Abstract
Stress is an inevitable part of life that can profoundly impact social and emotional functioning, contributing to the development of psychiatric disease. One key component of emotion and social processing is facial expressions, which humans can readily detect and react to even without conscious awareness. Facial expressions have been the focus of philosophic and scientific interest for centuries. Historically, facial expressions have been relegated to peripheral indices of fixed emotion states. More recently, affective neuroscience has undergone a conceptual revolution, resulting in novel interpretations of these muscle movements. Here, we review the role of facial expressions according to the leading affective neuroscience theories, including constructed emotion and social-motivation accounts. We specifically highlight recent data (Mayo et al, 2018) demonstrating the way in which stress shapes facial expressions and how this is influenced by individual factors. In particular, we focus on the consequence of genetic variation within the endocannabinoid system, a neuromodulatory system implicated in stress and emotion, and its impact on stress-induced facial muscle activity. In a re-analysis of this dataset, we highlight how gender may also influence these processes, conceptualized as variation in the “fight-or-flight” or “tend-and-befriend” behavioral responses to stress. We speculate on how these interpretations may contribute to a broader understanding of facial expressions, discuss the potential use of facial expressions as a trans-diagnostic marker of psychiatric disease, and suggest future work necessary to resolve outstanding questions.
Keywords: Facial expression, Affect, Emotion, Stress, Anandamide, Gender differences
Highlights
-
•
Affective neuroscience theories disagree on the meaning of facial expressions.
-
•
They may be used in social communication or to express affective state.
-
•
The effect of stress on facial expressions varies across individuals.
-
•
Facial expressions are sensitive to pharmacological intervention.
-
•
Facial expressions may serve as a trans-diagnostic biomarker of psychiatric disease.
1. Introduction
Stress- and affect-related psychiatric disorders are often co-morbid, resulting in heterogeneous psychiatric populations with similarly varied treatment needs (Craske and Stein, 2016; Griebel and Holmes, 2013). Understanding the interplay between stress and affect in healthy humans can provide a benchmark for investigating the dysregulation of these processes in mental illnesses, potentially pointing to novel therapeutics. The behavioral and physiological components of the stress response have been well-studied (Godoy et al., 2018), but less is known about the behavioral and physiological mechanisms of affect in humans (Rubinow and Schmidt, 2018). This dearth of knowledge has been recognized and addressed in the domain of affective neuroscience over the last several years. In the course of the development of this discipline, prior conceptualizations of affect have been brought to the forefront and criticized (Barrett, 2006), refined (Hutto et al., 2018), or replaced all together (Barrett, 2017). Our renewed interest in emotional functioning in the context of the brain has reignited age-old debates over how, or even if, affect can be studied in humans in a meaningful and reproducible way (Ekman, 2016). In both experimental and clinical settings, researchers and clinicians often rely on self-reported assessments of affective functioning. Self-report may not be as reliable as we think (Nisbett and Wilson, 1977), but what are the alternatives? One potential candidate may be staring us in the face.
Facial expressions have captivated scientists and philosophers alike for centuries. From Darwin (1872), all the way back to the physiognomists, or “face-readers”, of ancient Egypt and Arabia (circa 340 B.C.) (Fridlund and Fridlund, 1994), facial expressions have elicited fascination and speculation. Modern research has shown that humans are primed to recognize and react to facial expressions, at times even without explicit awareness (Dimberg et al., 2000; Dimberg and Petterson, 2000). Thus, the face serves as a powerful social communication tool. But what is it that facial expressions are actually signaling? At first glance, the relation between affect and facial expressions may seem obvious. Children learn early that smiling faces are “happy” while furrowed brows signal “anger.” However, without a scientific consensus on a conceptualization of affect, interpreting this relationship becomes tenuous. Recently, trans-diagnostic reports of dysregulated facial expressions (Davies et al., 2016) have contributed to the inclusion of facial expression production within the National Institute of Mental Health (NIMH)'s Research Domain Criteria (RDoC) initiative. At the same time, automated facial expression recognition software, based on Paul Ekman's facial action coding system (FACS (Ekman and Rosenberg, 1997); discussed more below) has been used across disciplines (Whitehill et al., 2013; Gilmore, 2017) with some proposing to implement facial expression-detecting algorithms to aid in psychotherapy (Gay et al., 2013; Kumazaki et al., 2017). Thus, understanding the role of facial expressions in social and emotional functioning is critical to elucidating clinically relevant biomarkers, understanding their functional consequence, and implementing related therapies in an effective, evidence-based manner.
Here, we will briefly highlight how current perspectives on affective functioning suggest we understand facial expressions. While an exhaustive description of these theories is beyond the scope of the current manuscript, we will instead place emphasis on the role of facial expressions within these models. Across theoretical frameworks, context has become increasingly hailed as an integral component in the interpretation of facial expressions. Consequently, we will concentrate our discussion on facial expressions within the context of a particularly relevant social situation: acute stress exposure. As an illustrative example, we will focus on recently published data (Mayo et al., 2018) highlighting individual factors that influence how stress shapes facial expressions. We will discuss in depth how genetic variation in the endocannabinoid system, a neuromodulatory system implicated in stress and emotion processing, influences stress-induced facial muscle activity. In addition, we will introduce a re-analysis of this dataset that implicates gender as a factor that modulates stress-induced facial expressions. In both cases, we will frame these results in the context of the leading theories of affect in an effort to determine whether this dataset can help us disentangle the role of facial expressions in subjective affective experience versus social-motivation terms. We will close by outlining the real-world implications of these alternative views of facial expressions, the potential clinical relevance, and future directions that may provide the information needed to move towards a consensus on this issue.
1.1. Finding a theoretical place for the face
Darwin proposed a homology in facial expressions across individuals of different cultures, as well as between humans and animals, based on systematic investigation of facial expressions across the age span, cultures, and disease states (Darwin, 1872). This work served as the foundation for the predominant neurocultural theory of facial expressions (Izard, 1971; Ekman and Friesen, 1969). Nearly ubiquitous in introductory psychology courses, this essentialist view of emotion has guided decades of research (Ekman, 1984). According to the most notable example of this view, formalized by Ekman as the Basic Emotion Theory (BET (Ekman, 1984)), facial expressions convey fixed, generalizable emotion states. From this perspective, the emotion “anger” is displayed by a prototypical anger face that includes furrowed brows and a scowl. Based on this formulation, Ekman created FACS as a standardized method to score minute facial muscle movements indicative of these proposed fixed emotion states (Ekman and Rosenberg, 1997). This system has left a significant footprint not only in academia, but also in industry and popular culture. FACS is used by software companies (Whitehill et al., 2013), has been capitalized on by marketing companies (Neto and Filipe, 2016), and even incorporated into popular media (e.g. the Fox series “Lie to Me”).
