Abstract
Background
Pharmacokinetic (PK) and pharmacodynamic (PD) data on perioperative antibiotic prophylaxis or antibiotic therapy are rare in patients suffering from morbid obesity. Furthermore, dosing regimens should be based on PK/PD models that ensure effective antibiotic exposure not in plasma, but primarily at the site of infection, mostly in the interstitial fluid (ISF). The aim of this trial is to investigate whether current dosing regimens of various antibiotics lead to effective concentrations in the ISF of morbidly obese patients.
Methods
We designed a prospective, parallel group, open-labeled, controlled single center trial to investigate the plasma and tissue pharmacokinetics of the antibiotics linezolid, meropenem, tigecycline, piperacillin/tazobactam, fosfomcyine, cefazolin, metronidazole and as secondary aim the analgesics metamizole and acetaminophen. Inclusion criteria comprise body mass index ≥35 kg/m2 for obese or between 18.5 and 30 kg/m2 for non-obese patients scheduled for elective abdominal surgery. For PK analysis, blood and microdialysate samples of subcutaneous tissue were collected 0–8 h after study drug administration. The primary endpoint is to investigate a possible dependency of the area-under-the-curve (AUC0-8) in the interstitial fluid on body weight and obesity with population based pharmacokinetic analysis.
Discussion
Inadequate dosing regimes of antibiotics may be a relevant factor for morbidity and mortality of patients, as well as for the development of bacterial antibiotic resistance. The measurement of plasma and tissue concentrations will provide information necessary for PK/PD-modelling. These data about antibiotic PK/PDcharacteristics in soft tissue and their dependence on weight should help to develop weight-dependent models for calculation of patient's individual doses of different antibiotics.
Trial registration
EU clinical trials register (EudraCT-No. 2012-004383-22) and German Clinical trials Register (DRKS00004776);
Keywords: Obesity, Antibiotic dosing, Pharmacokinetics, Pharmacodynamics, Microdialysis
1. Introduction
Surgical wound infections constitute the most common source of postoperative complications. Perioperative antibiotic prophylaxis decreases infectious morbidity and mortality, length of stay, and cost of care, and is particularly important in obese patients who are at higher risk of postoperative wound infections [[1], [2], [3], [4]]. Standard dosing regimens, usually developed in healthy normal weighted volunteers, typically comprise a fixed, single antibiotic dose about 1 h prior to surgical incision. For patients suffering from morbid obesity, however, pharmacokinetic (PK) and pharmacodynamic (PD) data for antibiotic prophylaxis are rare. Appropriate dosing is essential to provide sufficient perioperative antibiotic prophylaxis or antibiotic effect in obese patients. Dosing regimens should consider PK/PD models not only in plasma but, in particular, in the interstitial tissue fluid as target site where bacteria frequently reside [3,5].
Microdialysis is an established tool to measure interstitial unbound drug concentrations in virtually every given tissue and organ in animals and humans [6,7]. So far, only few studies with a limited number of subjects have focused on soft tissue concentrations of antibiotics in obese patients [5,8] and consequently, data for appropriate dose adjustments in this population are lacking.
The aim of this trial is to investigate the hypothesis that for individual antibiotics, current dosing regimens do not lead to effective concentrations in soft tissues of morbidly obese patients. Thus, we designed a trial to characterize the plasma and tissue pharmacokinetics of antibiotics after a single short intravenous infusion in surgical morbidly obese patients compared to non-obese patients. In addition, we aim at generating data on methodological variability of the microdialysis method in this clinical setting.
