Abstract
Mild cognitive impairment (MCI) is a common neurological disorder. This study aims to investigate the modulation effect of Baduanjin (a popular mind-body exercise) on MCI. 69 patients were randomized to Baduanjin, brisk walking, or an education control group for 24 weeks. The Montreal Cognitive Assessment (MoCA) and Magnetic Resonance Imaging scans were applied at baseline and at the end of the experiment. Compared to the brisk walking and control groups, the Baduanjin group experienced significantly increased MoCA scores. Amplitude of low-frequency fluctuations (ALFF) analysis showed significantly decreased ALFF values in the right hippocampus (classic low-freqency band, 0.01‐0.08 Hz) in the Baduanjin group compared to the brisk walking group and increased ALFF values in the bilateral anterior cingulate cortex (ACC, slow-5 band, 0.01-0.027 Hz) in the Baduanjin group compared to the control group. Further, ALFF value changes in the right hippocampus and bilateral ACC were significantly associated with corresponding MoCA score changes across all groups. We also found increased gray matter volume in the Baduanjin group in the right hippocampus compared to the brisk walking group and in the bilateral ACC compared to the control group. In addition, there was an increased resting state functional connectivity between the hippocampus and right angular gyrus in the Baduanjin group compared to the control group. Our results demonstrate the potential of Baduanjin for the treatment of MCI.
Keywords: Mind-body intervention, Baduanjin, Resting state functional connectivity, Voxel-based morphometry, Mild cognitive impairment, Hippocampus, Anterior cingulate cortex
Abbreviations: MCI, Mild Cognitive Impairment; MoCA, Montreal Cognitive Assessment; ALFF, low-frequency fluctuations; rsFC, resting state functional connectivity; AD, Alzheimer's disease; fMRI, functional Magnetic Resonance Imaging; ACC, anterior cingulate cortex; mPFC, medial prefrontal gyrus; DLPFC, dorsal lateral prefrontal cortex
Highlights
-
•
Six months of Baduanjin training can improve cognitive function in patients with MCI.
-
•
Baduanjin can modulate the ALFF value and GMV of the hippocampus and ACC.
-
•
Baduanjin can increased rsFC between hippocampus and angular gyrus.
-
•
The ALFF values change of the hippocampus are associated with corresponding MoCA score changes across all groups.
1. Introduction
Mild cognitive impairment (MCI), a prevalent and burdensome disease in elderly adults, is believed to be the first cognitive expression of Alzheimer's disease (AD). A study that followed individuals with MCI older than 65 for 2 years found a cumulative dementia incidence of 14.9%. Nevertheless, pharmacologic treatment for MCI is far from satisfactory (Petersen et al., 2018), highlighting the urgent need for the development of effective non-pharmacologic treatments.
Previous studies have suggested that mind-body exercise (movement-based mind-body intervention) that combines physical exercise postures with breathing and deep relaxation (Wells et al., 2019) can significantly improve cognitive function in individuals with MCI (Shuo et al., 2018; Sungkarat et al., 2017, Sungkarat et al., 2018). As a popular mind-body exercise, Baduanjin is unique and much simpler to learn than other mind-body exercises such as Tai Chi (Liao et al., 2015), making it an optimal choice for older individuals with cognitive decline (Ho et al., 2017). Previous studies have suggested that compared to jogging, Baduanjin can prevent normal age-related memory decline, delaying the effects of aging (Fiatarone et al., 1994). Literature suggests that Baduanjin can also improve executive function in both young and older healthy populations, improve memory function in older adults, and modulate brain function and structure associated with cognition (Liu et al., 2019; Tao et al., 2016, Tao et al., 2017a, Tao et al., 2017b, Tao et al., 2017c). These findings further endorse the potential of Baduanjin in treating MCI. However, no randomized controlled clinical trial has been conducted to compare the treatment effects of Baduanjin and its underlying mechanism.
Recently, resting state amplitude of low-frequency fluctuations (ALFF), an index of low-frequency oscillations, has gained increased attention (Han et al., 2011; Liang et al., 2014; Tomasi et al., 2016). Although still under investigation, studies have linked ALFF with cerebral blood flow (Zou et al., 2009) and task-evoked activation (Yuan et al., 2013) in human subjects. Unlike distant connectivity methods that explore the association between different brain regions / networks, an advantage of ALFF is that it can help target key regions involved in mind-body interventions (Tao et al., 2017b; Wei et al., 2017). In addition, ALFF has demonstrated good test-retest reliability and replicability among commonly used resting state-fMRI metrics (Chen et al., 2018).
The low frequency fluctuations between 0.01 and 0.08 Hz are of particular relevance for resting state fMRI (rs-fMRI) (Biswal et al., 1995). This low frequency range has been further divided into several distinct bands (Buzsáki and Draguhn, 2004), such as slow-5 (0.01–0.027 Hz) and slow-4 (0.027–0.073 Hz). Studies suggest that spontaneous fluctuations at slow-5 (0.01–0.027 Hz) and slow-4 (0.027–0.073 Hz) may be linked with different neural processes and cognitive functions (Birn, 2007; Buzsáki and Draguhn, 2004; Knyazev, 2007; Petersen, 2004; Veldsman et al., 2017) and are altered in patients with MCI (Han et al., 2011).
In the present study, we investigated the treatment effects of 24 weeks of Baduanjin, brisk walking (a physical exercise of low to high intensity that depends primarily on the aerobic energy-generating process), and a non-exercise, health education control in patients with MCI. We also examined the modulation effect of Baduanjin on ALFF values in the classic low frequency bands (0.01–0.08 Hz) as well as in the slow-4 and slow-5 bands. Finally, we investigated morphometry and resting state functional connectivity of key regions showing ALFF changes after Baduanjin.
2. Material and methods
2.1. Patients
This brain-imaging study was nested in a randomized controlled trial with three parallel groups (Baduanjin, brisk walking, and a non-exercise health education control). Of all subjects enrolled in the clinical trial, approximately half of the subjects in each group were randomly selected to undergo MRI/fMRI brain scans. This manuscript focuses on the participants with MRI/fMRI data. Ethics approval was obtained from the Medical Ethics Committee of the Second People's Hospital of Fujian Province. We also registered this study in the Chinese Clinical Trial Registry (ChiCTR) (ChiCTR-ICR-15005795). All subjects provided informed consent before the initiation of study procedures. Details of the study protocol can be found in our published manuscript (Zheng et al., 2016).
MCI patients aged 60 years or older from Fuzhou City that had not participated in regular physical exercise for at least half a year (exercise with a frequency of at least twice a week and 20 min per session) were enrolled. All MCI diagnoses were determined by study physicians based on the Petersen diagnostic criteria (Petersen, 2004). Inclusion criteria included: memory problems with Montreal Cognitive Assessment (MoCA) score < 26 (if years of education ≤ 12, one point was added to the patient's score), cognitive decline in accordance with age and education, intact activities of daily living (ADL) (Lawton–Brody ADL score < 18), and absence of dementia (Global Deterioration Scale score at 2 or 3).
