Abstract
Chronic stress can lead to psychiatric illness characterized by impairments of executive function, implicating the prefrontal cortex as a target of stress-related pathology. Previous studies have shown that various types of chronic stress paradigms reduce dendritic branching, length and spines of medial prefrontal cortex (mPFC) pyramidal neurons. However, these studies largely focused on layer II/III pyramidal neurons in adult male rats with less known about layer V, the site of projection neurons. Because the prefrontal cortex develops throughout adolescence, stress during adolescence may have a greater impact on structure and function than stress occurring during adulthood. Furthermore, females display greater risk of stress-related psychiatric disorders, indicating sex-specific responses to stress. In this study, male and female adolescent (42–48 days old, 4 rats per group) or adult (68–72 days old, 4 rats per group) Sprague-Dawley rats were exposed to 5 days of repeated social stress in the resident-intruder paradigm or control manipulation. We examined dendritic morphology of cells in the mPFC in both layer II/III and Layer V. Repeated social stress resulted in decreased dendritic branching in layer II/III apical dendrites regardless of sex or age. In apical layer V dendrites, stress increased branching in adult males but decreased it in all other groups. Stress resulted in a decrease in dendritic spines in layer V apical dendrites for male adolescents and female adults, and this was mostly due to a decrease in filopodial and mushroom spines for male adolescents, but stubby spines for female adults. In sum, these results suggest that repeated stress reduces complexity and synaptic connectivity in adolescents and female adults in both input and output layers of prelimbic mPFC, but not in male adults. These changes may represent a potential underlying mechanism as to why adolescents and females are more susceptible to the negative cognitive effects of repeated or chronic stress.
Keywords: Stress, Development, Morphology, Prefrontal cortex, Sex, Golgi stain
1. Introduction
The medial prefrontal cortex (mPFC) regulates executive functions including attentional focus and direction, decision-making, behavioral inhibition and cognitive/behavioral flexibility (Goldman-Rakic, 1996). Many psychiatric illnesses such as depression, anxiety, schizophrenia, and post-traumatic stress disorder manifest with impairments in executive function. Thus, the mPFC has been implicated as a potential contributor to the pathophysiology of these disorders (Gorman et al., 1989; Grillon et al., 1996; Lewis, 1997; Zubieta et al., 1999; Brody et al., 2001; Arnsten, 2011; Veeraiah et al., 2014). Many of these disorders have also been associated with exposure to chronic stress, which impairs cognitive performance (Mazure, 1995; Kessler, 1997; Marin et al., 2011; Carr et al., 2013). Various stressors produce distinct morphological changes to neuronal populations within the rat mPFC. Short- or long-term daily restraint stress result in decreased arborization and dendritic spines in layer II/III mPFC pyramidal neurons (Brown et al., 2005; Liston et al., 2006; Radley et al., 2006). Daily injection of corticosterone resulted in similar changes, indicating that the glucocorticoids may play a role in mPFC morphological changes following stress (Wellman, 2001; Seib and Wellman, 2003). Most studies have focused on examining morphological changes in layer II/III neurons. However, the mPFC has distinct laminar organization, and layer V pyramidal neurons may be of particular interest to understanding how chronic stress impairs mPFC control of executive functions. Layer V pyramidal neurons constitute the primary output neurons of the PFC, receiving information from the more superficial layers and projecting out to subcortical structures (Barbas and Pandya, 1989; Berendse et al., 1992; Yeterian and Pandya, 1994; Ding et al., 2001). They also possess a unique composition of NMDA receptors that imparts greater plasticity and the ability for persistent firing, thought to be the neuronal correlate of working memory (Wang et al., 2008). A few studies have indicated that chronic restraint stress results in decreased dendritic spine density in layer V pyramidal neurons, but these studies have been performed only on adult male rats (Liu and Aghajanian, 2008; Ramkumar et al., 2012). Thus, morphological alterations in layer V may have different functional consequences than in layer II/III.
The mPFC may be particularly vulnerable to long-term effects of stress due to its slow maturation, which often extends into the beginning of the third decade of life in humans (Kolb et al., 2012). This affords an extended period of high plasticity to the mPFC and the potential for early-life experiences to cause lasting alterations in mPFC. Indeed, rats exposed to repeated social stress during adolescence show greater locus coeruleus (LC) activation, reduced coherence of mPFC and LC, reduced neuronal excitability and synaptic transmission in mPFC, and later impairments on an mPFC-dependent task (Snyder et al., 2014; Zitnik et al., 2015; Urban and Valentino, 2016). These studies identify the adolescent period as a period of heightened vulnerability to stress.
In addition to age, sex plays a role in the vulnerability to stress. Females display a higher risk of stress-related psychiatric disorders, with depression and anxiety having a two-to three-fold greater prevalence (Heller, 1993; Blehar, 1995; Pigott, 1999). Rodent studies have revealed that female rats have a higher baseline level of corticosterone and greater sensitivity to the stress-related hormone corticosteroid-releasing factor (CRF) which leads to increased activation of the hypothalamic-pituitary adrenal (HPA) axis in response to stress (Kitay, 1961; Kirschbaum et al., 1992; Handa et al., 1994; Bangasser et al., 2010; Bangasser and Valentino, 2014; Grafe et al., 2017). This greater hormonal reactivity to stress may underlie the increased vulnerability of females to stress-related psychiatric disorders, although how exactly neuronal function and structure may be altered in females compared to males is still unclear.
For humans, stressors of a social nature are most common, such as peer bullying and domestic abuse (Brown and Prudo, 1981; Prudo et al., 1981; Adler et al., 1994; Sapolsky, 2005). Social stress is especially relevant during adolescence, as this developmental stage is one of dynamic social growth and interaction. We previously demonstrated that repeated social stress has sex- and age-specific effects on strategy-shifting, a task requiring cognitive flexibility and mPFC function (Snyder et al., 2014; Snyder et al., 2015), and that it also results in decreased neuronal excitability and synaptic transmission in layer V pyramidal neurons in the mPFC of adolescent male and female rats (Urban and Valentino, 2016).
Given the evidence that chronic stress targets the mPFC to impair cognitive functions, resulting in symptoms of psychiatric disorders, the present study examined the effects of repeated social stress (resident-intruder stress) occurring either during adolescence or adulthood on the morphology of mPFC pyramidal neurons of male and female Sprague-Dawley rats. In order to determine if stress effects are laminar-specific, neurons of both layer II/III and layer V were included. This study is, to the best of our knowledge, the first to systematically examine age-, sex-, and layer-dependent effects of a relevant social stressor on neuronal morphology. The working hypothesis was that adolescent and female rats would be more susceptible to the effects of stress, resulting in greater dendritic retraction and/or spine loss.
2. Materials and methods
2.1. Animals
Male and female Sprague-Dawley rats (Charles River, Wilmington, Massachusetts) served as social stress “intruders” (experimental rats) or age-matched controls. Retired breeder male Long-Evans rats (Charles River, Wilmington, Massachusetts) served as residents to defeat experimental males, and lactating female Sprague-Dawley rats served residents to defeat experimental females as per our previous studies (Ver Hoeve et al., 2013). Residents were singly housed in a double-sized shoebox rat cage, and intruders were initially pair-housed, then separated 24 h before testing began. Animals were given one week to acclimate upon arrival and access to food and water was ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia.
2.2. Experimental design
Social stress or control manipulation was begun during one of two developmental periods: adolescence (PD 42–46) or adulthood (PD 69–73). Social stress and control rats were exposed to 5 days of their respective experimental manipulation. Twenty-four hours after completion of the final manipulation, rats were sacrificed and brains were prepared for Golgi staining.
2.3. Social stress
The social stress was a modification of the resident-intruder paradigm, as previously described (Wood et al., 2010; Chaijale et al., 2013, 2014; Ver Hoeve et al., 2013; Snyder et al., 2014; Chen et al., 2015; Snyder et al., 2015; Pearson-Leary et al., 2017). Intruders were individually placed in the home cage of a resident rat, and the resident and intruder were allowed to freely interact until either the intruder assumed a submissive defeat posture (>3 s in a frozen supine position), 5 attacks occurred, or 15 min had elapsed. For females, pups were removed from the resident prior to testing and returned after testing completed. Upon satisfaction of one of the criteria above, a mesh barrier was placed in the cage to separate the animals. This mesh barrier allowed for visual, olfactory, and auditory contact for the remainder of the 30 min period. Immediately following the end of the resident-intruder exposure period, intruders were then returned to their home cages. This was repeated for 5 consecutive days with the intruder being randomly placed into the home cage of a different resident rat on each testing day. For all rats subjected to social stress, latency to assume defeat, number of attacks by the resident, and latency to first attack were recorded. Control rats were removed from the home cage and placed into a clean, empty large rat cage for 15 min, and then a mesh barrier was placed in the middle of the cage for an additional 15 min.