2. Methods/design
2.1. Design and sample size
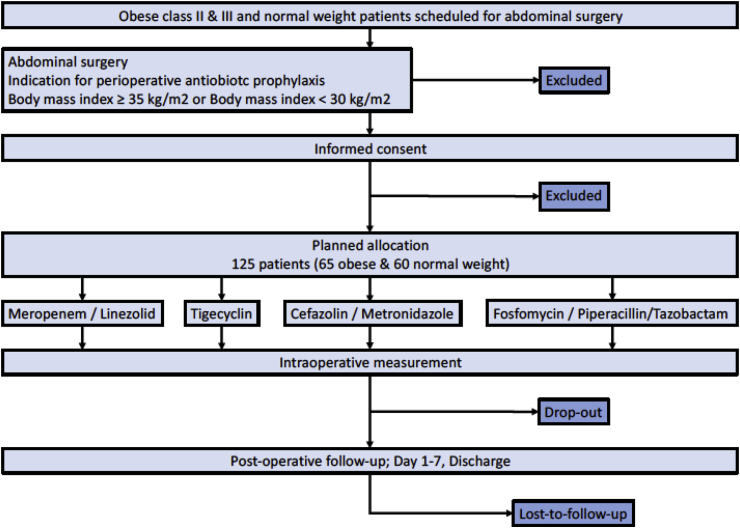
This prospective, parallel group, open-labeled, controlled single center trial is located at the Leipzig University Hospital. Approval for the trial was granted by the Leipzig University ethics committee (121/13-ff) and the Federal Institute for Drugs and Medical Devices of Germany (BfArM) and was registered in the European Union clinical trials register (EudraCT No. 2012-004383-22). Sample size calculations are based on the assumption that area-under-the-curve (AUC) for drug concentrations on a log-scale is roughly linear with weight [3,5]. Simulations taking into account the expected weight distribution in the patients showed that 13 patients per treatment group have to be analyzed to show a statistically significant difference in AUC with 80% power. To allow for drop-outs and missing data, 15 patients per weight category and drug combination were included. In total, 120 patients were to be allocated to the trial (see consolidated Standards of Reporting Trial [CONSORT] diagram, Fig. 1). Five additional patients were included for feasibility testing of the newly established microdialysis method. The non-obese patients were to be matched within each treatment group (see Table 1) based on age and sex.
Fig. 1.
Consort diagram.
Table 1.
Treatment groups.
| Group | Study Medication |
|---|---|
| Group A | Meropenem 1 g (AstraZeneca GmbH, Wedel, Germany) Linezolid 600 mg, (Pfizer, New York, USA) |
| Group B | Tigecycline 100 mg (Pfizer, New York, USA) |
| Group C | Cephazolin 2 g (Fresenius Kabi, Bad Homburg, Germany) Metronidazole 500 mg (Fresenius Kabi, Bad Homburg, Germany) |
| Group D | Fosfomycin 8 g (Infectopharm, Heppenheim, Germany) Piperacillin/tazobactam 4.0/0.5 g (Stragen Pharma, Cologne, Germany) |
Additional in every group: acetaminophen 1 g (Fresenius Kabi, Bad Homburg, Germany) or metamizole 1 g (Novaminsulfon-ratiopharm, Ratiopharm, Ulm, Germany).
After obtaining results of our in-vitro trials [11], microdialysis pump flow settings were optimized and changes of the retrodialysis protocol (Table 2) were implemented. An amendment of the study protocol (version 3.0; November 2014) for group D is provided in additional files. There are no changes in endpoints.
Table 2.
Retroperfusate concentration used for calibration.
| Group | Study Medication | Retroperfusate Concentration |
|---|---|---|
| Group A | Meropenem 1000 mg | 20 mg/L |
| Linezolid 600 mg | 150 mg/L | |
| Group B | Tigecycline 100 mg | 500 mg/L |
| Group C | Cephazolin 2000 mg | 50 mg/L |
| Metronidazole 500 mg | 50 mg/L | |
| Group D | Fosfomycin 8 g | 200 mg/L |
| Piperacillin/tazobactam 4.0/0.5 g | 200 mg/L | |
| Analgetics | acetaminophen 1 g | 20 mg/L |
| metamizole 1 g | 10 mg/L | |
2.2. Study population
Patients scheduled for elective abdominal surgery at the Leipzig University Hospital were screened for eligibility. Inclusion criteria comprised age ≥18 years and body mass index (BMI) ≥ 35 kg/m2 for obese patients or between 18.5 and 30 kg/m2 for non-obese patients. Exclusion criteria were pregnancy or breastfeeding, known allergic reaction to one of the investigated medications, severe liver disease, severe kidney disease, arterial hypotension, failure of the bone marrow function, acute hepatic porphyria, phenylketonuria, hereditary fructose intolerance, intake of monoaminoxidase A or B inhibitors, intake of one of the study medications in the last three days or participation in another clinical trial with medications.