The exclusion criteria were: resistant hypertension, severe visual or hearing loss, evidence of severe psychiatric conditions (such as active suicidal ideation, schizophrenia), Geriatric Depression Scale (GDS) score ≥ 10, current severe medical conditions for which exercise is contraindicated, history of alcohol or drug abuse, and participation in other clinical studies. Individuals taking any medications that affect cognitive function were excluded from the study.
2.2. Intervention
2.2.1. Baduanjin
Participants in the Baduanjin group received 24 weeks of Baduanjin training (based on the Health Qigong Baduanjin Standard; Health Qigong Management Center of General Administration of Sport of China, 2003) with a frequency of 3 days/week, 60 min/day. The Baduanjin exercise consisted of 10 postures: (1) preparation posture, (2) prop up the sky with two hands to regulate tri-jiao, (3) draw a bow on both sides like shooting a vulture, (4) raise a single arm up to regulate spleen and stomach, (5) look back to treat five strains and seven impairments, (6) shake head and buttocks to expel heart (xin)-fire, (7) pull toes with both hands to reinforce the kidney (shen) and waist, (8) clench one's fist and glare to increase strength, (9) rise and fall on tiptoe seven times to treat all disease, and (10) ending posture. Each session included a 15-min warm-up, 40-min Baduanjin training, and 5-min cool down. Two professional coaches with over 5 years of Baduanjin teaching experience at the Fujian University of Traditional Chinese Medicine were employed to guide participants' training. A research staff member also participated in all training sessions to record the attendance of each subject. In addition, subjects received health education as described below.
2.2.2. Brisk walking
Participants in the brisk walking group received 24 weeks of brisk walking training at a community center with a frequency of 3 days a week for 60- min a day (15-min warm up, 40- min of brisk walking, and 5-min cool down). Professional coaches were employed to guide participants' training. The intensity of exercise was controlled by monitoring participants' heart rates at 55–75% of their heart rate reserve through the Polar Heart Rate Monitor. Similar to the Baduanjin group, a research staff member also participated in all training sessions to record the attendance of each subject. Subjects also received health education as described below.
2.2.3. Non-exercise health education control
Subjects in the non-exercise control group were required to maintain their original physical activity levels and did not receive any specific exercise interventions. In addition, similar to the other two groups, health education was provided every 8 weeks for 30 min per session. In these sessions, participants were educated on ways to prevent the development of MCI.
2.3. Cognitive function measurement
We used the Chinese Beijing version of the MoCA scale, a cognitive screening instrument created and validated to detect MCI, as our primary outcome measure. MoCA is a brief (about 10-min) test that evaluates visuospatial/executive functions, naming, verbal memory registration and learning, attention, abstraction, 5-min delayed verbal memory, and orientation with a total score of 0–30 (a higher score equates to better function) (Yu et al., 2012; Zheng et al., 2016). The MoCA was measured at baseline and within 1 week after the 24-week intervention.
2.4. Structural and functional MRI data acquisition
We applied two identical fMRI scanning sessions for each participant, one at baseline and another after the end of the 24-week intervention. The fMRI data were acquired on a 3.0-T GE scanner (General Electric, Milwaukee, WI, USA) with an eight-channel phased-array head coil. Subjects were asked to stay awake and remain motionless during the scan with their eyes closed and ears plugged. The scanning parameters were as follows: resting state functional MRI, TR = 2100 ms, TE = 30 ms, flip angle = 90°, voxel size = 3.125 mm × 3.125 mm × 3.6 mm, 42 axial slices, field of view (FOV) = 200 mm × 200 mm, phases = 160; T1-weighted images: voxel size 1 × 1 × 1 mm3, 240 mm FOV, and 164 slices in acquisition.
2.5. Behavioral data analysis and statistics
Behavioral data analysis was performed using IBM SPSS Statistics software (IBM Inc., Armonk, NY, USA). One-way ANOVA and chi-square tests were applied to compare the baseline characteristics of subjects. Analysis of covariance (ANCOVA) was applied to compare changes (post-treatment minus pre-treatment) in MoCA scores across all groups with age (years), gender, education, and baseline MoCA scores included as covariates (Dimitrov and Rumrill, 2003).
2.6. ALFF value calculation
Resting-state fMRI data preprocessing and ALFF value calculation were performed using Data Processing & Analysis of Brain Imaging (DPABI) (http://rfmri.org/dpabi) (Yan et al., 2016) in MATLAB (Mathworks Inc., Natick, MA, USA) based on Statistical Parametric Mapping (SPM 12). The data preprocessing was similar to our previous study (Tao et al., 2017b). The first 5 volumes of functional data for each subject were discarded for signal equilibrium and subject adaptation to the imaging noise. The remaining volumes were slice-timing corrected, within-subject spatially realigned, co-registered to the respective structural images for each subject, and then segmented. Subjects were excluded if head movement was >3 standard deviations from the mean image intensity. To perform subject-level head motion correction, the Friston 24-parameter model (Friston et al., 1996; Yan et al., 2013) was used. Images were normalized using structural image unified segmentation and then re-sampled to 3-mm cubic voxels. After smoothing with an 8 mm full width at half maximum (FWHM) kernel, the linear and quadric trends of the time courses were removed. Similar to previous studies, no temporal filtering was implemented during preprocessing (Han et al., 2011).
In this study, we applied three frequency bands: classic low-frequency (0.01–0.08 Hz), slow-4 (0.027–0.073 Hz), and slow-5 (0.01–0.027 Hz). Since the classic low frequency band is widely applied in resting state functional connectivity analysis, we defined the classic low frequency band as the primary frequency band and slow-4 and slow-5 bands as exploratory frequency bands in data analysis.
To explore the differences among the three groups after treatment, we used a flexible factorial module in SPM12 with two factors for group analysis. The first factor (group) had three levels (Baduanjin, brisk walking, and non-exercise), and the second factor (time points) had two levels (pre- and post-treatment). Age (years), gender, education, and baseline MoCA scores were also included in the analysis as covariates of non-interest. A threshold of voxel-wise p < .005 uncorrected with 20 continuous voxels was used for the main effect of group analysis, and voxel-wise p < .005 uncorrected with cluster-level p < .05 false discovery rate (FDR) corrected was applied for the post-hoc analysis.
To explore the association between the ALFF value changes observed above and cognitive function changes, we extracted the ALFF values of some key regions (brain regions involved in cognition and memory) that showed significant differences after Baduanjin exercise as compared with other groups and subsequently performed a multiple regression analysis, including age (years), gender, education, group, and baseline MoCA scores as covariates across all groups.
2.7. Region-of-interest Voxel-Based Morphometry (VBM) analysis
We found significantly decreased ALFF values in the right hippocampus in the Baduanjin group compared to the brisk walking groups in our primary outcome band (classic low frequency band). Further, we found significantly increased ALFF values in the bilateral ACC in the slow-4 band in the Baduanjin group compared to the control group, and the ALFF value changes in the right hippocampus and bilateral ACC were significantly associated with corresponding MoCA score-changes (see Results for more details). To investigate whether there were structural changes in the two brain regions (right hippocampus and bilateral ACC), similar to the methods of Andrew et al. (Andrew et al., 2018), we conducted a region-of-interest voxel-based morphometry (VBM) analysis.
