Abstract
The antimicrobial resistance to Acinetobacter baumannii is significantly high and continues to grow; it has become a global health issue, particularly in regards to carbapenem resistance. The expression of efflux pumps is one of the major mechanisms of antibiotic resistance in A. baumannii by, most prevalently, adeABC of the resistance/nodulation/division family. The detection rate of adeB was the highest in clinical isolates compared to others (adeFGH, adeIJk), although it varied among other strains. In this minireview, we explain the adeABC efflux gene in A. baumannii causing antibiotic resistance and compare adeABC with other efflux genes in order to discern the function of adeABC in A. baumannii resistance, which may help in the discovery of new antibacterial agents.
Keywords: Acinetobacter baumannii, adeABC efflux gene, antibiotic resistance, efflux pumps, infection
Introduction
Acinetobacter baumannii is a common Gram-negative opportunistic pathogen. In recent decades, it has successfully evolved from an ordinary bacterium to an important pathogen of nosocomial infection, causing ventilator-associated pneumonia, bacteraemia, urinary tract infection and secondary meningitis [1]. At present, the infection of A. baumannii is widespread, especially in intensive care units. Statistical data estimated that about 45 000 (41 400–8300) cases of Acinetobacter infections occurred in the United States each year, and about 1 000 000 (600 000–1 400 000) around the world [2]. Moreover, the emergence of multidrug-resistant A. baumannii, extensively drug-resistant A. baumannii and even pandrug-resistant A. baumannii brings about great challenges to global healthcare workers. Therefore, further research is needed to investigate the resistance mechanism and related genes in order to offer more information for the development of new sensitive antibiotics.
The major mechanisms of resistance generally include producing antimicrobial-inactivating enzymes, modifying targets, reducing the membrane permeability and forming biofilm and overexpression of the membrane active efflux system [3]. Antimicrobial inactivating enzymes hydrolyze drugs and confer resistance against drugs. However, the substrates of the inactivated enzyme are often selective. For example, β-lactamases cause the inactivation of β-lactams, and aminoglycosides-modifying enzymes induce the resistance to aminoglycosides [4]. Compared to other resistance mechanisms, active efflux pumps are more widely distributed and have a wider substrate, resulting in more kinds of drug resistance [1]. In addition, recent research has suggested that biofilm formation of A. baumannii is potentially associated with the genes encoding efflux pumps [5]. Finally, minocycline and tigecycline are broad-spectrum antibiotics which show effective activity against multidrug-resistant Acinetobacter. However, with the mutation leading efflux pump overexpression, the susceptibility of A. baumannii to these drugs is limited [4], [6].
Efflux Pump in A. baumannii
The first efflux pump of A. baumannii, AdeABC, regulated by AdeRS, was found in multidrug-resistant A. baumannii BM4454 by Magnet et al. in 2001 [7]. The study of the efflux pump system in Acinetobacter subsequently developed. adeDE [8] and adeXYZ [9] were detected in Acinetobacter genomic DNA group 3 (GDG3) in 2004 and 2006, respectively. In 2008, the AdeIJk efflux pump was found in BM4454 by Damierpiolle et al. [10]. The adeFGH efflux pump was discovered in BM4664 in 2010 [11].
The membrane-active efflux system generally consists of three parts: outer membrane protein (adeC), multidrug transporter (adeB) and membrane fusion protein (adeA). According to the homology of the amino acid sequence, the membrane efflux pump is divided into five superfamilies: ATP-binding cassette (ABC), small multidrug resistance (SMR), multiantimicrobial extrusion (MATE), major facilitator (MFS) and resistance/nodulation/division (RND). The ABC family mainly exists in Gram-positive bacteria, which rely on ATP to provide energy, while for the SMR, MATE, MFS and RND family the proton driving force acts as the energy source [12]. The RND efflux pump superfamily, including adeABC, adeDE, adeFGH, adeIJK and adeXYZ, is prevalent in A. baumannii, and its substrate is the most extensive. At present, adeABC, as the pump gene in A. baumannii discovered first, is the most studied pump gene; some researchers have even proposed that adeABC be used as a sign of resistance of A. baumannii.
