scale; F values were computed using Kenward-Roger degrees of freedom; p-values were adjusted for multiple comparisons using single step procedure.
Data were expressed as mean ± standard error of the mean (S.E.M.). Statistical analysis was carried out using GraphPad Prism6 (GraphPad Software, La Jolla, CA, USA) or R version 3.5.1 (R Foundation).
3. Results
3.1. Sucrose preference test allows deeming rats resilient or vulnerable to chronic mild stress. Ketamine rapidly restores anhedonic behavior in vulnerable rats
The CMS group showed a significant reduction in sucrose preference already after 3 weeks of stress (t = 3.87) (Fig. 1b). Applying a 55% cut-off (Strekalova et al., 2011), rats with a sucrose preference higher than 55% were considered resilient to CMS (CMS-R), while rats showing preference lower than 55% were considered vulnerable (CMS-V) (F2,144 = 143.9, p < 0.001) (Fig. 1c). This was not due to preexisting differences in preference for sucrose (Supplementary Fig. S1).
After 5 weeks of CMS, half of the CMS-V rats (n = 25) were injected with KET (CMS-V-KET), and SPT was repeated 23 h later, 1 h before sacrifice. We found that, while saline-treated CMS-V rats still showed anhedonic behavior, sucrose preference was significantly increased in CMS-V + KET compared to CMS-V, and restored at the level of CMS-R rats (F3,104 = 23.08, p < 0.001) (Fig. 1d). Previous work has shown that subanesthetic KET has no effect on sucrose intake in naïve animals, as opposed to animals subjected to CMS (Autry et al., 2011). Moreover, in a separate set of animals, we found no effect of KET in SPT in CMS-R rats (Supplementary Fig. S2). Taken together, these results show that ketamine affects hedonic behavior only in animals subjected to chronic stress.
3.2. Chronic mild stress induces major phenotypic changes in vulnerable rats
Two-way ANOVA revealed a significant effect of CMS (F2,1036 = 264.7, p < 0.001), time (F11,1036 = 151.8, p < 0.001) and interaction between CMS and time (F22,1036 = 8.17, p < 0.001) on body weight gain (Fig. 1e). TPHT showed that, starting from Day +5 of stress, CMS significantly decreased body weight gain in both CMS-R and CMS-V in comparison to CNT. The decrease of weight gain was more evident in CMS-V in comparison to CMS-R, with a difference that reached significance between Day +19 and Day +26. No effect of KET on body weight gain was detected at Day +35 (F3,23 = 12.61, p < 0.001) (Supplementary Fig. S3).
Weight of adrenal glands was increased in CMS-V, but not in CMS-R, while KET had no significant effect on this readout (F3,144 = 8.42, p < 0.001) (Fig. 1f). CMS also increased serum CORT levels, although the increase was significantly higher in CMS-V compared to CMS-R, and again no effect of KET was revealed (F3,77 = 21.52, p < 0.001) (Fig. 1g).
3.3. Chronic mild stress reduces presynaptic release of glutamate and GABA in hippocampus of vulnerable rats. Ketamine partly restores the changes
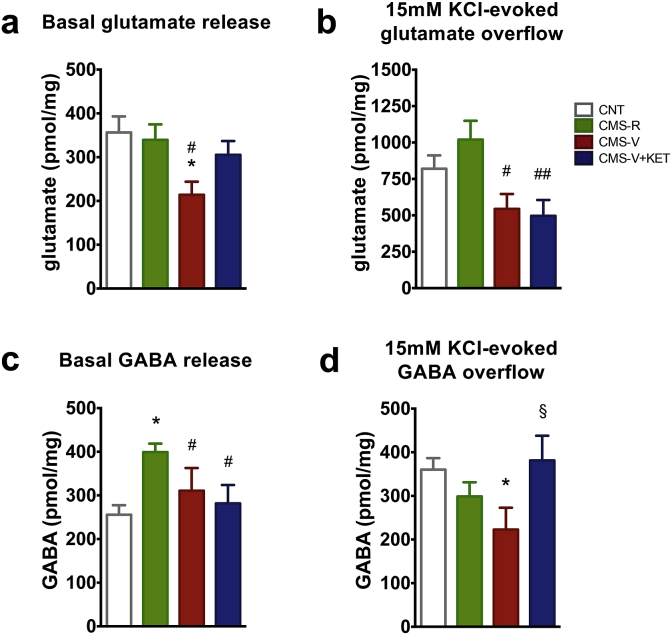
As stress is known to induce changes in glutamate/GABA release and synaptic transmission, we measured the release of these two amino acid neurotransmitters (Bonanno et al., 2005; Bonifacino et al., 2016; Popoli et al., 2012). Both basal and depolarization-evoked release of endogenous glutamate in CMS-R were similar to CNT (Fig. 2a and b). CMS significantly reduced basal glutamate release from superfused synaptosomes selectively in CMS-V animals, compared to both CNT and CMS-R (F3,43 = 2.18, p < 0.05) (Fig. 2a). Interestingly, KET affected only basal release in CMS-V, with a strong trend toward normalization, and no significant differences between CMS-V + KET group and CNT or CMS-R. Instead, depolarization-evoked release of glutamate was significantly reduced in HPC synaptosomes from both CMS-V and CMS-V + KET rats compared to CMS-R, suggesting no effect of KET at this level (F3,43 = 4.00, p < 0.05) (Fig. 2b).