The MRI data preprocessing in this study was identical to that of our previous study (Tao et al., 2017c) Voxel-based morphometry (VBM) analysis was performed with SPM12. After T1 images of all participants were segmented into gray matter, white matter, and cerebrospinal fluid, a group-specific template was created. The images were then normalized using the high dimensional DARTEL algorithm into the standard Montreal Neurological Institute (MNI) space. Spatial smoothing was conducted with an 8 mm FWHM kernel. To investigate gray matter volume (GMV) differences among the three groups, a flexible factorial design module with two factors (i.e., group with three levels and time with two levels) was applied. Age (years), gender, education, and baseline MoCA score were also included in the model as covariates. An absolute threshold of 0.1 was used for masking. Total intracranial volume was obtained by summing up the overall volumes of gray matter, white matter, and cerebrospinal fluid. A threshold of voxel-wise p < .005 uncorrected with 20 continuous voxels was used for the main effect of group analysis, and voxel-wise p < .005 uncorrected with cluster-level p < .05 false discovery rate (FDR) corrected was applied for the post-hoc analysis. The two ROIs of right hippocampus and bilateral ACC were defined from the ALFF results. We extracted participant-specific raw GMV from the smoothed gray matter images for all voxels within each ROI and performed ANCOVA analysis to compare group changes (post-treatment minus pre-treatment) across all groups with age (years), gender, education, baseline ROI volume, and baseline MoCA scores as covariates.
2.8. Seed-to-voxel correlational analyses
We found a significant association between the ALFF values and corresponding MoCA scores in the right hippocampus in the 0.01–0.08 Hz band and in the bilateral ACC in the 0.01–0.027 Hz band. We thus used the peak MNI coordinates of the right hippocampus (24,−12,-21), which had significantly decreased values in the Baduanjin group compared to the brisk walking group, and the peak MNI coordinates of the bilateral ACC (3,12,36), which had significantly increased ALFF values in the Baduanjin group compared to the control group, as seeds with 4 mm diameter for seed-to-whole brain voxel correlational analysis to investigate the effects of Baduanjin exercise on long-range functional connectivity.
Seed-to-voxel correlational analyses were calculated by applying the functional connectivity (CONN) toolbox v17.C (http://www.nitrc.org/projects/conn) in MATLAB (Mathworks, Inc., Natick, MA, USA), similar to our previous studies (Egorova et al., 2015; Liu et al., 2016, Liu et al., 2019; Song et al., 2017; Tao et al., 2017a). The preprocessing included slice-timing, realignment, coregistration to subjects' respective structural images, normalization, and smoothing with an 8-mm FWHM kernel. We employed segmentation of gray matter, white matter, and cerebrospinal fluid (CSF) areas for the removal of temporal confounding factors (Whitfield and Nieto, 2012). Band-pass filtering was performed with a frequency window of 0.01–0.089 Hz. ART was used to identify outlier time points in the motion parameters and global signal intensity. We treated images as outliers if composite movement from a preceding image exceeded 0.5 mm, or if the global mean intensity was >3 SDs from the mean image intensity for the entire resting scan. Outliers were included as regressors in the first-level general linear model along with motion parameters.
First-level correlation maps were produced by extracting the residual BOLD time course from each seed and by computing Pearson's correlation coefficients between that time course and the time courses of all other voxels in the brain. Correlation coefficients were Fisher transformed into “Z” scores to increase normality and improve second-level General Linear Model analyses. Whole-brain group analysis was applied using ANCOVA to compare the right hippocampus/bilateral ACC functional connectivity changes between different groups adjusted for age, gender, education, and baseline MoCA score. A threshold of voxel-wise p < .005 uncorrected with 20 continuous voxels was used for the main effect of group analysis, and voxel-wise p < .005 uncorrected with cluster-level p < .05 false discovery rate (FDR) corrected was applied for the post-hoc analysis.
Region-of-interest functional connectivity analysis: In our previous study (Tao et al., 2016), we found significantly increased rsFC between the left/right/bilateral hippocampus and medial prefrontal cortex (mPFC) in Tai Chi Chuan (another popular mind-body exercise) and Baduanjin groups (albeit at a lower threshold) compared to a health education control group after 12 weeks of training in older adults. Using the mPFC as a seed, we also found that compared with a health education control, 12-week Baduanjin training can significantly alter rsFC with other brain regions related to cognitive function, such as the ACC and supramarginal gyrus in older adults (Liu et al., 2019). In this study, we defined the mPFC as an a priori region of interest (ROI) using Automated Anatomical Labeling (AAL) (bilateral superior and orbital medial frontal) based on a threshold of p < .005 to investigate whether the rsFC between the hippocampus and mPFC can be modulated after Baduanjin intervention in patients with MCI. To correct for multiple comparisons, Monte Carlo simulations using 3dFWHMx and 3dClustSim (AFNI (https://afni.nimh.nih.gov)) were applied for the ROI.
3. Results
Sixty-nine MCI patients completed the baseline behavioral test and fMRI scan (Baduanjin group n = 23, brisk walking group n = 23, and non-exercise group n = 23). 57 subjects finished the whole study and were included in data analysis (Baduanjin group n = 20, brisk walking group n = 17, and non-exercise group n = 20). Three subjects were dropped from the Baduanjin group (one subject withdrew voluntarily and two were unwilling to participate in the second MRI scan). Six subjects were dropped from the brisk walking group (one withdrew voluntarily, one was unwilling to participate in the second MoCA test, one was lost to follow-up, and three had poor-quality MRI data). Three subjects were dropped from the control group (one was lost to follow-up and two were unwilling to participate in the second MoCA test). No subject reported taking additional pharmacological treatment during the training.
3.1. Behavioral results
Baseline characteristics and MoCA scores of participants are listed in Table 1. There were no significant differences in age, gender, education, GDS scores, or MoCA scores among the three groups at baseline. The attendance rate (mean ± SD) for the Baduanjin and brisk walking groups were 0.842 ± 0.100 and 0.838 ± 0.116, respectively. There were no significant differences in attendance during training between the two exercise groups (p = .927).
Table 1.
Demographics of study participants and clinical outcome results.
| Characteristics | Baduanjin (n = 20) | Walking (n = 17) | Control (n = 20) | F or chi-square value | p value |
|---|---|---|---|---|---|
| Agea (mean (SD)) | 66.17(4.17) | 64.32(2.60) | 65.97(5.66) | 0.945 | 0.395 |
| Genderb (male/female) | 5/15 | 7/10 | 6/14 | 1.148 | 0.563 |
| Educationb (1/2/3/4) | 3/6/6/5 | 2/5/9/1 | 3/4/8/5 | 4.016 | 0.675 |
| GDSa (mean (SD)) | 6.05(2.84) | 4.18(2.65) | 5.50(2.76) | 2.204 | 0.120 |
| Pre-MoCAa (mean (SD)) | 22.45(2.16) | 21.47(2.27) | 21.00(2.36) | 2.035 | 0.141 |
| MoCA changec (mean (SD)) | 2.10(2.25) | 0.88(1.96) | 1.10(1.48) | 4.311 | 0.019 |
p values were calculated with one-way analysis of variance.
p values were calculated with a chi-square test.
p values were calculated with ANCOVA. Education 1: primary school; 2: middle school; 3: high school; 4: college and above. Baduanjin: Baduanjin group; Walking: brisk walking group; Control: non-exercise group; MoCA change: post-treatment MoCA score minus pre-treatment MoCA score.