The ubiquity of this essentialist viewpoint is not confined to laypeople or novice psychology students. Only a few years ago, a poll of prominent emotion scientists found that approximately 80% endorsed the view that the face conveys universal signals of emotion (Ekman, 2016). The universality of facial expressions has been promoted largely based on data suggesting that remote, small-scale societies with limited exposure to Western cultural norms have, nonetheless, similar facial expressions and similar interpretations of these expressions. The first wave of these studies took place decades ago and used confirmation-based research methods designed to support universality (Ekman et al., 1987; Ekman and Friesen, 1971). For instance, participants were shown a picture of a posed emotion face and asked to label it using a limited number of discrete emotion categories (Ekman et al., 1987). Even then, support was moderate, at best (Nelson and Russell, 2013). Increasingly innovative methods, including more objective, data-driven approaches have called into question the recognition of facial expressions as universal, discrete emotions (Chen et al., 2018; Gendron et al., 2018). A second wave of studies in small-scale, indigenous samples, discussed more below, have recently provided even stronger contrary evidence. Moreover, evidence from studies assessing the neural and physiological correlates of facial expressions fail to support the existence of distinct emotions categories (Barrett, 2006; Lindquist et al., 2012). Thus, accumulating evidence (Shariff and Tracy, 2011; Barrett and Satpute, 2017) strongly suggests that facial expressions are more than reflections of hardwired, discrete emotions that many classic essentialist theories claim.
An alternative functional account of facial expressions derives from constructivist emotion theories. From this perspective, emotions are not unique perception-action programs with stereotyped behavioral and physiological outputs, and as a result, facial expressions are not indicative of discrete emotion categories (Barrett, 2006; Posner et al., 2005). Instead, constructivist accounts describe an affective state as comprised of the orthogonal dimensions of valence (positive versus negative) and arousal (activated versus deactivated). Consequently, facial expressions serve as indices of individuals' affective state, but they do so only at the level of “core affect” indicating points on the dimensions of valence and arousal. Core affect can be characterized as the totality of information regarding an organism's neurophysiological state and any perturbations as a consequence of internally or externally changing events (Barrett, 2006). Thus, objects and events have affective meaning to the extent that they can influence the homeostatic (or “core affective”) state of the individual. Our conscious representation of core affect is thus experienced as feeling states described in terms of affective valence and arousal (Russell and Barrett, 1999).
Thinking in terms of core affect, as opposed to basic emotion states, modifies our interpretation of facial expressions. Instead of basic emotion states such as “happy” or “sad”, varying levels of valence and arousal combine to produce affective states to which emotional category terms can be applied. Thus, the term “happy” has been attributed to the state of high positive valence and moderate arousal. This extends to the interpretation of facial expressions. For instance, activation of the corrugator muscle to furrow the brow would signal an increase in negative-valenced affect, while activation of the zygomatic muscle to pull up the corners of the mouth into a smile would convey enhanced positive-valenced affect (Lang et al., 1993). Increased corrugator activity alone, however, does not tell you which of the approximately six basic emotions hypothesized by essentialist accounts is being experienced. More recent studies conducted within small-scale societies (Crivelli et al., 2017; Gendron et al., 2014) (for review, see (Gendron et al., 2018)) have also cast doubt on essentialist emotion theories proposing universality of emotional expression and experience (Gendron et al., 2018; Chen and Jack, 2017). For instance, many individuals from small, isolated societies fail to accurately label faces conveying the prototypical emotions proposed by essentialist accounts, while the same images elicit near universal agreement on affective valence of the face in question (Crivelli et al., 2017). Neuroimaging, physiological, and psychological data are supportive of a more general distinction of affect in terms of valence and arousal rather than discrete emotion states (for review, see (Barrett, 2006; Lindquist et al., 2012)). Thus, facial expressions may depict the internal affective state of an individual, effectively broadcasting this state to others, but not in as specific of terms as suggested by universalist models.
Both essentialists and constructivists propose that perceivers of facial expressions use them as a means to infer the mental state of the expresser. However, it is possible that facial expressions are instead playing a role in action identification (Dewey, 1894; Kozak et al., 2006), particularly in the contexts of social influence and communication (Parkinson, 1996; Crivelli and Fridlund, 2018; Krebs et al., 1984). One of the most well-developed of these motivation-communication views, the behavioral ecology view of facial displays (Fridlund, 1994), hypothesizes a role for facial expressions as tools to communicate behavioral intentions or social motives, rather than express one's current affective state. Accordingly, in a series of observational studies, it was found that athletes often do not smile upon winning a given event, even though emotional valence and intensity are expected to be at relative extremes (Crivelli et al., 2015; Kraut and Johnston, 1979; Fernández-Dols and Ruiz-Belda, 1995; Ruiz-Belda et al., 2003). Instead, smiles were displayed by athletes upon turning to face an audience or when otherwise engaged in a social interaction. Facial expression are thus signs for promoting contingent action in social negotiation, the interpretation of which relies on the context, the individuals engaged in the negotiation, and their histories (Crivelli and Fridlund, 2018). For instance, an expresser displaying an “anger” face is providing a cue that signals the potential for a hostile interaction, allowing the receiver to make a choice to either continue and face the impending antagonistic interaction or to flee and avoid the hostile situation (Parkinson, 1996). Similarly, a smile (“happy” face) facilitates affiliative behavior, while scrunching the nose (“disgust” face) signals a rejection of the current interaction trajectory (Crivelli and Fridlund, 2018). In agreement, new methods used to study small, indigenous societies suggest that facial expressions are more associated with behaviors than with discrete emotions (Gendron et al., 2018; Crivelli et al., 2017). Even in children less than a year old, facial expressions appear to be motivated more by social context than subjective state (Jones et al., 1991). Social-motivation accounts of facial expressions even receive support from animal studies, as recent evidence suggests that macaques can predict social outcomes from facial expressions (Waller et al., 2016). Clinically, social anxiety disorder is associated with reduced positive facial expressions during social interaction, potentially as a means to discourage social approach (Pearlstein et al., 2019). Thus, facial expressions may be a tool to express social motives, such as eliciting approach or promoting avoidance from others in a social setting.
A common critique of the behavioral ecology view of facial expressions and other social-motivation accounts is the implied necessity of the social setting. Facial expressions are frequently made in the absence of other social interactants, such as those directed towards animals (e.g. our pets), non-living things (e.g. cellphones), or even ourselves (e.g. talking to oneself). One may argue that there is no need for social signaling in these situations and that these examples would provide weight to the constructivist view of facial expressions displaying a measure of internal affect. The counterargument, as described in detail elsewhere (Crivelli and Fridlund, 2018), is that while physically alone, we are not necessarily psychologically alone. Production of facial expressions is influenced by social relationship status of those in the vicinity (Wagner and Smith, 1991) and potentiated by the presence of real or imagined audiences (Fridlund et al., 1990; Fridlund, 1991). Thus, the context does not only include the physical context, but perhaps the psychological context, as well.