2.3. Anesthesia
Patients did not receive any premedication on the day of surgery. Anesthesia was performed according to clinical standards and left at the discretion of the anaesthetist. The local clinical standard included either balanced anesthesia with propofol and sufentanil or remifentanil followed by desflurane/isoflurane, or total intravenous anesthesia (TIVA). Endotracheal intubation was facilitated by a single shot of rocuronium. Routine perioperative monitoring in the participating patients included invasive measurement of arterial blood pressure, pulse oximetry, and electrocardiogram (SC9000, Siemens, Erlangen, Germany). All patients received infusion of crystalloid fluid, with or without additional vasopressors such as theodrenaline + cafedrine and/or norepinephrine as required to assure a mean arterial pressure >60 mmHg.
2.4. Perioperative antibiotic prophylaxis and analgesic study medication
After allocation to one treatment group (A-D, Table 1), patients got a single dose of the respective antibiotic study drug and analgesic co-medication after induction of anesthesia through an additional vein access as a single dose over 30 min using an automated infusion system (Infusomat® Space, B. Braun Melsungen AG, Melsungen, Germany) 60 to 30 min prior to incision. A repetitive dose of antibiotics for perioperative prophylaxis was allowed according to the clinical pathway at our hospital using cefuroxime 1500 mg after 3 h of surgery.
2.5. Blood and microdialysate samples
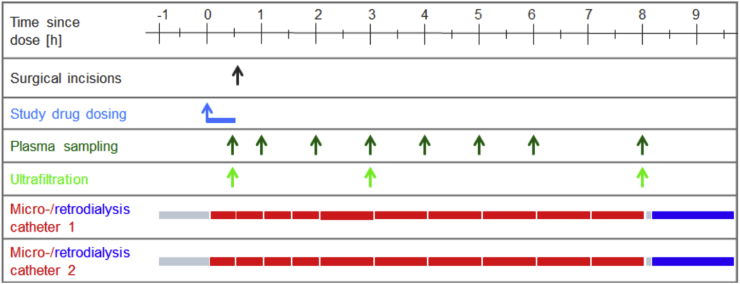
After skin disinfection and under sterile conditions, two microdialysis catheters (CMA 63 microdialysis probe, membrane length 30 mm, cut-off 20,000 Da, CMA, Kista, Sweden) were placed into the subcutaneous tissue of both upper arms (one in each arm) assuring that the complete length was subcutaneous, but without using ultrasound for anatomical landmarks and without using local anesthesia. The catheters were placed 90 min before beginning anesthesia and administration of the study drugs (Fig. 2). The probes were perfused with 0.9% saline at 1 or 2 μL/min. After equilibration (30 min) microdialysate were collected for 1 h and a baseline blood sample was taken. Immediately after induction of general anesthesia, the study antibiotics were infused for exactly 30 min. Additional blood samples were taken at 0.5 (end of infusion), 1, 2, 3, 4, 5, 6 and 8 h. Microdialysates were collected from 0 to 0.5, 0.5–1, 1–1.5, 1.5–2, 2–3, 3–4, 4–5, 5–6, 6–7 and 7–8 h.
Fig. 2.
Schematic representation of drug dosing and sampling schedule.
Blood samples were centrifuged within 30 min after sampling (3000 g, 4 °C, 10 min). Plasma and dialysate samples were frozen immediately at approximately −25 °C and then stored at −80 °C until bioanalysis.
2.6. Microdialysis
Microdialysis was performed at a constant flow rate of 2 μl/min in group A-C and at a flow rate of 1 μl/min in group D. To obtain interstitial concentrations from microdialysate concentrations (CμD), the retrodialysis method was used [9]. After a washing step, retrodialysis was started with the retroperfusate (RP), a perfusion solution containing a defined concentration of the respective study drug (Table 2). Retrodialysate (RD) was collected as described in the retrodialysis protocol (Table 3) [10,11]. Drug concentrations were determined in the retroperfusate (CRP) and retrodialysate (CRD) to determine the in vivo relative recovery as follows: relative recovery = (1 – CRD/CRP) x 100%. The concentration of the drugs in the interstitial fluid (ISF) was then given by CISF = CμD x 100/relative recovery.
