Abstract
Background:
Νeuroendocrine tumors of the lungs are rare arising in the thymus and gastro-entero-pancreatic tract and belonging to foregut of neuroendocrine tumors. The aim of the present prospective study was to estimate the potential impact of single-photon emission computed tomography somatostatin receptor scintigraphy using 99mTc-Tektrotyd on diagnosis, treatment response, and prognosis in patients with neuroendocrine tumors of the lungs.
Methods:
Thirty-six patients with neuroendocrine tumors of the lungs were evaluated by using 99mTc-HYNIC-TOC scintigraphy. The scintigraphic results were compared to liver tissue uptake (Krenning score). Likewise, the functional imaging results were compared with biochemical indices including chromogranin A, neuroendocrine-specific enolase, and insulin-like growth factor 1 at the time of diagnosis (baseline) and disease progression.
Results:
The number of somatostatin receptors, expressed with Krenning score, did not show any correlation with the survival of patients both at baseline (P = .08) and at disease progression (P = .24), and scintigraphy results did not relate significantly to progression-free survival. Comparing the results of 99mTc-HYNIC-TOC scintigraphy according to the response of patients in the initial treatment, a statistically significant negative correlation was observed both in the first and in the second scintigraphy with patients’ response (P = .001 and P < .001, respectively). The concentrations of biochemical markers were in accordance with scintigraphy results in the diagnosis.
Conclusion:
This study indicates that 99mTc-HYNIC-TOC scintigraphy appears to be a reliable, noninvasive technique for detection of primary neuroendocrine tumors and their locoregional or distant metastases, although it cannot be used as a neuroendocrine tumors of the lungs predictive technique.
Keywords: Tektrotyd, lung neuroendocrine tumors, prognostic factors, predictive factors
Introduction
Neuroendocrine tumors (NETs) encompass a genetically heterogeneous group of malignancies arising from the diffuse neuroendocrine system cells. Tumors homologous with NET of the lungs (Lu-NETs) are rare and arise in the thymus, gastro-entero-pancreatic tract, and the bronchopulmonary system, possibly because of their common origin from endoderm-derived precursors/stem cells of the foregut according to certain molecular gene pathway changes.1-3 The Lu-NETs are a very inhomogeneous group of malignancies,4 representing 20% to 25% of all cancers of the respiratory system.5,6 They are a particular subtype of tumors with similar morphological, immunohistochemical, and structural characteristics. The Lu-NETs are classified into the following histological variants: typical carcinoid (TC), atypical carcinoid, large-cell neuroendocrine carcinoma (LCNEC), and small-cell lung carcinoma (SCLC).7 The NETs are characterized by the overexpression of somatostatin receptors (SSTRs).8 Apart from malignant cells, these receptors are expressed by inflammatory cells and cells of the innate and adaptive immune system.9 The SSTRs signal to alter hormonal secretion, augment apoptosis, and reduce cellular proliferation. Their expression by endocrine tumors is critical for the use of the somatostatin analogs in pharmacotherapy. In this regard, somatostatin and analogs can slow their growth, and this antitumor activity appears to be related to both direct and indirect effects. Directly, activation of SSTRs in cancer cells causes cell cycle arrest or apoptosis, and indirectly, somatostatin reduces cancer growth by inhibiting malignant angiogenesis and the growth factors secretion10; somatostatin analog therapy is the principal first-line therapeutic regimen, particularly in well-differentiated NETs with increased SSTR expression.11
Specifically, SSTRs belong to the typical 7-transmembrane domain family of G-protein-coupled receptors that internalized after binding to specific ligands. This property of overexpression of SSTRs in NETs is helpful for their detection by some functional imaging modalities.8 In this respect, the somatostatin receptor scintigraphy (SRS) is the top option for detection of well-differentiated NETs.12
Recently, the SRS technique single-photon emission computed tomography (SPECT) computed tomography (CT) with 99mTc-EDDA/HYNIC-TOC (99mTc-Tektrotyd, Polatom, Otwock, Poland) has been approved for NET diagnosis.13-16 This tracer displays high affinity to SSTR2, whereas lower affinity to SSTR3 and SSTR5.14 It is introduced for the diagnosis of neoplasms with the overexpression of SSTRs, particularly subtype 2.17 The radiopeptide 99mTc- HYNIC-TOC contains the analog Tyr3-octreotide (TOC), the substituent 6-hydrazino nicotinic acid (HYNIC), and the complementary ethylenodiaminodioxide acid (EDDA) substituent for fitting of 99mTc. This radiopeptide had encouraging preclinical data: high binding affinity to SST2, high internalization capability to SST2 + cells, high specific uptake in SST2 + tumors AR4-2J, and low uptake in the kidneys.18,19
However, to date, only few studies evaluated the Tc-99m-EDDA/HYNIC-TOC scintigraphy use in the diagnosis of Lu-NETs.20-22 A vital need still unmet for these tumors is a clinically applicable biomarker development that captures NET behavior and can be introduced to guide clinical therapeutic schemes. The use of blood-based molecular data combined with functional imaging may offer noninvasive real-time multidimensional knowledge that could be used as a prognostic and predictive biomarker. The purpose of this prospective study was to estimate the potential impact of Tektrotyd on diagnosis, treatment response, and prognosis of patients with Lu-NETS.