2.4. Golgi staining
Twenty-four hours following the final defeat, whole brains were collected and placed in impregnation solution. The impregnation solution contained a mixture of potassium chloride, mercury chloride, and potassium dichromate, and was provided as two parts (A and B) to be mixed 1:1, per the manufacturer's instructions (FD Neurotechnologies, Columbia, Maryland). The impregnation solution was replaced after 24 h, and brains were then left for 14 days, with gentle agitation every 4 days to prevent precipitation of the impregnation chemicals. Following impregnation, the brains were placed in a rehydration solution (Solution C) for 7 days. Brains were then frozen and sliced into 180 μM slices on a cryostat. Slices containing prefrontal cortex were immediately mounted on gelatin-coated slides (FD Neurotechnologies, Columbia, Maryland). The staining procedure was followed as described in the FD Neurotechnologies Rapid-Golgi kit, a commercially available kit. Slides were left to dry in the dark for 2 h, stained, dehydrated with increasing concentrations of ethanol, and cleared with xylene. Finally, slides were coverslipped with Permount.
2.5. Imaging and neuronal reconstruction
Golgi-stained neurons in the prelimbic region of the medial prefrontal cortex were viewed using a Nikon Eclipse NI microscope (Nikon, Tokyo, Japan) configured with Neurolucida software package (MBF Bioscience, Williston, Vermont). For dendritic length and branch analysis, 3D z-stacks from sequential scans were taken at low zoom (40× lens) at 1 μm increments to include the entire apical and basal dendritic trees. Total dendritic length and number was determined within the 3D matrix of each Z-stack using Neurolucida 7 to trace and reconstruct each neuron.
2.6. Dendritic spine counting
Neurons were visualized using a 100× oil-immersion lens (Nikon, Tokyo, Japan). Spines were counted for layer II/III and layer V using the following criteria: basal dendrite 40 μm segments were sampled at midway between the soma and the distal termination (medial) and at the distal termination of the dendrite branch and combined, apical 50 μm segments were sampled at midway between the soma and the distal termination (medial) and at the distal termination of the dendrite branch (distal), and combined. Spines were classified as spiny, filopodial, or mushroom (Hering, 2001b).
2.7. Data analysis and statistics
Number of cells assessed and number of animals are provided in the Figure Legends. Apical and basal dendrite lengths, branching, and complexity were recorded. Sholl analysis was also performed on all reconstructions, measuring intersections at successive 20 μm concentric circles. JMP Statistics (SAS, Cary, North Carolina) was used to run repeated measures ANOVA on Sholl intersections and three-way full factorial ANOVAS with sex, age, and stress as factors on dendritic length, branch numbers, and dendritic spine number. Posthoc comparisons were by Tukey's HSD unless otherwise noted.
3. Results
3.1. Stress effects on basal dendritic branching
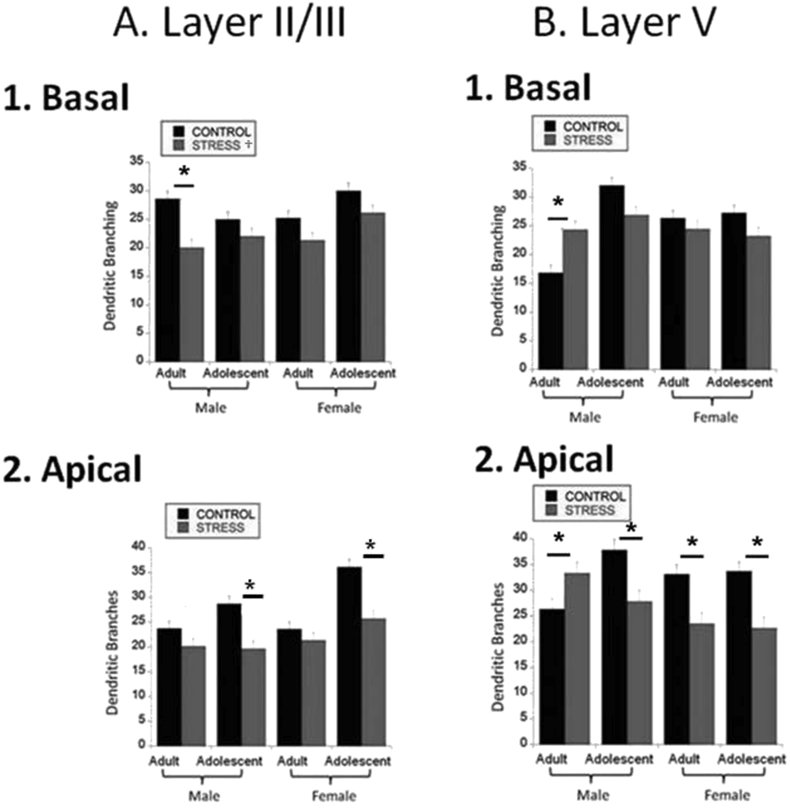
We first examined dendritic branching in basal dendrites of layer II/III and layer V neurons. There was a main effect of stress to decrease branching in layer II/III [F(1,238) = 26, p < 0.0001] suggesting stress decreased branching regardless of age or sex. In addition, stress reduced layer II/III basal dendritic branching in male adults only [Fig. 1A.1; Age*Sex*Stress interaction: F(1,238) = 6.6, p = 0.01]. In layer V, stress increased branching in male adults [Fig. 1B.1; Age*Sex*Stress interaction: F(1,257) = 12.4, p = 0.0005]. There was an interaction of Age*Stress whereby stress reduced branching in adolescents and increased it in adults as well [F(1,257) = 16.2, p < 0.0001]. However, this was due mostly to the increase in stressed adult males as the post-doc between adolescent control and adolescent stressed animals was not significant.
Fig. 1.
Dendritic Morphology. Dendritic branching in both apical and basal dendrites was assessed in adolescent and adult males and females in layer II/III and layer V. (* = p < 0.05, post-hoc 3-way interaction; ‡ = p < 0.05, age effect; # = p < 0.05, sex effect; † = p < 0.05, stress effect). A) Layer II/III.1. Basal Dendritic Branching. Five days of social stress decreased branching, regardless of age or sex. Furthermore, stress reduced branching in male adults. Group sizes were: n = 32 cells/4 rats for male adult controls, 25 cells/4 rats for male adult defeats, 28 cells/4 rats for male adolescent controls, 28 cells/4 rats for male adolescent defeats, 31 cells/4 rats for female adult controls, 36 cells/4 rats for female adult defeats, 25 cells/4 rats for female adolescent controls, 34 cells/4 rats for female adolescent defeats.2. Apical Dendritic Branching. Social stress decreased apical branching in adolescents regardless of sex. Group sizes were: n = 32 cells/4 rats for male adult controls, 25 cells/4 rats for male adult defeats, 28 cells/4 rats for male adolescent controls, 28 cells/4 rats for male adolescent defeats, 31 cells/4 rats for female adult controls, 36 cells/4 rats for female adult defeats, 25 cells/4 rats for female adolescent controls, 34 cells/4 rats for female adolescent defeats).* = p < 0.05.B) Layer V.1. Basal Dendritic Branching. Social stress increases dendritic branching in adult males. Group sizes were: n = 36 cells/4 rats for male adult controls, 37 cells/4 rats for male adult defeats, 34 cells/4 rats for male adolescent controls, 30 cells/4 rats for male adolescent defeats, 30 cells/4 rats for female adult controls, 30 cells/4 rats for female adult defeats, 31 cells/4 rats for female adolescent controls, 30 cells/4 rats for female adolescent defeats.2. Apical Dendritic Branching. Social stress increased branching in adult males but decreased branching in female adolescents, male adolescents, and female adults. Group sizes were: n = 36 cells/4 rats for male adult controls, 37 cells/4 rats for male adult defeats, 34 cells/4 rats for male adolescent controls, 30 cells/4 rats for male adolescent defeats, 30 cells/4 rats for female adult controls, 30 cells/4 rats for female adult defeats, 31 cells/4 rats for female adolescent controls, 30 cells/4 rats for female adolescent defeats.* = p < 0.05.