Structure and Regulator of adeABC
Structure of adeABC
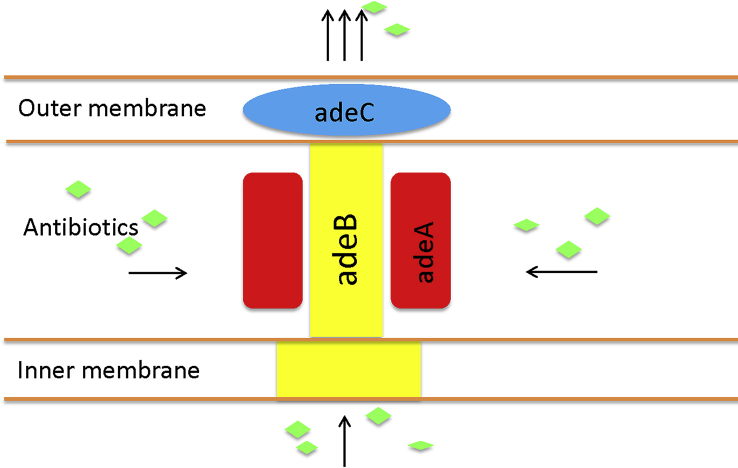
At present, the understanding of the molecular mechanisms and functions of the adeABC complex are primarily based on the study of the resistant strain A. baumannii BM4454 [7]. adeABC is located in the chromosome genome of A. baumannii. adeA, adeB and adeC are continuous, encoding membrane fusion protein, multidrug transporter and outer membrane channel protein structure, respectively. Their function can be simply explained by the fact that adeB captures substrates in the inner membrane of phospholipids bilayer or the cytoplasm, then transports the substrates by adeC (membrane channel protein). Therefore, these structural genes can promote drug discharge (Fig. 1).
Fig. 1.
Function of adeABC efflux pump in cell wall of Acinetobacter baumannii. adeA acts as membrane fusion protein, adeB as multidrug transporter and adeC as outer membrane protein. adeB captures antibiotics in inner membrane of phospholipids bilayer or cytoplasm, then transports substrates out by adeC (membrane channel protein).
The expression of adeABC is regulated by adeRS, and the expression levels of adeA, adeB and adeC are inconsistent. PCR amplification showed that the detection rate of adeB was highest in clinical isolates, but the detection rates varied in these studies. In some studies the adeB gene was found in all clinically isolated strains, while in other studies the rate just was 70% to 75% [13], [14]. By contrast, some studies found that adeA has the highest detection rate in clinically isolated strains [15], [16]. More researchers incline to the view that adeB is the most important gene in adeABC and is most associated with the resistance of A. baumannii [17]. In all studies, the detection rate of adeC was the lowest [13], [14], [15], [16], with the lowest rate of 42% in some studies [13]. However, compared to adeC-positive and adeC-negative strains, we found that the adeC-positive group had more strain resistance to all six antibiotics in the study [13]. It demonstrated that although adeC is not necessary in adeABC efflux-mediated drug resistance, the presence of adeC is more likely to result in multidrug resistance or pan-drug resistance.
adeRS regulates expression of adeABC
adeRS is located upstream of adeABC, separated by a 133 bp intercistronic spacer between the adeRS and adeABC operons [18] and the expression direction reverse to adeABC. The adeRS bicomponent regulation system consists of sensor kinase adeS and responsive regulator adeR. adeS consists of histidine kinase that receives environmental signals and cause autophosphorylation, then transfers the phosphoric acid to the output responder, adeR. adeR has been recognized as a recognition response factor and acts as a transcriptional activator [19]. In the study of Hassan et al. [20], adeB and adeR were discovered in mutant cell populations by the fluorescence technique. adeB and adeR have similar fluorescence intensity higher than that of parents. When Hornsey et al. [6] analysed the nucleotide sequence of the carbendazone-resistant clone strain South-East and OXA-23 clone 1, they found that at position 62 of the AdeS sensor histidine kinase, there was a difference in amino acid, which was methionine in the OXA-23 clone 1 strain and isoleucine in the South-East clone strain. The amino acid sequence of AdeR was not different, however.