ANCOVA showed a significant difference in MoCA score changes (post-treatment minus pre-treatment) among the three groups (F2,50 = 4.311, p = .019). Post-hoc analysis showed the Baduanjin group had a significant increase in MoCA scores compared to the non-exercise group (p = .050) and the brisk walking group (p = .037), and there was no significant difference between the brisk walking group and the non-exercise group after SIDAK correction (Table 1).
3.2. Resting-state fMRI data analysis results (ALFF value)
3.2.1. ALFF value in the classic low-frequency band (0.01–0.08 Hz)
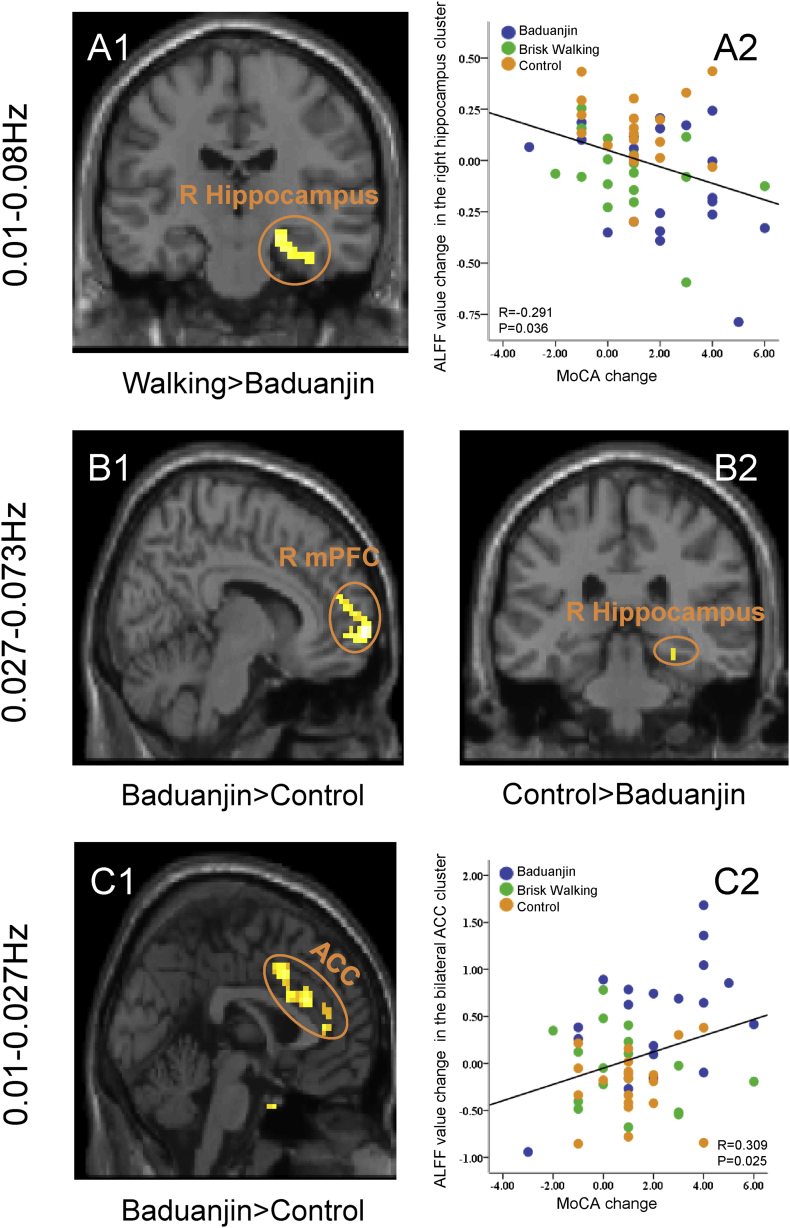
We found a significant main effect of group in the left medial prefrontal cortex (mPFC), right hippocampus, and left dorsal lateral prefrontal cortex (DLPFC). Post-hoc analysis revealed that the Baduanjin group had significantly decreased ALFF values in the right hippocampus compared to the brisk walking group and increased ALFF values in the left mPFC compared to the brisk walking group. No other significant group difference was found above the threshold we set (Fig. 1 A1, Table 2, and Supplementary Table 1).
Fig. 1.
Brain regions with significant ALFF value change in different bands and scatter plots showing the association between ALFF values of the significant cluster and corresponding MoCA scores. (A) ALFF value changes in the classic-low frequency band (0.01–0.08 Hz). (A1) Compared to the brisk walking group, Baduanjin significantly decreased ALFF value in the right hippocampus. (A2) Scatter plots showing the association between ALFF value change of the right hippocampus and corresponding MoCA score changes adjusted for age, gender, education, groupID, and baseline MoCA score. (B) ALFF value changes at 0.027–0.073 Hz. (B1) Baduanjin significantly increased ALFF value in the right mPFC compared to the control group; (B2) Baduanjin significantly decreased ALFF value in the right hippocampus compared to the control group. (C) ALFF value changes at 0.01–0.027 Hz. (C1) Compared to the control group, Baduanjin significantly increased ALFF value in the bilateral anterior cingulate cortex. (C2) Scatter plots showing the association between ALFF value change of the anterior cingulate cortex and corresponding MoCA score changes adjusted for age, gender, education, groupID, and baseline MoCA score.
Table 2.
Comparison of ALFF values at different bands among experimental groups.
| Band | Contrast | Brain region | Cluster | T value | Z value | MNI coordinates |
||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| 0.01–0.08 Hz (classic low-frequency band) | Baduanjin > Control | No brain region above the threshold | ||||||
| Control > Baduanjin | No brain region above the threshold | |||||||
| Baduanjin > Walking | Left mPFC | 166 | 4.26 | 3.94 | -12 | 45 | 30 | |
| Walking > Baduanjin | Right hippocampus | 127 | 3.86 | 3.61 | 24 | -12 | −21 | |
| Walking > Control | No brain region above the threshold | |||||||
| Control > Walking | No brain region above the threshold | |||||||
| 0.027–0.073 Hz (Slow-4 band) | Baduanjin > Control | Right mPFC | 76 | 4.49 | 4.12 | 9 | 66 | 0 |
| Left DLPFC | 80 | 3.95 | 3.68 | −18 | 33 | 48 | ||
| Control > Baduanjin | Right lingual gyrus/hippocampus | 111 | 4.87 | 4.42 | 21 | −45 | −9 | |
| Left lingual gyrus | 65 | 5.8 | 5.09 | −21 | −48 | −9 | ||
| Right superior temporal gyrus | 64 | 3.75 | 3.52 | 54 | −30 | 9 | ||
| Baduanjin > Walking | Left DLFPC | 80 | 3.86 | 3.61 | −21 | 54 | 9 | |
| Walking > Baduanjin | No brain region above the threshold | |||||||
| Walking > Control | No brain region above the threshold | |||||||
| Control > Walking | No brain region above the threshold | |||||||
| 0.01–0.027 Hz (Slow-5 band) | Baduanjin > Control | Bilateral ACC | 172 | 4.94 | 4.46 | 3 | 12 | 36 |
| Control >Baduanjin | No brain region above the threshold | |||||||
| Baduanjin > Walking | Left DLFPC | 182 | 4.52 | 4.14 | −36 | 45 | 27 | |
| Walking > Baduanjin | No brain region above the threshold | |||||||
| Walking > Control | No brain region above the threshold | |||||||
| Control > walking | No brain region above the threshold | |||||||
mPFC: medial prefrontal cortex; ACC: anterior cingulate cortex; DLPFC: dorsal lateral prefrontal cortex; Baduanjin: Baduanjin group; Walking: Brisk walking group; Control: non-exercise group.