3.2. Stress effects on apical dendritic branching
In layer II/III there was no main effect; however, stress reduced apical dendritic branching in adolescents compared to adults regardless of sex [Fig. 1A.2; Age*Stress interaction: F(1,238) = 12, p = 0.0006]. In layer V, there was a main effect of stress to decrease branching [F(1,257) = 8.3, p < 0.005]. Stress decreased apical dendritic branching in females but not in males regardless of age [Fig. 1B.2; Sex*Stress interaction: F(1,257) = 9.8, p < 0.005]. Stress increased branching in adult males and decreased it in all other groups [Fig. 1B.2; Age*Sex*Stress interaction: F(1,257) = 7.2, p < 0.01; Student's t-test p < 0.05]. The sum of all the significant effects indicated that stress has opposing effects on branching in adult males vs. all other groups, increasing branching in adult males but decreasing branching in all other groups.
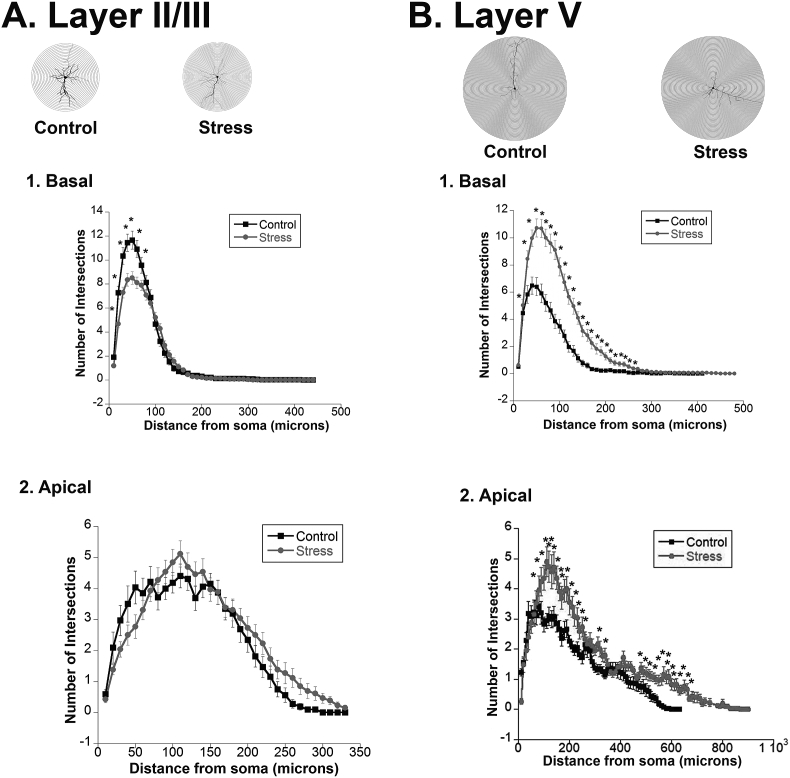
The unique alterations of dendritic complexity in adult males were further examined via Sholl analysis specifically in the control and stressed adult males. Stress decreased intersections in layer II/III basal dendrites [Fig. 2A.1; F(1,58) = 5.99, p = 0.0184], and increased intersections in layer V basal dendrites [Fig. 2B.1; F(1,62) = 41.43, p < 0.0001]. There was no effect in layer II/III apical dendrites [Fig. 2A.2]. However, stress also increased intersections in layer V apical dendrites [Fig. 2B.2; F(1,62) = 23.78, p < 0.0001]. Overall, the results from branching and sholl analysis showed a consistent picture of stress reducing branching in basal but not apical dendrites of Layer II/III but an increase in both basal and apical dendrites in Layer V. The changes produced by stress in adolescent groups were similar to those produced in adult females.
Fig. 2.
Dendritic Arborization and Complexity in Adult Males. Sholl analysis was run on adult male neurons in layer II/III and layer V, using 20 μm concentric rings. Intersections of dendrites with each concentric ring were recorded. Group size = 34 cells/4 rats for controls, 30 cells/4 rats for defeats. A. Layer II/III.1. Basal Dendrites. Repeated social stress decreased the complexity as measured by number of intersections. 2. Apical Dendrites. There was no effect of stress. B. Layer V.1. Basal Dendrites. Repeated social stress increased the complexity as measured by number of intersections. 2. Apical Dendrites. Repeated social stress increased the complexity as measured by number of intersections.
3.3. Stress effects on dendritic spines
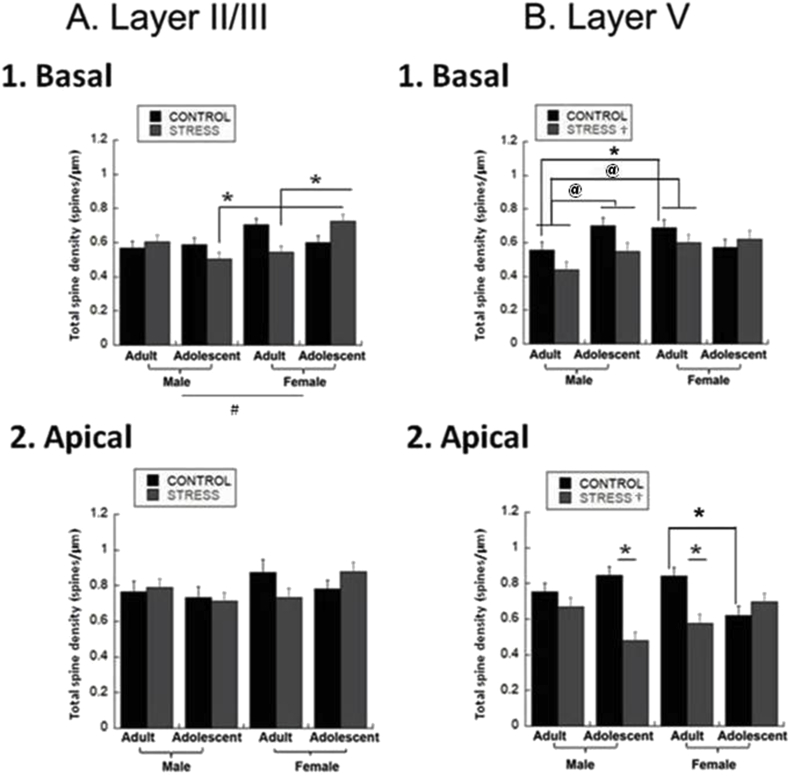
In order to understand if dendritic structural changes were related to alterations in synaptic connectivity, we examined dendritic spines in layer II/III and layer V on both apical and basal dendrites. We examined dendritic spines in a medial and distal 40 μm segment for basal dendrites in both layer II/III and layer V neurons, combined medial and distal portions, and recorded the average spine density (number of spines per μm) for each dendrite. In basal layer II/III dendrites, there was no main effect of stress, but females had greater dendritic spine density than males regardless of age of stress [Sex Effect, Fig. 3A.1; F(1,79) = 7.19, p = 0.0091]. Post-hoc tests from the significant three way interaction indicated that stressed adolescent females have increased spine density compared to stressed female adults and stressed adolescent males [Fig. 3A.1; Sex*Age*Stress Interaction, F(1,79) = 12.75, p = 0.0006]. In layer V basal dendrites, stress reduced spine density regardless of age or sex [Fig. 3B.1; F(1,79) = 4.92, p = 0.03]. In addition, adult males had lower spine density than adult females or adolescent males regardless of stress [Age*Sex Interaction, F(1,79) = 6.59, p = 0.01]. Adult male controls had reduced dendritic spine density compared to adult female controls.
Fig. 3.