1.2. Quantifying the faces of stress
Many scientists have explored how acute stress influences the perception or detection of facial expressions. Stress exposure is generally associated with enhanced sensitivity in detecting facial expressions (Daudelin-Peltier et al., 2017), though findings can vary based on factors such as age (Everaerd et al., 2017), gender (Duesenberg et al., 2016; Quas et al., 2000), and psychiatric diagnosis (Ahs et al., 2017). Stimuli used in these studies typically consist of standardized pictures of faces manipulated to display a prototypical emotion face (e.g. “fear” or “anger”) that participants are asked to match, given a limited set of emotion words (Ekman et al., 1987). Other approaches assess neural or psychophysiological responses to these facial displays (Morris et al., 1996; Breiter et al., 1996). The ability to detect and respond to facial expressions likely has profound impact on one's social capabilities. Across modalities, these efforts are almost exclusively focused on the response of the perceiver, or the person who is trying to decode the facial expression. However, social communication is a two-way street; it is not only dependent upon one's ability to detect facial expressions, but also on the ability to express via the face. Stress may influence traffic on this street heading in both directions; that is, stress may not only influence one's ability to detect facial expressions, but may also influence the production of facial expressions.
In contrast, few studies have asked how stress influences facial expressions of the expresser: the individual being stressed who is sending out the signal that is to be perceived. This is a particularly interesting scenario given that the face is a primary channel of social communication and may convey subtle messages apart from more typical stress assessments, i.e. cortisol response, skin conductance, or subjective self-report ratings. Moreover, several psychiatric disease states are associated with dysregulation in the production of facial expressions (Davies et al., 2016), suggesting that facial expressions may serve as biomarkers of both acute and chronic disease states. Thus, understanding how stress shapes facial expressions has the potential to provide a more nuanced picture of the consequences of acute and chronic stress on this proposed biomarker and its utility as an RDoC subconstruct.
Recently, we developed a paradigm to assess facial muscle reactivity to emotional images, as well as stress-induced changes in facial muscle responses (Mayo et al., 2018) (for a detailed description of the methods, see supplemental materials). To do so, we measured facial electromyography (EMG) of the corrugator (“frown”) muscle and the zygomatic (“smile”) muscle in response to emotional images from the International Affective Picture System (IAPS (Lang et al., 1993)). Next, participants are subjected to a standardized stress task, the Maastricht Acute Stress Task (MAST (Smeets et al., 2012)), consisting of alternating trials of hand immersion in ice cold water and mental arithmetic performed aloud with negative socio-evaluative feedback. They then complete another IAPS task to assess how stress influences facial EMG activity at rest and in response to emotional images. On a separate day (in counterbalanced order), participants also complete a control task, again flanked by IAPS image presentation and EMG measurement. As a result, we obtain a baseline readout of facial muscle activity in response to emotional images, and we can then determine how stress modulates these responses. Importantly, with EMG we obtain continuous data whereas FACS supplies only discrete, categorical output. With this approach, we can begin to understand how stress changes facial expressions and what individual factors modulate this behavior.
1.3. Is the “bliss molecule” an emotional buffer?
While we are beginning to resolve questions about what facial expressions tell us about the architecture and function of emotion, we are only recently starting to understand relations between molecular and expressive levels. That is, do pharmacological compounds that influence how we “feel” also influence what our faces show? Psychoactive drugs are consumed recreationally, often to increase mood or enhance sociability (Bogt and Engels, 2005; Boys et al., 2001; TART, 1970), and psychiatric disorders hallmarked by dysregulation of social or affective processing are currently, or may soon be, treated by these same substances (Mithoefer et al., 2016). Notably, marijuana is the most commonly used illicit drug (UNODC, 2015) and has been used for centuries to both enhance mood and reduce stress or anxiety (Cheung et al., 2010) and has recently gained medicinal or recreational use in several regions. The primary psychoactive component of marijuana, Δ-9-tetrahydrocannabinol (THC), is an agonist at cannabinoid type 1 (CB1) receptors. This receptor is also activated by endogenous cannabinoid ligands, such as anandamide. Anandamide (AEA), whose name derives from word ananda, the Sanskrit word for “joy, bliss, or happiness”, is often referred to as the “bliss molecule” in reference to its positive-mood enhancing properties (Devane et al., 1992). AEA is proposed to act as an emotional buffer (Morena and Campolongo, 2014), and preclinical evidence suggests that enhanced AEA via inhibition of its main degradative enzyme, fatty acid amide hydrolase (FAAH), protects against the anxiogenic effects of stress (Haller et al., 2009). FAAH inhibitors are proposed as novel treatments for stress-related psychopathology (Gunduz-Cinar et al., 2013a, Gunduz-Cinar et al., 2013b; Patel et al., 2017), but are not currently approved for human use. However, initial insights into the consequences of elevated AEA come from human behavioral genetics studies (Hariri et al., 2009; Gunduz-Cinar et al., 2013a, Gunduz-Cinar et al., 2013b; Dincheva et al., 2015), including our recently published work (Mayo et al., 2018).
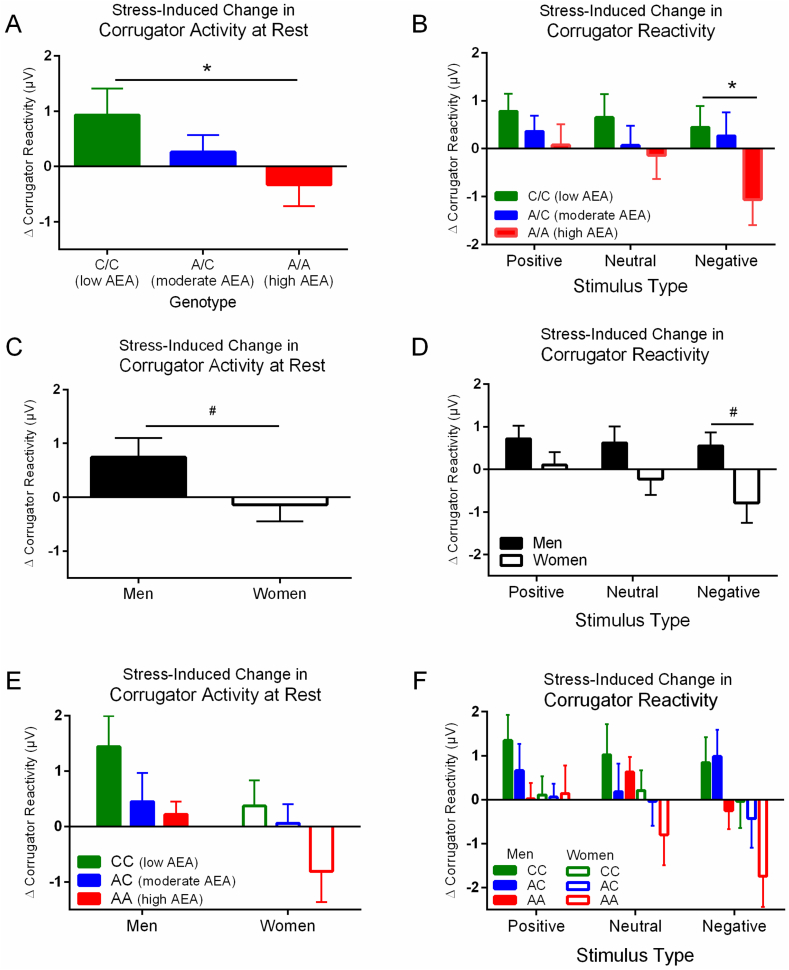
Using the aforementioned stress and affect paradigm, we assessed how enhanced AEA influenced stress and stress-induced facial muscle activity (Mayo et al., 2018) (see Fig. 1; Fig. 2A and B). We found that individuals homozygous for the loss-of-function FAAH 385A allele, whom we confirmed to have elevated peripheral AEA levels at baseline, are protected against stress-induced decreases in circulating AEA. Peripheral AEA levels, at baseline or in response to stress, were not associated with any differences in subjective or neuroendocrine stress response. Consequently, this allowed us to determine if this stress-induced decrease in AEA influences facial muscle activity apart from any confounding factors implicating differences in stress reactivity as assessed via common stress-related outcome measures (e.g. neuroendocrine, autonomic). Put more simply, would retaining this “emotional buffer” indeed buffer the negative affective consequences of stress? We found that stress produced an increase in corrugator activity at rest (e.g. in the absence of any stimulus) in a gene-dose-dependent