Partial correlation analysis revealed a significant negative association between the ALFF value change in the right hippocampus and the corresponding MoCA score changes across all groups adjusted for age, gender, education, groupID, and baseline MoCA score (r = −0.291, p = .036; Fig. 1 A2).
3.2.2. ALFF value in the slow-4 band (0.027–0.073 Hz)
We found a significant main effect of group in the bilateral lingual gyrus, left cerebellum, right angular gyrus, right mPFC, and left DLFPC. Post-hoc analyses revealed that the Baduanjin group had significantly increased ALFF values in the right mPFC and left DLPFC and decreased ALFF values in the right lingual gyrus/hippocampus, left lingual gyrus, and right superior temporal gyrus compared to the control group and increased ALFF values in the left DLPFC compared to the walking group. No other significant group difference was observed above the threshold we set (Fig. 1 B1 and B2, Table 2, and Supplementary Table 1).
No significant association was detected between the ALFF values of the hippocampus and corresponding MoCA score changes across all subjects adjusted for age, gender, education, groupID, and baseline MoCA score.
3.2.3. ALFF Value in the Slow-5 Band (0.01–0.027 Hz)
We found a significant main effect of group in the bilateral ACC, bilateral DLPFC, and right insula. Post-hoc analysis showed that the ALFF values in the Baduanjin group were significantly increased in the bilateral ACC compared to the non-exercise group and in the left DLFPC compared to the brisk walking group. No other significant group difference was observed above the threshold we set (Fig. 1 C1, Table 2, and Supplementary Table 1).
Regression analysis revealed a significant positive association between ALFF value change in the cluster of the bilateral ACC and corresponding MoCA score changes adjusted for age, gender, education, groupID, and baseline MoCA score across all subjects (r = 0.309, p = .025; Fig. 1 C2).
3.3. Region-of-interest voxel-based morphometry (VBM) analysis results
For whole-brain VBM analysis, we found a significant main effect of group in the left middle temporal gyrus, right middle orbital gyrus, left superior parietal gyrus, bilateral DLPFC, right supramarginal gyrus, left cerebellum, bilateral orbital prefrontal gyrus, right precentral gyrus, right inferior temporal gyrus, and right precuneus (Supplementary Table 2). For post-hoc analysis, we did not find any significant group difference above the threshold we set among the three groups.
For region-of-interest VBM analysis, we found significantly GMV changes in the right hippocampus among the three groups (p = .018, F2,50 = 4.31). Post-hoc analysis showed significantly increased GMV in the Baduanjin group compared to the brisk walking group (p = .015) after intervention, and no other significant group difference was found after SIDAK correction [GMV change (post-treatment minus pre-treatment), mean (SD), in Baduanjin group: 0.009(0.005); brisk walking group: −0.013(0.05); control group: −0.002(0.005)]. There was also significant GMV changes in the bilateral ACC among the three groups (p = .002, F2,50 = 7.32). Post-hoc analysis showed significantly increased GMV in the Baduanjin group compared to the control group (p = .001), and no other significant group difference was found after SIDAK correction in the bilateral ACC [GMV change, mean (SD), in Baduanjin group: 0.229(0.005); brisk walking group: 0.214(0.06); control group: 0.201(0.005)].
In addition, we explored the association between the VBM results and clinical outcomes and found no significant association between baseline hippocampal/ACC volume and corresponding MoCA scores and between the percentage changes of hippocampal/ACC volume and corresponding MoCA scores (association between hippocampal volume and corresponding MoCA score at baseline, p = .13; association between the percentage changes of hippocampal volume and corresponding MoCA score, p = .85; association between ACC volume and corresponding MoCA score at baseline, p = .51; association between the percentage changes of ACC volume and corresponding MoCA score, p = .29). Age, gender, education, and groupID were also adjusted for during the analysis.
3.4. Seed-based resting state functional connectivity results
3.4.1. Results using right hippocampus as seed
Seed-to-voxel rsFC analysis using the right hippocampus as a seed showed a significant main effect of group in the left insula, left inferior temporal gyrus, and right angular gyrus among the three groups (Supplementary Table 3). Post-hoc analysis showed increased rsFC between the hippocampus and right angular gyrus in the Baduanjin group compared to the control group, and no other significant group difference was found above the threshold we set (Table 3). We did not find significant rsFC changes between the mPFC and hippocampus among the three groups above the threshold we set. At a lower threshold (voxel-wise p < .005 uncorrected), we found significantly increased rsFC between the left mPFC and hippocampus in the Baduanjin group compared to the control group (peak MNI coordinate: -6 69 24, 6 continuous voxels).
Table 3.
Brain regions showing significant functional connectivity changes (post-treatment minus pre-treatment) with right hippocampus and bilateral ACC as seeds.
| Seed | Contrast | Brain region | Cluster | T value | Z value | MNI coordinates |
||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Hippocampus | Baduanjin > Control | Right angular gyrus | 185 | 4.59 | 4.02 | 54 | −51 | 24 |
| Control > Baduanjiin | No brain region above the threshold | |||||||
| Baduanjin > Walking | No brain region above the threshold | |||||||
| Walking > Baduanjin | No brain region above the threshold | |||||||
| Walking >Control | No brain region above the threshold | |||||||
| Control >Walking | No brain region above the threshold | |||||||
| Bilateral ACC | Baduanjin >Control | No brain region above the threshold | ||||||
| Control > Baduanjin | No brain region above the threshold | |||||||
| Baduanjin >Walking | No brain region above the threshold | |||||||
| Walking >Baduanjin | No brain region above the threshold | |||||||
| Walking > Control | No brain region above the threshold | |||||||
| Control > Walking | No brain region above the threshold | |||||||
We did not find any significant association between the significantly increased rsFC and corresponding MoCA scores adjusted for age, gender, education, groupID, and baseline MoCA score.
3.4.2. Results using bilateral ACC as seed
We found a significant main effect of group in the left central operculum and bilateral cerebellum among the three groups (Supplementary Table 3). For post-hoc analysis, we did not find any group difference above the threshold we set using the bilateral ACC as a seed.