On the basis of the above evidence, we suspect that AdeS may play a leading role in the regulation of AdeABC. Some studies have shown that amino acid substitutions at AdeRS of clinical isolates resulted in overexpression of the adeABC efflux pump. Hornsey et al. [6] found Ala-94 → Val substitution in adeS in AdeB-overexpressing tigecycline-resistant strains; Coyne et al. [12] found Asp-30 → Gly substitution in the AdeS-sensing domain in the multidrug-resistant strain; Chang et al. [21] found Met-197 → Ile substitution and Gly-200 → Cys substitution in adeS DNA combination domain in tigecycline-resistant isolates. In addition, insertion sequence (IS) in adeRS also can affect the resistance of A. baumannii. Sun et al. [22] found that inserting ISAbaI into adeS can produce N-terminal truncated free forms of adeS and messenger RNA transcripts. The truncated AdeS then enhances adeABC gene overexpression. Lopes et al. [23] also demonstrated that the insertion of ISAbaI in AdeS enhanced the expression of adeABC efflux pump and reduced the susceptibility of A. baumannii to tigecycline. In short, the mutation of adeRS or the insertion sequence in AdeRS can cause the overexpression of adeABC, resulting in resistance of A. baumannii.
How do adeRS regulate the overexpression of adeABC efflux pumps? Some studies showed that phosphorylated regulators bind to adeABC promoter regions and regulate the expression of adeABC operon [24], [25]. However, Chang et al. [21] explored the interaction between adeR and adeABC by electrophoresis mobility shift analysis; they found that AdeR and adeABC promoters did not interact. Even if adeS was present, adeR was not found to bind to the promoter region of adeABC. Their further studies discovered that adeR binds to a direct-repeat motif region between adeR and adeABC, then regulated adeABC expression. They argued that amino acid substitutions of adeRS changes the binding ability of AdeR to the direct-repeat motif region, thereby leading to adeABC overexpression. However, additional research is required to support this notion. adeRS regulating the expression of adeABC is defined, but the mode of action is still difficult to specify. In addition, the regulation of the expression of the adeABC gene is complex. Under some conditions, the ISAbaI insertion does not lead to the overexpression of this pump [26], indicating that other regulators may be involved. One study showed that the other two-component system, BaeSR, can regulate adeA and adeB [27]. More research is needed to explore the regulation mechanism of adeABC, and more direct evidence of the association of mutations and regulators involved in antibiotic resistance is required.
adeABC in drug-resistant A. baumannii
adeABC has a wide range of substrates, including β-lactams, fluoroquinolones, tetracycline (tigecycline), macrolide (linamides) and chloramphenicol; it confers the clinical resistance of aminoglycosides. Among these, netilmicin and gentamycin appear to be the best substrates for efflux pump adeABC [7]. Efflux pumps such as adeABC play an important role in the resistance mechanisms of tigecycline by throwing drugs away from the target biding site [28], [29]. Studies have also found that the adeABC pump has a synergistic effect with carbapenems and aminoglycosidases on drug resistance [30]. A study in China showed a close association between overexpression of AdeABC efflux pump genes and carbapenem (meropenem) resistance in A. baumannii without mutation of its regulatory genes [31]. It has been noted that the presence of both Int1 and 16S ribosomal RNA methylases confers resistance to aminoglycosides [32].