manner (effect of genotype: F (2,69) = 4.83, p = 0.031, partial η (Griebel and Holmes, 2013) = 0.064), such that those with the lowest AEA (C-allele homozygotes) show the greatest increase in resting corrugator activity (e.g. more “frowning”) following stress. When exposed to affective stimuli, those with stress-induced decreases in AEA showed a net increase in corrugator reactivity in response to stimuli across all stimulus categories (positive, neutral, and negative; F (2,138) = 3.75, p = 0.050, partial η (Griebel and Holmes, 2013) = 0.053). Perhaps unexpectedly, individuals whose AEA levels were impervious to stress failed to show this increase, and in fact demonstrate reduced corrugator activity specifically to negative stimuli following stress (response to negative stimuli: F (2,69) = 4.53, p = 0.037, partial η (Griebel and Holmes, 2013) = 0.061). It is important to note that we found no difference in self-reported ratings of image valence or arousal. Consequently, our participants did not differ in how positively or negatively they perceived the images, but only differed in their reactions to the images. Moreover, these effects were only apparent following the stress task, as we did not see any effect of genotype on baseline EMG activity or self-report ratings (see supplemental materials).
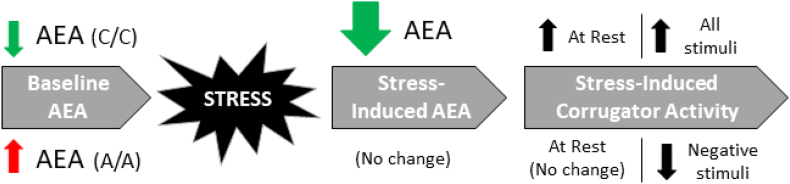
Fig. 1.
Summary of FAAH C385A genotype-based differences in anandamide and stress-induced corrugator activity. Individuals with low baseline levels of peripheral AEA (e.g. C/C) demonstrated a stress-induced reduction in AEA that coincided with an increase in corrugator reactivity at rest and in response to affective stimuli (positive, neutral, and negative). Those with higher levels of AEA (e.g. A/A) demonstrated no change in AEA following stress. In addition, they were protected against stress-induced changes in corrugator activity at rest, and even showed reduced corrugator activity in response to negative stimuli. Schematic based on data presented in (Mayo et al., 2018) and Fig. 2A and B.
Fig. 2.
Anandamide and gender-based differences in stress-induced changes in corrugator reactivity. Participants completed an affective image task before and after stress and control procedures. We calculated the net change due to stress of the corrugator (“frown”) muscle as follows: ([Post – Pre] stress corrugator activity – [Post – Pre] control corrugator activity). This was assessed at rest (e.g. in the absence of any stimulus), and in response to emotional images with positive, neutral, or negative emotional content. Enhanced anandamide conferred via FAAH 385A produced a gene-dose-dependent reduction in stress-induced corrugator reactivity at rest (A) and in response to emotional images, particularly those with negative emotional content (B). Following stress, men show a greater increase in corrugator reactivity at rest (C), as well as in response to emotional images (D), as compared to women. Combinatory effects of genotype and gender on corrugator activity at rest (E) and in response to emotional images (F) are in the expected directions. *p < 0.05 effect of genotype; #p < 0.05 effect of gender.
A straightforward interpretation of these findings is that elevated AEA, a putative emotional buffer (Morena and Campolongo, 2014) protects against stress-induced increases in negative affect as measured via corrugator activity. When stress depletes this emotional buffer, we see increased corrugator activity, signaling increased internal negative affect. However, for those whose AEA levels are unaffected by stress, we instead see a reduction in negative affect. This could be a particularly advantageous response in the face of environmental aversiveness: while our stimuli (e.g. pictures of snakes, spiders, etc.) elicit an increase in negative affect, these are rendered less affectively salient following the stressor. This finding is in line with accumulating neuroimaging evidence showing that this same variant is associated with enhanced fronto-amygdala connectivity at rest and during emotion regulation, suggesting a greater capacity for emotion regulation in individuals carrying this allele (Gunduz-Cinar et al., 2013a, Gunduz-Cinar et al., 2013b; Dincheva et al., 2015; Gartner et al., 2018). Together, these data suggest that AEA functions as an emotional buffer, protecting against the negative effects of stress, including enhanced negative affect demonstrated via enhanced corrugator reactivity.
Alternatively, our findings could be interpreted as an advantageous adaptation in social functioning following stress. Recently, the eCB system has been implicated as an integral component in both social anxiety and social reward, suggesting that modulation of the eCB system may bi-directionally mediate social behavior (Wei et al., 2017). In rodent models, elevations in AEA via FAAH inhibition are associated with increased social interaction (Cassano et al., 2011), increased social play following increased environmental aversiveness (Manduca et al., 2014), and amelioration of deficits in social functioning (Wei et al., 2016). In humans, psychiatric diagnoses associated with lower circulating AEA (Karhson et al., 2018) are also associated with consistent dysregulation in production of facial expressions, resulting in atypical regulation of social interactions (Trevisan et al., 2018). Consequently, in regards to the data at hand, a reduction in AEA following stress may mediate social behaviors accordingly, promoting increased corrugator as signal to dissuade social approach. Conversely, elevated AEA during stress may promote prosocial behavior, promoting reduced corrugator as a signal to approach. Unfortunately, from these data alone, it is unclear whether AEA is buffering stress-induced negative affect or influencing social behavior following stress.
1.4. To fight-or-flight or tend-and-befriend?
The canonical portrayal of the stress response is that of a “fight-or-flight” response, which includes an array of physiological and behavioral responses meant to promote coping with a real or perceived threat. This terminology accurately reflects the physiological characteristics of the stress response, such as increased cortisol or heightened cardiovascular activity, but it is does not fully reflect the variation in behavioral responses to stress, particularly the striking differences between genders. As an alternative to the fight-or-flight response, women may instead adopt a so-called “tend-and-befriend” response (Taylor et al., 2000). According to this hypothesis, stress does not always elicit antagonistic behaviors such as fighting or fleeing, but can actually promote social affiliation under stress, a behavioral profile that is particularly pronounced in women (Taylor et al., 2000). This is supported by evidence that, following stress, women are more likely to demonstrate social cooperation and accept unfair economic offers (Youssef et al., 2018). Moreover, men and women engage distinct patterns of neural resources in response to stress (Seo et al., 2017), further highlighting gender divergence in stress responsivity.