Materials and Methods
Patients’ Cohort
During February 2015 to November 2016, 38 patients with recently detected Lu-NETS were enrolled for relative treatment and monitoring in the lung cancer unit of our department. The study was approved by the hospital’s ethics committee (registration number: 359/15), and all participants gave written informed consent.
Following completion of their treatment, 2 of 38 patients died and finally 36 patients (23 SCLC, 8 LCNEC, and 5 TCs) were included. Selection criteria included performance status 0-1; age <75 years; stage IIIb-IV, histologically confirmed Lu-NETS (SCLC, LCNEC, and carcinoids); relative immunohistochemical markers, that is, CD56, neuroendocrine-specific enolase (NSE), chromogranin A (CgA), and synaptophysin; absence of cardiovascular disease; and normal renal and hepatic function. Complete staging was performed with CT scans (chest, abdomen, and brain) and bone scintigraphy.
Imaging Analysis
All patients underwent scintigraphy Tektrotyd at baseline and at relapse, detected by chest CT. The whole-body scintigraphy was achieved 1 and 3 hours after 740 MBq 99mTc-Tektrotyd, Polatom intravenous injection, followed by whole-body and static, planar and SPECT images. It was achieved using 360° orbits, step and shoot mode, at 40 seconds per view. The obtained records were collected in a 128 × 128 computer matrix and reconstructed by applying filtered back-projections with a Butterworth filter (cutoff 0.6 cycles/pixel, order 5) and iterative reconstruction. The study was achieved by SOPHA gamma camera and computer (XELERIS), with high-resolution collimator and 1 photopeak activity (140 keV ± 20%). The aforementioned images were assessed independently by 2 relative experts (V.Ch. and G.G.). Pathologic uptake was graded with a semiquantitative visual scoring system consisting of the Krenning scale score from 0 to 4 and uses liver and spleen as reference organs.23-25
Patients’ Protocol
All patients were treated with chemotherapy (4-6 cycles) with platinum-based regimens, depending on their histological type. As first-line chemotherapy, patients with LCNEC received taxane–cisplatin and patients with SCLC received VePesid–cisplatin combination. Patients with carcinoid tumors, all centrally located, underwent surgical resection of the tumor, and they have been under surveillance ever since. Restaging of patients with chest CT took place after 4 cycles of chemotherapy, and the response evaluation was performed at this time for patients with LCNEC, SCLC, and carcinoid too. Overall survival (OS) was defined as the time from initial diagnosis till mortality and progression-free survival (PFS) as the time from initial diagnosis till objective tumor progression or death.26 In case of disease progression, according to RECIST criteria v.1.1,27 scintigraphy with Tektrotyd was carried out again, at least 2 weeks after the last chemotherapy, and was compared with the initial examination. All patients with disease progression continued with second-line chemotherapy, pemetrexed–cisplatin for patients with LCNEC and taxane–carboplatin for patients with SCLC. Moreover, patients with SCLC who responded to the initial treatment had radiation therapy of the primary site and prophylactic cranial irradiation. After 1 year of follow-up, patients with carcinoid tumors had no progression confirmed by the surveillance scintigraphy at that time (Krenning score = 0).
Neuroendocrine Markers in Patients’ Serum Samples Analysis
The NSE was measured by Quantikine Human Enolase 2 Immunoassay (Catalog No. DENL20; R&D Systems, Minneapolis, Canada), and insulin-like growth factor 1 (IgF-1) was measured by Quantikine Human IGF-I Immunoassay (Catalog No. DG100; R&D Systems), according to the manufacturer’s protocol. The CG-A was measured by human CgA enzyme-linked immunosorbent assay (ELISA) kit (catalog No. CSB-E09153 h; Cusabio, Wuhan, China), according to the manufacturer’s protocol. The values for the abovementioned assays were recorded at a wavelength of 450 nm, and a standard curve was plotted on a 4-parameter logistic curve fit for NSE and CgA, whereas for IgF-1 a log/log curve fit was applied as a standard curve. The values were then calculated from the standard curve. Blood samples were collected from all patients at baseline and at radiologically confirmed disease progression. The second blood sample was collected from patients with carcinoid tumors without recurrence at 1-year follow-up period. To measure serum hormone concentrations, blood samples were centrifuged after collection and frozen at −80°C until they were measured by ELISA.