A study by Sun et al. [22] found the RNA transcripts of the adeA, adeF and adeI genes in the isolates resistance to ticarcillin were 8.18 (±14.60), 0.03 (±0.07) and 1.65 (±1.64) times, respectively, as those of the reference strain A. baumannii ATCC 15151. The expression of the adeABC efflux pump gene therefore changes more than adeFGH and AdeIJk in ticarcillin-resistant A. baumannii isolates. In addition, adeB gene expression was not observed in any of the initially sensitive strains. In the study of Rumbo et al. [33], clinical isolates overexpressed the adeABC efflux system (expression level 30 to 45 times those of A. baumannii ATCC 17978) were resistant to tigecycline, minocycline and gentamycin, and other biological functions were significantly correlated. The high-expression adeIJk efflux system (expression level as eight to ten times those of reference A. baumannii ATCC 17978) were related to only tigecycline and minocycline resistance.
In an article published in 2010, Coyne et al. [11] pointed out that adeABC was detected in 80% (reported rate, 53–97%) in clinically isolated resistant strains and was the most frequently involved in the multidrug-resistant RND system in the clinical setting. Hornsey et al. [6] found a correlation between higher minimum inhibitory concentration (MIC) values and elevated adeABC expression. Furthermore, overexpression of AdeABC efflux pump genes is a common mechanism to decrease susceptibility to tigecycline, which is supported by the presence of efflux pump inhibitor (EPI) to reverse the resistance pattern [34]. Further, differences in expression of adeABC contributed to both inter- and intraclone variation in tigecycline MICs in A. baumannii [34]. One study in China compared tigecycline-susceptible A. baumannii and tigecycline-sensitive A. baumannii isolates; the study found that overexpression of adeABC is the main mechanism for the decrease in resistance to tigecycline [35]. In the study of adeB, adeB was found to be related to aminoglycoside resistance and mediated tetracycline, chloramphenicol, erythromycin, trimethoprim and ethidium bromide sensitivity levels [36]. The resistance range was similar to all expressions of adeABC, which further illustrates the fact that the adeB gene plays an important role in adeABC pump resistance mechanism.
The presence of the adeABC gene in sensitive strains is therefore low, and is prevalent in the drug-resistant strains, so some researchers support the notion that adeABC can be used as a sign of resistance of A. baumannii [14]. To date, however, A. baumannii is not particularly sensitive to many drugs, but its sensitivity to colistin remains high [37], [38]. One study showed the contribution of adeABC in colistin heteroresistance when exposed to colistin by overexpression of adeB in clinical isolate [39]. In the study of Gholami et al. [40], the clinical isolates are all sensitive to colistin.
Other Efflux Pump Genes
adeDE (containing unidentified outer membrane constituent genes), belonging to the RND family, was first detected in Acinetobacter stage GDG3 [8], then was detected separately in GDG13TU and -17 [9]. GDG3 and did not appear with adeABC [14], but adeB and adeE were found in the study of Hou et al. [41] in isolates of resistant strains of A. baumannii, indicating that adeB and adeE can be expressed simultaneously in parts of A. baumannii. The expression and the role of adeDE in A. baumannii remains to be studied. adeFGH, RND family; and LysR transcription factor adeL are responsible for transcription of adeFGH. adeFGH overexpression was found in chloramphenicol-resistance–acquired mutant strains, and adeF is not associated with resistance to ticarcillin. In all ticarcillin-based extensively drug-resistant A. baumannii isolates, the adeF gene was the lowest in the three major RND pump genes (adeABC, adeFGH, adeIJk) [22].
adeIJK also belongs to the RND family. Its expression is regulated by the TetR family transcriptional regulator AdeN. In sensitive and resistant strains, adeIJk has been detected. Studies have shown that adeIJk may only cause intrinsic resistance rather than adeABC, which will produce intrinsic resistance and acquired resistance. adeIJK is resistant to β-lactams, chloramphenicol, tetracycline, erythromycin, linamides, fluoroquinolons, fusidic acid, neonatal acid, rifampicin, trimethoprim, acridine (dyes), coke (dyes) and sodium dodecyl sulfate. Although the average expression level of adeJ is relatively low, as long as the expression of the adeIJk carried by the plasmid occurs, it can significantly increase the MIC level of cloxacillin, oxacillin, nitrothromine and ethidium bromide [10]. On this basis, it is speculated that the physiologic effect of adeIJK efflux pump may be stronger than that of adeABC as well as the properties of adeABC and adeIJk (Table 1).