In a re-analysis of our original dataset, we found distinct gender differences in stress-induced facial muscle activity. Following stress, men showed an increase in corrugator reactivity or “frowning” at rest (e.g. in the absence of any stimulus; main effect of gender: F (1,70) = 4.12, p = 0.049 partial η (Griebel and Holmes, 2013) = 0.54; Fig. 2C). Moreover, men demonstrated a net increase in corrugator reactivity to all stimuli, regardless of emotional content (main effect of gender: F (1,70) = 4.51, p = 0.036, partial η (Griebel and Holmes, 2013) = 0.061; Fig. 2D). Meanwhile, we observed that this stress-induced increase in resting corrugator activity was absent in women. In fact, women tended to display a reduction in corrugator reactivity specifically in response to negative stimuli (effect of gender on response to negative stimuli: (F1,70) = 5.52, p = 0.022, partial η (Griebel and Holmes, 2013) = 0.073; Fig. 2D). Our original finding—that enhanced anandamide protects against stress-induced increases in corrugator reactivity—still applies. Specifically, men show greater stress-induced corrugator reactivity than women, but AEA shifts all groups towards less corrugator activity to a similar degree. The study was not powered to detect genotype-by-gender interactions when both factors were entered into the model, but did nevertheless find trend level effects on both resting corrugator (effect of gender F (1,66) = 3.88, p = 0.053, partial η (Griebel and Holmes, 2013) = 0.054; effect of genotype F (2,66) = 4.57, p = 0.036, partial η (Griebel and Holmes, 2013) = 0.065; Fig. 2E) and response to negative stimuli (effect of gender F (1,66) = 2.16, p = 0.069, partial η (Griebel and Holmes, 2013) = 0.070; effect of genotype F (2,66) = 4.18, p = 0.027, partial η (Griebel and Holmes, 2013) = 0.071; Fig. 2F). Thus, our data suggest that men demonstrate greater corrugator reactivity following stress as compared to women, and enhanced AEA is associated with reduced corrugator activity in both genders. Current efforts are underway to replicate and extend this finding using pharmacology.
If corrugator activity is interpreted as a proxy of internal affect, our data suggest that women experience less negative affect following stress. This may indeed be true, though we failed to find any difference in stress response (e.g. cortisol response, skin conductance, cardiovascular activity) or self-reported affect. Alternatively, women may be demonstrating a prosocial behavioral profile akin to the tend-and-befriend response, using facial expressions to elicit approach in an effort to soothe social unease (Tamres et al., 2002). Men, in turn, may behave within a fight-or-flight response model, displaying a threat response that promotes avoidance. This interpretation is corroborated by evidence that in men, stress-induced increases in cortisol are associated with more angry facial expressions, as determined via FACS (Lupis, Lerman, Wolf, 2014). Stress was not associated with displays of anger (or fear) in women, though they did self-report greater anger than men (Lupis, Lerman, Wolf, 2014). Thus, even when women report feeling more angry, it does not show on their face. In addition, while women are more sensitive in detecting aggression at baseline, stress abolishes this advantage (DeDora et al., 2011), perhaps increasing the likelihood of social approach under stressful conditions.
Efforts to understand the biochemical underpinnings of the tend-and-befriend behavioral profile have primarily focused on oxytocin (Taylor, 2006). In general, stress increases oxytocin release (Crockford et al., 2018), though contextual factors moderate this effect in humans (Brown et al., 2016). Oxytocin appears to gate social behavior via interactions with dopamine (Hung et al., 2017), a finding which has recently been extended to include modulation via CB1 receptor activity (Xiao et al., 2018). Preclinical evidence also shows that oxytocin drives AEA activity at CB1 receptors, promoting prosocial behavior (Wei et al., 2015). In humans, stress-induced prosocial behavior is primarily studied in women (von Dawans et al., 2018), but recent evidence suggests that men may engage in similar behaviors (von Dawans et al., 2012; Heinrichs et al., 2003). This is particularly apparent following exogenous oxytocin administration and in the presence of social support (Heinrichs et al., 2003). Hence, the behavioral response to stress may lie along a spectrum of increasing sociality, ranging from fight-or-flight (antagonistic) to tend-and-befriend (prosocial), with male distributions skewed towards the former and females towards the latter. However, these phenotypes are not fixed; environmental factors (e.g. social support) or individual factors (e.g. anandamide, oxytocin levels) may shift an individual towards a profile of increased prosociality. As such, exploring the extensive individual variation in the behavioral consequences of stress, including facial expressions, has the potential to shed new light on how such responses can become dysregulated.
1.5. Face value in psychiatric research
We have provided evidence regarding stress-induced changes in facial muscles in healthy humans, suggesting that canonical views of facial expressions as indices of internal affect may instead be more appropriately interpreted as social signaling tools, based both on empirical data and theoretical discourse (Crivelli and Fridlund, 2018). While conceptually and theoretically interesting to affective neuroscientists, a fair response may be to question the real-world implications of this debate. That is, from a clinical perspective, will a functional understanding of facial expressions provide any advantages in psychiatric diagnosis, prognosis, or treatment?
Psychiatric disorders are syndromes, i.e. clusters of co-occurring symptoms, many of which are characterized in part by dysregulation of emotional and social functioning. As psychiatric symptoms are reshuffled and syndromes rename in each iterative version of the major diagnostic manual (APA, 2000; APA, 2013), an attractive alternative approach to gain mechanistic insight into behaviors that span diagnoses is the NIMH's RDoC matrix. Within this framework, clinically-relevant, biologically-based constructs are highlighted, providing a footing for both clinical and preclinical research efforts. The production of facial expressions is listed within the RDoC matrix, under the construct of social communication, perhaps due to the fact that dysregulation of facial expressions appears to be a trans-diagnostic trait of psychiatric disease (Davies et al., 2016). Thus, a proper understanding of the functional significance of facial expressions would facilitate the development of this RDoC domain as a useful research and clinical tool, including the development of novel therapeutic strategies.
