Abstract
The gut microbiota is involved in the maintenance of the homeostasis of the human body and its alterations are associated with the development of different pathological conditions. The liver is the organ most exposed to the influence of the gut microbiota, and recently important connections between the intestinal flora and hepatocellular carcinoma (HCC) have been described. In fact, HCC is commonly associated with liver cirrhosis and develops in a microenvironment where inflammation, immunological alterations, and cellular aberrations are dramatically evident. Prevention and diagnosis in the earliest stages are still the most effective weapons in fighting this tumor. Animal models show that the gut microbiota can be involved in the promotion and progression of HCC directly or through different pathogenic mechanisms. Recent data in humans have confirmed these preclinical findings, shedding new light on HCC pathogenesis. Limitations due to the different experimental design, the ethnic and hepatological setting make it difficult to compare the results and draw definitive conclusions, but these studies lay the foundations for a pathogenetic redefinition of HCC. Therefore, it is evident that the characterization of the gut microbiota and its modulation can have an enormous diagnostic, preventive, and therapeutic potential, especially in patients with early stage HCC.
Keywords: bile acids, cirrhosis, gut microbiota, hepatocellular carcinoma, immune system, inflammation, microenvironment
Background
Hepatocellular carcinoma (HCC) is a heterogeneous tumor usually arising in an inflammatory environment. Indeed, in the vast majority of cases, HCC is diagnosed in patients with chronic liver disease, in whom it represents one of the leading causes of death despite surveillance programs for early diagnosis.1 Liver cirrhosis is the paradigm of inflammatory diseases, as it frequently develops in association with chronic viral or alcohol-related hepatitis, or in patients with nonalcoholic fatty liver disease (NAFLD); the hepatocellular damage produced by various etiologic agents eventually results in tissue repair up to the development of fibrosis. Persistent hepatocellular proliferation in this inflammatory microenvironment promotes genetic mutations that trigger hepatocarcinogenesis.2
The tumor microenvironment (TME), which includes stromal cells, such as angiogenic cells, immune cells, fibroblasts, and their products, is crucial in the pathogenesis of HCC. Particular importance has been given to the surrounding nontumor tissue. Indeed, oncogenic signals in the cirrhotic liver seem to have a prognostic relevance even more than HCC cells genomic profile, and this has been demonstrated also for early stage tumors.3–8
Interestingly, inflammation seems not to be extinguished by the elimination of the pathogenic noxa from the cirrhotic liver. Histological data after the cure of hepatitis C virus (HCV) infection have shown a significant improvement in fibrosis, periportal and lobular inflammation, but with the persistence of damage in the portal area.9 In addition, HCC can occur in subjects without liver cirrhosis and this is particularly common in patients with NAFLD.10,11 These findings suggest the existence of a pro-inflammatory mechanism that acts regardless of the etiologic agent of liver injury and of the presence of cirrhosis, which may involved in the development of HCC.
The gut microbiota is a crucial element in the progression of liver disease. Although the overall community of our intestinal bacteria is generally well structured in physiological conditions, being in some way influenced by age, diet, and geography, and resilient to perturbations, it can be strongly affected by various pathological conditions.12,13 In cirrhotic patients, the gut microbiota fingerprint is characterized by the reduction of beneficial microbes and the increase of those potentially pathogenic, this being associated with a marked systemic inflammation.14,15 In addition, the gut barrier is deranged leading to the translocation of bacteria and their products to the liver concurring to the persistence of an inflammatory microenvironment.16 The gut microbiota therefore can act as primer, being involved in the origination or maintenance of the inflammatory background that promotes the development of HCC.