Table 1.
Basic properties of adeABC and adeIJk
| Property | adeABC | adeIJk |
|---|---|---|
| Distribution strains | Almost always mutant strains, seldom wild | Mutant and wild |
| Regulator | AdeRS (positive regulation) | adeN (negative regulation) |
| Kind of antibiotic resistance | Intrinsic antibiotic resistance and acquired antibiotic resistance | Intrinsic antibiotic resistance |
| Drug resistance | β-Lactams, fluoroquinolons, tetracycline, linamides, chloramphenicol, aminoglycosides | β-Lactams, fluoroquinolons, tetracycline, linamides, chloramphenicol, erythromycin, fusidic acid, neonatal acid, rifampicin, trimethoprim, acridine (dyes), coke (dyes) and sodium dodecyl sulfate |
Comparison of adeABC and adeIJk of A. baumannii with those of the AcrAB-TolC system of Escherichia coli showed that under similar conditions, adeABC was more effective than the similar level of AcrAB-TolC in the resistance to tetracycline but was less effective in lipophilic β-lactams, novobiocin and ethidium bromide. Interestingly, adeIJK was more effective than AcrAB-TolC in lipophilic β-lactams, novobiocin and ethidium bromide, although less effective in tetracycline [42]. However, there are no studies directly comparing the effect of adeABC and adeIJk. Therefore, further study is needed to understand the important effect of adeIJk on A. baumannii. Genes with unknown mechanisms and drug-resistant efflux pump genes are being found; these genes seem to be related to resistance to certain drugs. For example, in a study of AbeM by the MATE family, it was revealed that it can cause resistance to aminoglycosides and quinolones [43]. TetA is related to tetracycline resistance, while MdfA contributes to ciprofloxacin and chloramphenicol resistance [44]. CraA [45] and CmlA [12] play an important role in chloramphenicol resistance. AmvA [46] has an effect on erythromycin resistance.
Conclusion
Although the efflux gene of A. baumannii has been studied for decades, many things remain unclear. Coyne et al. [47] evaluated the expression of the A. baumannii efflux pump gene; their microarray chip contained 205 gene probes, including 47 efflux systems, 55 resistance determinants and 35 housekeeping genes. Therefore, the efflux genes of A. baumannii are more than those have studied. Except for the above-mentioned genes, many genes remain unclear. A. baumannii is also an easy-to-carry drug-resistant gene that moves elements such as plasmids, transposon and insert sequence, leading to its more efficient and complex mechanism. adeABC has been widely implicated; other related genes have been less studied. Some literature has suggested that the expression of adeIJK may be a potential gene associated with resistance to A. baumannii. What genes play a more extensive and powerful role in drug resistance? Regulation of efflux pump gene expression and whether there are other regulatory genes are also concerns.
It is noteworthy that the current studies on the resistance mechanism of efflux pumps are focused on in vitro studies. The detection of expression and resistance of efflux pump genes are also carried out in vitro. Therefore, some genes such as adeC are thought to be unnecessary in mediating drug resistance, but in, vivo, its specific role has not been studied; whether it plays a role in the interaction of the strain and the host is not understood. The efflux pump inhibitor is a drug class that does not itself have a bactericidal effect but that can inhibit the efflux pump in combination with antibiotics to reduce the MIC, just as sulbactam in combination with cefoperazone. However, the existing efflux pump inhibitors have a wide range of substrates, and the toxicity is high. If these characters can lead to further resistance is not known. Using more rational drug according to the efflux pump, and developing less toxic and more selective drugs are extremely urgent.
Conflict of Interest
None declared.
Acknowledgements
Supported in part by the National Natural Science Foundation of China (81270726), Natural Science Foundation of Liaoning Province (20170541023) and National Natural Science Foundation of China (81771621).