Statistical Analysis
Statistical analysis was performed with Cox regression, 1-way analysis of variance, and post hoc analysis (SPSS, version 21.00) for Windows (Microsoft). Statistical significance was defined at P < .05.
Results
Patients’ characteristics according to their histological type of bronchial NET are shown in Table 1. In the first restaging, disease progression was reported in 11 (30%) of 36 patients, stable disease in 10 (28%) of 36 patients, partial response in 8 (22%) of 36 patients, and complete response in 7 (19%) of 36 patients.
Table 1.
Patients Characteristics.
| SCLC, n = 23 | LCNEC, n = 8 | Carcinoid, n = 5 | ||||
|---|---|---|---|---|---|---|
| Number of patients | % | Number of patients | % | Number of patients | % | |
| Age (mean) | 65 | 64 | 47 | |||
| Stage | LD 13 | 57% | IIIb 3 | 38% | Ib 4 | 80% |
| ED 10 | 43% | IVb 5 | 62% | IIa 1 | 20% | |
| NSE, mean in ng/mL | 24.63 | 22.08 | – | |||
| CgA, mean in ng/mL | 29.04 | 25.08 | – | |||
| IgF1, mean in ng/mL | 10.39 | 7.93 | – | |||
| Response | ||||||
| PD | 6 | 26% | 5 | 62% | 0 | 0% |
| SD + OR | 17 | 74% | 3 | 38% | 5 | 100% |
Abbreviations: CGA, chromogranin A; ED, extensive disease; IgF-1, insulin-like growth factor 1; LCNEC, large-cell neuroendocrine carcinoma; LD, limited disease; NSE, neuroendocrine-specific enolase; OR, objective response; PD, progressive disease; SCLC, small-cell lung carcinoma; SD, stable disease.
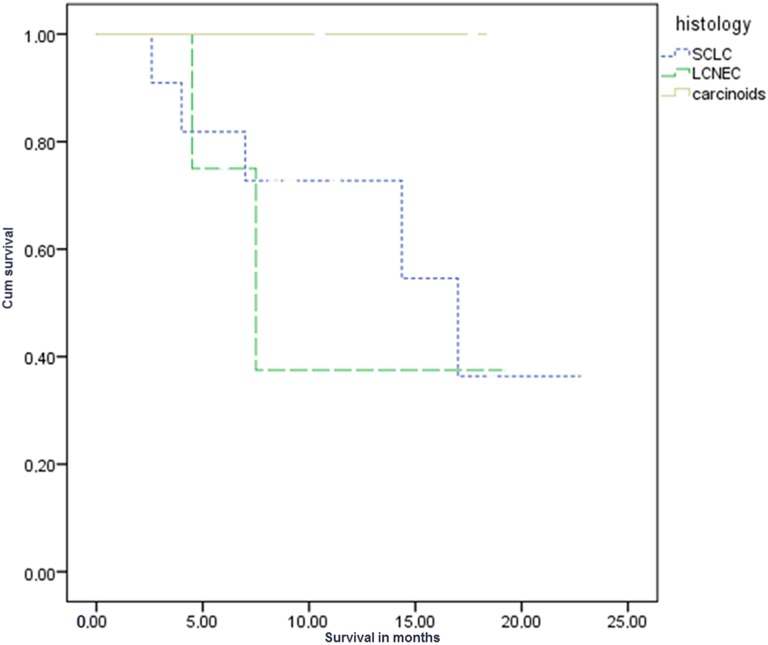
The mean OS for all patients studied was 11.6 months (standard deviation [SD]: 6 ± 1 month). Survival was different among the 3 different groups of patients as expected due to differences in biological behavior of tumors per their histological subtype. The OS of patients with SCLC was 11 months (SD: 6.2 ± 1.3 months), patients with LCNEC was 9.3 months (SD: 6.2 ± 2.2 months), and patients with carcinoid was 15.6 months (SD: 3.9 ± 1.6 months) at the time of the last follow-up. Regarding the survival rate, no significant difference was observed in patients with SCLC and LCNEC (P > .05), whereas significant difference was observed when compared to patients with carcinoid (P = 0036 and P = .028, respectively; Figure 1).
Figure 1.
