Abstract
Background:
There are limited data regarding prolonged extracorporeal membrane oxygenation (ECMO) support, despite increase in ECMO use and duration in patients with respiratory failure. The objective of this study was to investigate the outcomes of severe acute respiratory failure patients supported with prolonged ECMO for more than 28 days.
Methods:
Between January 2012 and December 2015, all consecutive adult patients with severe acute respiratory failure who underwent ECMO for respiratory support at 16 tertiary or university-affiliated hospitals in South Korea were enrolled retrospectively. The patients were divided into two groups: short-term group defined as ECMO for ⩽28 days and long-term group defined as ECMO for more than 28 days. In-hospital and 6-month mortalities were compared between the two groups.
Results:
A total of 487 patients received ECMO support for acute respiratory failure during the study period, and the median support duration was 8 days (4–20 days). Of these patients, 411 (84.4%) received ECMO support for ⩽28 days (short-term group), and 76 (15.6%) received support for more than 28 days (long-term group). The proportion of acute exacerbation of interstitial lung disease as a cause of respiratory failure was higher in the long-term group than in the short-term group (22.4% versus 7.5%, p < 0.001), and the duration of mechanical ventilation before ECMO was longer (4 days versus 1 day, p < 0.001). The hospital mortality rate (60.8% versus 69.7%, p = 0.141) and the 6-month mortality rate (66.2% versus 74.0%, p = 0.196) were not different between the two groups. ECMO support longer than 28 days was not associated with hospital mortality in univariable and multivariable analyses.
Conclusions:
Short- and long-term survival rates among patients receiving ECMO support for more than 28 days for severe acute respiratory failure were not worse than those among patients receiving ECMO for 28 days or less.
Keywords: acute respiratory failure, extracorporeal life support, long-term care, outcomes
Introduction
Extracorporeal membrane oxygenation (ECMO) is a therapeutic modality for treating severe acute respiratory failure that has been refractory to conventional support.1 Continuous survival improvements have been reported for patients receiving ECMO support; these encouraging clinical results led to increased use of ECMO.2 Meanwhile, technological advances in circuit components and overall improvement in patient management reduced the rate of adverse events during ECMO support.3 In addition, ECMO support can be maintained safely for a relatively long period. There have been several reports of patients who received prolonged ECMO support.4 However, there is often a concern about whether ECMO is futile in patients with a very low chance of recovery, especially if prolonged ECMO is required. This is partly due to the limited prognostic data for acute respiratory failure patients that require prolonged ECMO, despite the increase in ECMO use and duration. The aim of this study was to investigate the characteristics and clinical outcomes of patients supported with prolonged ECMO for severe acute respiratory failure.
Methods
Study design
This retrospective observational cohort study was conducted using a multicenter registry that collected data from 16 tertiary or university-affiliated hospitals in South Korea between January 2012 and December 2015 (Supplementary Table S1). All adult patients age 16 years or over who treated with ECMO for severe acute respiratory failure were eligible for inclusion. Participating centers registered a total of 491 patients during the study period; among them, we excluded 4 patients with no data on total duration of ECMO support.
There were no predefined criteria for indications and contraindications of ECMO and protocol for patients and circuit management during ECMO. The decisions were left to the discretion of each participating center, but usually followed general recommendations in the extracorporeal life support organization guidelines and practices reported by previous studies and delivered modern critical care.
This study was approved by the institutional review board of each participating hospital, and the requirement for informed consent was waived because of the study’s noninterventional nature.