If facial expressions are indeed a biomarker of psychiatric disease, then it would be ideal to elucidate therapeutic avenues that can modulate this indicator. Pharmacological interventions used to treat various psychiatric disorders have been shown to modulate facial expressions in healthy populations. For example, amphetamine is a prototypical stimulant drug often prescribed for ADHD that, in healthy adults, modulates facial muscle reactivity to emotional stimuli in a manner distinct from more general effects of drug on mood (Wardle and de Wit, 2012; Wardle et al., 2012). MDMA, a stimulant drug with unique prosocial effects (Wardle et al., 2014; Bedi et al., 2010) has recently garnered attention as a potential adjunct to psychotherapy for various psychiatric populations (Mithoefer et al., 2016; Danforth et al., 2018). In healthy humans, MDMA enhances facial muscle reactions to positive social stimuli (e.g. happy faces) (Wardle, de Wit, 2014) and influences perceptions of social interactions (Frye et al., 2014). Moreover, naltrexone, an antagonist at endogenous opioid receptors, increases negatively-valenced facial expressions to positive faces, interpreted by the authors as an increase in negative responses to affiliative cues (Meier et al., 2016). Finally, the data presented hererin (Mayo et al., 2018) highlights a role for elevated AEA in shaping facial expressions, while the same mechanism has been proposed as a treatment for stress-related psychopathologies (Gunduz-Cinar et al., 2013a, Gunduz-Cinar et al., 2013b; Patel et al., 2017). Thus, facial expression may represent a potential trans-diagnostic biomarker of psychiatric disorders, which is sensitive to psychiatric medication. As a result, understanding what we are modulating (e.g. via pharmacology) is paramount to understanding which specific clinical conditions would benefit from these treatments. Furthermore, our finding of gender differences in the consequences of stress on facial expressions that may relate to gender differences in the prevalence of psychiatric disease, particularly those relating to social and emotional processing (Li and Graham, 2017), which may further instantiate facial expressions as a biomarker of interest. Consequently, a more definitive functional account of the role of facial expressions is critical to the use of this biomarker in a clinically informative way.
In summary, interpretations of facial expressions vary across theoretical accounts nearly as much as the expressions they aim to describe. Of the leading theories, one interpretation is that facial expressions are an outlet of expression for the internal affective state of an individual. Alternatively, they may be social signaling tools used to communicate social motivations. In regards to the discussed dataset, we originally interpreted our findings in the contexts of facial expressions as a measure of internal affect, but a more thorough analysis suggests that a behavioral role in social communication may be more likely. To date, there have been few instances that have pitted the latter two theories against each other in mutually exclusive way. Moreover, it remains to be seen if the gender differences we describe are related to inherent biological sex differences or a result of culturally-mediated expectations (Chen and Jack, 2017). In the future, the constraints of laboratory-based studies may be overcome by more naturalistic experimental settings (Fernández-Dols and Crivelli, 2013), allowing us to determine whether organically occurring facial expressions are truly predictive of social behavior, which may be key to elucidating the functional significance of facial expressions in health and disease.
Acknowledgements
This research was supported by Swedish Research Council grant 2013-7434 (MH). The authors are grateful to J. Paul Hamilton for thoughtful comments on an earlier version of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2019.100166.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahs F., Gingnell M., Furmark T., Fredrikson M. Within-session effect of repeated stress exposure on extinction circuitry function in social anxiety disorder. Psychiatry Res. Neuroimaging. 2017;261:85–90. doi: 10.1016/j.pscychresns.2017.01.009. [DOI] [PubMed] [Google Scholar]
- APA . 2000. DSM-IVT-R: Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- APA . fifth ed. American Psychiatric Publishing, Inc.; 2013. Diagnostic And Statistical Manual of Mental Disorders: DSM-5TM. [Google Scholar]
- Barrett L.F. Are emotions natural kinds? Perspect. Psychol. Sci. 2006;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Barrett L.F. The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cognit. Affect Neurosci. 2017;12:1–23. doi: 10.1093/scan/nsw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F., Satpute A.B. Historical pitfalls and new directions in the neuroscience of emotion. Neurosci. Lett. 2017 doi: 10.1016/j.neulet.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G., Hyman D., de Wit H. Is ecstasy an ‘empathogen’? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol. Psychiatry. 2010;68:1134–1140. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogt, T. F M ter, Engels R.C.M.E. ‘Partying’ hard: party style, motives for and effects of MDMA use at rave parties. Subst. Use Misuse. 2005;40:1479–1502. doi: 10.1081/JA-200066822. [DOI] [PubMed] [Google Scholar]
- Boys A., Marsden J., Strang J. Understanding reasons for drug use amongst young people: a functional perspective. Health Educ. Res. 2001;16:457–469. doi: 10.1093/her/16.4.457. [DOI] [PubMed] [Google Scholar]
- Breiter H.C. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brown C.A., Cardoso C., Ellenbogen M.A. A meta-analytic review of the correlation between peripheral oxytocin and cortisol concentrations. Front. Neuroendocrinol. 2016;43:19–27. doi: 10.1016/j.yfrne.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Cassano T. Evaluation of the emotional phenotype and serotonergic neurotransmission of fatty acid amide hydrolase-deficient mice. Psychopharmacol. Berl. 2011;214:465–476. doi: 10.1007/s00213-010-2051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Jack R.E. Discovering cultural differences (and similarities) in facial expressions of emotion. Curr. Opin. Psychol. 2017;17:61–66. doi: 10.1016/j.copsyc.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Chen C. Distinct facial expressions represent pain and pleasure across cultures. Proc Natl Acad Sci U A. 2018 doi: 10.1073/pnas.1807862115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J.T. Anxiety and mood disorders and cannabis use. Am. J. Drug Alcohol Abuse. 2010;36:118–122. doi: 10.3109/00952991003713784. [DOI] [PubMed] [Google Scholar]
- Craske M.G., Stein M.B. Anxiety. Lancet. 2016;388:3048–3059. doi: 10.1016/S0140-6736(16)30381-6. [DOI] [PubMed] [Google Scholar]
- Crivelli C., Fridlund A.J. Facial displays are tools for social influence. Trends Cognit. Sci. 2018;22:388–399. doi: 10.1016/j.tics.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Crivelli C., Carrera P., Fernández-Dols J.-M. Are smiles a sign of happiness? Spontaneous expressions of judo winners. Evol. Hum. Behav. 2015;36:52–58. [Google Scholar]
- Crivelli C., Russell J.A., Jarillo S., Fernandez-Dols J.M. Recognizing spontaneous facial expressions of emotion in a small-scale society of Papua New Guinea. Emotion. 2017;17:337–347. doi: 10.1037/emo0000236. [DOI] [PubMed] [Google Scholar]
- Crockford C., Deschner T., Wittig R.M. The role of oxytocin in social buffering: what do primate studies add? In: Hurlemann R., Grinevich V., editors. Behavioral Pharmacology of Neuropeptides: Oxytocin. Springer International Publishing; 2018. pp. 155–173. [DOI] [PubMed] [Google Scholar]