Kaplan-Meier curve: Survival in the 3 different groups, SCLC, LCNEC and carcinoids, statistically significant between SCLC carcinoids (P = .036) and LCNEC carcinoids (P = .028) but not between SCLC–LCNEC (P > .05). LCNEC indicates large-cell neuroendocrine carcinoma; SCLC, small-cell lung carcinoma
Concerning SSTRs expressed with Krenning score, no correlation was observed in the patients’ survival at baseline (P = .08), whereas correlation was observed at the time of progression (P = .003). The higher the Krenning score was in the progression, the worse the survival. Likewise, scintigraphy results did not relate significantly to PFS initially (P < .05), whereas there was a significant relation between scintigraphy results and PFS at the time of relapse (P = .02).
Regarding the results of scintigraphy according to the response of patients in the initial treatment, there was a statistically significant negative correlation both in the first and in the second scintigraphy with patients’ response (P = .001 and P < .001, respectively), that is, the excess of SSTRs was associated with negative response (Table 2, Figure 2). Moreover, the difference in scintigraphy uptake in the 2 measurements correlated significantly with patients’ response (P < .001, linear regression).
Table 2.
Tektrotyd Results Before and After Treatment According to Patients Response.a
| N | Mean | Standard Deviation | Standard Error | 95% CI for Mean | Minimum | Maximum | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| First Tektrotyd | PD | 11 | 2.60 | 0.843 | 0.267 | 2.00 | 3.20 | 2 | 4 |
| SD | 10 | 2.40 | 0.516 | .163 | 2.03 | 2.77 | 2 | 3 | |
| PR + CR | 15 | 2.00 | 0.535 | 0.189 | 1.05 | 2.95 | 1 | 3 | |
| Total | 36 | 2.28 | 0.741 | 0.124 | 2.03 | 2.53 | 1 | 4 | |
| Second Tektrotyd | PD | 11 | 2.60 | 0.843 | 0.267 | 2.00 | 3.20 | 2 | 4 |
| SD | 10 | 1.60 | 0.843 | 0.267 | 1.00 | 2.20 | 1 | 3 | |
| PR + CR | 15 | 1.50 | 0.535 | 0.189 | 0.27 | 1.95 | 0 | 2 | |
| Total | 36 | 1.61 | 1.076 | 0.179 | 1.25 | 1.98 | 0 | 4 | |
Abbreviations: CI, confidence interval; CR, complete response; SD, stable disease; PD, progressive disease; PR, partial response.
aComplete response defined as the proportion of patients who achieved a complete response (disappearance of all target tumors) or a partial response (≥30% decrease in the sum of the longest diameters of target tumors; objective response), based on modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Figure 2.
Bar diagram with Tektrotyd results before and after treatment according to patients’ response.
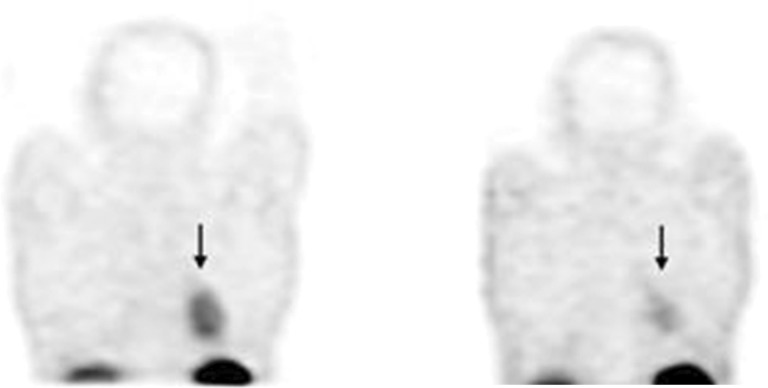
The Krenning score before therapy was significantly higher (2.28 ± 0.741, range: 2.03-2.53) than that at posttherapy time (1.61 ± 1.076, range: 1.0-1.98; P = .01). As shown in Figure 3, a patient with SCLC in limited stage is presented before (Krenning score = 2) and after chemotherapy (Krenning score = 1), suggesting a reduction in SSTRs during the course of disease.
Figure 3.
Reduction in primary tumor uptake before (left) and after (right) chemotherapy in patient with small-cell lung carcinoma (SCLC; arrows).
Biomarkers levels were estimated in 36 patients at the first visit and when the disease radiologically progressed. With regard to CgA, the concentration ranged from 10 to 108.326 ng/mL, with a median value of 27.91 ng/mL at the first and 31.18 ng/mL at the second estimation. A significant negative relation was demonstrated between serum levels of CgA and disease progression and patients’ survival (P = .01), but not at baseline (P = .18; Figure 4). Elevated CgA levels were also correlated with high SSTRs expression at baseline (P = .045). Patients with Krenning score 4 had mean CgA level 70 ng/mL (Figure 5).
Figure 4.
Chromogranin A levels at diagnosis (first measurement) and progression of the disease (second measurement).
Figure 5.