Features of the TME associated with early HCC
The complex network of interactions that can be found in the HCC microenvironment, even in the early stage, involves humoral factors and cellular components that together concur in fostering inflammation and neoangiogenesis.17
The necroinflammatory damage caused by several external agents, such as hepatitis viruses, alcohol or fat accumulation, converges in the activation of molecular pathways and transcription factors leading to the production of cytokines and chemokines, growth factors, and proangiogenic signals, resulting in inflammation, cell regeneration, and fibrosis.18 Several studies have shown that similar biological processes and genes are dysregulated in liver regenerating tissue and in early stage HCC.19,20 In particular, liver cirrhosis appears to be linked with the development of HCC due to (a) genetic aberrations triggered by the damage/proliferation mechanism associated with persistent inflammation and (b) abnormal secretion of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), from hepatic stromal cells and fibroblasts, promoted by the capillarization of hepatic sinusoids, which is a consequence of portal hypertension.17 The increased expression of VEGF, PDGF, and FGF has been reported even in dysplastic nodules, and is associated with HCC progression in advanced stages.21,22 Nevertheless, the aberrant vascular structure of the cirrhotic liver is inefficient, causing hypoxia in the tumor tissue and the production of hypoxia-inducible factor 1 (HIF-1), which amplifies angiogenesis and further tumor progression.23,24
The most well-characterized molecular pathways that closely associate inflammation with HCC are the nuclear factor kB (NF-kB) and the interleukin (IL)-6/signal transducer activator of transcription 3 (STAT-3), both involved in the transcription of cytokines and chemokines-related genes, cell survival and proliferation, angiogenesis, tumor invasion, metastasis, and oxidative stress3,25–32 NF-kB can also exert antitumorigenic functions,33–37 and these two molecular systems are connected by a mutual crosstalk,35,38–40 making more complex the understanding of their involvement in liver cancer pathogenesis.
Although inflammation is the first hit that promotes the development of HCC lesions and the perpetuation of an oncogenic stimulation, the suppression of the immune system seems to be crucial. The cytokine profile associated with HCC metastatic potential and aggressiveness is related to Th2 lymphocytes (IL-4, IL-8, IL-10, and IL-5) rather than to Th1 ones (IL-1-alpha and beta, IL-2, and tumor necrosis factor (TNF)-alpha).41–46 T regulatory cells (Tregs),47 myeloid-derived the suppressor cells (MDSCs),48 tumor-associated macrophages (TAMs),49 invariant natural killer T cells (iNKT),50 and regulatory dendritic cells (DCs), which are involved in immune escape mechanisms, can be found not only in HCC tissue but also in the bloodstream and correlate with tumor progression. In particular, TAMs present in the tumor tissue are M2 macrophages, which have a weak antigen presentation potential and are rather involved in the promotion of angiogenesis, tissue remodeling, and activation of Th2 lymphocytes.49 The switch from M1 to M2 macrophages can be induced by tumor cells and by TME.
Co-inhibitory molecules are also part of the TME; in particular, the Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4), Programmed death-ligand 1 (PD-L1), and Programmed cell death protein 1 (PD-1) checkpoint inhibitors are the most well-characterized and are associated with immune-system exhaustion and immune tolerance in HCC.51–57 Their expression is enhanced in inflammatory conditions such as chronic liver disease, and this may promote oncogenic mechanisms as shown in experimental models.58–63
The role of the gut microbiota in hepatocarcinogenesis
In addition to the consideration that HCC is usually a tumor arising in a diseased organ, it is now clear that it also develops in a diseased intestinal environment. Therefore, the gut microbiota-driven hepatic inflammatory stimulation is additional to that derived from chronic liver disease and, together, they fuel the process of hepatocarcinogenesis.
Translocated bacterial products can trigger an inflammatory response by activating Toll-like receptors (TLRs). In particular, lipopolysaccharides (LPS) from Gram-negative bacteria can bind TLR-4, whereas TLR-9 is a ligand for DNA containing unmethylated CpG motifs and TLR-2 and TLR-5 recognize peptidoglycan and lipoteichoic acid, which are elements of the Gram-positive bacteria cell wall or flagellin, respectively.64–67 The final downstream effect is the production of inflammatory cytokines, such as TNF-alpha, IL-1-beta, and IL-6, via the NF-kB pathway.27,68 This links the gut microbiota with proliferation and immortalization of HCC cells, either directly or via the Janus kinase (JAK) or the STAT3 pathway, mainly activated by IL-6.25,69