Table 3.
| Begin of the clinical trial | After in vitro study [11] | |
|---|---|---|
| Group A-C |
|
|
| Group D |
|
2.7. Study visits and data collection
Patients were visited preoperatively, intraoperatively and daily between postoperative day 1 and 7 or until discharge (Table 4). A complete participant time line, including all variables as well as interventions, is available as a supplementary file (crf).
Table 4.
Study schedule.
| study schedule | screening visit | study day | post- operative days 1–7 |
|---|---|---|---|
| written informed consent | x | ||
| Demographics | x | ||
| medical history, concomitant diseases | x | ||
| inclusion/exclusion criteria | x | ||
| adverse event monitoring | x | X | |
| evaluations/measurements according to table below | x | ||
| insertion of two microdialysis probes and equilibration | x | ||
| in-vivo calibration of microdialysis probes | x | ||
| administration of study drug | x | ||
| plasma for pharmacokinetics | x | ||
| microdialysate for pharmacokinetics | x | ||
| removal of microdialysis probes | x | ||
| monitoring incidence of surgical site infection within 1st week | X |
All preoperative patients were screened for eligibility. After providing informed consent, baseline variables were collected, including birth date, sex, age, weight, height, medical history, indication for operation, antibiotic prescriptions within the last 3 months, current medication and blood work (for example for liver and kidney function).
On the day of the operation, the type of surgery was documented as well as duration of surgery, type of anesthesia, intravenous medication, perioperative complications (hypotonia, hypoxia, allergic reaction, anaphylaxia, bleedings), use of vasoactive medication, transfusions, blood loss and fluid balance. In addition, detailed information regarding the microdialysis was documented, including time and placement of the catheters, time and duration of administration of the study drugs, time of taking the samples, as well as any problems with the study procedures.
Antibiotics, analgesics and other medication were recorded on day seven or at discharge, whichever came first. The incidence of wound infections, postoperative pain (using NRS), and adverse events were recorded.
Patient data were collected in pseudonymous form using a patient identification number. Study data were collected and managed using an Oracle-DBMS database with eResearch Network. Electronic data capture tools are hosted at the Clinical Trials Center of the University of Leipzig, Germany.
2.8. Bioanalytical measurements
Study drug concentrations in microdialysates, plasma and plasma ultrafiltrate samples were analyzed at the Department of Pharmacology, University of Regensburg, Regensburg, Germany, using HPLC methods with photometric detection [11].
2.9. Study endpoints
The primary endpoint is the dependence of the AUC0-8 in the interstitial fluids on body weight. Further analysis will explore the dependence of AUC0-8 in the interstitial fluid (ISF) on further covariates, such as BMI and compare obese with non-obese patients. Analogous analyses will be performed for the secondary outcomes AUC0-8 in plasma, maximum concentration (Cmax), Cmax/MIC (MIC = minimal inhibitory concentration), AUC/MIC in the microdialysate and plasma after single dose, time over MIC (T > MIC) AUCISF/AUCplasma ratio, PK/PD parameters and half-life (t1/2). Originally foreseen endpoints such as concentration in surgical tissue specimen, incidence of wound infections, and postoperative pain scoring and the length of stay at hospital are deemed uninformative and will not be analyzed formally, though summary statistics of the corresponding data will be presented.
2.10. Statistical analysis
2.10.1. Analyses using linear models
Plasma and ISF concentrations will be treated on a logarithmic scale. AUC will be determined by taking the mean of ISF concentrations from the two catheters and using a trapezoid technique with the midpoint of the collection interval as the time point. AUC will be taken as the dependent variable in linear models with covariates such as weight, BMI and sex. When analyzing longitudinal data, a linear mixed-model will be used with a random effects term allowing an intercept and slope for each patient. Obesity status, catheter and time will be taken as fixed effects along with an interaction term between obesity status and time. Parsimonious models based on Akaike's Information Criterion will be used. Estimates with 95% confidence intervals will be presented and p-values ≤ 0.05 will be considered significant.