Total Dendritic Spine Densities. Dendritic spines were counted in 40 μm segments midway down the basal dendrites and at the distal termination, and in 50 μm segments midway and at the distal termination for apical dendrites and averaged to create a final spines/μm density for adolescent and adult males and females. (* = p < 0.05, post-hoc 3-way interaction; @ = p < 0.05, post-hoc 2-way interaction; ‡ = p < 0.05, age effect; # = p < 0.05, sex effect; † = p < 0.05, stress effect). A. Layer II/III.1. Basal Dendrites. Females had greater spine density than males regardless of age or stress. There is an interaction of Age*Sex*Stress such that stressed adolescent females have spine density greater than stressed adolescent males or stressed female adults. The group sizes were: n = 9 cells/4 rats for male adult controls, 10 cells/4 rats for stressed male adults, 9 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 7 cells/4 rats for female adult controls, 14 cells/4 rats for stressed female adults, 11 cells/4 rats for female adolescent controls, 10 cells/4 rats for stressed female adolescents. 2. Apical Dendrites. There was no effect on apical dendritic spine density. The group sizes were: n = 9 cells/4 rats for male adult controls, 10 cells/4 rats for stressed male adults, 9 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 7 cells/4 rats for female adult controls, 14 cells/4 rats for stressed female adults, 11 cells/4 rats for female adolescent controls, 10 cells/4 rats for stressed female adolescents]. B. Layer V.1. Basal Dendrites. Adult males had lower spine density than adult females or adolescent males regardless of stress. Adult male controls had lower spine density than adult female controls. Stress reduced dendritic spine density overall regardless of sex or age. Group sizes were: n = 10 cells/4 rats for male adult controls, 9 cells/4 rats for male adult defeats, 11 cells/4 rats for male adolescent controls, 10 cells/4 rats for male adolescent defeats, 9 cells/4 rats for female adult controls, 10 cells/4 rats for female adult defeats, 10 cells/4 rats for female adolescent controls, 11 cells/4 rats for female adolescent defeats. 2. Apical Dendrites. Stress decreased spine density overall regardless of age or sex. This was driven by adult females and adolescent males. Adult female controls had greater spine density than adolescent female controls. Group sizes were: n = 10 cells/4 rats for male adult controls, 9 cells/4 rats for stressed male adults, 11 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 9 cells/4 rats for female adult controls, 10 cells/4 rats for stressed female adults, 10 cells/4 rats for female adolescent controls, 11 cells/4 rats for stressed female adolescents.
We examined medial (50 μm midway down the dendritic length), and distal (final 50 μm) segments of apical dendrites in layer II/III and layer V, combined medial and distal portions, and recorded the average spine density (number of spines per μm) for each dendrite. There was no effect on layer II/III apical dendrites. However, in layer V apical dendrites, stress reduced dendritic spine density regardless of sex or age [main effect of Stress F(1,79) = 22.55, p < 0.0001], and this was driven by adolescent males and adult females [Fig. 3B.2; Age*Sex*Stress Interaction, F(1,79) = 21.21, p < 0.0001]. Additionally, adult female controls had greater spine density than adolescent female controls.
In order to more thoroughly examine how dendritic spine and expected synaptic function may change following repeated stress, we classified dendritic spines into three subtypes: filopodial, mushroom, and stubby (Rochefort and Konnerth, 2012).
3.4. Filopodial spine density
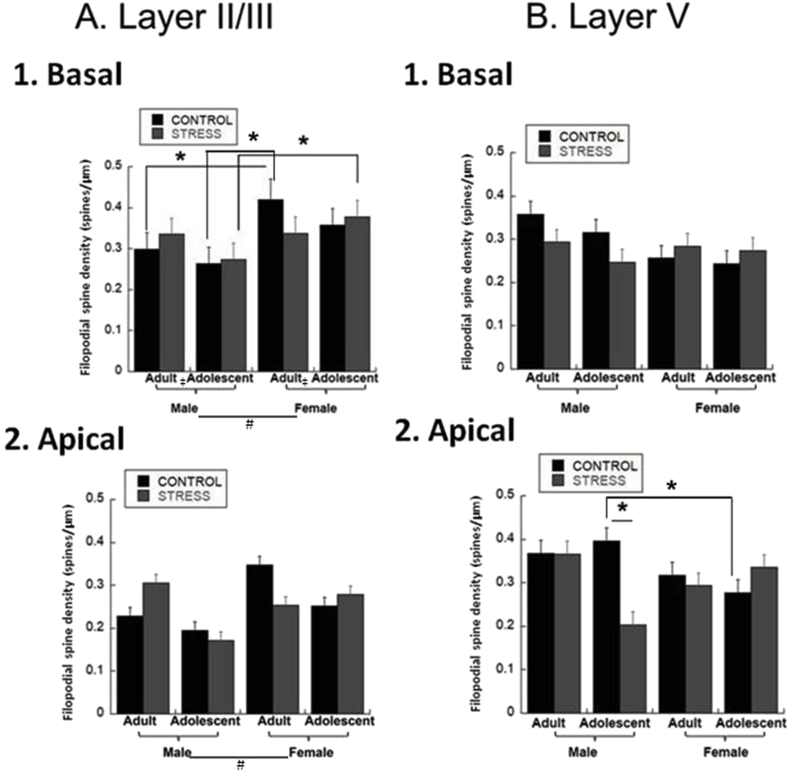
We first examined filopodial spines, characterized as thin protuberances lacking a bulbous head (Hering, 2001b). In layer II/III basal dendrites, age increased filopodial spine density [Fig. 4A.1; significant effect of Age: F(1,79) = 12.83, p = 0.0006]. Females had greater filopodial spine density than males regardless of age or stress [Fig. 4A.1; Sex Effect, F(1,79) = 11.78, p = 0.001]. There was no main effect of stress, but female stressed adolescents had greater spine density than stressed male adolescents, and female adult controls had greater spine density than male adult controls and adolescent male controls [Fig. 4A.1; Age*Sex*Stress Interaction, F(1,79) = 11.09, p = 0.0014]. There was no effect in layer V basal dendrites [Fig. 4B.1].
Fig. 4.
Filopodial Spine Densities. Filopodial spines were counted in 40 μm segments midway down the basal dendrites and at the distal termination, and in 50 μm segments midway and at the distal termination for apical dendrites and averaged to create a final spines/μm density for adolescent and adult males and females. (* = p < 0.05, post-hoc 3-way interaction; ‡ = p < 0.05, age effect; # = p < 0.05, sex effect; † = p < 0.05, stress effect). A. Layer II/III.1. Basal Dendrites. Spine density increased with age overall regardless of stress or sex. Females had more filopodial spines than males regardless of age or stress. There was an interaction of Age*Sex*Stress such that female stressed adolescents had greater spine density than stressed male adolescents, and female adult controls had greater spine density than male adult controls and adolescent male controls. Group sizes were: n = 9 cells/4 rats for male adult controls, 10 cells/4 rats for stressed male adults, 9 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 7 cells/4 rats for female adult controls, 14 cells/4 rats for stressed female adults, 11 cells/4 rats for female adolescent controls, 10 cells/4 rats for stressed female adolescents. 2. Apical Dendrites. Females had greater spine density than males regardless of age or stress. Group sizes were: n = 9 cells/4 rats for male adult controls, 10 cells/4 rats for stressed male adults, 9 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 7 cells/4 rats for female adult controls, 14 cells/4 rats for stressed female adults, 11 cells/4 rats for female adolescent controls, 10 cells/4 rats for stressed female adolescents. B. Layer V.1. Basal Dendrites. There was no effect on filopodial spine density. Group sizes were: n = 10 cells/4 rats for male adult controls, 9 cells/4 rats for stressed male adults, 11 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 9 cells/4 rats for female adult controls, 10 cells/4 rats for stressed female adults, 10 cells/4 rats for female adolescent controls, 11 cells/4 rats for stressed female adolescents. 2. Apical Dendrites. Male controls had greater spine density than female controls and stressed males regardless of age. Stress decreased filopodial spine density in male adolescents. Male adolescent controls have greater spine density than female adolescent controls. Group sizes were: n = 10 cells/4 rats for male adult controls, 9 cells/4 rats for stressed male adults, 11 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 9 cells/4 rats for female adult controls, 10 cells/4 rats for stressed female adults, 10 cells/4 rats for female adolescent controls, 11 cells/4 rats for stressed female adolescents.
There was no overall effect in layer II/III apical dendrites, but females had greater filopodial spine density than males [Fig. 4A2; Sex Effect: F(1,79) = 7.57, p = 0.0075]. However, in layer V apical dendrites, male controls had greater filopodial spine density than stressed males and female controls regardless of age [Fig. 4B.2; Sex*Stress Interaction, F(1,79) = 6.86, p = 0.01]. There was no main effect of stress, but stress decreased filopodial spine density in male adolescents, and male adolescent controls had greater spine density than female adolescent controls [Fig. 4B.2; Age*Sex*Stress Interaction, F(1,79) = 9.8, p = 0.0025].