Pathogen-associated molecular patterns (PAMPs) derived from the gut microbiota can activate the NADPH oxidase (NOX) 1–NOX4 complex in enterocytes, stimulating the generation of reactive oxygen species (ROS). This leads to the activation of inflammasomes, the release of cytokines such as IL-18 and IL-1-beta and the degranulation of goblet cells. Furthermore, oxidative signaling may up-regulate the activity of NF-κB interfering with the activation of IκB ubiquitin ligase through neddylation by Ubc12, a Nedd8 ligase, with a covalent modification on the cullin-1 (Cul-1) regulatory subunit.70–72
The interaction of gut bacteria with intestinal epithelium leads to the blockage of this process.73
Overall, this mechanism is protective and necessary for physiological functions, such as cell growth, differentiation, and regulation of ROS-sensitive enzymatic reactions and transcription of cytoprotective elements.74–76
However, an imbalance in the redox status, possibly related to microbial imbalance, can alter this machinery and enhance inflammation, causing DNA damage, lipid peroxidation, and protein oxidation, as well as disrupt the physiological process of cell proliferation and differentiation.77,78
Recent data have also shown that gut bacteria can regulate the bioavailability of glycine, which is essential for glutathione synthesis, reducing the antioxidant potential in the small intestine, liver, and colon.79 Glycine is also involved in DNA/histone methylation and in the metabolism of proteins and purines, playing a key role in the proliferation of cancer cells.80
Furthermore, the gut microbiota can modulate the farnesoid X receptor (FXR) activation81; mice knocked-out for the FXR gene in both the liver and the intestine spontaneously develop HCC82 but, most importantly, this can be avoided if FXR expression in the enterocytes is restored, being this effect is dependent on the level of bile acids.83 The increase in bile acids accumulation in the liver caused by FXR downregulation leads to damage of hepatocyte plasma membrane, triggering an inflammatory response through the protein kinase C (PKC)-p38 mitogen-activated protein kinase (MAPK) pathway and the increased production of ROS, which both eventually activate NF-kB.84,85 The accumulation of bile acids is further amplified by the inhibitory effect exerted by the heterodimer NF-kB p50/p65 on FXR.86
The gut microbiota may also be involved in the suppression of immune surveillance on HCC cells. Chronic inflammation derived from intestinal bacteria, particularly in a dysbiotic condition such as liver cirrhosis, can actually lead to exhaustion of the immune system.87,88 To confirm this, the chronic activation of TLRs, which can be driven by the gut microbiota, can lead macrophages to M2 polarization with consequent immunosuppressive effects.89–92 Nevertheless, Th17 lymphocytes are rarely found in the liver and come mainly from the gut, where they are produced through interaction with the microbiota.93 They have been found in serum and tumor tissue of HCC patients and are associated with poor prognosis, probably due to abnormal IL-17A IL17A secretion that enhances angiogenesis and the production of inflammatory cytokines.94–96
The gut microbiota and HCC
Animal models
Several studies on animal models provided evidence that the gut microbiota is involved in the pathogenesis of HCC.
In a pioneering study, Yu and colleagues97 reported an increase in plasma LPS levels in diethylnitrosamine (DEN)-treated rats during tumor progression, which were reduced by antibiotic treatment. Furthermore, the number and size of tumors, as well as tumor cell proliferation and liver weight were reduced after antibiotic administration. To confirm the role of endotoxin in the development of HCC, rats knocked-out for TLR-4, the LPS receptor, showed a reduction in HCC incidence by 25%, smaller diameters of nodules and more frequent evidence of cell apoptosis. This was associated with a reduced infiltration of macrophages and expression of TNF-alpha and IL-6, along with an attenuation in the detection of NF-kB in the liver tissue. Bone marrow transplantation from the TLR-4 mutant to wild-type rats led the lowest production of inflammatory mediators, whereas the opposite could be observed when wild-type bone marrow was transferred to knock-out animals.
Dapito and colleagues98 later demonstrated that the number and size of tumors was lower in mice knocked-out for TLR-4 than in wild-type controls, although tumor incidence did not differ significantly, and that this was associated with reduced inflammation. The same result was observed after antibiotic treatment or in germ-free rats. Interestingly, the effects of gut sterilization on HCC development seem to be preemptive: indeed, antibiotic treatment showed the maximum benefit when administered in the late phase of hepatotoxic stimulation and had no efficacy when tumor lesions were already established. Subcutaneous administration of low-dose LPS was also able to induce the development of HCC by triggering the expression of inflammatory genes. TLR-4 mutation in resident liver cells was necessary to reduce the number and size of HCC lesions, regardless of the TLR-4 expression in bone marrow cells.