Data collection and clinical outcomes
Data from ECMO-treated patients was collected by trained study coordinators using a standardized form.5,6 The database included the following patient information. (a) Patient’s clinical characteristics and therapeutic management before ECMO initiation: age, sex, body weight and height, primary diagnosis of respiratory failure classified into eight categories, sequential organ failure assessment (SOFA) before ECMO initiation, respiratory extracorporeal membrane oxygenation survival prediction (RESP) score (6), predicting death for severe ARDS on VV-ECMO (PRESERVE) score (7), mechanical ventilation and adjunctive/rescue therapies used before ECMO initiation, hemodynamic and ventilatory parameters, laboratory variables. (b) Clinical course during ECMO support: mode of ECMO, equipment, membrane oxygenator, number of membrane changes, and ECMO support duration. (c) Clinical outcomes: weaning from mechanical ventilation and ECMO, length of stay, hospital discharge status, and 6-month mortality after ECMO initiation.
In this study, we defined short-term support as ECMO for ⩽28 days and the long-term support as ECMO for more than28 days. The primary outcome in this study was in-hospital mortality. Secondary outcomes were 6-month mortality after ECMO initiation and intensive-care unit and hospital stay lengths.
Statistical analysis
To compare characteristics and clinical outcomes between the two groups, we analyzed categorical variables using the χ2 test or Fisher’s exact test, where applicable; we presented data as numbers and percentages. We used the Mann-Whitney U test for analyses of continuous variables, which we presented as medians with interquartile ranges. Logistic regression analysis was performed to evaluate the effect of long-term ECMO support and to identify the risk factors for hospital mortality. Variables that had significant association with hospital mortality in the univariable regression analysis at a p value less than 0.05 were eligible for entry into a multivariable regression model. Odds ratios (ORs) of each variable are reported with their 95% confidence intervals (CIs). A p value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS 18.0 for Windows (Chicago, IL, USA).
Results
During the study period, a total of 487 patients received ECMO support for acute respiratory failure, and the median ECMO support duration among all patients was 8 days (4–20 days). Of these patients, 411 (84.4%) received ECMO support for 28 days or less, and 76 (15.6%) received support for more than 28 days.
The baseline clinical characteristics for the two groups are shown in Table 1. Age, sex, and disease severity scores at ECMO initiation were not different between the two groups. However, the proportion of acute exacerbation of interstitial lung disease as a cause of respiratory failure was higher in the long-term group than in the short-term group (22.4% versus 7.5%, p < 0.001). The ventilator parameters before ECMO were similar, but the duration of mechanical ventilation was significantly longer in the long-term group than in the short-term group (4 days versus 1 day, p < 0.001). In arterial blood gas analysis, the short-term group had lower pH, bicarbonate level, and oxygen saturation. And the short-term group had higher level of creatinine (1.05 mg/dl versus 0.73 mg/dl, p < 0.001) and lactate (2.8 mmol/l versus 1.6 mmol/l, p < 0.001) than the long-term group.