Fig. 2.
(a) Basal glutamate release from HPC synaptosomes in superfusion. n = CNT 14; CMS-R 16; CMS-V 7; CMS-V + KET 9. (b) 15 mM KCl-evoked glutamate release from HPC synaptosomes in superfusion. n = CNT 11; CMS-R 16; CMS-V 6; CMS-V + KET 8. (c) Basal GABA release from HPC synaptosomes in superfusion. n = CNT 18; CMS-R 21; CMS-V 10; CMS-V + KET 9. (d) 15 mM KCl-evoked GABA release from HPC synaptosomes in superfusion. n = CNT 9; CMS-R 13; CMS-V 6; CMS-V + KET 6. TPHT: *p < 0.05 vs CNT; **p < 0.001 vs CNT; #p < 0.05 vs CMS-R; ##p < 0.05 vs CMS-R; §p < 0.05 vs CMS-V.
With regard to endogenous GABA release, the basal release was found increased in CMS-R compared to CNT, CMS-V and CMS-V + KET (F3,43 = 6.10, p < 0.05) (Fig. 2c). Conversely, we found that CMS reduced the depolarization-evoked release of GABA selectively in CMS-V compared to CNT, an effect that was fully restored to CNT levels by KET (F3,43 = 2.58, p < 0.05) (Fig. 2d).
3.4. Chronic mild stress reduces the expression of BDNF mRNA and protein in hippocampus. Ketamine does not restore the changes
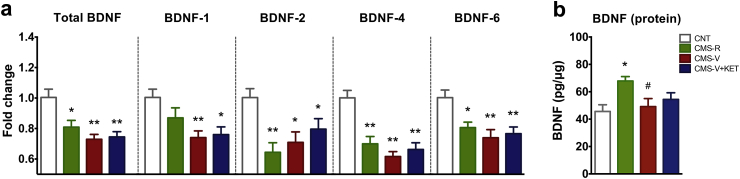
Total BDNF mRNA levels in whole HPC were significantly reduced by CMS in both CMS-V and CMS-R, and KET treatment had no effect (F3,34 = 8.72, p < 0.001) (Fig. 3a).
Fig. 3.
(a) Levels of total BDNF transcripts and of BDNF splice variant transcripts containing exon 1 (BDNF-1), 2 (BDNF-2), 4 (BDNF-4) or 6 (BDNF-6) in HPC homogenate. n = CNT 8; CMS-R 9; CMS-V 10; CMS-V + KET 10. (b) BDNF protein levels in HPC homogenate. n = CNT 13; CMS-R 13; CMS-V 14; CMS-V + KET 14. TPHT: *p < 0.05 vs CNT; **p < 0.001 vs CNT; #p < 0.05 vs CMS-R.
We also analyzed the effect of CMS and KET on the transcription of BDNF splice variants BDNF-1, BDNF-2, BDNF-4 and BDNF-6, the major splice variants expressed in rat brain (Aid et al., 2007). We found that CMS caused a general reduction of the expression of BDNF transcripts in all stressed rats. BDNF-1 mRNA levels were significantly reduced in HPC of CMS-V and CMS-V + KET, but not in CMS-R rats (F3,34 = 4.97, p < 0.05) (Fig. 3a). BDNF-2 mRNA levels were significantly reduced in CMS-R, CMS-V, and CMS-V + KET (F3,34 = 7.89, p < 0.001), and the same was observed for BDNF-4 (F3,34 = 13.85, p < 0.001), and BDNF-6 levels (F3,34 = 5.74, p < 0.05) (Fig. 3a). As for total BDNF, KET did not restore the levels of the splice variants.
We also measured the BDNF protein expression levels in whole HPC and found a significant increase of BDNF selectively in CMS-R (F3,50 = 3.90, p < 0.05) (Fig. 3b). BDNF protein level of CMS-V (with or without KET) was not different from controls.