4. Discussion
In this study, we investigated the effects of 24 weeks of Baduanjin, brisk walking, and a non-exercise control in patients with MCI. We found that, compared to brisk walking and no exercise, Baduanjin significantly increased cognitive function as measured by MoCA. Resting state functional MRI data analysis showed that Baduanjin can produce frequency-specific ALFF changes. Specifically, Baduanjin significantly decreased ALFF value in the right hippocampus compared to the brisk walking group in the classic low-frequency band and increased ALFF value in the bilateral ACC compared to the control group in the slow-5 band. Brain activity changes at the right hippocampus and bilateral ACC were associated with corresponding MoCA score changes across all groups. In addition, we found that Baduanjin produced significantly increased GMV in the right hippocampus compared to the brisk walking group and increased GMV in the bilateral ACC compared to the control group. Finally, seed-to-whole-brain voxel analysis showed significantly increased hippocampus-right angular gyrus rsFC in the Baduanjin group compared to the control group.
4.1. Baduanjin training can improve cognitive function in patients with MCI
Our finding of Baduanjin's ability to improve MoCA scores compared to other intervention groups is consistent with previous studies that demonstrated the benefits of Baduanjin on cognitive function. For instance, Chen et al. found that 8 weeks of Baduanjin was more effective than a relaxation exercise program in improving executive control in college students (Chen et al., 2017). Studies from our group also found that, compared to a health education control, 12 weeks of Baduanjin practice could significantly prevent memory decline in older adults (Liu et al., 2019; Tao et al., 2016, Tao et al., 2017a, Tao et al., 2017b, Tao et al., 2017c).
We did not find significant group differences between the brisk walking group and control group in MoCA score change. This may be related to the short duration of training, the frequency of the training, and the small sample size. An earlier meta-analysis from Colcombe and colleagues reported that aerobic fitness training enhances the cognitive vitality of healthy but sedentary older adults (Colcombe and Kramer, 2003). The modulation effect of physical training on cognition has also been recently investigated by several studies. However, the sample size of these studies are generally small, and results have been inconsistent (Baker et al., 2010; Colcombe et al., 2004; Smiley et al., 2008; Themanson et al., 2006). For instance, a study from Baker showing that aerobic exercise had sex-specific effects on cognition included only 29 adults in the primary analysis (n = 19 in the aerobic group and n = 10 in the control group)(Baker et al., 2010). Colcombe et al. found that 6-month aerobic exercise resulted in an 11% reduction in behavioral conflict while control participants demonstrated only a 2% reduction in interference (Colcombe et al., 2004). In a recent large-sample clinical trial, researchers found that compared to the usual care, 12 months of high intensity aerobic and strength exercise training resulted in no significant group differences in slowing cognitive impairment in people with mild to moderate dementia (Sarah et al., 2018). Further study is needed to confirm these results.
4.2. Baduanjin training significantly decreased ALFF value and increased gray matter volume in the hippocampus
Current literature suggests that the hippocampus plays a critical role in information storage and retrieval (Buckner et al., 2008). Studies have demonstrated that brain morphometry, functional activity, and connectivity at the hippocampus are altered in patients with MCI. For instance, Ni et al. reported that, compared to cognitively normal subjects, non-lacunar stroke patients with MCI have increased ALFF in the bilateral hippocampus (Ni et al., 2016). Hyperactivity in the hippocampal region during memory encoding was also found in task-related fMRI studies of MCI patients (Kolling et al., 2016; Mayada et al., 2017). We found that after 24 weeks of Baduanjin, hippocampal ALFF value was decreased compared to the brisk walking group, while hippocampal volume was increased. In addition, the decreased ALFF value in the right hippocampus was significantly associated with the corresponding MoCA score. This result is in line with the results of the aforementioned studies, as well as a previous study in which we found that 12 weeks of Baduanjin increased functional connectivity between the bilateral hippocampus and other brain regions (Tao et al., 2016). These results suggest that Baduanjin improves cognitive function by enhancing the intrinsic function of the hippocampus, increasing hippocampal volume, and further normalizing (decreasing) the abnormal hyperactivity of the hippocampus, as indicated by a decrease in ALFF value.
4.3. Baduanjin training increased rsFC between the hippocampus and angular gyrus
We found increased rsFC between the hippocampus and angular gyrus in the Baduanjin group compared to the control group. Previous studies have suggested that the angular gyrus plays a critical role in episodic simulation and episodic memory (Thakral et al., 2017), efficient automatic retrieval of specific semantic information (Davey et al., 2015), and the encoding and retrieval of memory (Van et al., 2017). The angular gyrus is also a key target region in the progression of MCI. Griffith et al. reported that for amnestic MCI patients, angular gyrus volume can significantly predict financial abilities by modulating arithmetic ability (Griffith et al., 2010). A study on the conversion between MCI and AD also found that, compared to non-converters, converters showed significantly decreased regional cerebral blood flow in the left angular gyrus (Hirao et al., 2005). This result supports that Baduanjin may improve cognitive function in patients with MCI by enhancing the activity of the angular gyrus and hippocampus and increasing the resting state functional connectivity between the two areas.
4.4. Baduanjin training increased ALFF value in the ACC
We also found increased ALFF value in the Baduanjin group at the ACC, which showed a positive correlation with cognitive function improvement. In addition, we found significantly increased GMV in the ACC of the Baduanjin group compared to the control group. Neuroimaging research has indicated that the ACC plays an important role in cognition and attention (Bush et al., 2000). Lesion studies have also found that the ACC is required for learning instrumental tasks (Mayada et al., 2017) and is active during decision-making (Kolling et al., 2016). Neurophysiological and task-performance studies have suggested that physical activity exerts substantial influence on the ACC (Hillman et al., 2008). These findings are consistent with our results, indicating that Baduanjin can significantly enhance cognition in MCI patients by modulating the function and structure of the ACC.
4.5. Baduanjin training increased the ALFF value in the mPFC
Baduanjin increased mPFC ALFF value compared to the brisk walking group in the classic low-frequency band and compared to the control group in the slow-4 band. A series of studies have confirmed that the mPFC plays an important role in cognitive processes such as social cognitive processing (Bos et al., 2007), cognitive control, inhibition, and memory (Alejandro et al., 2016). Previous studies have suggested that, compared to healthy elderly controls, MCI patients demonstrate deactivation of the mPFC (Rombouts et al., 2005). This deactivation can be countered by increasing the resting-state functional connectivity between the mPFC and medial temporal lobe as well as the volume of the prefrontal cortex through exercise (Colcombe et al., 2006). These results provide direct support for our findings.
4.6. Limitations
There are several limitations to this study. First, the sample size is small, and future studies with larger sample sizes are needed to validate our results. Second, we only measured cognitive function via MoCA in our present study. Further research is needed to investigate the modulation effect of Baduanjin training on different cognitive domains, such as memory function and attention- in patients with MCI. Finally, we did not include the GDS score as a covariate in data analysis because we excluded patients with depression (GDS score ≥ 10), and there were no significant group differences among the three groups at baseline.
5. Conclusions
In summary, we found that 24 weeks of Baduanjin exercise can significantly improve cognitive function and modulate regional fluctuation and gray matter volume in the hippocampus and anterior cingulate cortex. In addition, we found increased hippocampal resting state functional connectivity with the angular gyrus. Our results demonstrate the potential of Baduanjin in preventing the progression of mild cognitive impairment.