2.10.2. Pharmacokinetic modelling and simulation
After designing and registering the trial, co-operation was established with a group with expertise in population pharmacokinetic modelling integrating total and unbound plasma as well as microdialysis data [[12], [13], [14]]. Because of the exploratory and pilot nature of the trial, it was deemed appropriate to treat these well-established methods on the same footing as those foreseen from the trial's outset.
Appropriate modelling and simulation approaches such as the nonlinear mixed-effects modelling approach (NONMEM, Icon Development Solutions, Ellicott City, MD, USA) will be used to characterize the PK including the associated variabilities and link the PK to the PD. For the development of the base PK model, total drug concentrations will be investigated using appropriate disposition models. If available, unbound plasma concentrations will be integrated into the model in order to characterize the plasma protein binding. The model will be extended by μD concentrations to evaluate the kinetics of tissue distribution. The statistical model focuses on quantifying both PK-related variability between the patients as well as variability related to the methodology of microdialysis. Covariates will be identified that explain the methodology- or PK-related variability between the patients. The focus of the analysis will lie on the quantification of the impact of body size descriptors (e.g. total body weight, BMI, lean body weight) on the PK. Ultimately, the final PK model will be used to perform Monte-Carlo simulations in order to evaluate the probability of PK/PD target attainment both in plasma and in the interstitial fluid to identify the patients at risk for sub-therapeutic antibiotic exposure and, if needed, to recommend new dosing regimens.
3. Discussion
With this study, we want to characterize the plasma and tissue pharmacokinetics of different antibiotics used for prophylaxis and treatment of tissue and surgical wound infections in morbidly obese and non-obese control patients. This arises from the clinical need to provide adequate prophylaxis and treatment options for a growing number of obese patients for whom standard doses may be inappropriate due to greater body mass and a higher proportion of body fat. As the second objective, we want to evaluate whether the pharmacokinetics can be predicted by body size descriptors (e.g. body mass index or total body weight) and to develop a population PK model for calculating the patient's individual dose.
To achieve these study goals, we designed a study with 4 groups to investigate different antibiotic classes used to treat soft tissue and wound infections. We compare 60 patients with WHO BMI classes II-III to 60 non-obese patients, making this the largest study using the microdialysis technique. With this technique it is possible to measure antibiotic concentrations directly in a potential site of action of bacteria. The perioperative setting with patients requiring antibiotic prophylaxis for surgery is a clinical model that is highly common, standardized and relevant. It also serves as a model for antibiotics, which are not approved for prophylaxis but for treatment of tissue infections.
To investigate precision and methodological aspects as well as intra-individual variability of the microdialysis method, we perform measurements in every patient using two microdialysis catheters inserted in the same subcutaneous tissue of the same patient at the same time in both upper arms.
Inadequate dosing regimes of antibiotics, leading to sub-therapeutic drug concentrations in the target tissue may be a relevant factor for individual morbidity and mortality of patients, as well as for the development of bacterial antibiotic resistance. The measurement of plasma concentrations of important classes of antibiotics will provide necessary information for PK/PD modelling and help contrast with the measurements typically used for dosage assessment. This can help us to ascertain the relevance of obesity in dosing regimens. We might also identify further patient characteristics impacting the drug concentrations in the ISF of the soft tissue using the population pharmacokinetic modelling approach. Finally, our goal is to identify the patients at risk for therapy failure and to suggest new dosing regimens, which will be validated in prospective dosing studies in future.
In conclusion, we aim to better characterize the pharmacokinetics of antibiotics in soft tissues and to quantify the impact of body size descriptors such as BMI. These data will help to develop BMI-dependent models for calculating the patient's individual optimal doses of different antibiotics.
Ethics approval and consent to participate
Approval for the trial was granted by the Leipzig University Ethics committee (No.: 121-13-28012013). The study was also approved by the Federal Institute of Drug and Medical Devices (BfArM – No.: 4038808) and registered in EU clinical trials register (Eudr-CT-No. 2012-004383-22) and German Clinical trials Register (DRKS00004776). The study is designed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from every enrolled patient upon request by the local German law.