3.5. Chronic mild stress reduces the dendritic trafficking of total BDNF mRNA and BDNF splice variants in hippocampus of vulnerable rats. Ketamine restores the changes
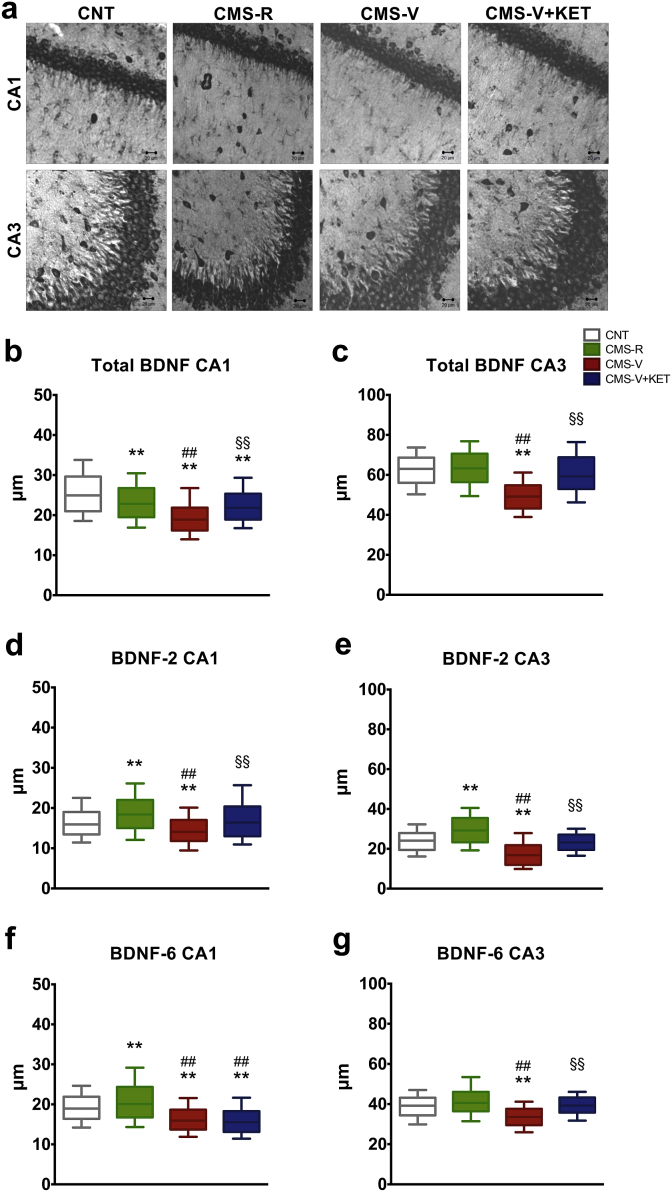
Local translation and release of BDNF at synapses, also dependent on dendritic transcripts, has been suggested to be central for the rapid antidepressant action of KET (Song et al., 2017). Therefore, we performed in situ hybridization studies (Fig. 4a) to assess changes in BDNF mRNA dendritic localization. A significant decrease in the trafficking of total BDNF mRNA was found in the CA1 region of HPC of both CMS-R and CMS-V (MCS,F4,16.88 = 5026, p < 0.001); this reduction was greater in CMS-V compared to CMS-R and restored to the levels of CMS-R by KET (Fig. 4b). Instead, in CA3 region, we found a significant decrease in the trafficking of total BDNF selectively in CMS-V (MCS,F4,20.17 = 6135, p < 0.001); this impairment was completely restored by KET (Fig. 4c).
Fig. 4.
(a) Representative images of in situ hybridization of total BDNF mRNA in CA1 and CA3 regions of HPC in CNT, CMS-R, CMS-V and CMS-V + KET rats. (b) Dendritic trafficking of total BDNF transcripts in CA1. (c) Dendritic trafficking of total BDNF transcripts in CA3. (d) Dendritic trafficking of BDNF splice variant transcripts containing exon 2 (BDNF-2) in CA1. (e) Dendritic trafficking of BDNF splice variant transcripts containing exon 2 (BDNF-2) in CA3. (f) Dendritic trafficking of BDNF splice variant transcripts containing exon 6 (BDNF-6) in CA1. (g) Dendritic trafficking of BDNF splice variant transcripts containing exon 6 (BDNF-6) in CA3. n = 3–4 rats/group, 2–3 slices/rat, 80–100 dendrites/slice. MCA: **p < 0.001 vs CNT; ##p < 0.001 vs CMS-R; §§p < 0.001 vs CMS-V.
We also analyzed the effect of CMS and KET on the trafficking of BDNF splice variants containing BDNF-2 or -6, the main BDNF transcripts transported to distal dendrites (Baj et al., 2011). In CA1, BDNF-2 dendritic trafficking was increased in CMS-R, while decreased in CMS-V rats (MCS,F4,20.58 = 3042, p < 0.001); KET treatment completely restored this change in CMS-V (Fig. 4d). Similar results were obtained in CA3 (MCS,F4,13.57 = 337, p < 0.001): BDNF-2 dendritic trafficking was increased in CMS-R, while decreased in CMS-V and restored to CNT levels by KET (Fig. 4e).
Concerning BDNF-6 mRNA, in CA1 its dendritic trafficking was increased in CMS-R and reduced in CMS-V; no effect of KET was detected (MCS,F4,15.92 = 5660, p < 0.001) (Fig. 4f). In CA3, BDNF-6 trafficking was reduced selectively in CMS-V; KET fully reversed this change (MCS,F4,7.85 = 5461, p < 0.001) (Fig. 4g).