Table 1.
Baseline patient characteristics of all consecutive patients treated with extracorporeal membrane oxygenation (ECMO) for severe respiratory failure according to ECMO duration.
| Characteristics | Short-term group (n = 411) |
Long-term group (n = 76) |
p value |
|---|---|---|---|
| Age, years | 59 (46–67) | 58 (44–65) | 0.389 |
| Male | 267 (65.0) | 54 (71.1) | 0.304 |
| Primary diagnosis | <0.001 | ||
| Viral pneumonia | 34 (8.3) | 9 (11.8) | |
| Bacterial pneumonia | 94 (22.9) | 20 (26.3) | |
| Asthma/COPD | 6 (1.5) | 1 (1.3) | |
| Trauma/Burn | 24 (5.8) | 0 (0.0) | |
| Asphyxia | 4 (1.0) | 0 (0.0) | |
| Interstitial lung disease | 31 (7.5) | 17 (22.4) | |
| Chronic respiratory failure | 16 (3.9) | 5 (6.6) | |
| Other respiratory failure | 202 (49.1) | 24 (31.6) | |
| SOFA scores before ECMO | 11 (8–14) | 11 (7–12) | 0.007 |
| RESP scores | 0 (−2–2) | 0 (−1–2) | 0.601 |
| PRESERVE scores | 5 (4–6) | 5 (3–6) | 0.111 |
| MV before ECMO | |||
| Duration of MV, days | 1 (0–6) | 4 (2–10) | 0.001 |
| MV settings | |||
| FiO2, % | 1.0 (0.9–1.0) | 1.0 (0.8–1.0) | 0.209 |
| PEEP, cmH2O | 10 (6–12) | 10 (5–12) | 0.541 |
| Peak inspiratory pressure, cmH2O | 28 (23–32) | 29 (25–32) | 0.345 |
| Minute ventilation, l/min | 9.6 (7.4–12.4) | 9.3 (7.0–13.2) | 0.947 |
| Arterial blood gas before ECMO | |||
| pH | 7.26 (7.15–7.37) | 7.34 (7.26–7.43) | <0.001 |
| PaCO2, mmHg | 51.3 (38.8–66.0) | 49.0 (38.0–59.7) | 0.284 |
| PaO2, mmHg | 61.0 (50.3–75.0) | 68.0 (53.3–82.6) | 0.065 |
| HCO3, mmol/l | 22.4 (18.5–28.0) | 24.9 (21.2–30.5) | 0.003 |
| SaO2, % | 87.6 (78.2–92.9) | 90.8 (84.0–94.4) | 0.005 |
| Laboratory variables before ECMO | |||
| C-reactive protein, mg/dl | 12.0 (4.8–21.4) | 8.9 (4.2–25.4) | 0.810 |
| Hemoglobin, g/dl | 10.6 (9.0–12.3) | 10.8 (9.1–12.8) | 0.475 |
| Platelets, 103/mm3 | 137 (77–226) | 140 (89–230) | 0.431 |
| Total bilirubin, mg/dl | 0.9 (0.5–1.9) | 0.8 (0.5–1.1) | 0.061 |
| AST, IU/l | 50 (30–115) | 41 (31–72) | 0.202 |
| ALT, IU/l | 31 (16–74) | 38 (17–72) | 0.687 |
| BUN, mg/dl | 23 (14–34) | 25 (17–32) | 0.682 |
| Creatinine, mg/dl | 1.05 (0.73–1.61) | 0.73 (0.51–1.13) | <0.001 |
| LDH, IU/l | 703 (407–1116) | 754 (679–966) | 0.389 |
| Lactate, mmol/l | 2.8 (1.5–6.3) | 1.6 (1.0–2.8) | 0.001 |
| Initial mode of ECMO | 0.442 | ||
| Veno-venous | 362 (88.1) | 63 (82.9) | |
| Veno-arterial | 36 (8.8) | 10 (13.2) | |
| Others* | 13 (3.2) | 3 (4.0) |
Values are given as the median (interquartile range) or n (%).
Others include veno-venous-arterial, veno-veno-venous-arterial, and veno-arteriovenous.
ALT, alanine aminotransferase; AST, aspartate transaminase; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; LDH, lactate dehydrogenase; MV, mechanical ventilation; PaCO2, partial pressure of carbon dioxide in arterial blood; PaO2, partial pressure of oxygen in arterial blood; PEEP, positive end-expiratory pressure; PRESERVE, predicting death for severe ARDS on VV-ECMO; RESP, respiratory extracorporeal membrane oxygenation survival prediction; SaO2, arterial oxygen saturations; SOFA, sequential organ failure assessment.