A subsequent study also sought to elucidate the compositional difference in intestinal bacteria possibly associated with the presence of HCC.99 The authors started by observing a small group of patients with hepatitis B virus (HBV)-related liver cirrhosis with or without HCC compared with 16 healthy subjects. Plasma levels of LPS and IL-6 were higher in cirrhotic patients, regardless of the presence of HCC, whereas the anti-inflammatory IL-10 was reduced. Thus, a mice model of DEN-induced hepatocarcinogenesis was reproduced; circulating levels of LPS were high because the intestinal mucosa was damaged, and there was an increased abundance of Gram-negative bacteria (Escherichia coli, Atopobium, Collinsella, Eggerthella, and Coriobacterium) after DEN treatment. In contrast, beneficial bacteria such as Lactobacillus, Bifidobacterium, and Enterococcus were deficient. Both antibiotic treatment and dextran sulfate sodium (DSS) administration increased LPS levels, the number and size of HCC lesions, and cell proliferation; this was mediated by an increased inflammation, as evidenced by the enhanced expression of NF-kB and phosphorylation of STAT3. Furthermore, in this model the administration of high doses of the probiotic #VSL3 reduced the number and size of tumors, as well as the incidence of lesions, compared with lower doses of probiotic or no treatment. This was associated with the reduction in intestinal permeability, circulating levels of LPS and IL-6, NF-kB translocation, phosphorylation of STAT3, and the abundance of Gram-negative bacteria in the gut. Further data have confirmed that probiotics can reduce the growth, size, and weight of HCC lesions, producing a shift towards bacteria with anti-inflammatory activity (Prevotella, Oscillibacter), which decreases Th17 polarization and production of IL-17 in the intestine promoting the differentiation of anti-inflammatory Treg/type 1 regulatory T (Tr1) cells.100
Yoshimoto and colleagues101 have further focused on the relationship between the gut microbiota and HCC in the context of obesity. They found that obese mice fed with a high-fat diet (HFD) developed HCC after treatment with dimethylbenz(a)anthracene (DMBA), a chemical carcinogen. In addition, hepatic stellate cells (HSCs) in the proximity of cancerous hepatocytes expressed a senescence associated secretory phenotype (SASP), which was induced by the caspase1/IL-1-beta pathway following the activation of the inflammasome.102 Antibiotic treatment reduced the development of HCC and SASP HSCs. Interestingly, vancomycin but not TLR-4 gene deletion was able to block the development of HCC, suggesting that in this model the contribution of Gram-positive intestinal bacteria, which are devoid of LPS, the ligand for TLR-4, was the most relevant for the pathogenesis of HCC. Antibiotic treatment reduced the production of deoxycholic acid (DCA) by intestinal bacteria, which was caused by HFD; DCA is known to induce DNA damage through the production of ROS and this mechanism is a key factor in the development of SASP. Thus, DCA produced by an altered gut microbiota as a consequence of HFD leads to SASP in HSCs, leading to the secretion of inflammatory cytokines and oncogenic factors promoting the development of HCC after the exposure to a chemical carcinogen.
A further study confirmed an altered profile of serum and hepatic bile acids in a mice model of HCC related to nonalcoholic steatohepatitis (NASH).103 The authors observed an increase of LPS in plasma, liver, and stools as well as a significant alteration in the main bacterial genera involved in bile acid synthesis (Clostridium, Bacteroides, Atopobium, Desulfovibrio, Parasutterella, Akker-mansia). Administration of cholestyramine reduced inflammation and incidence and size of HCC tumors. To confirm the link between HFD, gut microbiota derangement, and bile acids toxicity in the liver, the incubation of bile acids with HepG2 cells inhibited the tumor suppressor gene CCAAT Enhancer Binding Protein (CEBP) alpha, thus promoting the development of HCC.
Yamada et al.104 have recently reported on the gut microbiota composition of mice fed with steatohepatitis-inducing HFD (STHD)-01, an experimental model of HCC associated with NASH. In particular, after 41 weeks, they observed an increase in liver weight and the development of HCC in the STHD-01 group, whereas in the STHD-01 mice receiving antibiotic treatment the whole body weight was increased and the occurrence of HCC was reduced. Increased abundance of Bacteroides and Clostridium cluster XVIII and a reduction in Bifidobacterium, Prevotella, and Streptococcus was observed in STHD-01 mice. Since the STHD-01 diet was enriched with cholesterol, which accumulated in the liver, bile acids synthesis was enhanced with subsequent accumulation in liver, plasma, and feces. Antibiotics did not reduce the accumulation of bile acids, but produced a compositional shift, decreasing the conversion from primary to secondary. In particular, DCA, tauro-DCA (TDCA), and hyo-DCA (HDCA) accumulated in the liver of the STHD-01 group and were reduced in the STHD-01 mice treated with antibiotics; instead the concentration of urso-DCA (UDCA), tauro-UDCA (TUDCA), and 12-keto lithocholic acid (KLCA) was not affected by antibiotic treatment. When tested on HepG2 cell lines, primary or secondary bile acids showed no toxic effect, although DCA was able to activate the mammalian target of rapamycin (mTOR) pathway, which is known to be activated in HCC cells.105 Increased phosphorylation of mTOR was also detected in the liver of mice fed with STHD-01 diet, and was attenuated by antibiotic administration.