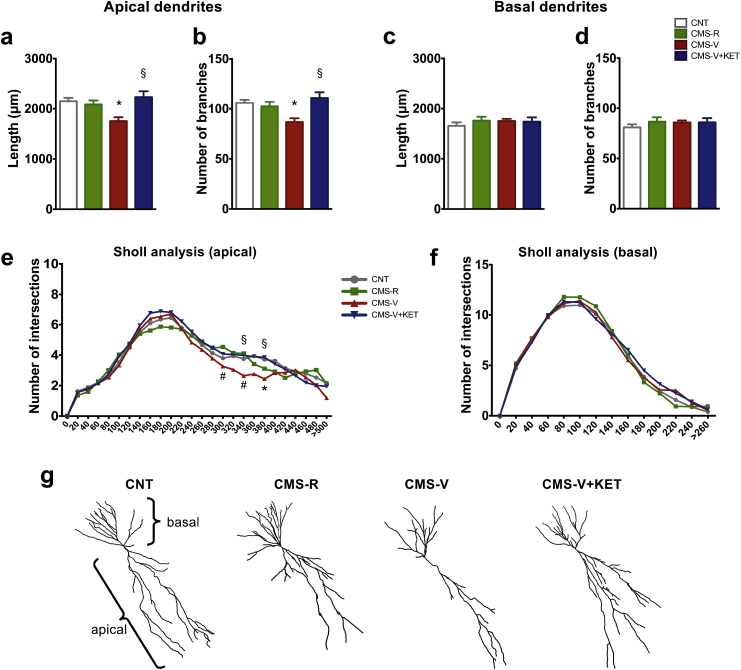
3.6. Chronic mild stress induces retraction of CA3 apical dendrites in hippocampus of vulnerable rats. Ketamine restores the changes
In CA1 we found no significant changes in length and branching of apical and basal dendrites, as confirmed by Sholl analysis (Supplementary Fig. S4).
Conversely, one-way ANOVA revealed that CMS significantly reduced the total length of apical dendrites in the CA3 of CMS-V (F3,38 = 5.67, p < 0.05) (Fig. 5a). This effect was completely restored to control levels by KET. Moreover, CMS induced dendritic simplification selectively in CMS-V rats, as shown by the reduction of the total number of branches of CA3 apical dendrites, which was completely restored by KET (F3,38 = 5.52, p < 0.05) (Fig. 5b). We found no significant difference between experimental groups in the total length (Fig. 5c) and branching number (Fig. 5d) of basal dendrites.
Fig. 5.
(a) Total dendritic length of CA3 apical dendrites. (b) Branching number of CA3 apical dendrites. (c) Total dendritic length of CA3 basal dendrites. (d) Branching number of CA3 basal dendrites. (e) Sholl analysis of CA3 apical dendrites. (f) Sholl analysis of CA3 basal dendrites. n = CNT 11; CMS-R 11; CMS-V 10; CMS-V + KET 10. TPHT: *p < 0.05 vs CNT; #p < 0.05 vs CMS-R; §p < 0.05 vs CMS-V. (g) Representative drawings of CA3 pyramidal neurons reconstructed with Imaris software.
Sholl analysis confirmed the CMS-induced retraction of apical dendrites in the CA3 of CMS-V (F3,90 = 10.89, p < 0.001). In particular, TPHT showed that CMS significantly decreased the number of intersections of distal apical dendrites (between 300 μm and 380 μm), selectively in the CA3 of CMS-V; KET rescued this effect in CMS-V (Fig. 5e). Instead, Sholl analysis showed no significant effect of CMS in basal dendrites (Fig. 5f).
4. Discussion
In this work we employed one of the most popular and reliable protocols of chronic stress, the CMS model (Willner, 2017), to analyze the stress-induced behavioral, cellular and molecular changes involved, and to understand what changes are restored by the single administration of a sub-anesthetic dose of the rapid-acting antidepressant KET. In most previous studies, the effects of KET were investigated in groups of stressed animals taken as a whole (Li et al., 2011; Ma et al., 2013; Papp et al., 2017; Sun et al., 2016). Here we distinguished resilient and vulnerable animals by using the SPT and, for the first time, investigated the effects of KET in the animals vulnerable to CMS.
After CMS, we observed a number of changes exclusive for vulnerable rats, namely: (1) anhedonic behavior; (2) hyperactivation of the HPA axis (adrenal gland weight and CORT level); (3) reduction of basal/depolarization-evoked glutamate release and evoked GABA release in HPC; (4) reduction of dendritic BDNF mRNA trafficking; (5) atrophy/remodeling of apical dendrites. Reduction of expression of total BDNF mRNA and BDNF splice variants was found in both resilient and vulnerable rats. Most of these putative maladaptive changes were completely restored by the KET treatment after 24 h, considered the peak of the rapid antidepressant effect of KET (Abdallah et al., 2015; Duman, 2014), with the exception of HPA axis hyperactivation, evoked glutamate release and expression of BDNF in HPC. The rapid restoration of neuroarchitecture compromised by stress was shown here for the first time in the HPC of CMS-V rats.