- Danforth A.L. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacology. 2018;235:3137–3148. doi: 10.1007/s00213-018-5010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. John Murray; 1872. On the Expression of the Emotions in Man and Animals. [Google Scholar]
- Daudelin-Peltier C., Forget H., Blais C., Deschenes A., Fiset D. The effect of acute social stress on the recognition of facial expression of emotions. Sci. Rep. 2017;7:1036. doi: 10.1038/s41598-017-01053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. Facial expression to emotional stimuli in non-psychotic disorders: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016;64:252–271. doi: 10.1016/j.neubiorev.2016.02.015. [DOI] [PubMed] [Google Scholar]
- DeDora D.J., Carlson J.M., Mujica-Parodi L.R. Acute stress eliminates female advantage in detection of ambiguous negative affect. Evol. Psychol. 2011;9:532–542. [PubMed] [Google Scholar]
- Devane W.A. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dewey J. The theory of emotion: I: emotional attitudes. Psychol. Rev. 1894;1:553–569. [Google Scholar]
- Dimberg U., Petterson M. Facial reactions to happy and angry facial expressions: evidence for right hemisphere dominance. Psychophysiology. 2000;37:693–696. [PubMed] [Google Scholar]
- Dimberg U., Thunberg M., Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychol. Sci. 2000;11:86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Dincheva I. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat. Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesenberg M. Does cortisol modulate emotion recognition and empathy? Psychoneuroendocrinology. 2016;66:221–227. doi: 10.1016/j.psyneuen.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Ekman P. Expression and the nature of emotion. Approaches Emot. 1984;3:19–344. [Google Scholar]
- Ekman P. What scientists who study emotion agree about. Perspect. Psychol. Sci. 2016;11:31–34. doi: 10.1177/1745691615596992. [DOI] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. The repertoire of nonverbal behavior: categories, origins, usage, and coding. Semiotica. 1969;1:49–98. [Google Scholar]
- Ekman P., Friesen W.V. Constants across cultures in the face and emotion. J. Personal. Soc. Psychol. 1971;17:124. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- Ekman P., Rosenberg E.L. Oxford University Press; USA: 1997. What the Face Reveals: Basic and Applied Studies of Spontaneous Expression Using the Facial Action Coding System (FACS) [Google Scholar]
- Ekman P. Universals and cultural differences in the judgments of facial expressions of emotion. J. Personal. Soc. Psychol. 1987;53:712. doi: 10.1037//0022-3514.53.4.712. [DOI] [PubMed] [Google Scholar]
- Everaerd D., Klumpers F., Oude Voshaar R., Fernandez G., Tendolkar I. Acute stress enhances emotional face processing in the aging brain. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:591–598. doi: 10.1016/j.bpsc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Fernández-Dols J.-M., Crivelli C. Emotion and expression: naturalistic studies. Emot. Rev. 2013;5:24–29. [Google Scholar]
- Fernández-Dols J.-M., Ruiz-Belda M.-A. Are smiles a sign of happiness? Gold medal winners at the Olympic Games. J. Personal. Soc. Psychol. 1995;69:1113–1119. [Google Scholar]
- Fridlund A.J. Sociality of solitary smiling: potentiation by an implicit audience. J. Personal. Soc. Psychol. 1991;60:229–240. [Google Scholar]
- Fridlund A.J. Academic Press; 1994. Human Facial Expression: an Evolutionary View. [Google Scholar]
- Fridlund A.J. 1 - PRE-DARWINIAN VIEWS ON FACIAL EXPRESSION. In: Fridlund A.J., editor. Human Facial Expression. vols. 1–12. Academic Press; 1994. [Google Scholar]
- Fridlund A.J. Audience effects on solitary faces during imagery: displaying to the people in your head. J. Nonverbal Behav. 1990;14:113–137. [Google Scholar]
- Frye C.G., Wardle M.C., Norman G.J., de Wit H. MDMA decreases the effects of simulated social rejection. Pharmacol. Biochem. Behav. 2014;117:1–6. doi: 10.1016/j.pbb.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A. Impact of FAAH genetic variation on fronto-amygdala function during emotional processing. Eur. Arch. Psychiatry Clin. Neurosci. 2018 doi: 10.1007/s00406-018-0944-9. [DOI] [PubMed] [Google Scholar]
- Gay V., Leijdekkers P., Wong F. Using sensors and facial expression recognition to personalize emotion learning for autistic children. Stud. Health Technol. Inf. 2013;189:71–76. [PubMed] [Google Scholar]
- Gendron M., Roberson D., van der Vyver J.M., Barrett L.F. Perceptions of emotion from facial expressions are not culturally universal: evidence from a remote culture. Emotion. 2014;14:251–262. doi: 10.1037/a0036052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron M., Crivelli C., Barrett L.F. Universality reconsidered: diversity in making meaning of facial expressions. Curr. Dir. Psychol. Sci. 2018;27:211–219. doi: 10.1177/0963721417746794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore G. 2017. Facial Recognition AI Will Use Your Facial Expressions to Judge Creditworthiness. [Google Scholar]
- Godoy L.D., Rossignoli M.T., Delfino-Pereira P., Garcia-Cairasco N., de Lima Umeoka E.H. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front. Behav. Neurosci. 2018;12:127. doi: 10.3389/fnbeh.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G., Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O., Hill M.N., McEwen B.S., Holmes A. Amygdala. FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol. Sci. 2013;34:637–644. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatr. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacol. Berl. 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol. Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hung L.W. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357:1406–1411. doi: 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutto D.D., Robertson I., Kirchhoff M.D. A new, better BET: rescuing and revising basic emotion theory. Front. Psychol. 2018;9:1217. doi: 10.3389/fpsyg.2018.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard C.E. 1971. The Face of Emotion. [Google Scholar]
- Jones S.S., Collins K., Hong H.-W. An audience effect on smile production in 10-month-old infants. Psychol. Sci. 1991;2:45–49. [Google Scholar]
- Karhson D.S. Plasma anandamide concentrations are lower in children with autism spectrum disorder. Mol. Autism. 2018;9:18. doi: 10.1186/s13229-018-0203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M.N., Marsh A.A., Wegner D.M. What do I think you’re doing? Action identification and mind attribution. J. Soc. Psychol. 2006;90:543–555. doi: 10.1037/0022-3514.90.4.543. [DOI] [PubMed] [Google Scholar]
- Kraut R.E., Johnston R.E. Social and emotional messages of smiling: an ethological approach. J. Personal. Soc. Psychol. 1979;37:1539–1553. [Google Scholar]
- Krebs J.R., Dawkins R. Animal signals: mind-reading and manipulation. In: Krebs J.R., Davies N.B., editors. Behavioural Ecology: an Evolutionary Approach. Blackwell Scientific; 1984. [Google Scholar]