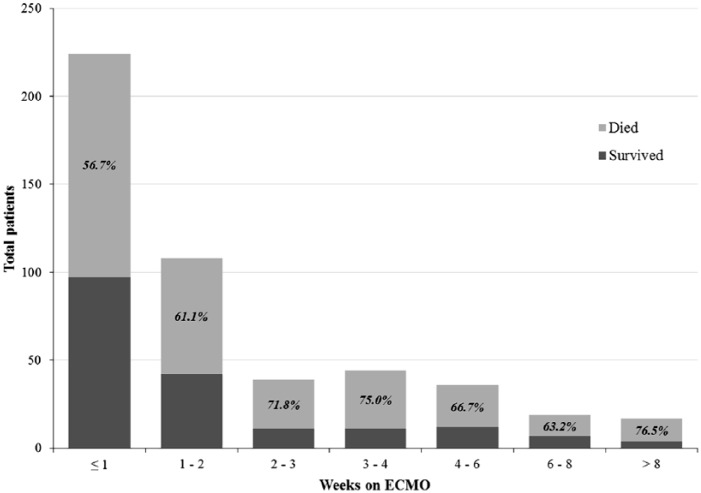
Overall, 303 patients (62.2%) died during hospitalization. When calculated according to ECMO discontinuation week (Figure 1), mortality increased from 56.7% (95% CI 50.1–63.1) in the 1st week and peaked in the 4th week at 73.5% (95% CI 60.0–85.7). The mortality rate appeared to decrease after this point, but the CIs were wide due to decreasing numbers of patients at risk.
Figure 1.
Mortality by week of extracorporeal membrane oxygenation (ECMO) discontinuation.
The clinical outcomes of patients are presented in Table 2. There was no significant difference in hospital mortality rate between the short-term and long-term groups (60.8% versus 69.7%, p = 0.141). These results did not differ among centers and time periods (Supplementary Table S2, S3, and S4). In addition, 6-month mortality rate was not significantly different between the two groups (66.2% versus 74.0%, p = 0.196). The median ECMO support duration (39 days versus 7 days, p < 0.001), length of ICU stay (46 days versus 16 days, p < 0.001), and length of hospital stay (61 days versus 31 days, p < 0.001) were significantly longer in the long-term group than in the short-term group.
Table 2.
Comparisons of clinical outcomes between the short-term (⩽28 days) and the long-term (>28 days) groups.
| Clinical outcomes | Short-term group (n = 411) |
Long-term group (n = 76) |
p value |
|---|---|---|---|
| Duration of ECMO support, days | 7 (3–13) | 39 (34–55) | <0.001 |
| Mortality | |||
| Hospital mortality | 250 (60.8) | 53 (69.7) | 0.141 |
| Six-month mortality | 257 (66.2) | 54 (74.0) | 0.196 |
| Length of stays | |||
| ICU length of stay, days | 16 (8–28) | 46 (37–61) | <0.001 |
| Hospital length of stay, days | 31 (15–51) | 61 (45–93) | <0.001 |
Values are given as the median (interquartile range) or n (%).
ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Nonsurvivors received longer duration of ECMO support than survivors (9 days versus 7 days, p = 0.011) (Supplementary Table S5), but logistic regression analysis revealed that ECMO support longer than 28 days was not associated with hospital mortality in patients treated with ECMO for respiratory failure (Table 3). Only four pre-ECMO factors including age, RESP score, peak inspiratory pressure, and platelet counts were independent predictor of hospital mortality in multivariable logistic regression analysis.
Table 3.
Predictors of hospital mortality in patients treated with extracorporeal membrane oxygenation for severe respiratory failure.
| Variables | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | Adjusted OR | 95% CI | p value | |
| ECMO support longer than 28 days | 1.48 | 0.88–2.52 | 0.143 | 1.91 | 0.94–3.90 | 0.074 |
| Age | 1.04 | 1.03–1.06 | <0.001 | 1.04 | 1.02–1.06 | <0.001 |
| SOFA score before ECMO | 1.09 | 1.04–1.15 | 0.001 | 0.98 | 0.91–1.06 | 0.616 |
| RESP score | 0.83 | 0.78–0.88 | <0.001 | 0.88 | 0.80–0.96 | 0.004 |
| Peak inspiratory pressure | 1.07 | 1.03–1.1 | <0.001 | 1.05 | 1.01–1.10 | 0.011 |
| Hemoglobin | 0.84 | 0.77–0.92 | <0.001 | 0.91 | 0.81–1.02 | 0.121 |
| Platelet counts | 1.00 | 1.00–1.00 | 0.007 | 1.00 | 0.99–1.00 | 0.021 |
| BUN | 1.02 | 1.00–1.03 | 0.007 | 1.01 | 1.00–1.02 | 0.127 |
BUN, blood urea nitrogen; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; OR, odds ratio; RESP, respiratory extracorporeal membrane oxygenation survival prediction; SOFA, sequential organ failure assessment.