Mean values of neuroendocrine-specific enolase (NSE), chromogranin A (CGA), and insulin-like growth factor (IgF-1) in 4 different Tektrotyd levels expressed in Krenning score (0-4) at the time of diagnosis.
The NSE values varied from 3.45 to 51.67 ng/mL, with a median value of 23.90 ng/mL at the first and 19.41 ng/mL at the second measurement, without statistically significant relation to survival (P = .54 and .21, respectively). The NSE levels increased proportional to Krenning score (P = .018; Figure 5). Patients with Krenning score 4 had mean NSE levels 51.67 ng/mL.
Levels of IgF-1 ranged between 10.281 and 9 ng/mL before and after treatment, without any significant correlation with survival. First high measurements of IgF-1 were correlated statistically with high expression of SSTRs (P = .009; Figure 5).
Discussion
The results of our series confirmed the published relative data by other investigators, indicating that SRS identified all Lu-NETs.14,28 The SSTR expression as detected by Tektrotyd is an accurate and safe modality imaging, although it is not predictive of OS or PFS in patients with Lu-NETs. However, Tektrotyd could predict patient response to chemotherapeutic regimens, as higher uptake is consistent with poorer response. Tumor uptake, expressed in Krenning scale, depicts a rather aggressive biological behavior of the primary tumor in the pretreatment setting.
Another study29 reported an apparent decrease in the primary neoplasm somatostatin analogs accumulation during regression and uptake differences in patients assessed earlier and/or following chemotherapy. It was suggested that, apart from the reduced neoplasm burden, the tumor surface SSTRs could be decreased by therapy. However, this study included only SCLC and focused mainly on the diagnostic procedure, while the assessment tool was “in-pentetreotide.”
A more recent study assessed the prognostic value of SSTR2 expression in SCLC measured by 68Ga-DOTATATE-positron emission tomography (PET)/CT compared to histopathological SSTR2a expression and the response to peptide receptor radionuclide therapy (PRRT). The authors concluded that SSTR-directed PET/CT cannot be used as a noninvasive prognostic indicator, although it could be introduced as a theranostic mean for PRRT evaluation.30
The PET imaging with different radiotracers is a valuable functional imaging technique for several endocrine tumors.31 Recent data indicated that in metastatic NETs of unknown primary (Carcinoma of Unknown Primary site Neuroendocrine Tumors [CUP-NETs]), the pattern of uptake on dual-tracer PET (68Ga-DOTATATE and 18F-fluorodeoxyglucose [18F-FDG]) is linked to neoplasm proliferation index with a few outliers; combined dual tracer PET/CT with MIB-1/Ki-67 index is useful for better evaluation of the whole-body tumor biology in CUP-NETs.32 Moreover, introducing PET measurements of the 18F-FDG uptake (SUVmax) in the Lu-NETs, corrected for partial volume effects, proved achievable to distinguish the carcinoids from the hamartomas but not the clinically more aggressive atypical from the TCs.33 Both 18F-FDG PET and PET/CT, generally used as powerful imaging techniques in oncology, are limited in carcinoid neoplasms recognition, owing to their small proliferative activity.34
Nevertheless, other positron-emitting radionuclides are recommended, including 11C-5-hydroxytryptophan, 11C- or 18F dihydroxyphenylalanine, 18F-fluorodopamine, 11C-hydroxyephedrine, and others as well as and the most broadly used, 68Ga-labeled somatostatin analogs.35-37
Although there are several advantages concerning the affinity and resolution of the receptor, the cost and the question whether these pharmaceutical products are available, especially in our country, still do not permit their wide application.
Although our understanding has exponentially increased, there is still the need for more detailed research to clarify how SSTR signaling affects cell proliferation, especially in malignant tissue where mutated cells might exhibit a different response to SSTR signaling than normal cells.
Since somatostatin is able to exert antiproliferative effects and reduce tumor growth by suppressing the synthesis and secretion of growth factors, including IgF-1, an important modulator of many neoplasms,38,39 we measured IgF-1 levels before and after treatment, without nonetheless finding any statistically significant impact on patients’ survival.
In our study, high-functional imaging results combined with high levels of biochemical biomarkers predicted poor response to the treatment. Moreover, CgA levels at the time of disease progression exhibited an impact on patients’ survival. Serum CgA appears to be a useful noninvasive test, allowing the NETS’ recognition and management.40,41 The clinical usefulness of CgA as an indicator for NETS is established, and CgA concentrations are documented as a diagnostic and early relapse marker.42 Decrease in CgA concentration can be introduced as a common marker of response to treatment43; elevated levels of CgA are strongly correlated with tumor volume.44
However, caution in the interpretation of these data is warranted because of the limitations of our study. Median follow-up in our study is only 15 months and the number of patients is limited in order to establish a diagnostic tool for treatment prognosis. Another question is if all kinds of Lu-NETs have the same behavior to therapeutic management, as surgical resection in carcinoid tumors is the choice treatment and no other chemotherapy is usually needed.