Interestingly, a role of fermented fibers in the pathogenesis of bile acid-mediated hepatocarcinogenesis has been recently proposed.106 The authors used the T5KO mouse model that presents the deletion of TLR-5, the flagellin receptor, and develops a dysregulated innate immune response promoting dysbiosis (increased intestinal bacterial load and increased abundance of Proteobacteria), intestinal/systemic inflammation and metabolic syndrome. Feeding the T5KO mice an inulin containing diet (ICD) reduced the incidence of obesity by 40%, but these animals surprisingly developed cholestasis. Mice with hyperbilirubinemia showed higher liver enzymes and fibrosis markers, and reduced synthetic and detoxifying ability of the liver compared with mice fed with ICD, and all of them developed HCC. Histological analyses revealed that mice with high bilirubin developed a chronic liver disorder, characterized by steatosis, inflammation and fibrosis, increased hepatocyte proliferation and cell death. Pattern recognition receptors (PRR) such as Nucleotide-binding and oligomerization domain (NOD)-like receptor family card-containing-4 (NLRC4) and TLR-2 were upregulated as well as TLR-4 and NOD-like receptor pyrin domain-containing-3 (NLRP3) but to a lesser extent. The administration of a diet enriched in other soluble fibers such as pectin and fructo-oligosaccharide recapitulated the occurrence of hyperbilirubinemia, liver injury, and HCC, although at a lower rate (about 13%), whereas this was not observed when cellulose, a nonfermentable fiber, was administered. Feeding HFD enriched with inulin (HFD-I) attenuated the incidence of metabolic syndrome but increased the incidence of HCC from 40 to 65% in T5KO mice, and the same diet induced metabolic syndrome in all except 10% of wild-type animals, which also developed HCC. However, the first tumors were characterized by multinodular diffusion, the latter were small well-differentiated lesions. Mice that developed hyperbilirubinemia upon ICD diet displayed loss in gut bacteria richness and diversity, reduced Tenericutes, and increased abundance of Proteobacteria and Clostridia, which are capable of producing butyrate and secondary bile acids. Notably, butyrate is involved in the promotion or inhibition of cell proliferation based on the amount and duration of exposure and the type of target cell107 and excessive doses may exert oncogenic effects, promote liver steatosis and intestinal inflammation,108–112 whereas secondary bile acids have known hepatotoxic activity.113 The metabolomic analysis showed that an increase in butyrate cecal content characterized mice with hyperbilirubinemia and HCC. Butyrate administration to T5KO mice induced hyperbilirubinemia, liver inflammation, fibrosis, and upregulation of HCC markers without the development of evident tumor lesions; the depletion of butyrate-producing bacteria by metronidazole was able to reduce the incidence of HCC in T5KO mice fed with ICD and inhibition of bacterial fermentation by beta-acids abolished the development of tumors. Early switching of T5KO mice from inulin to cellulose containing diet when hyperbilirubinemia was initial protected from the development of HCC. Finally, the authors observed that ICD mice with hyperbilirubinemia presented atypical elevation of serum primary and secondary bile acids and reduced fecal excretion; treatment with cholestyramine reduced bilirubin and transaminases, and no tumor lesions were detected. To confirm the close correlation between the development of HCC and gut dysbiosis, specific T5KO mice models not developing dysbiosis and liver-specific T5KO mice, having a normal intestinal expression of TLR-5, did not develop hyperbilirubinemia and HCC. Furthermore, cohousing with T5KO mice induced hyperbilirubinemia and HCC in wild-type mice; in contrast, antibiotics reduced the occurrence of the phenotype in ICD T5KO mice and the same was observed for germ-free T5KO mice fed with an irradiated ICD.
Taken together, the results of this study demonstrate that, in the presence of gut dysbiosis, an excess of soluble fibers can cause butyrate overload and alterations in the bile acid pool, with consequent accumulation of lipids in the liver, inflammation, and tumorigenesis.