Discussion
In this study, we investigated the clinical outcomes in patients receiving ECMO support for more than 28 days for severe acute respiratory failure. Our results indicated that in-hospital mortality among long-term ECMO support patients (>28 days) was not significantly different from that among short-term ECMO support patients (<28 days). There was also no significant difference in 6-month mortality rate between the two groups.
The international report from the Extracorporeal Life Support Organization (ELSO) registry showed that the mean ECMO duration in adult patients with respiratory failure was 177 h (7 days) in 2012.7 Only 4 years later, however, the mean duration increased to 256–325 h (10–14 days) varying according to diagnosis.2 In conjunction with increases in support duration, the absolute number of patients receiving prolonged ECMO support increased, and approximately 15–20% of all ECMO runs are prolonged, although the definition of prolonged ECMO varies according to study.8–11 Despite continuous improvements in survival for adults supported with ECMO, prolonged ECMO often causes physicians to question the utility of this costly treatment.2 Posluszny and colleagues showed that prolonged ECMO cases between 2007 and 2013 had better clinical outcomes than those between 1989 and 2006, but even the improved survival rate of prolonged ECMO in the later period was significantly lower than that from overall ECMO-support cases or short-term ECMO-support cases, which supports physicians’ futility concerns.8
However, in two recent single-center studies, the survival rate of patients receiving ECMO support for 21 days or more was not different from that of patients receiving ECMO for less than 21 days.10,11 In addition, Camboni and colleagues divided 127 patients who required veno-venous ECMO support into short-term (⩽10 days), intermediate-term (11–20 days), and long-term (>21 days) groups and found that the survival to discharge rate in the long-term group was not worse than that of the short-term or intermediate-term group.9
Considering that lungs have vast recovery potential after acute injury, depending on both the underlying pulmonary function before ECMO and the primary cause of acute respiratory failure, the difference in duration of native lung recovery might depend on prognosis regardless of short- or long-term ECMO support.12 Several studies reported that patients receiving prolonged ECMO had a longer duration of mechanical ventilation before ECMO,8,10,11 in consistent with this study. In addition, the proportion of patients with acute respiratory failure due to acute exacerbation of interstitial lung disease was much higher among those receiving prolonged ECMO. Even now, it is difficult to accurately conclude whether patients with these characteristics actually require a longer recovery time and whether the repair process will continue after a specific time period. Further studies focusing on these questions are needed to identify which patients are expected to require prolonged ECMO.
Although this study provides additional information on prolonged ECMO for respiratory support with relatively large sample size from a national registry, our study has some limitations that should be considered. First, because it was conducted as a retrospective cohort study, there is always the possibility of selection bias influencing the significance of our findings. However, the data were collected from all patients consecutively treated with ECMO for respiratory failure at the majority of ECMO centers in Korea. Thus, our cohort is more likely to reflect the patients in routine ECMO practice in Korea. Nonetheless, a registry-based dataset has the inherent limitation of reflecting the pooled submitted data of multicenters with different treatment practices. Second, we arbitrarily choose ECMO support longer than 28 days for the long-term support, but there is no consensus on the definition of long-term ECMO support yet. As our interest was not merely to identify the survival of patients receiving ECMO over a long period of time, but rather whether maintaining long-term ECMO support is futile, we thought that it would be reasonable to define long-term ECMO based on consideration of clinical course of ARDS and role of ECMO support. In previous studies, the mortality rate of patients with ARDS was reached a plateau after 3–4 weeks.13–15 Respiratory support and minimization of ventilator induced lung injury via ECMO may theoretically provide opportunity for recovery in patients with progressive lung injury during this period. Therefore, we defined the patients who required ECMO more than 28 days as a long-term group. Third, the mortality rate in our cohort was higher than the reported mortality rate of patients received ECMO for respiratory support in ELSO. We think that difference in knowledge and experience of medical treatment and circuit management for patient receiving ECMO by country may partly affect the difference in mortality rates. A recent study showed that the survival rates of ECMO supported respiratory failure patients were gradually improved over time in Korea, and this result supports our assumptions.16 Last, ethical issues in patients requiring prolonged ECMO support, such as determining futility or maintenance of ECMO support, is different among countries, our findings have limitations in their generalizability to other population. However, our data suggest that timing for determining futility could be delayed.