Similar to other predictive biomarkers, overexpression of vascular endothelial growth factor (VEGF) suggests a poor prognosis for patients with NSCLC and SCLC but not the response to the treatment,45 and SSTRs, expressed by scintigraphy, are linked with clinical outcomes for patients with Lu-NETs. This expression profile offers a prognostic signature for patients with Lu-NETs, especially for those with neuroendocrine characteristics. In this regard, neuroendocrine cancer cell lines are used to evaluate therapeutic targets in NETs and have been helpful in the design of clinical trials targeting, apart from somatostatin analogs, the VEGF inhibitors.46
Management of Lu-NETs remains a therapeutic challenge. Due to poor prognosis, especially for SCLC, works are made to develop a prognostic tool to follow the appropriate treatment options. In particular, relative knowledge seems to be inadequate to predict early and accurately progressive or stable disease. Furthermore, there is an agreement concerning a clinically actionable, biologically significant biomarker introduced in treatment response valuations.47
Conclusion
In the personalized medicine period, upcoming success in diagnosing and treating Lu-NETs might incorporate the exploitation of SSTRs and signaling pathways. The Tektrotyd scintigraphy could be of prognostic value for the response of patients with Lu-NETs, though not for their survival.
Abbreviations
- CgA
chromogranin A
- CT
computed tomography
- EDDA
ethylenodiaminodioxide acid
- ELISA
enzyme-linked immunosorbent assay
- HYNIC
6-hydrazino nicotinic acid
- IgF-1
insulin-like growth factor 1
- LCNEC
large-cell neuroendocrine carcinoma
- Lu-NETs
NET of the lungs
- NET
neuroendocrine tumors
- NSE
neuroendocrine-specific enolase
- OS
overall survival
- PET
positron emission tomography
- PFS
progression-free survival
- PRRT
peptide receptor radionuclide therapy
- SCLC
small-cell lung carcinoma
- SD
standard deviation
- SPECT
single-photon emission computed tomography
- SRS
the somatostatin receptor scintigraphy
- SSTR
somatostatin receptors
- TC
typical carcinoid
- Tektrotyd
99mTc-HYNIC-TOC
- TOC
Tyr3-octreotide
- VEGF
vascular endothelial growth factor.
Footnotes
Authors’ Note: All procedures performed in this study involving human participants were in accordance with the ethical standards of our institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As it has been highlighted in the manuscript, the study was approved by G. Papanikolaou Hospital’s ethics committee (Registration number: 359/15). Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by IKY Fellowships of Excellence for Postgraduate Studies in Greece-Siemens Program (grant number: SR 22911).
ORCID iD: Efimia Boutsikou, PhD  https://orcid.org/0000-0003-3430-6916
https://orcid.org/0000-0003-3430-6916
References
- 1. Klimstra DS, Modlin IR, Adsay NV, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34(3):300–313. [DOI] [PubMed] [Google Scholar]
- 2. Klimstra DS. Pathologic classification of neuroendocrine neoplasms. Hematol Oncol Clin North Am. 2016;30(1):1–19. [DOI] [PubMed] [Google Scholar]
- 3. Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: european neuroendocrine tumor society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604–1620. [DOI] [PubMed] [Google Scholar]
- 4. Ploenes T, Aigner C. Resection concepts for early stage neuroendocrine tumors of the lungs and bronchi [in German]. Chirurg. 2018;89(6):440–447. [DOI] [PubMed] [Google Scholar]
- 5. Hilal T. Current understanding and approach to well differentiated lung neuroendocrine tumors: an update on classification and management. Ther Adv Med Oncol. 2017;9(3):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134(11):1628–1638. [DOI] [PubMed] [Google Scholar]
- 7. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pruthi A, Pankaj P, Verma R, Jain A, Belho ES, Mahajan H. Ga-68 DOTANOC PET/CT imaging in detection of primary site in patients with metastatic neuroendocrine tumours of unknown origin and its impact on clinical decision making: experience from a tertiary care centre in India. J Gastrointest Oncol. 2016;7(3):449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuccurullo V, Di Stasio GD, Mansi L. Radioguided surgery with radiolabeled somatostatin analogs: not only in GEP-NETs. Nucl Med Rev Cent East Eur. 2017;20(1):49–56. [DOI] [PubMed] [Google Scholar]