In our hands, HPA axis hyperactivation does not seem to be a target of KET, which suggests that KET targets are downstream of this readout, as found previously for traditional antidepressants (Musazzi et al., 2010). As for glutamate/GABA release there is little or no previous data in the literature.
4.1. The impairment in glutamate and GABA release induced by CMS is partly restored by ketamine
A novel result of this work is the finding that CMS induces a major impairment of presynaptic release of endogenous glutamate in the HPC, only in CMS-V rats (see Fig. 2). Dysfunction of the glutamate system, a major outcome of stress in HPC and cortical areas, is considered a main feature of neuropsychiatric pathophysiology (Duman and Aghajanian, 2012; Lener et al., 2017; Murrough et al., 2017; Musazzi et al., 2017, 2010; Sanacora et al., 2012). The present findings are in line with previous studies showing that chronic stress reduces excitatory transmission in HPC and cortical areas (Kallarackal et al., 2013; Marrocco et al., 2014; Yuen et al., 2012). There is a general consensus in the literature on the hypothesis that dysfunction of excitatory transmission (reduction in HPC and in parts of the PFC) is a reason for impaired top-down control of subcortical areas, such as reward circuitry and amygdala, implicated in anhedonia, lowered mood and dysregulation of emotional control, typical of MDD (Price and Drevets, 2010; Workman et al., 2018). Strong support for this hypothesis comes from the compelling evidence of volumetric changes in these brain areas and of changes in functional connectivity in MDD patients (Abdallah et al., 2017). It is conceivable that the maladaptive changes in neuroarchitecture, extensively described after chronic stress, are accompanied by a reduction of glutamate release and impairment of synaptic homeostasis. Homeostatic synaptic mechanisms restore stability to brain circuitry in response to long-term changes in neuronal activity (Turrigiano, 2012); impairment of these mechanisms is likely to be involved in pathophysiology, whereas manipulation of homeostatic plasticity could be necessary for therapeutic action (Workman et al., 2018). Intriguingly, we found that KET seems to affect only basal but not depolarization-evoked glutamate release in CMS-V rats. Although at present it is unknown how this selective action of KET is connected to its antidepressant effect, it is interesting that basal glutamate release at synapses is a key regulator of homeostatic plasticity through local protein synthesis (Kavalali, 2015).
With regard to endogenous release of GABA, we found that basal release, similar to CNT in CMS-V, was enhanced in resilient rats, while depolarization-evoked GABA release was impaired in CMS-V rats and completely restored by KET treatment. Previous work has shown that CMS reduces the number of GABAergic interneurons in HPC, while impairing GABA synthesis, uptake and inhibitory synaptic transmission in PFC (Czéh et al., 2015; Wang et al., 2016). Interestingly, a higher number of GABAergic interneurons has been found in the PFC of resilient rats (Varga et al., 2017). Instead, the enhancement of basal GABA release induced by KET cannot be ascribed to a change in the number of interneurons and is likely due to changes in the parameters of GABA release. Our finding that KET completely restores evoked release of GABA in the HPC of CMS-V rats adds to its action on glutamate release and suggests a more complex action of KET on synaptic dysfunction and excitation/inhibition balance.
4.2. The impairment in dendritic BDNF trafficking induced by CMS is completely restored by ketamine
While the reduction of BDNF expression after CMS was expected, the complete lack of effect of KET on this deficiency was somewhat a surprise. Also, it was intriguing to find a higher BDNF protein expression in CMS-R rats, which is in line with the idea that resilience to stress is not a simple reversal of vulnerability mechanisms and involves pro-adaptive neuroplasticity (Musazzi et al., 2017; Russo et al., 2012). Recent studies have shed more light on some aspects of BDNF synthesis and release at synapses, and on their involvement in MDD pathophysiology and rapid-antidepressant action of KET (Song et al., 2017) and refs. therein). First, hybridization studies have reliably detected endogenous BDNF mRNA in neuronal dendrites (in both cell cultures and brain). Second, functional studies have shown that dendritic BDNF release is required for certain synaptic events, including enhancement of glutamate release and homeostatic synaptic plasticity, while disruption of dendritic BDNF localization leads to deficiencies in synaptic function. As a consequence, the purported role of BDNF in the rapid action of KET (Autry and Monteggia, 2012; Li et al., 2011) could be linked to selective and rapid trafficking, synthesis and release of dendritic pools of BDNF. This mechanism could be common to other glutamatergic rapid-acting antidepressants, and may be the basis of a future strategy for antidepressant drug development (Song et al., 2017).