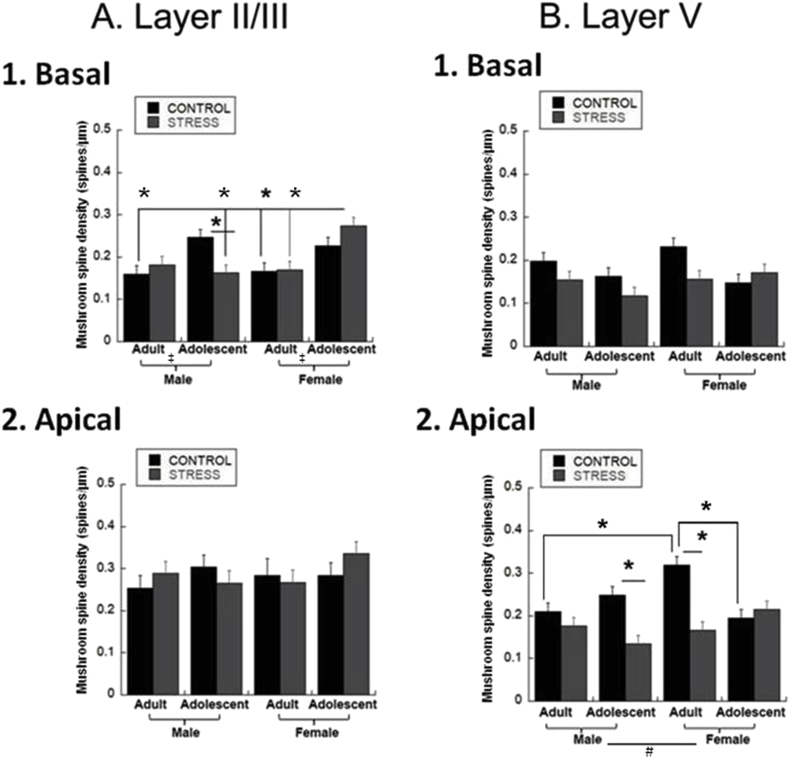
3.5. Mushroom spine density
We next examined mushroom spines, characterized by a thin neck with a bulbous head (Hering, 2001b). In basal layer II/III dendrites, age decreased mushroom spine density regardless of sex or stress [Fig. 5A.1; F(1,79) = 16.5, p = 0.0002]. There was no main effect of sex or stress. There was an interaction of Age*Sex*Stress such that stressed female adolescents had greater mushroom spine density than stressed male adolescent, control or stressed adult females and control and stressed adult males [Fig. 5A.1; F(1,79) = 6.66, p = 0.0012]. Furthermore, stress reduced density in adolescent males. In layer V basal dendrites, age increased mushroom spine density regardless of sex or stress [Fig. 5B.1; F(1,79) = 6.98, p = 0.01], and stress reduced mushroom spine density regardless of age or sex [Fig. 5B.1; F(1,79) = 7.14, p = 0.009].
Fig. 5.
Mushroom Spine Densities. Mushroom spines were counted in 40 μm segments midway down the basal dendrites and at the distal termination, and in 50 μm segments midway and at the distal termination for apical dendrites and averaged to create a final spines/μm density for adolescent and adult males and females. (* = p < 0.05, post-hoc 3-way interaction; ‡ = p < 0.05, age effect; # = p < 0.05, sex effect; † = p < 0.05, stress effect). A. Layer II/III.1. Basal Dendrites. Age decreased spine density. There was an interaction of Age*Sex*Stress such that stressed female adolescents had greater spine density than stressed male adolescents, stressed female adults, female adult controls, adult male controls and stressed adolescent males. Stress reduced spine density in male adolescents. Group sizes were: n = 9 cells/4 rats for male adult controls, 10 cells/4 rats for stressed male adults, 9 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 7 cells/4 rats for female adult controls, 14 cells/4 rats for stressed female adults, 11 cells/4 rats for female adolescent controls, 10 cells/4 rats for stressed female adolescents]. 2. Apical Dendrites. There was no effect on mushroom spine density. Group sizes were: n = 9 cells/4 rats for male adult controls, 10 cells/4 rats for stressed male adults, 9 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 7 cells/4 rats for female adult controls, 14 cells/4 rats for stressed female adults, 11 cells/4 rats for female adolescent controls, 10 cells/4 rats for stressed female adolescents. B. Layer V.1. Basal Dendrites. Spine density increased with age overall regardless of sex or stress. Stress decreased spine density. Group sizes were: n = 10 cells/4 rats for male adult controls, 9 cells/4 rats for stressed male adults, 11 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 9 cells/4 rats for female adult controls, 10 cells/4 rats for stressed female adults, 10 cells/4 rats for female adolescent controls, 11 cells/4 rats for stressed female adolescents. 2. Apical Dendrites. Females had greater spine density than males regardless of stress or age. Stress also decreased spine density regardless of age or sex. This was driven by stress decreasing spine density in male adolescents and female adults. Adult female controls had greater spine density than adult male controls or adolescent female controls. Group sizes were: n = 10 cells/4 rats for male adult controls, 9 cells/4 rats for stressed male adults, 11 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 9 cells/4 rats for female adult controls, 10 cells/4 rats for stressed female adults, 10 cells/4 rats for female adolescent controls, 11 cells/4 rats for stressed female adolescents.
There was no effect on apical layer II/III dendrites [Fig. 5A.2]. In layer V apical dendrites, stress decreased spine density regardless of sex or age [F(1,79) = 17.49, p < 0.0001]. Females overall had greater mushroom spine density than males regardless of age or stress [Fig. 5B.2; Sex effect, F(1,79) = 4.3, p = 0.04]. Stress reduced mushroom spine density in male adolescents and female adults, and adult female controls had greater spine density than adult male controls or adolescent female controls [Fig. 5B.2; Age*Sex*Stress Interaction, F(1,79) = 17.5, p < 0.0001].
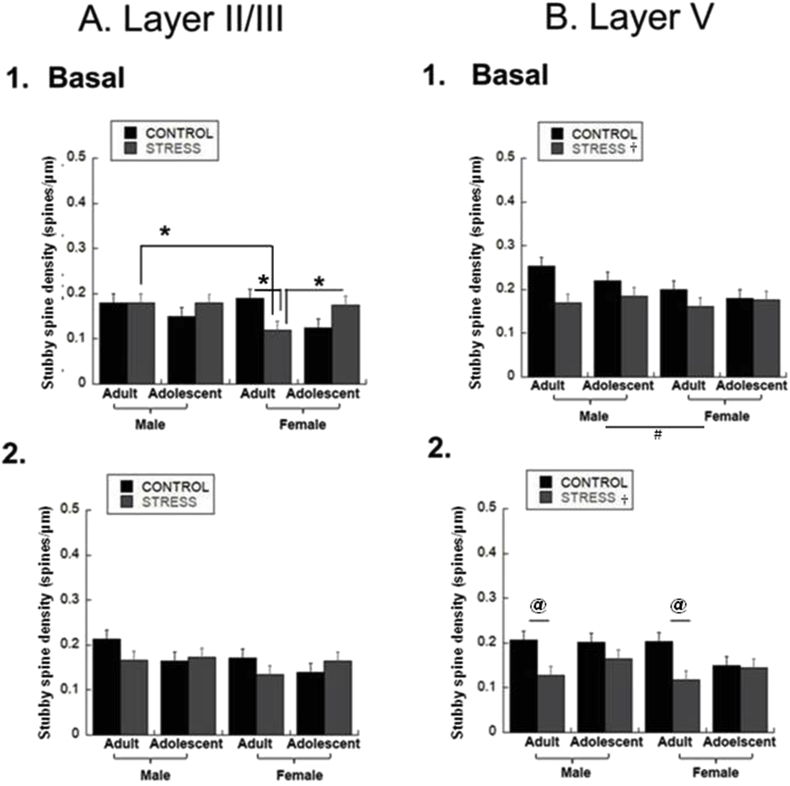
3.6. Stubby spine density
Finally, we examined stubby dendritic spines, which are short structures that lack a defined neck (Hering, 2001b). In layer II/III basal dendrites, there was no main effect, but adult controls had greater stubby spine density than adolescent controls [Fig. 6A.1; Age*Stress Interaction, F(1,79) = 10.3, p = 0.002]. Stress reduced stubby spine density in adult females and adult stressed females had lower stubby spine density than stressed adolescent females and adult males [Fig. 6A.1; Age*Sex*Stress Interaction, F(1,79) = 4.8, p = 0.03]. In layer V basal dendrites, stress reduced stubby spine density regardless of age or sex [Fig. 6B.1; Stress Effect: F(1,79) = 11, p = 0.0014]. Males had greater stubby spine density than females regardless of age or stress [Fig. 6B.1; Sex Effect, F(1,79) = 5.3, p = 0.024].