Another model of hepatocarcinogenesis involving the gut microbiota and induced by arsenic has been described.114 Germ-free mice showed higher urinary excretion of arsenic and its metabolites, with an increased monomethylarsonic acid (MMA)/dimethylarsinic acid (DMA) ratio, compared with conventional mice, as well as decrease in the fecal concentration of arsenic. The authors showed in a culture model that the gut microbiota is involved in the uptake of arsenic and also that germ-free mice present a downregulation of enzymes involved in arsenic methylation. Furthermore, gut microbiota depletion enhanced the toxic effect of arsenic on the liver by altering the expression of genes related to the p53 pathway and others related to hepatocarcinogenesis (StAR-related lipid transfer (START) domain containing 13 (STARD13), VEGF A, antizyme Inhibitor 1 (AZIN1), secreted phosphoprotein 1 (SPP1), HIV-1 Tat interactive protein 2 (HTATIP2), oxidative stress induced growth inhibitor 1 (OSGIN1), activated leukocyte cell adhesion molecule (ALCAM), prothymosin-alpha (PTMA), tribbles homolog 2 (TRIB2), and Atonal BHLH Transcription Factor 8 (ATOH8), suggesting an increased risk of developing HCC in germ-free mice.
Therefore, preclinical data show that intestinal dysbiosis coexists with the process of hepatocarcinogenesis. The gut microbiota could be involved through (a) the exacerbation of inflammation, (b) reduced conversion of primary bile acids with the accumulation of toxic compounds such as DCA, (c) the production of potentially harmful metabolites at high concentrations such as short chain fatty acids, and (d) altered metabolism of xenobiotics with carcinogenic effect. In particular, dysbiosis-related inflammation seems to play a strong pathogenetic role in all the experimental settings, whereas the toxic effect of bile acids and short chain fatty acids has been better studied in HCC models associated with hepatic steatosis. However, it is likely that these mechanisms can act synergistically in the complex process of hepatocarcinogenesis. For all these reasons, the modulation of the gut microbiota using antibiotics or probiotics seems to be a promising tool to interfere with the development or progression of neoplastic lesions.
Human studies
There is little evidence on the potential mechanistic relationship between the gut microbiota and HCC in humans.
A small prospective controlled study based on culture techniques analyzed the gut microbiota of 15 cirrhotic patients with HCC awaiting liver transplantation.115 Higher fecal counts of E. coli were significantly associated with HCC and showed a predictive accuracy of 0.742, with a bacterial count cut-off of 17.728 having a sensitivity of 66.7% and a specificity of 73.3%. However, the study was not based on metagenomic sequencing analyses, and only the stool counts of Enterococcus, E. coli, Proteus, Klebsiella, Enterobacter, Citrobacter, Serratia, Pseudomonas, Bifidobacterium, Bacteroides, Lactobacillus, Clostridium, and yeasts were taken into account.
More recently, the gut microbiota profile was evaluated in 20 Child Pugh A cirrhotic patients with NAFLD and early HCC and compared with counterparts without evidence of liver tumor, matched for age and severity of portal hypertension, and controls.116 This prospective study recognized an increase in intestinal permeability, measured by high circulating levels of zonulin-1 (ZO-1) and LPS, and in fecal calprotectin, which is a marker of intestinal inflammation, in patients with cirrhosis compared with healthy subjects. However, among cirrhotic patients, those with HCC presented a marked increase in fecal calprotectin without significant differences in permeability and LPS. This was associated with a specific increase in systemic cytokines and chemokines milieu, which was characterized by an increase in IL-8, IL-13, CCL-3, CCL-4, and CCL-5, and correlated with circulating activated monocytes and monocytic MDSC (mMDSC) in the HCC group. Metagenomic analysis showed a lower abundance of Akkermansia and an increase in Enterobacteriaceae in NAFLD cirrhotic patients compared with controls, and an additional depletion in Bifidobacterium and enrichment in Bacteroides and Ruminococcus in those affected by HCC compared with those without evidence of tumor. To draw a complete picture of the complex connections between all the microbial, inflammatory, and immunological elements, a correlation network displayed that the beneficial bacteria Akkermansia and Bifidobacterium were inversely associated with intestinal inflammation, which in turn was related to the expression of cytokines and chemokines, connecting the gut to the immune system compartment and the presence of HCC. The abundance of Bacteroides was correlated with pro-inflammatory cytokines such as IL-8 and IL-13, being involved in the development of HCC through inflammation probably due to the Western-type diet that is common in NAFLD cirrhotic patients.