Consent to publish
Not applicable.
Availability of data and materials
The datasets analyzed during the present study may be provided from the corresponding author upon request.
Conflicts of interest
HW received grants from Pfizer (Investigator Initiated Trial Program, Berlin, Germany) and InfectoPharm (Heppenheim, Germany), both for the clinical microdialysis trial. HW reports lecture fees from InfectoPharm (Heppenheim, Germany), MSD (Konstanz, Germany), and consultant honoraria from Dräger Medical (Lübeck, Germany). PS received grants from Pfizer (Investigator Initiated Trial Program, Berlin, Germany) and InfectoPharm (Heppenheim, Germany), both for the clinical microdialysis trial. PS reports lecture fees from InfectoPharm (Heppenheim, Germany). CK reports research grants from an industry consortium (AbbVie Deutschland GmbH & Co. KG, Boehringer Ingelheim Pharma GmbH & Co. KG, Grünenthal GmbH, F. Hoffmann-La Roche Ltd, Merck KGaA and SANOFI), the Innovative Medicines Initiative-Joint Undertaking (‘DDMoRe’), Diurnal Ltd and the Federal Ministry of Education and Research within the Joint Programming Initiative on Antimicrobial Resistance Initiative (JPIAMR). The other authors declare that they have no competing interests.
Funding
This trial was funded by the Federal Ministry of Education and Research, Germany (Integrated Research and Treatment Center IFB “Adiposity Diseases'', FKZ: 01E01001), and by departmental funding. The governmental funding included a peer-review process in the proposal phase with an international review board of the Federal Ministry of Education and Research. This funding contains financial support for study personal, planning, implementation of the study, fees for materials, analysis, approvals by ethics committee and federal institute, and professional study management including statistical analysis from the Clinical Trials Center of the University of Leipzig, Germany.
Authors contributions
PS, DP, CD, LE, CK, CP, AD, MZ, FK and HW all fulfilled the International Committee of Medical Journal Editors (ICMJE) guidelines, to qualify as an author by making substantial contributions to conception and design of the study, were involved in the drafting and/or critical revision of the manuscript, gave approval to the final submitted version and agree to be accountable for all respective aspects of the work.
Author's information
Not applicable.
Trial status
This is an ongoing trial. Patient recruitment is completed and pharmacological analysis is still ongoing after which data analysis including pharmacokinetic modelling and simulation will be performed.
Acknowledgements
We thank the team of the Clinical Trial Center of the University of Leipzig for organizational support, study promotion, and on-site monitoring.
Footnotes
This study is being conducted at the Department of Anesthesiology and Intensive Care Medicine, University of Leipzig, Germany.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2019.100375