In summary, short- and long-term survival rates among patients receiving ECMO support for more than 28 days for severe acute respiratory failure were not worse than those among patients receiving ECMO for 28 days or less. Our findings indicate that, if the patient was being appropriately supported with ECMO while also receiving support to prevent additional lung injury, extrapulmonary organ failure, or ECMO-related complications for a period of time sufficient for native lung recovery, then long ECMO duration itself does not indicate poor prognosis. Therefore, ECMO duration should not be used as a guide to determine when treatment has become futile and should be withdrawn.
Supplemental Material
Supplemental material, TAR-18-100_R1_Supplementary_tables for Clinical outcomes of patients receiving prolonged extracorporeal membrane oxygenation for respiratory support by Soo Jin Na, Jae-Seung Jung, Sang-Bum Hong, Woo Hyun Cho, Sang-Min Lee, Young-Jae Cho, Sunghoon Park, So-My Koo, Seung Yong Park, Youjin Chang, Byung Ju Kang, Jung-Hyun Kim, Jin Young Oh, So Hee Park, Jung-Wan Yoo, Yun Su Sim and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease
Acknowledgments
Authors SJ Na and JS Jung contributed equally.
Footnotes
Funding: This study was supported the Korea Health Technology R & D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare, Republic of Korea (grant number HC15C1507).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Youjin Chang  https://orcid.org/0000-0002-4838-466X
https://orcid.org/0000-0002-4838-466X
Kyeongman Jeon  https://orcid.org/0000-0002-4822-1772
https://orcid.org/0000-0002-4822-1772
Contributor Information
Soo Jin Na, Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Jae-Seung Jung, Department of Thoracic and Cardiovascular Surgery, Korea University Anam Hospital, Seoul, Republic of Korea.
Sang-Bum Hong, Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Woo Hyun Cho, Department of Internal Medicine, Pusan National University Yangsan Hospital, Gyeongsangnam-do, Republic of Korea.
Sang-Min Lee, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea.
Young-Jae Cho, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, Gyeonggi-do, Republic of Korea.
Sunghoon Park, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Hallym University Sacred Heart Hospital, Gyeonggi-do, Republic of Korea.
So-My Koo, Division of Pulmonary and Allergy Medicine, Department of Internal Medicine, Soonchunhyang University Hospital, Seoul, Republic of Korea.
Seung Yong Park, Department of Internal Medicine, Chonbuk National University Hospital, Jeollabuk-do, Republic of Korea.
Youjin Chang, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Inje University Sanggye Paik Hospital, Seoul, Republic of Korea.
Byung Ju Kang, Division of Pulmonology, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Republic of Korea.
Jung-Hyun Kim, Division of Pulmonary and Critical Care Medicine, Department of Medicine, CHA Bundang Medical Center, Gyeonggi-do, Republic of Korea.
Jin Young Oh, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Dongguk University Ilsan Hospital, Gyeonggi-do, Republic of Korea.
So Hee Park, Department of Pulmonary and Critical Care Medicine, Kyung Hee University Hospital at Gangdong, Seoul, Republic of Korea.