Fig. 6.
Stubby Spine Densities. Stubby spines were counted in 40 μm segments midway down the basal dendrites and at the distal termination, and in 50 μm segments midway and at the distal termination for apical dendrites and averaged to create a final spines/μm density for adolescent and adult males and females. (* = p < 0.05, post-hoc 3-way interaction; @ = p < 0.05, post-hoc 2-way interaction; ‡ = p < 0.05, age effect; # = p < 0.05, sex effect; † = p < 0.05, stress effect). A. Layer II/III.1. Basal Dendrites. Stress decreased spine density in female adults, and these stressed female adults had lower spine density than female stressed adolescents and male adult stressed rats. Group sizes were: n = 9 cells/4 rats for male adult controls, 10 cells/4 rats for stressed male adults, 9 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 7 cells/4 rats for female adult controls, 14 cells/4 rats for stressed female adults, 11 cells/4 rats for female adolescent controls, 10 cells/4 rats for stressed female adolescents.
2. Apical Dendrites. There was no effect on stubby spine density. Group sizes were: n = 9 cells/4 rats for male adult controls, 10 cells/4 rats for stressed male adults, 9 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 7 cells/4 rats for female adult controls, 14 cells/4 rats for stressed female adults, 11 cells/4 rats for female adolescent controls, 10 cells/4 rats for stressed female adolescents. B. Layer V.1. Basal Dendrites. Overall, males have greater spine density than females regardless of age or stress. Stress decreases spine density regardless of sex or age. Group sizes were: n = 10 cells/4 rats for male adult controls, 9 cells/4 rats for male adult defeats, 11 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 9 cells/4 rats for female adult controls, 10 cells/4 rats for stressed female adults, 10 cells/4 rats for female adolescent controls, 11 cells/4 rats for stressed female adolescents. 2. Apical Dendrites. Stress decreased spine density overall and this was driven by significant effects of stress in adult males and females but not in adolescents. Stress decreases spine density in adults. Group sizes were: n = 10 cells/4 rats for male adult controls, 9 cells/4 rats for stressed male adults, 11 cells/4 rats for male adolescent controls, 10 cells/4 rats for stressed male adolescents, 9 cells/4 rats for female adult controls, 10 cells/4 rats for stressed female adults, 10 cells/4 rats for female adolescent controls, 11 cells/4 rats for stressed female adolescents.
In layer II/III apical dendrites, there was no effect [Fig. 6A.2]. However, stress reduced stubby spine density in layer V apical dendrites [Fig. 6B.2; Stress Effect: F(1,79) = 18.2, p < 0.0001], and this was driven by adults, both male and female [Fig. 6B.2; Age*Stress Interaction, F(1,79) = 6.5, p = 0.013].
4. Discussion
The present study is the first to characterize the effects of repeated social stress on the morphology of prefrontal cortical neurons of both layer II/III and layer V in an age- and sex-dependent manner. Repeated social stress reduced dendritic branching in adolescents (both males and females) in apical dendrites of neurons in layer II/III. In contrast, stress increased branching in adult males in layer V neurons, both in the basal and apical dendrites. These results were supported by Sholl analysis, which revealed increased complexity in neurons of adult males following stress. Total dendritic spine density was decreased following repeated social stress in adolescent males and adult females, and this was mostly due to decreases in filopodial and mushroom spine density for adolescent males but stubby spine density for adult females.
4.1. Relationship to previous studies
There are multiple studies examining the effects of stress on prefrontal cortical neurons, although the majority of these have focused on layer II/III neurons only or did not distinguish layer, and used adult rats as experimental subjects. Chronic restraint stress has been shown to reduce apical dendritic arborization in layer II/III in prefrontal cortex in males (Cook and Wellman, 2004; Brown et al., 2005; Liston et al., 2006). Chronic uncontrollable stress paradigms also reduce branching in apical mPFC neurons (Izquierdo et al., 2006). However, not all stress paradigms reduce dendritic branching and spines. Prenatal stress and maternal separation increase dendritic spines in apical and basilar neurons in mPFC, but decrease these measures in orbital frontal cortex (Muhammad et al., 2012). Despite the prevalence of literature on stress effects on layer II/III PFC neurons, comparatively little is known about morphological changes in layer V neurons. Layer V pyramidal neurons in the PFC are the primary output neurons, and are uniquely plastic and sensitive to perturbations due to their high concentrations of NR2B-containing NMDA receptors (Wang et al., 2008). In one of the few studies examining layer V neurons, repeated restraint stress was shown to reduce arborization and spine density in distal tufts of apical dendrites (Liu and Aghajanian, 2008).
The present study substantially increased the current state of knowledge by systematically examining the effects of repeated social stress on both layer II/III and layer V neurons, both apical and basal dendrites, in both sexes over development. Resident-intruder stress was selected because for humans, stressors of a social nature are most prevalent and this paradigm can be considered a relevant model of human social stress (Bjorkqvist, 2001; Blanchard et al., 2001). Despite the relevance of this model to human stress, the effects of resident-intruder stress on the morphology of mPFC neurons has not been explored.
4.2. Social stress has opposing effects on dendritic branching in adult males versus adolescents and females
Repeated social stress reduced branching in layer II/III apical dendrites in adolescents of both sexes and, in addition, in layer V apical dendrites of female adults. Dendritic branching was also decreased in basal dendrites of layer II/III neurons in adult males. In contrast, stress resulted in increased branching in adult males in layer V neurons, both apical and basal dendrites. In a previous study on the functional effects of repeated social stress, we noted reduced excitability of layer V PFC pyramidal neurons in adolescents of both sexes as well as female adults, but not in adult males (Urban and Valentino, 2016). The morphological changes in layer V apical dendrites of adolescents and adult females corroborate the functional results of our previous study. However, we noted no functional changes in adult males despite seeing morphological alterations in this study. Here in adult males, we see an increase in branching in layer V basal and apical dendrites, but a decrease in layer II/III basal dendrites. The mPFC has a distinct laminar organization, with layer II/III neurons receiving inputs from subcortical and cortical structures. These layer II/III neurons send projections deeper to layer V, and layer V neurons project out to PFC target structures (Yeterian and Pandya, 1994; Ding et al., 2001). Thus, it is possible that in adult males, the reductions in basal branching in layer II/III result in reduced responsiveness to inputs from stress-related structures like amygdala and nucleus accumbens, but that increased branching in layer V neurons compensates for this reduced connectivity in layer II/III, resulting in no note change in excitability in the mPFC. In all other groups, male adolescents, female adolescents, and female adults, we see decreased branching in either or both layer II/III and layer V apical dendrites, which likely underlies the net decrease in excitability and synaptic transmission in layer V neurons seen in our previous study and may suggest reduced mPFC output to subcortical structures (Urban and Valentino, 2016).
4.3. Stress effects on dendritic spines
In this study, repeated social stress resulted in decreased total dendritic spine density in apical dendrites of layer V neurons of adolescent males and adult females but not in adult males. Dendritic spines represent locations for synaptic connections, and changes in total spine density are thought to index the state of synaptic connectivity. However, there are multiple classes of dendritic spines, each with its own morphological and synaptic characteristics. Filopodial spines are thin structures lacking a defined head and neck. They are transient, common on developing neurons, and may represent precursors to mature spines (Hering, 2001a; Rochefort and Konnerth, 2012). Mushroom spines have a thin neck and bulbous head and are thought to indicate mature synapses. Stubby spines are short and devoid of a neck and prominent between postnatal developmental stages (Rochefort and Konnerth, 2012). Spines with larger heads are thought to indicate stronger synaptic connections (Hering, 2001b). Interestingly, in our study adults had increased filopodial spine density in layer II/III basal dendrites and mushroom spines in basal layer V neurons following stress compared to adolescents overall. Unlike other cortical regions, prefrontal cortex maintains a high degree of plasticity throughout life. As new synaptic connections must form constantly, a high number of filopodial spines is not unexpected (Wang et al., 2008). A reduction in filopodial spines following exposure to stress suggests that following repeated social stress, male adolescents may have reduced connectivity between layer II/III and layer V neurons, which could result in impaired prefrontal cortical control of cognitive processing.