Contributor Information
P. Simon, Email: philipp.simon@medizin.uni-leipzig.de.
D. Petroff, Email: david.petroff@zks.uni-leipzig.de.
C. Dorn, Email: christoph.dorn@ur.de.
L. Ehmann, Email: lisa.ehmann@fu-berlin.de.
C. Kloft, Email: charlotte.kloft@fu-berlin.de.
C. Prettin, Email: christiane.prettin@zks.uni-leipzig.de.
A. Dietrich, Email: arne.dietrich@medizin.uni-leipzig.de.
M. Zeitlinger, Email: markus.zeitlinger@meduniwien.ac.at.
F. Kees, Email: frieder.kees@web.de.
H. Wrigge, Email: hermann.wrigge@bergmannstrost.de.
List of Abbreviations
- AUC
area under the curve
- BfArM
Federal Institute for Drug and Medical Devices of Germany
- BMI
body mass index
- C
concentration
- Crf
case report form
- Da
Dalton
- EudraCT
European Union clinical trials register
- ISF
interstitial fluid
- MIC
minimal inhibitory concentration
- NRS
numerical rating scale
- PD
pharmacodynamic(s)
- PK
pharmacokinetic(s)
- RD
retrodialysis
- T
time
- TIVA
total intravenous anesthesia
- WHO
World Health Organisation
- μD
microdialysate concentrations
Appendix A. Supplementary data
The following is/are the supplementary data related to this article:
References
- 1.Centers for Disease Control and Prevention National Health and Nutrition Examination Survey 2007-2008. http://www.cdc.gov/nchs/nhanes.htm 2009
- 2.Dindo D., Muller M.K., Weber M., Clavien P.A. Obesity in general elective surgery. Lancet. 2003;361:2032–2035. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 3.Toma O., Suntrup P., Stefanescu A., London A., Mutch M., Kharasch E. Pharmacokinetics and tissue penetration of cefoxitin in obesity: implications for risk of surgical site infection. Anesth. Analg. 2011 Oct;113(4):730–737. doi: 10.1213/ANE.0b013e31821fff74. Epub 2011 Jun 3. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A., Schmidt S., Rout W.R., Ben-David K., Burkhardt O., Derendorf H. Soft tissue penetration of cefuroxime determined by clinical microdialysis in morbidly obese patients undergoing abdominal surgery. Int. J. Antimicrob. Agents. 2009;34:231–235. doi: 10.1016/j.ijantimicag.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Hollenstein U.M., Brunner M., Schmid R., Muller M. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. Int. J. Obes. Relat. Metab. Disord. 2001;25:354–358. doi: 10.1038/sj.ijo.0801555. [DOI] [PubMed] [Google Scholar]
- 6.Crommelin Daan J.A., Lipper Robert A. Springer; New York: 2013. Microdialysis in Drug Development. [Google Scholar]
- 7.Plock N., Kloft C. Microdialysis – theoretical background and recent implementation in applied life-sciences. Eur. J. Pharm. Sci. 2005;25:1–24. doi: 10.1016/j.ejps.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Barbour A., Schmidt S., Rout W.R., Ben-David K., Burkhardt O., Derendorf H. Soft tissue penetration of cefuroxime determined by clinical microdialysis in morbidly obese patients undergoing abdominal surgery. Int. J. Antimicrob. Agents. 2009;34:231–235. doi: 10.1016/j.ijantimicag.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Bouw M.R., Hammarlund-Udenaes M. Methodological aspects of the use of a calibrator in in vivo microdialysis–further development of the retrodialysis method. Pharm. Res. (N. Y.) 1998;15:1673–1679. doi: 10.1023/a:1011992125204. [DOI] [PubMed] [Google Scholar]
- 10.Karjagin J., Lefeuvre S., Oselin K., Kipper K., Marchand S., Tikkerberi A., Starkopf J., Couet W., Sawchuk R.J. Pharmacokinetics of meropenem determined by microdialysis in the peritoneal fluid of patients with severe peritonitis associated with septic shock. Clin. Pharmacol. Ther. 2008;83:452–459. doi: 10.1038/sj.clpt.6100312. [DOI] [PubMed] [Google Scholar]
- 11.Burau D., Simon P., Petroff D., Ehmann L., Weiser C., Dorn C., Kratzer A., Wrigge H., Kloft C. Drug combinations and impact of experimental conditions on relative recovery in in vitro microdialysis investigations. Eur. J. Pharm. Sci. 2019;127:252–260. doi: 10.1016/j.ejps.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Minichmayr I.K., Schaeftlein A., Kuti J.L., Zeitlinger M., Kloft C. Clinical determinants of target non-attainment of linezolid in plasma and interstitial Space fluid: a pooled population pharmacokinetic analysis with focus on critically ill patients. Clin. Pharmacokinet. 2017;56:617–633. doi: 10.1007/s40262-016-0463-7. [DOI] [PubMed] [Google Scholar]
- 13.Schaeftlein A., Minichmayr I.K., Kloft C. Population pharmacokinteics meets microdialysis: benefits, pitfits, pitfalls and necessities of new analysis approaches for human microdialysis data. Eur. J. Pharm. Sci. 2014;57:68–73. doi: 10.1016/j.ejps.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Buerger C., Plock N., Dehghanyar P., Joukhadar C., Kloft C. Pharmacokinteics of unbound linezolid in plasma and tissue interstitium of critical ill patients after multiple dosing using microdialysis. Antimicrob. Agents Chemother. 2006;50(7):2455–2463. doi: 10.1128/AAC.01468-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the present study may be provided from the corresponding author upon request.