Jung-Wan Yoo, Department of Internal Medicine, College of Medicine, Gyeongsang National University Hospital, Gyeonsangnam-do, Republic of Korea.
Yun Su Sim, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Hallym University Kangnam Sacred Heart Hospital, Seoul, Republic of Korea.
Kyeongman Jeon, Department of Critical Care Medicine and Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Republic of Korea.
References
- 1. Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011; 365: 1905–1914. [DOI] [PubMed] [Google Scholar]
- 2. Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal life support organization registry international report 2016. ASAIO J 2017; 63: 60–67. [DOI] [PubMed] [Google Scholar]
- 3. Kotani Y, Honjo O, Davey L, et al. Evolution of technology, establishment of program, and clinical outcomes in pediatric extracorporeal membrane oxygenation: the “sickkids” experience. Artif Organs 2013; 37: 21–28. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg AA, Haft JW, Bartlett R, et al. Prolonged duration ECMO for ARDS: futility, native lung recovery, or transplantation? ASAIO J 2013; 59: 642–650. [DOI] [PubMed] [Google Scholar]
- 5. Baek MS, Chung CR, Kim HJ, et al. Age is major factor for predicting survival in patients with acute respiratory failure on extracorporeal membrane oxygenation: a Korean multicenter study. J Thorac Dis 2018; 10: 1406–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho WH, Oh JY, Yeo HJ, et al. Obesity survival paradox in pneumonia supported with extracorporeal membrane oxygenation: analysis of the national registry. J Crit Care 2018; 48: 453–457. [DOI] [PubMed] [Google Scholar]
- 7. Paden ML, Conrad SA, Rycus PT, et al. Extracorporeal life support organization registry report 2012. ASAIO J 2013; 59: 202–210. [DOI] [PubMed] [Google Scholar]
- 8. Posluszny J, Rycus PT, Bartlett RH, et al. Outcome of adult respiratory failure patients receiving prolonged (⩾14 days) ECMO. Ann Surg 2016; 263: 573–581. [DOI] [PubMed] [Google Scholar]
- 9. Camboni D, Philipp A, Lubnow M, et al. Support time-dependent outcome analysis for veno-venous extracorporeal membrane oxygenation. Eur J Cardiothorac Surg 2011; 40: 1341–1346;discussion 1346–1347. [DOI] [PubMed] [Google Scholar]
- 10. Kon ZN, Dahi S, Evans CF, et al. Long-term venovenous extracorporeal membrane oxygenation support for acute respiratory distress syndrome. Ann Thorac Surg 2015; 100: 2059–2063. [DOI] [PubMed] [Google Scholar]
- 11. Menaker J, Rabinowitz RP, Tabatabai A, et al. Veno-venous extracorporeal membrane oxygenation for respiratory failure: how long is too long? ASAIO J 2018; 64: 497–501. [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez-Lopez A, Albaiceta GM. Repair after acute lung injury: molecular mechanisms and therapeutic opportunities. Crit Care 2012; 16: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 14. Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354: 1671–1684. [DOI] [PubMed] [Google Scholar]
- 15. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354: 2564–2575. [DOI] [PubMed] [Google Scholar]
- 16. Baek MS, Lee SM, Chung CR, et al. Improvement in the survival rates of extracorporeal membrane oxygenation-supported respiratory failure patients: a multicenter retrospective study in Korean patients. Crit Care 2019; 23: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TAR-18-100_R1_Supplementary_tables for Clinical outcomes of patients receiving prolonged extracorporeal membrane oxygenation for respiratory support by Soo Jin Na, Jae-Seung Jung, Sang-Bum Hong, Woo Hyun Cho, Sang-Min Lee, Young-Jae Cho, Sunghoon Park, So-My Koo, Seung Yong Park, Youjin Chang, Byung Ju Kang, Jung-Hyun Kim, Jin Young Oh, So Hee Park, Jung-Wan Yoo, Yun Su Sim and Kyeongman Jeon in Therapeutic Advances in Respiratory Disease