References
- 1.Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spellberg B., Rex J.H. The value of single-pathogen antibacterial agents. Nat Rev Drug Discov. 2013;12:963. doi: 10.1038/nrd3957-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon N.C., Wareham D.W. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int J Antimicrob Agents. 2010;35:219–226. doi: 10.1016/j.ijantimicag.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Lee C.R., Lee J.H., Park M., Park K.S., Bae I.K., Kim Y.B. Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front Cell Infect Microbiol. 2017;7:55. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He X., Lu F., Yuan F., Jiang D., Zhao P., Zhu J. Biofilm formation caused by clinical acinetobacter baumannii isolates is associated with overexpression of the AdeFGH efflux pump. Antimicrob Agents Chemother. 2015;59:4817. doi: 10.1128/AAC.00877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hornsey M., Ellington M.J., Doumith M., Thomas C.P., Gordon N.C., Wareham D.W. AdeABC-mediated efflux and tigecycline MICs for epidemic clones of Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:1589–1593. doi: 10.1093/jac/dkq218. [DOI] [PubMed] [Google Scholar]
- 7.Magnet S., Courvalin P., Lambert T. Resistance–nodulation–cell division–type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob Agents Chemother. 2001;45:3375. doi: 10.1128/AAC.45.12.3375-3380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chau S.L., Chu Y.W., Houang E.T. Novel resistance–nodulation–cell division efflux system AdeDE in Acinetobacter genomic DNA group 3. Antimicrob Agents Chemother. 2004;48:4054–4055. doi: 10.1128/AAC.48.10.4054-4055.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu Y.W., Chau S.L., Houang E.T. Presence of active efflux systems AdeABC, AdeDE and AdeXYZ in different Acinetobacter genomic DNA groups. J Med Microbiol. 2006;55(pt 4):477–478. doi: 10.1099/jmm.0.46433-0. [DOI] [PubMed] [Google Scholar]
- 10.Damier-Piolle L., Magnet S., Bremont S., Lambert T., Courvalin P. AdeIJK, a resistance–nodulation–cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob Agents Chemother. 2008;52(2):557–562. doi: 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyne S., Rosenfeld N., Lambert T., Courvalin P., Périchon B. Overexpression of resistance–nodulation–cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:4389–4393. doi: 10.1128/AAC.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyne S., Courvalin P., Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother. 2011;55:947–953. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieczorek P., Sacha P., Czaban S., Hauschild T., Ojdana D., Kowalczuk O. Distribution of AdeABC efflux system genes in genotypically diverse strains of clinical Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2013;77:106. doi: 10.1016/j.diagmicrobio.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Lin L., Ling B.D., Li X.Z. Distribution of the multidrug efflux pump genes, adeABC, adeDE and adeIJK, and class 1 integron genes in multiple-antimicrobial-resistant clinical isolates of Acinetobacter baumannii-Acinetobacter calcoaceticus complex. Int J Antimicrob Agents. 2009;33(1):27–32. doi: 10.1016/j.ijantimicag.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Modarresi F., Azizi O., Shakibaie M.R., Motamedifar M., Valibeigi B., Mansouri S. Effect of iron on expression of efflux pump (adeABC) and quorum sensing (luxI, luxR) genes in clinical isolates of Acinetobacter baumannii. APMIS. 2015;123:959–968. doi: 10.1111/apm.12455. [DOI] [PubMed] [Google Scholar]