The prefrontal cortex undergoes a final wave of synaptic pruning and maturation following puberty (Arain et al., 2013). As mushroom spines are considered to represent mature spines with active robust synapses, changes in mushroom spine density are likely to reflect established, static synapses (Hering, 2001b). Mushroom spines are increased with age in basal dendrites of layer V neurons but decreased with age in basal dendrites of layer II/III neurons, indicating differential pruning in these two layers. Stress decreased mushroom spine density in adolescents and adult females in layer V, suggesting that any stress-induced cognitive impairment in these groups may be due to decreased mature synapses in the mPFC output neurons.
Stress decreased stubby spine density in basal dendrites of layer V neurons in general, and in apical dendrites of layer V neurons for adults. The function of stubby spines is less clear. They have been proposed to indicate older, dying synapses, but have also been proposed to represent mature synapses with faster kinetics than mushroom synapses due to their shorter length (Hering, 2001b; Rochefort and Konnerth, 2012). Thus, these results may represent ongoing synaptic retraction, or may indicate that in adolescents, there is a selective loss of fast kinetic synapses, resulting in slower signal transmission in prefrontal cortex, whereas in female adults both fast and slow kinetic synapses are affected.
4.4. Baseline age and sex effects
Our study reveals striking age- and sex-dependent effects of repeated social stress. However, morphological differences between adolescents and adults and between males and females without stress are also of importance. Post hoc analyses revealed several interesting differences among non-stressed control groups. Male adult controls had reduced branching in layer V basal dendrites compared to female controls of both ages and male adolescent controls, and reduced branching in layer V apical dendrites compared to male adolescent controls. Adult male controls also had lower total spine density in layer V basal dendrites than female adult controls, and adult female controls had greater spine density than adolescent female controls in layer V apical dendrites. The majority of baseline spine density differences seemed to be attributable to filopodial spines, with adult female controls having greater filopodial spine density in layer II/III basal dendrites than male controls of both ages, and adolescent male controls having higher filopodial spine density than female adolescent controls in layer V apical dendrites. Adult female controls also had higher density of mushroom spines in layer V apical dendrites compared to both adult male and adolescent female controls. These differences indicate that sex and age-dependent differences in morphology exist regardless of stress experience. Overall, adult females seemed to have higher baseline levels of branching and spine density than adult males, perhaps indicating reduced synaptic pruning in females.
4.5. Functional implications
Repeated social stress altered prefrontal cortical pyramidal neuron morphology in a sex-, age-, and layer-dependent manner. In our previous study, we noted that repeated social stress reduced neuronal excitability and synaptic transmission in layer V pyramidal neurons of adolescents of both sexes and female adults, but not male adults (Urban and Valentino, 2016). In this study, repeated social stress reduced apical dendritic branching in adolescents and adult females, indicating reduced connectivity between deep cortical output layers and more superficial input layers. We noted reductions in density of filopodial and mushroom spines in male adolescents and stubby spines in female adults. Taken together, these results suggest that following repeated social stress, the prefrontal cortex of adolescents and female adults undergoes remodeling that decreases both connectivity and responsiveness to interlaminar inputs. This would result in decreased top-down cognitive processing following stress, allowing for not only increased amygdalar drive, but potentially the emergence of executive function deficits, a hallmark of stress-related psychiatric and cognitive disorders (Arnsten, 2009, 2011). Indeed, dendritic spine loss has been observed in human patients and animal models of depression, schizophrenia, and bipolar disorder (Konopaske et al., 2014; Qiao et al., 2016; Csabai et al., 2018).
Also of interest is the finding that in adult males, dendritic branching and arborization is decreased in basal dendrites of layer II/III but increased in apical and basal dendrites of layer V. This may represent a compensatory mechanism that underlies the resilience of adult males to many of the negative cognitive effects of a variety of social stressors (Weintraub et al., 2010; Snyder et al., 2014). Furthermore, male rats develop territorial aggression towards other males as a natural sexual behavior as they routinely combat for access to territory and females in the wild (Thor and Flannelly, 1976). Thus, the unique morphological profile seen in adult male rats following social stress may indicate that the stressor is subjectively different for these animals than it is for adolescents or females, groups that do not naturally fight for mates. In addition, the experience of social defeat may differ between males and females because males are fighting for territory/dominance while female residents are primarily engaging in aggressive interactions to protect their offspring (Blanchard et al., 2001).
The resident-intruder paradigm has strong relevance to common social stressors in humans, and may therefore provide more easily translatable results. However, there is great variability among the effects of various types of stress in rodents. Furthermore, the majority of these studies have focused on adult males and layer II/III neuronal morphology (Cook and Wellman, 2004; Brown et al., 2005; Liston et al., 2006; Perez-Cruz et al., 2007; Luczynski et al., 2015). Interestingly, Cook and Wellman found that only apical dendritic arbors were reduced by stress, whereas our study revealed reductions in layer II/III apical and basal arbors in adult males (Cook and Wellman, 2004). Perez-Cruz found a hemispheric difference as well, with restraint stress reducing dendritic arbors in right hemisphere more than in left, abolishing a baseline hemispheric bias of longer arbors in right mPFC (Perez-Cruz et al., 2007). These differences highlight the importance of examining multiple stressors, and suggest that comprehensive developmental and sex difference morphology studies such as ours should be completed using several stressors to confirm the translational strength and the influence of type of stressor.
In conclusion, this study revealed distinct age-, sex- and layer-dependent alterations in pyramidal neuron morphology following repeated social stress. It extends previous knowledge by systematically examining both layer II/III and layer V neurons, and both apical and basal dendrites in males and females over development. Reductions in complexity and dendritic spine density in layer V apical dendrites of adolescents and female adults were consistent with reductions in neuronal function previously revealed, and suggest reduced connectivity between the mPFC and subcortical structures following stress. However, in adult male mPFC, reductions in basal dendritic branching in layer II/III was met with increased branching in both basal and apical dendrites of layer V, and overall spine density was not altered, indicating that in adult males, connectivity of mPFC to subcortical structures may increase following stress, buffering the cognitive impacts of social stress. These distinct changes may begin to elucidate how the sexes differ with their vulnerability to stress-related disorders.
Acknowledgements
This work was funded by The National Institutes of Health Grants R01MH 093981 to Rita J. Valentino and Seema Bhatnagar and 5T32NS077413 to Kimberly R. Urban).
Contributor Information
Kimberly R. Urban, Email: urbank@email.chop.edu.
Rita J. Valentino, Email: Rita.valentino@nida.gov.