Competing interests
JK has a disclosure to report (holding equity in a startup company, MNT, and pending patents to develop a new brain stimulation device) but declares no conflict of interest. All other authors declare no competing interests.
Funding and acknowledgments
This study is supported by the National Natural Science Foundation of China (grant no. 81574045), the Collaboration Innovation Center for Rehabilitation Technology (Fujian province, China, grant no. 2015003-Collaboration), the Fujian Provincial Rehabilitation Industrial Institution of China, and the Fujian Key Laboratory of Rehabilitation Technology of China. The funders have no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank instructors Hongmei Yi and Qiu Lin from the Fujian University of Traditional Chinese Medicine for the Baduanjin training.
Author contributions
Experimental design: Guohua Zheng, Lidian Chen, and Jian Kong; data collection: Rui Xia, Moyi Li, Maomao Huang, and Shuzhen Li; data analysis: Jing Tao and Jiao Liu; manuscript preparation: Jing Tao, Jiao Liu, Xiangli Chen, Courtney Lang, Joel Park, Guanli Xie, Georgia Wilson, and Binlong Zhang.
All authors contributed to manuscript preparation and have read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101834.
Contributor Information
Guohua Zheng, Email: zhenggh@sumhs.edu.cn.
Lidian Chen, Email: 1984010@fjtcm.edu.cn.
Jian Kong, Email: kongj@nmr.mgh.harvard.edu.
Appendix A. Supplementary data
Supplementary material
References
- Alejandro V., Chang L., Banich M., Wager T., Yarkoni T. Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. J. Neurosci. 2016;36:6553–6562. doi: 10.1523/JNEUROSCI.4402-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew S., Chelsea K., Eric I., Tony L., Steven H., Richard H., Alison M., Gordon W., Daniel C., Neil B. A multi-modal MRI study of the central response to inflammation in rheumatoid arthrit. Nat. Commun. 2018;9:2243. doi: 10.1038/s41467-018-04648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L., Frank L., Foster K., Green P., Wilkinson C., McTiernan A., Plymate S., Fishel M., Watson S., Cholerton B., Duncan G., Mehta P., Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R. The behavioral significance of spontaneous fluctuations in brain activity. Neuron. 2007;56:8–9. doi: 10.1016/j.neuron.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin Z., Haughton M., Hyde S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bos W., McClure S., Harris L., Fiske S., Cohen J. Dissociating affective evaluation and social cognitive processes in the ventral medial prefrontal cortex. Cogn. Affect. Behav. Neurosci. 2007;7:337–346. doi: 10.3758/cabn.7.4.337. [DOI] [PubMed] [Google Scholar]
- Buckner R., Andrews J., Schacter D. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Chen T., Yue G., Tian X., Jiang H. Baduanjin mind-body intervention improves the executive control function. Front. Psychol. 2017;7 doi: 10.3389/fpsyg.2016.02015. 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Lu B., Yan G. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum. Brain Mapp. 2018;39:300–318. doi: 10.1002/hbm.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S., Kramer A. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe S., Kramer A., Erickson K., Scalf P., McAuley E., Cohen N., Webb A., Jerome G., Marquez D., Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S., Erickson K., Scalf P., Kim S., Prakash R., McAuley E., Elavsky S., Marquez X., Hu L., Kramer A. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. doi:61/11/1166. [DOI] [PubMed] [Google Scholar]
- Davey J., Cornelissen P., Thompson H., Sonkusare S., Hallam G., Smallwood J., Jefferies E. Automatic and controlled semantic retrieval: TMS reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. J. Neurosci. 2015;35:15230–15239. doi: 10.1523/JNEUROSCI.4705-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D., Rumrill P. Pretest-posttest designs and measurement of change. Work. 2003;20:159–165. [PubMed] [Google Scholar]
- Egorova N., Gollub R., Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. NeuroImage: Clin. 2015;9:430–435. doi: 10.1016/j.nicl.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiatarone M., O'Neill E., Ryan N., Clements K., Solares G., Nelson M., Roberts S., Kehayias J., Lipsitz L., Evans W. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- Friston K., Williams S., Howard R., Frackowiak R., Turner R. Movement-related effects in fMRI time-series. Mag. Res. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Griffith R., Stewart C., Stoeckel L., Okonkwo O., Den J., Martin R., Belue K., Copeland J., Harrell L., Brockington J., Clark D., Marson D. Magnetic resonance imaging volume of the angular gyri predicts financial skill deficits in people with amnestic mild cognitive impairment. J. Am. Geriatr. Soc. 2010;58:265–274. doi: 10.1111/j.1532-5415.2009.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Wang J., Zhao Z., Min B., Lu J., Li K., He Y., Jia J. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. NeuroImage. 2011;55:287–295. doi: 10.1016/j.neuroimage.2010.11.059. [DOI] [PubMed] [Google Scholar]
- Health Qigong Management Center of General Administration of Sport of China . People's Sports Publishing House of China; Beijing: 2003. Health Qigong-Baduanjin. [Google Scholar]
- Hillman C., Erickson K., Kramer A. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hirao K., Ohnishi T., Hirata Y., Yamashita F., Mori T., Moriguchi Y., Matsuda H., Nemoto K., Imabayashi E., Yamada M., Iwamoto T., Arima K., Asada T. The prediction of rapid conversion to Alzheimer's disease in mild cognitive impairment using regional cerebral blood flow SPECT. NeuroImage. 2005;28:1014–1021. doi: 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Ho R., Wan A., Chan J., Ng S., Chung K., Chan C. Study protocol on comparative effectiveness of mindfulness meditation and qigong on psychophysiological outcomes for patients with colorectal cancer: a randomized controlled trial. BMC Complement. Altern. Med. 2017;17 doi: 10.1186/s12906-017-1898-6. 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007;31:377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kolling N., Wittmann M., Behrens T., Boorman E., Mars R., Rushworth M. Value, search, persistence and model updating in anterior cingulate cortex. Nat. Neurosci. 2016;19:1280–1285. doi: 10.1038/nn.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Xiang J., Liang H., Qi Z., Li K. Altered amplitude of low-frequency fluctuations in early and late mild cognitive impairment and Alzheimer's disease. Curr. Alzheimer Res. 2014;11:389–398. doi: 10.2174/1567205011666140331225335. [DOI] [PubMed] [Google Scholar]
- Liao Y., Lin Y., Zhang C., Xue L., Mao X., Zhang Y., Dai G., Wang F. Intervention effect of Baduanjin exercise on the fatigue state in people with fatigue-predominant subhealth: a cohort study. J. Altern. Complement. Med. 2015;21:554–562. doi: 10.1089/acm.2014.0395. [DOI] [PubMed] [Google Scholar]