Finally, Ren and colleagues117 explored the role of the gut microbiota as a potential biomarker for early HCC in a Chinese population of HBV cirrhotic patients. In the training cohort, Actinobacteria phylum and other 13 genera including Gemmiger, Parabacteroides, and Paraprevotella were enriched in cirrhotic patients with early HCC compared with those without. Other differences included a reduction in Verrucomicrobia and an increase in 12 genera including Alistipes, Phascolarctobacterium, and Ruminococcus and the reduction in six other genera, including Klebsiella and Haemophilus in HCC patients compared to controls. A probability of disease (POD) index was calculated using the best 30 operational taxonomic unit (OTU) markers for early HCC. In the validation phase, the POD showed an AUC of 76.80% between early HCC and controls and of 80.40% between advanced HCC and controls. However, it was not able to differentiate patients with early HCC from those with advanced tumors.
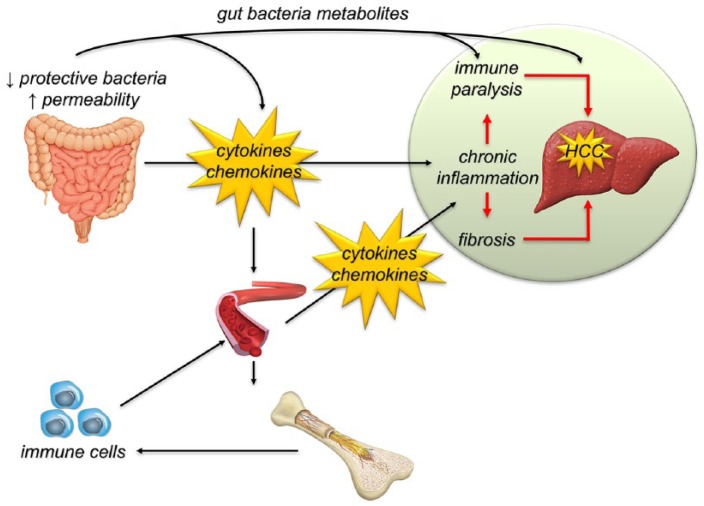
Taken together, the few studies available in humans show that during liver disease the gut microbiota is involved in the process of hepatocarcinogenesis, mainly feeding the pro-inflammatory microenvironment and interfering with the immune system (Figure 1).
Figure 1.
The lack of beneficial bacteria and the increase of intestinal permeability trigger a condition of chronic inflammation with consequent immunological activation, which in the long term can depress the immune system. Metabolic products of the gut microbiota, such as bile acids and short chain fatty acids, are involved in this process at multiple levels, through direct or indirect effects. This leads to the development of a pro-oncogenic microenvironment that promotes hepatocarcinogenesis and sustains tumor progression.
HCC, hepatocellular carcinoma.
Diagnostic and therapeutic potential of the gut microbiota for HCC
Several attempts have been made to identify key molecular or metabolic alterations able to characterize the transition from regenerating liver tissue to dysplasia and early HCC.2,118–121 However, HCC is a highly heterogeneous tumor, and this severely compromises our possibility to select the optimal biomarker for its early detection in patients at risk.
The gut microbiota could harbor a high diagnostic and therapeutic potential in this context. This may be associated with both the gut bacteria composition and metabolic functions. In fact, preclinical and clinical studies have shown a direct correlation between Gram-negative bacteria and inflammatory changes related to the development of HCC; in contrast, anti-inflammatory and beneficial bacteria seem to be depleted. These data suggest that the assessment of the proportion of harmful to beneficial bacteria can be considered as a prognostic indicator for the development of HCC. However, as demonstrated in the real-life, the gut microbiota compositional differences between patients with HCC and controls can be blurred when comparisons are made between cirrhotic patients.116 As a surrogate marker of the derangement of the gut–liver axis, fecal calprotectin can be used reliably to identify those patients with an enhanced intestinal inflammation and at higher risk of developing HCC. Furthermore, the gut microbiota metabolites can be considered as promising tools in the early diagnosis of HCC. The analysis of biliary acids composition or the quantification of fecal butyrate can help to identify those patients at high risk, and may be integrated with the assessment of the gut microbiota metagenomic profile to further stratify patient’s risk.