In light of these aspects, the present novel findings that CMS induces impairment of BDNF trafficking into dendrites, and KET rapidly restores this critical supply, may open new paths to understand better the role of BDNF in MDD pathophysiology and therapy. It is interesting that the reduction of BDNF trafficking implicates the two splice variants (BDNF-2 and BDNF-6) previously shown to be transported to distal dendrites (Baj et al., 2012, 2011). Intriguingly, the action of KET here seems to distinguish between BDNF-2 (whose translocation is restored in both CA1 and CA3) and BDNF-6 (restored only in CA3). It has been shown that BDNF contributes to the homeostatic plasticity of neuronal firing rates (Rutherford et al., 1998). Stabilization of homeostatic plasticity, compromised in MDD pathophysiology by chronic stress and other factors, has been purported as a major aspect of therapy, whether by pharmacological treatments or other means (Workman et al., 2018).
Different mechanisms have been proposed for the action of ketamine, including NMDA receptor-independent mechanisms (see Zanos et al., 2016; Williams et al., 2018; Wray et al., 2018; Zanos et al., 2018; Kadriu et al., 2019). Current hypotheses on the nature of rapid antidepressant mechanism of KET include rapid increase of glutamate release and rapid translation/release of BDNF at excitatory synapses (Autry and Monteggia, 2012; Li et al., 2011). However, it is not clear if the surge of glutamate release induces BDNF rapid translation or vice versa. It is likely that the two phenomena are related, but how?
There is convergent evidence in the literature that neuronal activation can lead to detectable increases of mRNAs in dendrites, including BDNF. Indeed, activation of selected synapses by glutamate uncaging can drive changes in the localization and local translation of endogenous mRNA in dendrites of HPC neurons (Yoon et al., 2016). On the other hand, BDNF may orchestrate presynaptic changes in a state-dependent manner. It has been shown that blockade of AMPA receptors in cultured HPC neurons induces rapid enhancement of presynaptic function, requiring synthesis and release of BDNF, locally translated in dendrites, as a retrograde messenger (Jakawich et al., 2010). Thus, changes of glutamate release and BDNF could be interconnected in the rapid action of KET. Future studies are bound to shed more light on the involvement of these aspects in rapid antidepressant action.
5. Conclusions
In conclusion, we have shown that KET rapidly reverses anhedonic behavior in rats vulnerable to CMS, and this is paralleled by partial restoration of glutamate/GABA release in the HPC, complete restoration of BDNF dendritic trafficking and of apical dendrites atrophy. Therefore, this work suggests a relationship between vulnerability/resilience to chronic stress, glutamate/GABA release and availability of BDNF mRNA at synapses. Overall, the present results are consistent with a mechanism of ketamine involving restoration of synaptic homeostasis, through re-equilibration of glutamate/GABA release and dendritic BDNF for synaptic translation and reversal of synaptic and circuitry impairment.
Conflict of interest
The authors declare no conflict of interests.
Funding
This work was supported by grants from the Ministry of Education, University and Research (MIUR) (PRIN, 2012 prot. 2012A9T2S9; PRIN, 2015 prot. 2015HRE757_002), Cariplo Foundation (biomedical science for young scientists, Prog. 2014-1133), NARSAD (2014 Young Investigator Grant), and supported by Centre for Stochastic Geometry and Advanced Bioimaging, funded by Villum Foundation.
GT is supported by a DFF grant from the Danish Council for Independent Research (DFF-5053-00103).