- Liu J., Fang J., Wang Z., Rong P., Hong Y., Fan Y., Wang X., Park J., Jin Y., Liu C., Zhu B., Kong J. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J. Affect. Disord. 2016;205:319–326. doi: 10.1016/j.jad.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Liu J., Tao J., Liu L., Huang J., Xue H., Li M., Yang G., Zhu F., Lang C., Park J., Tu H., Wilson G., Chen D., Kong J. Different modulation effects of tai chi Chuan and Baduanjin on resting state functional connectivity of the default mode network in older adults. Soc. Cogn. Affect. Neurosci. 2019;14:217–224. doi: 10.1093/scan/nsz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayada A., Carlier P., Sherine S., Marie M., Esclapez M., Boussaoud D. Role of anterior cingulate cortex in instrumental learning: blockade of dopamine D1 receptors suppresses overt but not covert learning. Front. Behav. Neurosci. 2017;11 doi: 10.3389/fnbeh.2017.00082. 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Liu R., Yin Z., Zhao H., Nedelska Z., Hort J., Zhou F., Wu W., Zhang X., Li M., Yu H., Zhu B., Xu Y., Zhang B. Aberrant spontaneous brain activity in patients with mild cognitive impairment and concomitant lacunar infarction: a resting-state functional MRI study. J. Alzheimers Dis. 2016;50:1243–1254. doi: 10.3233/JAD-150622. [DOI] [PubMed] [Google Scholar]
- Petersen R. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen R., Lopez O., Armstrong M., Getchius T., Ganguli M., Gloss D., Gronseth G., Marson D., Pringsheim T., Day G., Sager M., Stevens J., Rae A. Practice guideline update summary:mild cognitive impairment. Neurology. 2018;90:126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts B., Barkhof F., Goekoop R., Stam J., Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum. Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarah L., Bart S., Nicky A., Vivien N., Helen C., Dipesh M., Dosanjh S., Anne M., Ifekhar K., Stavros P., Ranjit L. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361 doi: 10.1136/bmj.k1675. k1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuo W., Huiru Y., Yong J., Lijing Z., Lisheng W., Li C. Effects of mind-body exercise on cognitive function in older adults with cognitive impairment: a systematic review and meta-analysis. J. Nerv. Ment. Dis. 2018;206:913–924. doi: 10.1097/NMD.0000000000000912. [DOI] [PubMed] [Google Scholar]
- Smiley A., Lowry K., Francois S., Kohut M., Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the “selective improvement” and “cardiovascular fitness” hypotheses. Ann. Behav. Med. 2008;36:286–291. doi: 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Cao J., Lang C., Dai M., Xuan L., Lv K., Cui Y., Jorgenson K., Xu S., Kong J. Disrupted functional connectivity of striatal sub-regions in Bell's palsy patients. NeuroImage: Clin. 2017;14:122–129. doi: 10.1016/j.nicl.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungkarat S., Boripuntakul S., Chattipakorn N., Watcharasaksilp K., Lord S. Effects of Tai Chi on cognition and fall risk in older adults with mild cognitive impairment: a randomized controlled trial. J. Am. Geriatr. Soc. 2017;65:721–727. doi: 10.1111/jgs.14594. [DOI] [PubMed] [Google Scholar]
- Sungkarat S., Boripuntakul S., Kumfu S., Lord S., Chattipakorn N. Tai chi improves cognition and plasma BDNF in older adults with mild cognitive impairment: a randomized controlled trial. Neurorehabil. Neural Repair. 2018;32:142–149. doi: 10.1177/1545968317753682. [DOI] [PubMed] [Google Scholar]
- Tao J., Liu J., Egorova N., Chen L., Sun S., Xue H., Huang J., Zheng H., Wang Q., Chen D., Kong J. Increased hippocampus-medial prefrontal cortex resting-state functional connectivity and memory function after Tai Chi Chuan practice in elder adults. Front. Aging Neurosci. 2016;8 doi: 10.3389/fnagi.2016.00025. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Chen L., Egorova N., Liu J., Xue H., Wang Q., Zheng H., Li Y., Hong J., Sun S., Chen D., Kong J. Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Sci. Rep. 2017;7 doi: 10.1038/srep41581. 41581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Chen L., Liu J., Egorova N., Xue H., Liu L., Zheng H., Li M., Wu S., Hu K., Wang J., Chen D., Kong J. Tai Chi Chuan and Baduanjin mind-body training changes resting-state low-frequency fluctuations in the frontal lobe of older adults: a resting-state fMRI study. Front. Hum. Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00514. 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Liu J., Liu L., Huang J., Xue H., Chen L., Wu S., Zheng H., Chen B., Li M., Sun S., Jorgenson K., Lang C., Hu K., Chen J., Chen D., Kong J. Tai Chi Chuan and Baduanjin increase grey matter volume in older adults: a brain imaging study. J. Alzheimers Dis. 2017;60:389–400. doi: 10.3233/JAD-170477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral P., Madore K., Schacter D. A role for the left angular gyrus in episodic simulation and memory. J. Neurosci. 2017;37:8124–8149. doi: 10.1523/JNEUROSCI.1319-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themanson J., Hillman C., Curtin J. Age and physical activity influences on action monitoring during task switching. Neurobiol. Aging. 2006;27:1335–1345. doi: 10.1016/j.neurobiolaging.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Shokri E., Volkow D. Temporal changes in local functional connectivity density reflect the temporal variability of the amplitude of low frequency fluctuations in gray matter. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154407. e0154407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van M., Berkers J., Morris M., Fernández G. Angular gyrus involvement at encoding and retrieval is associated with durable but less specific memories. J. Neurosci. 2017;37:9474–9485. doi: 10.1523/JNEUROSCI.3603-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldsman M., Egorova N., Singh B., Mungas D., DeCarli C., Brodtmann A. Low-frequency oscillations in default mode subnetworks are associated with episodic memory impairments in Alzheimer's disease. Neurobiol. Aging. 2017;59:98–106. doi: 10.1016/j.neurobiolaging.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Wei X., Zhu G., Zhi Y., Zuo N. Mind-body practice changes fractional amplitude of low frequency fluctuations in intrinsic control networks. Front. Psychol. 2017;8 doi: 10.3389/fpsyg.2017.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells E., Laura G., Brielle P. Springer; 2019. Complementary and Alternative Approaches to Chronic Daily Headache: Part I-Mind/Body. [Google Scholar]
- Whitfield S., Nieto A. Conn : a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Yan G., Cheung B., Kelly C., Colcombe S., Craddock C., Di A., Li Y., Zuo N., Castellanos X., Milham M. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G., Wang D., Zuo N., Zang F. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yu J., Li J., Huang X. The Beijing version of the Montreal cognitive assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. 2012;12 doi: 10.1186/1471-244X-12-156. 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R., Di X., Kim E., Barik S., Rypma B., Biswal B. Regional homogeneity of resting-state fMRI contributes to both neurovascular and task activation variations. Magn. Reson. Imaging. 2013;31:1492–1500. doi: 10.1016/j.mri.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Huang M., Li S., Li M., Xia R., Zhou W., Tao J., Chen L. Effect of Baduanjin exercise on cognitive function in older adults with mild cognitive impairment: study protocol for a randomised controlled trial. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010602. e010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Wu C., Stein E., Zang F., Yang H. Static and dynamic characteristics of cerebral blood flow during the resting state. NeuroImage. 2009;48:514–522. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material