- 16.Wei J., Li C., Zhang H., Li G., Liu X., Wei J. Prevalence of genes of OXA-23 carbapenemase and AdeABC efflux pump associated with multidrug resistance of Acinetobacter baumannii isolates in the ICU of a comprehensive hospital of Northwestern China. Int J Environ Res Public Health. 2015;12:10079–10092. doi: 10.3390/ijerph120810079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon E.J., Balloy V., Fiette L., Chignard M., Courvalin P., Grillotcourvalin C. Contribution of the Ade resistance–nodulation–cell division–type efflux pumps to fitness and pathogenesis of Acinetobacter baumannii. MBio. 2016;7 doi: 10.1128/mBio.00697-16. e00697-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchand I., Damierpiolle L., Courvalin P., Lambert T. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother. 2004;48:3298. doi: 10.1128/AAC.48.9.3298-3304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West A.H., Stock A.M. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biol Sci. 2001;26:369–376. doi: 10.1016/s0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- 20.Hassan K.A., Cain A.K., Huang T.T., Liu Q., Elbourne L.D.H., Boinett C.J. Fluorescence-based flow sorting in parallel with transposon insertion site sequencing identifies multidrug efflux systems in Acinetobacter baumannii. MBio. 2016;7:e01200–e01216. doi: 10.1128/mBio.01200-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang T.Y., Huang B.J., Sun J.R., Perng C.L., Chan M.C., Yu C.P. AdeR protein regulates adeABC expression by binding to a direct-repeat motif in the intercistronic spacer. Microbiol Res. 2016;183:60. doi: 10.1016/j.micres.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Sun J.R., Perng C.L., Lin J.C., Yang Y.S., Chan M.C., Chang T.Y. AdeRS combination codes differentiate the response to efflux pump inhibitors in tigecycline-resistant isolates of extensively drug-resistant Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis. 2014;33:2141. doi: 10.1007/s10096-014-2179-7. [DOI] [PubMed] [Google Scholar]
- 23.Lopes B.S., Amyes S.G. Insertion sequence disruption of adeR and ciprofloxacin resistance caused by efflux pumps and gyrA and parC mutations in Acinetobacter baumannii. Int J Antimicrob Agents. 2013;41:117–121. doi: 10.1016/j.ijantimicag.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Cheung J., Hendrickson W.A. Sensor domains of two-component regulatory systems. Curr Opin Microbiol. 2010;13:116. doi: 10.1016/j.mib.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornsey M., Loman N., Wareham D.W., Ellington M.J., Pallen M.J., Turton J.F. Whole-genome comparison of two Acinetobacter baumannii isolates from a single patient, where resistance developed during tigecycline therapy. J Antimicrob Chemoth. 2011;66:1499. doi: 10.1093/jac/dkr168. [DOI] [PubMed] [Google Scholar]
- 26.Sun J.R., Perng C.L., Chan M.C., Morita Y., Lin J.C., Su C.M. A truncated AdeS kinase protein generated by ISAba1 insertion correlates with tigecycline resistance in Acinetobacter baumannii. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin M.F., Lin Y.Y., Lan C.Y. The role of the two-component system BaeSR in disposing chemicals through regulating transporter systems in Acinetobacter baumannii. PLoS One. 2014;10 doi: 10.1371/journal.pone.0132843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh H., Thangaraj P., Chakrabarti A. Acinetobacter baumannii: a brief account of mechanisms of multidrug resistance and current and future therapeutic management. J Clin Diagn Res. 2013;7:2602–2605. doi: 10.7860/JCDR/2013/6337.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manchanda V., Sanchaita S., Singh N. Multidrug resistant acinetobacter. J Glob Infect Dis. 2010;2:291–304. doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon E.J., Chabane Y.N., Goussard S., Snesrud E., Courvalin P., Dé E. Contribution of resistance–nodulation–cell division efflux systems to antibiotic resistance and biofilm formation in Acinetobacter baumannii. MBio. 2015;6:e00309–e00315. doi: 10.1128/mBio.00309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou Q., Zou M., Li J., Wang H., Hu Y., Liu W. AdeABC efflux pump and resistance of Acinetobacter baumannii against carbapenem. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42:426–433. doi: 10.11817/j.issn.1672-7347.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Chen F., Wang L., Wang M., Xie Y., Xia X., Li X. Genetic characterization and in vitro activity of antimicrobial combinations of multidrug-resistant Acinetobacter baumannii from a general hospital in China. Oncol Lett. 2018;15:2305–2315. doi: 10.3892/ol.2017.