- 10. Florio T. Molecular mechanisms of the antiproliferative activity of somatostatin receptors (SSTRs) in neuroendocrine tumors. Front Biosci. 2008;13:822–840. [DOI] [PubMed] [Google Scholar]
- 11. Lo Russo G, Pusceddu S, Prinzi N, et al. Peptide receptor radionuclide therapy: focus on bronchial neuroendocrine tumors. Tumour Biol. 2016;37(10):12991–13003. [DOI] [PubMed] [Google Scholar]
- 12. Callison JC, Jr, Walker RC, Massion PP. Somatostatin receptors in lung cancer: from function to molecular imaging and therapeutics. J Lung Cancer. 2011;10(2):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parisella MG, Chianelli M, D’Alessandria C, et al. Clinical indications to the use of (99 m)Tc-EDDA/HYNIC-TOC to detect somatostatin receptor-positive neuroendocrine tumors. Q J Nucl Med Mol Imaging. 2012;56(1):90–98. [PubMed] [Google Scholar]
- 14. Sergieva S, Robev B, Dimcheva M, Fakirova A, Hristoskova R. Clinical application of SPECT-CT with 99mTc-Tektrotyd in bronchial and thymic neuroendocrine tumors (NETs). Nucl Med Rev Cent East Eur. 2016;19(2):81–87. [DOI] [PubMed] [Google Scholar]
- 15. Werner RA, Bluemel C, Lassmann M, et al. SPECT- and PET-based patient-tailored treatment in neuroendocrine tumors: a comprehensive multidisciplinary team approach. Clin Nucl Med. 2015;40(5):e271–e277. [DOI] [PubMed] [Google Scholar]
- 16. de Herder WW, Mazzaferro V, Tavecchio L, Wiedenmann B. Multidisciplinary approach for the treatment of neuroendocrine tumors. Tumori. 2010;96(5):833–846. [DOI] [PubMed] [Google Scholar]
- 17. Decristoforo C, Melendez-Alafort L, Sosabowski JK, Mather SJ. 99mTc-HYNIC-[Tyr3]-octreotide for imaging somatostatin-receptor-positive tumors: preclinical evaluation and comparison with 111In-octreotide. J Nucl Med. 2000;41(6):1114–1119. [PubMed] [Google Scholar]
- 18. Pavlovic S, Artiko V, Sobic-Saranovic D, et al. The utility of 99mTc-EDDA/HYNIC-TOC scintigraphy for assessment of lung lesions in patients with neuroendocrine tumors. Neoplasma. 2010;57(1):68–73. [DOI] [PubMed] [Google Scholar]
- 19. Plachcinska A, Mikołajczak R, Maecke HR, et al. 99mTc-EDDA/HYNIC-TOC scintigraphy in the differential diagnosis of solitary pulmonary nodules. Eur J Nucl Med Mol Imaging. 2004;31(7):1005–1010. [DOI] [PubMed] [Google Scholar]
- 20. Artiko V, Afgan A, Petrović J, et al. Evaluation of neuroendocrine tumors with 99mTc-EDDA/HYNIC TOC. Nucl Med Rev Cent East Eur. 2016;19(2):99–103. [DOI] [PubMed] [Google Scholar]
- 21. Sobic-Saranovic DP, Pavlovic SV, Artiko VM, et al. The utility of two somatostatin analog radiopharmaceuticals in assessment of radiologically indeterminate pulmonary lesions. Clin Nucl Med. 2012;37(1):14–20. [DOI] [PubMed] [Google Scholar]
- 22. Plachcinska A, Mikołajczak R, Kozak J, Rzeszutek K, Kuśmierek J. Differential diagnosis of solitary pulmonary nodules based on 99mTc-EDDA/HYNIC-TOC scintigraphy: the effect of tumour size on the optimal method of image assessment. Eur J Nucl Med Mol Imaging. 2006;33(9):1041–1047. [DOI] [PubMed] [Google Scholar]
- 23. Krenning EP, Valkema R, Kooij PP, et al. Scintigraphy and radionuclide therapy with [indium-111-labelled-diethyl triamine penta-acetic acid-D-Phe1]-octreotide. Ital J Gastroenterol Hepatol. 1999;31(suppl 2):S219–S223. [PubMed] [Google Scholar]
- 24. Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35(2):500–516. [DOI] [PubMed] [Google Scholar]
- 25. Wetz C, Apostolova I, Steffen IG, et al. Predictive value of asphericity in pretherapeutic [111In]DTPA-Octreotide SPECT/CT for response to peptide receptor radionuclide therapy with [177Lu]DOTATATE. Mol Imaging Biol. 2017;19(3):437–445. [DOI] [PubMed] [Google Scholar]
- 26. Hamada T, Nakai Y, Isayama H, et al. Progression-free survival as a surrogate for overall survival in first-line chemotherapy for advanced pancreatic cancer. Eur J Cancer. 2016;65:11–20. [DOI] [PubMed] [Google Scholar]
- 27. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 28. Granberg D, Sundin A, Janson ET, Oberg K, Skogseid B, Westlin JE. Octreoscan in patients with bronchial carcinoid tumours. Clin Endocrinol (Oxf). 2003;59(6):793–799. [DOI] [PubMed] [Google Scholar]