Acknowledgments
The authors acknowledge Drs Francesco Brizzi, Luca Bramè and Ilaria Mazza for technical help.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2019.100160.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdallah C.G., Averill C.L., Salas R., Averill L.A., Baldwin P.R., Krystal J.H., Mathew S.J., Mathalon D.H. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol. psychiatry. Cogn. Neurosci. neuroimaging. 2017;2:566–574. doi: 10.1016/j.bpsc.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah C.G., Averill L.A., Krystal J.H. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann. N. Y. Acad. Sci. 2015;1344:66–77. doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid T., Kazantseva A., Piirsoo M., Palm K., Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. 2013. DSM V, Diagnostic and Statistical Manual of Mental Disorders. [DOI] [Google Scholar]
- Autry A.E., Monteggia L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj G., D'Alessandro V., Musazzi L., Mallei A., Sartori C.R., Sciancalepore M., Tardito D., Langone F., Popoli M., Tongiorgi E. Physical exercise and antidepressants enhance BDNF targeting in hippocampal CA3 dendrites: further evidence of a spatial code for BDNF splice variants. Neuropsychopharmacology. 2012;37:1600–1611. doi: 10.1038/npp.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj G., Leone E., Chao M.V., Tongiorgi E. Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proc. Natl. Acad. Sci. U.S.A. 2011;108:16813–16818. doi: 10.1073/pnas.1014168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bonanno G., Giambelli R., Raiteri L., Tiraboschi E., Zappettini S., Musazzi L., Raiteri M., Racagni G., Popoli M. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J. Neurosci. 2005;25:3270–3279. doi: 10.1523/JNEUROSCI.5033-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino T., Musazzi L., Milanese M., Seguini M., Marte A., Gallia E., Cattaneo L., Onofri F., Popoli M., Bonanno G. Altered mechanisms underlying the abnormal glutamate release in amyotrophic lateral sclerosis at a pre-symptomatic stage of the disease. Neurobiol. Dis. 2016;95:122–133. doi: 10.1016/j.nbd.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Chao M.V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chiaruttini C., Sonego M., Baj G., Simonato M., Tongiorgi E. BDNF mRNA splice variants display activity-dependent targeting to distinct hippocampal laminae. Mol. Cell. Neurosci. 2008;37:11–19. doi: 10.1016/j.mcn.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Czéh B., Varga Z.K.K., Henningsen K., Kovács G.L., Miseta A., Wiborg O. Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus. 2015;25:393–405. doi: 10.1002/hipo.22382. [DOI] [PubMed] [Google Scholar]
- Duman R.S. Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin. Neurosci. 2014;16:11–27. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Aghajanian G.K. Synaptic dysfunction in depression: potential therapeutic targets. Science (80-. ) 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Aghajanian G.K., Sanacora G., Krystal J.H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.-H., Nestler E.J. Neural substrates of depression and resilience. Neurotherapeutics. 2017;14:677–686. doi: 10.1007/s13311-017-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A., Mallei A., Popoli M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016:6212983. doi: 10.1155/2016/6212983. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee S.R., Price T.S. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol. Psychiatr. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakawich S.K., Nasser H.B., Strong M.J., McCartney A.J., Perez A.S., Rakesh N., Carruthers C.J.L., Sutton M.A. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143–1158. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B., Musazzi L., Henter I.D., Graves M., Popoli M., Zarate C.A. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int. J. Neuropsychopharmacol. 2019;22:119–135. doi: 10.1093/ijnp/pyy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallarackal A.J., Kvarta M.D., Cammarata E., Jaberi L., Cai X., Bailey A.M., Thompson S.M. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J. Neurosci. 2013;33:15669–15674. doi: 10.1523/JNEUROSCI.2588-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali E.T. The mechanisms and functions of spontaneous neurotransmitter release. Nat. Rev. Neurosci. 2015;16:5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- Kavalali E.T., Monteggia L.M. How does ketamine elicit a rapid antidepressant response? Curr. Opin. Pharmacol. 2015;20:35–39. doi: 10.1016/j.coph.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali E.T., Monteggia L.M. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am. J. Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- Kellner Y., Gödecke N., Dierkes T., Thieme N., Zagrebelsky M., Korte M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front. Synaptic Neurosci. 2014;6:5. doi: 10.3389/fnsyn.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Via L., Bonini D., Russo I., Orlandi C., Barlati S., Barbon A. Modulation of dendritic AMPA receptor mRNA trafficking by RNA splicing and editing. Nucleic Acids Res. 2013;41:617–631. doi: 10.1093/nar/gks1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener M.S., Niciu M.J., Ballard E.D., Park M., Park L.T., Nugent A.C., Zarate C.A. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatry. 2017;81:886–897. doi: 10.1016/j.biopsych.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Liu R.-J., Dwyer J.M., Banasr M., Lee B., Son H., Li X.-Y., Aghajanian G., Duman R.S. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.-C., Dang Y.-H., Jia M., Ma R., Wang F., Wu J., Gao C.-G., Hashimoto K. Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J., Reynaert M.-L., Gatta E., Gabriel C., Mocaër E., Di Prisco S., Merega E., Pittaluga A., Nicoletti F., Maccari S., Morley-Fletcher S., Mairesse J. The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders. J. Neurosci. 2014;34 doi: 10.1523/JNEUROSCI.4131-13.2014. 2015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann. N. Y. Acad. Sci. 2016;1373:56–64. doi: 10.1111/nyas.13020. [DOI] [PubMed] [Google Scholar]