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rumbo C., Gato E., López M., Ruiz dAC., Fernández-Cuenca F., Martínez-Martínez L. Contribution of efflux pumps, porins, and β-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57:5247. doi: 10.1128/AAC.00730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin M.F., Lan C.Y. Antimicrobial resistance in Acinetobacter baumannii: from bench to bedside. World J Clin Cases. 2014;2:787. doi: 10.12998/wjcc.v2.i12.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng M., Zhu M.H., Li J.J., Bi S., Sheng Z.K., Hu F.S. Molecular epidemiology and mechanisms of tigecycline resistance in clinical isolates of Acinetobacter baumannii from a Chinese university hospital. Antimicrob Agents Chemother. 2014;58:297–303. doi: 10.1128/AAC.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang T., Wang M., Xie Y., Li X., Dong Z., Liu Y. Active efflux pump adeB is involved in multidrug resistance of Acinetobacter baumannii induced by antibacterial agents. Exp Ther Med. 2017:1538–1546. doi: 10.3892/etm.2017.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savari M., Ekrami A., Shoja S., Bahador A. Plasmid borne carbapenem-hydrolyzing class D beta-lactamases (CHDLs) and AdeABC efflux pump conferring carbapenem–tigecycline resistance among Acinetobacter baumannii isolates harboring TnAbaRs. Microb Pathog. 2017;104:310–317. doi: 10.1016/j.micpath.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 38.Hua X., Liu L., Fang Y., Shi Q., Li X., Chen Q. Colistin resistance in Acinetobacter baumannii MDR-ZJ06 revealed by a multiomics approach. Front Cell Infect Microbiol. 2017;7:45. doi: 10.3389/fcimb.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado D., Antunes J., Simões A., Perdigão J., Couto I., Mccusker M. Contribution of efflux to colistin heteroresistance in a multidrug resistant Acinetobacter baumannii clinical isolate. J Med Microbiol. 2018;67:740–749. doi: 10.1099/jmm.0.000741. [DOI] [PubMed] [Google Scholar]
- 40.Gholami M., Hashemi A., Hakemi-Vala M., Goudarzi H., Hallajzadeh M. Efflux pump inhibitor phenylalanine-arginine beta-naphthylamide effect on the minimum inhibitory concentration of imipenem in Acinetobacter baumannii strains isolated from hospitalized patients in Shahid Motahari Burn Hospital, Tehran, Iran. Jundishapur J Microbiol. 2015;8 doi: 10.5812/jjm.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou P.F., Chen X.Y., Yan G.F., Wang Y.P., Ying C.M. Study of the correlation of imipenem resistance with efflux pumps AdeABC, AdeIJK, AdeDE and AbeM in clinical isolates of Acinetobacter baumannii. Chemotherapy. 2012;58:152–158. doi: 10.1159/000335599. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara E., Nikaido H. Properties of AdeABC and AdeIJK efflux systems of Acinetobacter baumannii compared with those of the AcrAB-TolC system of Escherichia coli. Antimicrob Agents Chemother. 2014;58:7250–7257. doi: 10.1128/AAC.03728-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su X.Z., Chen J., Mizushima T., Kuroda T., Tsuchiya T. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob Agents Chemother. 2005;49:4362. doi: 10.1128/AAC.49.10.4362-4364.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vila J., Martí S., Sánchez-Céspedes J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2007;59:1210–1215. doi: 10.1093/jac/dkl509. [DOI] [PubMed] [Google Scholar]
- 45.Roca I., Marti S., Espinal P., Martínez P., Gibert I., Vila J. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53:4013–4014. doi: 10.1128/AAC.00584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajamohan G., Srinivasan V.B., Gebreyes W.A. Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:1919–1925. doi: 10.1093/jac/dkq195. [DOI] [PubMed] [Google Scholar]
- 47.Coyne S., Guigon G., Courvalin P., Perichon B. Screening and quantification of the expression of antibiotic resistance genes in Acinetobacter baumannii with a microarray. Antimicrob Agents Chemother. 2010;54:333–340. doi: 10.1128/AAC.01037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]