- Kumazaki H. Tele-operating an android robot to promote the understanding of facial expressions and to increase facial expressivity in individuals with autism spectrum disorder. Am. J. Psychiatry. 2017;174:904–905. doi: 10.1176/appi.ajp.2017.17030257. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Greenwald M.K., Bradley M.M., Hamm A.O. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Li S.H., Graham B.M. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry. 2017;4:73–82. doi: 10.1016/S2215-0366(16)30358-3. [DOI] [PubMed] [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupis S.B., Lerman M., Wolf J.M. Anger responses to psychosocial stress predict heart rate and cortisol stress responses in men but not women. Psychoneuroendocrinology. 2014;49(7):84–95. doi: 10.1016/j.psyneuen.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca A. Strain- and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behavior in rats. Eur. Neuropsychopharmacol. 2014;24:1337–1348. doi: 10.1016/j.euroneuro.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Mayo L.M. Protective effects of elevated anandamide on stress and fear-related behaviors: translational evidence from humans and mice. Mol. Psychiatr. 2018 doi: 10.1038/s41380-018-0215-1. [DOI] [PubMed] [Google Scholar]
- Meier I.M. Naltrexone increases negatively-valenced facial responses to happy faces in female participants. Psychoneuroendocrinology. 2016;74:65–68. doi: 10.1016/j.psyneuen.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Mithoefer M.C., Grob C.S., Brewerton T.D. Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiatry. 2016;3:481–488. doi: 10.1016/S2215-0366(15)00576-3. [DOI] [PubMed] [Google Scholar]
- Morena M., Campolongo P. The endocannabinoid system: an emotional buffer in the modulation of memory function. Neurobiol. Learn. Mem. 2014;112:30–43. doi: 10.1016/j.nlm.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Morris J.S. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Nelson N.L., Russell J.A. Universality revisited. Emot. Rev. 2013;5:8–15. [Google Scholar]
- Neto J.C., Filipe J. A. Consumers economic behavior and emotions: the case of iphone 6 in neuromarketing. Int. J. Latest Trends Financ. Econ. Sci. 2016;5:1041–1047. [Google Scholar]
- Nisbett R.E., Wilson T.D. Telling more than we can know: verbal reports on mental processes. Psychol. Rev. 1977;84:231–259. [Google Scholar]
- Parkinson B. Emotions are social. Br. J. Psychol. 1996;87:663–683. doi: 10.1111/j.2044-8295.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- Patel S., Hill M.N., Cheer J.F., Wotjak C.T., Holmes A. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci. Biobehav. Rev. 2017;76:56–66. doi: 10.1016/j.neubiorev.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein S.L., Taylor C.T., Stein M.B. Facial affect and interpersonal affiliation: displays of emotion during relationship formation in social anxiety disorder. Clin. Psychol. Sci. 2019 doi: 10.1177/2167702619825857. 2167702619825857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Russell J.A., Peterson B.S. The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 2005;17:715–734. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quas J.A., Hong M., Alkon A., Boyce W.T. Dissociations between psychobiologic reactivity and emotional expression in children. Dev. Psychobiol. 2000;37:153–175. doi: 10.1002/1098-2302(200011)37:3<153::aid-dev4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Rubinow D.R., Schmidt P.J. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 2018 doi: 10.1038/s41386-018-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Belda M.A., Fernandez-Dols J.M., Carrera P., Barchard K. Spontaneous facial expressions of happy bowlers and soccer fans. Cognit. Emot. 2003;17:315–326. doi: 10.1080/02699930302288. [DOI] [PubMed] [Google Scholar]
- Russell J.A., Barrett L.F. Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J. Personal. Soc. Psychol. 1999;76:805–819. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Seo D., Ahluwalia A., Potenza M.N., Sinha R. Gender differences in neural correlates of stress-induced anxiety. J. Neurosci. Res. 2017;95:115–125. doi: 10.1002/jnr.23926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff A.F., Tracy J.L. What are emotion expressions for? Curr. Dir. Psychol. Sci. 2011;20:395–399. [Google Scholar]
- Smeets T. Introducing the Maastricht Acute Stress Test (MAST): a quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology. 2012;37:1998–2008. doi: 10.1016/j.psyneuen.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Tamres L.K., Janicki D., Helgeson V.S. Sex differences in coping behavior: a meta-analytic review and an examination of relative coping. Pers. Soc. Psychol. Rev. 2002;6:2–30. [Google Scholar]
- TART C.T. Marijuana Intoxication : common experiences. Nature. 1970;226:701–704. doi: 10.1038/226701a0. [DOI] [PubMed] [Google Scholar]
- Taylor S.E. Tend and befriend:biobehavioral bases of affiliation under stress. Curr. Dir. Psychol. Sci. 2006;15:273–277. [Google Scholar]
- Taylor S.E. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Trevisan D.A., Hoskyn M., Birmingham E. Facial expression production in autism: a meta-analysis. Autism Res. Off. J. Int. Soc. Autism Res. 2018;11:1586–1601. doi: 10.1002/aur.2037. [DOI] [PubMed] [Google Scholar]
- UNODC . United Nations Office on Drugs and Crime; 2015. World Drug Report. [Google Scholar]
- von Dawans B., Fischbacher U., Kirschbaum C., Fehr E., Heinrichs M. The social dimension of stress reactivity:acute stress increases prosocial behavior in humans. Psychol. Sci. 2012;23:651–660. doi: 10.1177/0956797611431576. [DOI] [PubMed] [Google Scholar]
- von Dawans B., Ditzen B., Trueg A., Fischbacher U., Heinrichs M. Effects of acute stress on social behavior in women. Psychoneuroendocrinology. 2018;99:137–144. doi: 10.1016/j.psyneuen.2018.08.031. [DOI] [PubMed] [Google Scholar]
- Wagner H.L., Smith J. Facial expression in the presence of friends and strangers. J. Nonverbal Behav. 1991;15:201–214. [Google Scholar]
- Waller B.M., Whitehouse J., Micheletta J. Macaques can predict social outcomes from facial expressions. Anim. Cognit. 2016;19:1031–1036. doi: 10.1007/s10071-016-0992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle M.C., de Wit H. Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacol. Berl. 2012;220:143–153. doi: 10.1007/s00213-011-2498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle M.C., de Wit H. MDMA alters emotional processing and facilitates positive social interaction. Psychopharmacology. 2014;231(December 4):4219–4229. doi: 10.1007/s00213-014-3570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle M.C., Garner M.J., Munafo M.R., de Wit H. Amphetamine as a social drug: effects of d-amphetamine on social processing and behavior. Psychopharmacol. Berl. 2012;223:199–210. doi: 10.1007/s00213-012-2708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle M.C., Kirkpatrick M.G., de Wit H. ‘Ecstasy’ as a social drug: MDMA preferentially affects responses to emotional stimuli with social content. Soc. Cognit. Affect Neurosci. 2014;9:1076–1081. doi: 10.1093/scan/nsu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci U A. 2015;112:14084–14089. doi: 10.1073/pnas.1509795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D. Enhancement of anandamide-mediated endocannabinoid signaling corrects autism-related social impairment. Cannabis Cannabinoid Res. 2016;1:81–89. doi: 10.1089/can.2015.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D., Allsop S., Tye K., Piomelli D. Endocannabinoid signaling in the control of social behavior. Trends Neurosci. 2017;40:385–396. doi: 10.1016/j.tins.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehill J., Bartlett M.S., Movellan J.R. Automatic facial expression recognition. Soc. Emot. Nat. Artifact. 2013;88 [Google Scholar]
- Xiao L., Priest M.F., Kozorovitskiy Y. Oxytocin functions as a spatiotemporal filter for excitatory synaptic inputs to VTA dopamine neurons. Elife. 2018;7 doi: 10.7554/eLife.33892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef F.F., Bachew R., Bissessar S., Crockett M.J., Faber N.S. Sex differences in the effects of acute stress on behavior in the ultimatum game. Psychoneuroendocrinology. 2018;96:126–131. doi: 10.1016/j.psyneuen.2018.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.