- Monteggia L.M., Kavalali E.T. Scopolamine and ketamine: evidence of convergence? Biol. Psychiatry. 2013;74:712–713. doi: 10.1016/j.biopsych.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Murrough J.W., Abdallah C.G., Mathew S.J. Targeting glutamate signalling in depression: progress and prospects. Nat. Rev. Drug Discov. 2017;16:472–486. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- Musazzi L., Milanese M., Farisello P., Zappettini S., Tardito D., Barbiero V.S., Bonifacino T., Mallei A., Baldelli P., Racagni G., Raiteri M., Benfenati F., Bonanno G., Popoli M. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008566. e8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L., Tornese P., Sala N., Popoli M. Acute stress is not acute: sustained enhancement of glutamate release after acute stress involves readily releasable pool size and synapsin I activation. Mol. Psychiatr. 2017;22:1226–1227. doi: 10.1038/mp.2016.175. [DOI] [PubMed] [Google Scholar]
- Musazzi L., Treccani G., Mallei A., Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol. Psychiatry. 2013;73:1180–1188. doi: 10.1016/j.biopsych.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Nava N., Treccani G., Alabsi A., Kaastrup Mueller H., Elfving B., Popoli M., Wegener G., Nyengaard J.R. Temporal dynamics of acute stress-induced dendritic remodeling in medial prefrontal cortex and the protective effect of desipramine. Cerebr. Cortex. 2017;27:694–705. doi: 10.1093/cercor/bhv254. [DOI] [PubMed] [Google Scholar]
- Papp M., Gruca P., Lason-Tyburkiewicz M., Willner P. Antidepressant, anxiolytic and procognitive effects of subacute and chronic ketamine in the chronic mild stress model of depression. Behav. Pharmacol. 2017;28:1–8. doi: 10.1097/FBP.0000000000000259. [DOI] [PubMed] [Google Scholar]
- Popoli M., Yan Z., McEwen B.S., Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I., Bonini D., Via L. La, Barlati S., Barbon A. AMPA receptor properties are modulated in the early stages following pilocarpine-induced status epilepticus. NeuroMolecular Med. 2013;15:324–338. doi: 10.1007/s12017-013-8221-6. [DOI] [PubMed] [Google Scholar]
- Russo S.J., Murrough J.W., Han M.-H., Charney D.S., Nestler E.J. Neurobiology of resilience. Nat. Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford L.C., Nelson S.B., Turrigiano G.G. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Treccani G., Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Martinowich K., Lee F.S. BDNF at the synapse: why location matters. Mol. Psychiatr. 2017;22:1370–1375. doi: 10.1038/mp.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T., Couch Y., Kholod N., Boyks M., Malin D., Leprince P., Steinbusch H.M. Update in the methodology of the chronic stress paradigm: internal control matters. Behav. Brain Funct. 2011;7:9. doi: 10.1186/1744-9081-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.-L., Zhou Z.-Q., Zhang G.-F., Yang C., Wang X.-M., Shen J.-C., Hashimoto K., Yang J.-J. Role of hippocampal p11 in the sustained antidepressant effect of ketamine in the chronic unpredictable mild stress model. Transl. Psychiatry. 2016;6:e741. doi: 10.1038/tp.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treccani G., Musazzi L., Perego C., Milanese M., Nava N., Bonifacino T., Lamanna J., Malgaroli A., Drago F., Racagni G., Nyengaard J.R., Wegener G., Bonanno G., Popoli M. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol. Psychiatr. 2014;19:433–443. doi: 10.1038/mp.2014.5. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a005736. a005736–a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R. Gene-Environment interactions in severe mental illness. Front. Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Csabai D., Miseta A., Wiborg O., Czéh B. Chronic stress affects the number of GABAergic neurons in the orbitofrontal cortex of rats. Behav. Brain Res. 2017;316:104–114. doi: 10.1016/j.bbr.2016.08.030. [DOI] [PubMed] [Google Scholar]
- Wang G.-Y., Zhu Z.-M., Cui S., Wang J.-H. Glucocorticoid induces incoordination between glutamatergic and GABAergic neurons in the amygdala. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse E.G., Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol. Cell. Neurosci. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N.R., Heifets B.D., Blasey C., Sudheimer K., Pannu J., Pankow H., Hawkins J., Birnbaum J., Lyons D.M., Rodriguez C.I., Schatzberg A.F. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am. J. Psychiatry. 2018 doi: 10.1176/appi.ajp.2018.18020138. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol. Stress. 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman E.R., Niere F., Raab-Graham K.F. Engaging homeostatic plasticity to treat depression. Mol. Psychiatr. 2018;23:26–35. doi: 10.1038/mp.2017.225. [DOI] [PubMed] [Google Scholar]
- Wray N.H., Schappi J.M., Singh H., Senese N.B., Rasenic k M.M. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol. Psychiatr. 2018 doi: 10.1038/s41380-018-0083-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y.J., Wu B., Buxbaum A.R., Das S., Tsai A., English B.P., Grimm J.B., Lavis L.D., Singer R.H. Glutamate-induced RNA localization and translation in neurons. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E6877–E6886. doi: 10.1073/pnas.1614267113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E.Y., Wei J., Liu W., Zhong P., Li X., Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Moaddel R., Morris P.J., Georgiou P., Fischell J., Elmer G.I., Alkondon M., Yuan P., Pribut H.J., Singh N.S., Dossou K.S., Fang Y., Huang X.P., Mayo C.L., Wainer I.W., Albuquerque E.X., Thompson S.M., Thomas C.J., Zarate C.A., Jr., Gould T.D. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. https://doi: 10.1038/nature17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Gould T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatr. 2018;23:801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C.A., Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatr. 2006;63:856. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.