- 29. Reisinger I, Bohuslavitzki KH, Brenner W, et al. Somatostatin receptor scintigraphy in small-cell lung cancer: results of a multicenter study. J Nucl Med. 1998;39(2):224–227. [PubMed] [Google Scholar]
- 30. Lapa C, Hänscheid H, Wild V, et al. Somatostatin receptor expression in small cell lung cancer as a prognostic marker and a target for peptide receptor radionuclide therapy. Oncotarget. 2016;7(15):20033–20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Treglia G, Kroiss AS, Piccardo A, Lococo F, Santhanam P, Imperiale A. Role of positron emission tomography in thyroid and neuroendocrine tumours. Minerva Endocrinol. 2017;43(3):341–355. [DOI] [PubMed] [Google Scholar]
- 32. Sampathirao N, Basu S. MIB-1 index-stratified assessment of dual-tracer PET/CT with (68)Ga-DOTATATE and (18)F-FDG and multimodality anatomic imaging in metastatic neuroendocrine tumors of unknown primary in a PRRT workup setting. J Nucl Med Technol. 2017;45(1):34–41. [DOI] [PubMed] [Google Scholar]
- 33. Uhlen N, Grundberg O, Jacobsson H, et al. 18F-FDG PET/CT diagnosis of bronchopulmonary carcinoids versus pulmonary hamartomas. Clin Nucl Med. 2016;41(4):263–267. [DOI] [PubMed] [Google Scholar]
- 34. Tatci E, Ozmen O, Gokcek A, et al. 18F-FDG PET/CT rarely provides additional information other than primary tumor detection in patients with pulmonary carcinoid tumors. Ann Thorac Med. 2014;9(4):227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nanni C, Fantini L, Nicolini S, Fanti S. Non FDG PET. Clin Radiol. 2010;65(7):536–548. [DOI] [PubMed] [Google Scholar]
- 36. Kratochwil C, Giesel FL, López-Benítez R, et al. Intraindividual comparison of selective arterial versus venous 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors. Clin Cancer Res. 2010;16(10):2899–2905. [DOI] [PubMed] [Google Scholar]
- 37. Kahn D, Menda Y, Kernstine K, et al. The utility of 99mTc depreotide compared with F-18 fluorodeoxyglucose positron emission tomography and surgical staging in patients with suspected non-small cell lung cancer. Chest. 2004;125(2):494–501. [DOI] [PubMed] [Google Scholar]
- 38. Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17(12):1733–1742. [DOI] [PubMed] [Google Scholar]
- 39. Domvri K, Bougiouklis D, Zarogoulidis P, et al. Could somatostatin enhance the outcomes of chemotherapeutic treatment in SCLC? J Cancer. 2015;6(4):360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Al-Risi ES, Al-Essry FS, Mula-Abed WS. Chromogranin A as a biochemical marker for neuroendocrine tumors: a single center experience at royal hospital, Oman. Oman Med J. 2017;32(5):365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pifano M, Garona J, Capobianco CS, Gonzalez N, Alonso DF, Ripoll GV. Peptide agonists of vasopressin V2 receptor reduce expression of neuroendocrine markers and tumor growth in human lung and prostate tumor cells. Front Oncol. 2017;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nisman B, Heching N, Biran H, Barak V, Peretz T. The prognostic significance of circulating neuroendocrine markers chromogranin a, pro-gastrin-releasing peptide and neuron-specific enolase in patients with advanced non-small-cell lung cancer. Tumour Biol. 2006;27(1):8–16. [DOI] [PubMed] [Google Scholar]
- 43. Gut P, Czarnywojtek A, Fischbach J, et al. Chromogranin A—unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci. 2016;12(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lv Y, Han X, Zhang C, et al. Combined test of serum CgA and NSE improved the power of prognosis prediction of NF-pNETs. Endocr Connect. 2018;7(1):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhan P, Wang J, Lv XJ, et al. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol. 2009;4(9):1094–1103. [DOI] [PubMed] [Google Scholar]
- 46. Boora GK, Kanwar R, Kulkarni AA, et al. Exome-level comparison of primary well-differentiated neuroendocrine tumors and their cell lines. Cancer Genet. 2015;208(7-8):374–381. [DOI] [PubMed] [Google Scholar]
- 47. Oberg K, Krenning E, Sundin A, et al. A Delphic consensus assessment: imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr Connect. 2016;5(5):174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]