References
- Adler N.E., Boyce T., Chesney M.A., Cohen S., Folkman S., Kahn R.L., Syme S.L. Socioeconomic status and health. The challenge of the gradient. Am. Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Arain M., Haque M., Johal L., Mathur P., Nel W., Rais A., Sandhu R., Sharma S. Maturation of the adolescent brain. Neuropsychiatric Dis. Treat. 2013;9:449–461. doi: 10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F. Stress signaling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int. J. Dev. Neurosci. 2011;29:215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Curtis A., Reyes B.A., Bethea T.T., Parastatidis I., Ischiropoulos H., Van Bockstaele E.J., Valentino R.J. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatr. 2010;15(877):896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.V., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H., Pandya D.N. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Berendse H.W., Galis-de Graaf Y., Groenewegen H.J. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol. Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blanchard R.J., McKittrick C.R., Blanchard D.C. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol. Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Blehar M.C. Gender differences in risk factors for mood and anxiety disorders: implications for clinical treatment research. Psychopharmacol. Bull. 1995;31:687–691. [PubMed] [Google Scholar]
- Brody A.L., Barsom M.W., Bota R.G., Saxena S. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin. Clin. Neuropsychiatry. 2001;6:102–112. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- Brown G.W., Prudo R. Psychiatric disorder in a rural and an urban population: 1. Aetiology of depression. Psychol. Med. 1981;11:581–599. doi: 10.1017/s0033291700052880. [DOI] [PubMed] [Google Scholar]
- Brown S.M., Henning S., Wellman C.L. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cerebr. Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Carr C.P., Martins C.M., Stingel A.M., Lemgruber V.B., Juruena M.F. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J. Nerv. Ment. Dis. 2013;201:1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Chaijale N.N., Snyder K., Arner J., Curtis A.L., Valentino R.J. Repeated social stress increases reward salience and impairs encoding of prediction by rat locus coeruleus neurons. Neuropsychopharmacology. 2014;40(2):513–523. doi: 10.1038/npp.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaijale N.N., Curtis A.L., Wood S.K., Zhang X.Y., Bhatnagar S., Reyes B.A., Van Bockstaele E.J., Valentino R.J. Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence. Neuropsychopharmacology. 2013;38:1833–1843. doi: 10.1038/npp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.J., Kelly G., Sengupta A., Heydendael W., Nicholas B., Beltrami S., Luz S., Peixoto L., Abel T., Bhatnagar S. MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience. 2015;305:36–48. doi: 10.1016/j.neuroscience.2015.07.045. [DOI] [PubMed] [Google Scholar]
- Cook S.C., Wellman C.L. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Csabai D., Wiborg O., Czeh B. Reduced synapse and axon numbers in the prefrontal cortex of rats subjected to a chronic stress model for depression. Front. Cell. Neurosci. 2018;12:24. doi: 10.3389/fncel.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D.C., Gabbott P.L., Totterdell S. Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain Res. 2001;917:81–89. doi: 10.1016/s0006-8993(01)02912-2. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gorman J.M., Liebowitz M.R., Fyer A.J., Stein J. A neuroanatomical hypothesis for panic disorder. Am. J. Psychiatry. 1989;146:148–161. doi: 10.1176/ajp.146.2.148. [DOI] [PubMed] [Google Scholar]
- Grafe L.A., Cornfeld A., Luz S., Valentino R., Bhatnagar S. Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol. Psychiatry. 2017;81:683–692. doi: 10.1016/j.biopsych.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Southwick S.M., Charney D.S. The psychobiological basis of posttraumatic stress disorder. Mol. Psychiatr. 1996;1:278–297. [PubMed] [Google Scholar]
- Handa RJB L.H., Kerr J.E., O'Keefe J.A. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Heller W. Gender differences in depression: perspectives from neuropsychology. J. Affect. Disord. 1993;29:129–143. doi: 10.1016/0165-0327(93)90028-i. [DOI] [PubMed] [Google Scholar]
- Hering HS M. Dendritic spines: structure, dynamics, and regulation. Nat. Rev. Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Hering HS M. Dentritic spines : structure, dynamics and regulation. Nat. Rev. Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Izquierdo A., Wellman C.L., Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C.W., Wüst S., Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med. 1992;54(6):648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kitay J.I. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Kolb B., Mychasiuk R., Muhammad A., Li Y., Frost D.O., Gibb R. Experience and the developing prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2012;109(Suppl. 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopaske G.T., Lange N., Coyle J.T., Benes F.M. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Liston C., Miller M.M., Goldwater D.S., Radley J.J., Rocher A.B., Hof P.R., Morrison J.H., McEwen B.S. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.J., Aghajanian G.K. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P., Moquin L., Gratton A. Chronic stress alters the dendritic morphology of callosal neurons and the acute glutamate stress response in the rat medial prefrontal cortex. Stress. 2015;18:654–667. doi: 10.3109/10253890.2015.1073256. [DOI] [PubMed] [Google Scholar]
- Marin M.F., Lord C., Andrews J., Juster R.P., Sindi S., Arsenault-Lapierre G., Fiocco A.J., Lupien S.J. Chronic stress, cognitive functioning and mental health. Neurobiol. Learn. Mem. 2011;96:583–595. doi: 10.1016/j.nlm.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Mazure C.M. Does stress cause psychiatric illness? In: Spiegel D., editor. Progress in Psychology. ed. American Psychiatric Press; Washington, D.C.: 1995. p. 270. [Google Scholar]
- Muhammad A., Carroll C., Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–109. doi: 10.1016/j.neuroscience.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Pearson-Leary J., Eacret D., Chen R., Takano H., Nicholas B., Bhatnagar S. Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl. Psychiatry. 2017;7:e1160. doi: 10.1038/tp.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cruz C., Muller-Keuker J.I., Heilbronner U., Fuchs E., Flugge G. Morphology of pyramidal neurons in the rat prefrontal cortex: lateralized dendritic remodeling by chronic stress. Neural Plast. 2007:46276. doi: 10.1155/2007/46276. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott T.A. Gender differences in the epidemiology and treatment of anxiety disorders. J. Clin. Psychiatry. 1999;60(Suppl. 18):4–15. [PubMed] [Google Scholar]
- Prudo R., Brown G.W., Harris T., Dowland J. Psychiatric disorder in a rural and an urban population: 2. Sensitivity to loss. Psychol. Med. 1981;11:601–616. doi: 10.1017/s0033291700052892. [DOI] [PubMed] [Google Scholar]
- Qiao H., Li M.X., Xu C., Chen H.B., An S.C., Ma X.M. Dendritic spines in depression: what we learned from animal models. Neural Plast. 2016;2016:8056370. doi: 10.1155/2016/8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Miller M., Janssen W.G., Liston C., Hof P.R., McEwen B.S., Morrison J.H. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebr. Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Ramkumar K., Srikumar B.N., Venkatasubramanian D., Siva R., Shankaranarayana Rao B.S., Raju T.R. Reversal of stress-induced dendritic atrophy in the prefrontal cortex by intracranial self-stimulation. J. Neural Transm. 2012;119:533–543. doi: 10.1007/s00702-011-0740-4. [DOI] [PubMed] [Google Scholar]
- Rochefort N.L., Konnerth A. Dendritic spines: from structure to in vivo function. EMBO Rep. 2012;13:699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Seib L.M., Wellman C.L. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci. Lett. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- Snyder K., Barry M., Plona Z., Ho A., Zhang X.Y., Valentino R.J. The impact of social stress during adolescence or adulthood and coping strategy on cognitive function of female rats. Behav. Brain Res. 2015;286:175–183. doi: 10.1016/j.bbr.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K.P., Barry M., Valentino R.J. Cognitive impact of social stress and coping strategy throughout development. Psychopharmacology (Berlin) 2014;232:185–195. doi: 10.1007/s00213-014-3654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor D.H., Flannelly K.J. Age of intruder and territorial-elicited aggression in male long-evans rats. Behav. Biol. 1976;17:237–241. doi: 10.1016/s0091-6773(76)90546-0. [DOI] [PubMed] [Google Scholar]
- Urban K.R., Valentino R.J. Age- and sex-dependent impact of repeated social stress on intrinsic and synaptic excitability of the rat prefrontal cortex. Cerebr. Cortex. 2016;27(1):244–253. doi: 10.1093/cercor/bhw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraiah P., Noronha J.M., Maitra S., Bagga P., Khandelwal N., Chakravarty S., Kumar A., Patel A.B. Dysfunctional glutamatergic and gamma-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol. Psychiatry. 2014;76:231–238. doi: 10.1016/j.biopsych.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Ver Hoeve E.S., Kelly G., Luz S., Ghanshani S., Bhatnagar S. Short-term and long-term effects of repeated social defeat during adolescence or adulthood in female rats. Neuroscience. 2013;249:63–73. doi: 10.1016/j.neuroscience.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Stradtman G.G., 3rd, Wang X.J., Gao W.J. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub A., Singaravelu J., Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Wellman C.L. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wood S.K., Walker H.E., Valentino R.J., Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian E.H., Pandya D.N. Laminar origin of striatal and thalamic projections of the prefrontal cortex in rhesus monkeys. Exp. Brain Res. 1994;99:383–398. doi: 10.1007/BF00228975. [DOI] [PubMed] [Google Scholar]
- Zitnik G.A., Curtis A.L., Wood S.K., Arner J., Valentino R.J. Adolescent social stress produces an enduring activation of the rat locus coeruleus and alters its coherence with the prefrontal cortex. Neuropsychopharmacology. 2015;41:1376–1385. doi: 10.1038/npp.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J.K., Chinitz J.A., Lombardi U., Fig L.M., Cameron O.G., Liberzon I. Medial frontal cortex involvement in PTSD symptoms: a SPECT study. J. Psychiatr. Res. 1999;33:259–264. doi: 10.1016/s0022-3956(98)00060-0. [DOI] [PubMed] [Google Scholar]