However, several limitations affecting the use of the gut microbiota for these purposes should be taken into account. First, cirrhotic patients are often subjected to antibiotic treatments that profoundly alter the composition and function of their gut microbiota.122–124 Second, comorbidities can affect the gut microbiota12 and, therefore, make its use unreliable. Finally, ethnic differences125 and the inclusion of patients with different etiologies of liver disease make it difficult to compare the results of the available studies. It is also to underline that all the studies published so far are very preliminary and need further confirmations in larger series. Moreover, metagenomic sequencing of the gut microbiota is still a technique available to a limited number of laboratories, and has not yet been implemented in clinical practice. Metabolites, especially bile acids, are very challenging to analyze and this may be expensive and difficult in clinical practice. Therefore, it is not yet clear how all this information can be managed and how it can be integrated into already established diagnostic algorithms. If high-risk patients were identified on the basis of the gut microbial or metabolomic profile, how the patient follow up should be adapted is still unknown.
Important considerations can also be made for therapeutic purposes. It is clear that changes in the gut microbiota, the intestinal environment, and the systemic mediators associated with HCC suggest an inflammatory setting, which can promote the development of neoplastic aberrations directly or, when persisting over time, through the exhaustion of the immune system. In this scenario, different interventions can be hypothesized at different levels. The modulation of the gut microbiota through antibiotics, eubiotics, and probiotics should be aimed at avoiding the development of a proinflammatory bacterial community, and consequently its negative influence on the hepatic microenvironment and the immune system. Probiotics seem to have a positive effect in animal models of HCC, reducing the tumor burden and the inflammatory milieu.99,100 Other effects of probiotics in cancer are related to the improvement of intestinal barrier function and immunomodulatory activity.126 Antibiotic treatment has also been associated with reduced occurrence and progression of HCC in several studies,97,98,101,104,106 although data on humans are lacking. Rifaximin, an eubiotic compound capable of inducing the overgrowth of beneficial bacteria such as Bifidobacterium, Faecalibacterium, and Lacto-bacillus, and of exerting an anti-inflammatory effect,127 has never been tested in preclinical models of HCC and may represent a promising approach, considering the almost ubiquitous depletion of beneficial bacterial species associated with the development of HCC. Finally, fecal microbial transplantation (FMT), which is a recognized therapeutic option for C. difficile infection,128 has been employed with promising results in many other conditions related to gut microbiota derangement, such as restoring its the balance after allogeneic hematopoietic stem cell transplantation.129 Preliminary experiences in the treatment of liver cirrhosis from different etiologies and of its complications have also been published.130–138 Therefore, its adoption in HCC management deserves further attention.
Another possible approach involves drugs with anti-inflammatory effects, that could be used to reduce the intestinal and systemic inflammatory environment associated with the development of HCC. Various drugs may have an impact on the gut microbiota composition, as demonstrated recently,139 and some of them may play a role in the prevention and treatment of HCC. Metformin has previously been associated with a reduced incidence of HCC,140,141 and recent data suggest that it could play a role in decreasing nonresolving inflammation in animal models of NAFLD-related HCC.142 Metformin alters gut microbiota of healthy mice143; Furthermore, it is a potent modulator of the gut microbiota.144,145 In particular, the relative abundance of Bifidobacterium145 and Akkermansia146 are increased by metformin administration, suggesting that the anti-inflammatory role of this compound may be partially mediated by a favorable change in the gut microbiota composition. Similar effects have been recently shown for aspirin.147–149 Mesalazine, a derivative of 5-aminosalicylic acid, can alter the gut microbiota through different mechanisms, acting on the intestinal microenvironment, the mucosa or directly on gut bacteria.150 Although this drug is mainly used for its anti-inflammatory effects in patients with inflammatory bowel diseases (IBDs), it could be potentially used in other disease conditions, such as HCC associated with liver cirrhosis. In addition statins, which are known modulators of the inflammatory response and of the gut microbiota, and their metabolites151–156 have been associated with a reduced incidence of HCC in the general population and in high-risk patients.157–162
As a final consideration, the lessons from other cancers clearly indicate that a harmful dysbiosis can modulate the immune response and reduce effectiveness of immunotherapy, whereas the opposite is observed when there is a positive balance in the gut microbial community.163 Therefore, the awakening of antitumor immune response, which can be stunned by persistent chronic stimulation by intestinal antigens, can be adopted as preemptive strategy or therapeutic approach to avoid disease progression or recurrence after treatment in patients with early HCC, and can be achieved through the modulation of the gut microbiota.
Acknowledgments
This is a paper from the Gut -Liver (GuLiver) Axis Study Group of the Fondazione IRCCS Agostino Gemelli Hospital, Rome, Italy.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Francesca Romana Ponziani  https://orcid.org/0000-0002-5924-6238
https://orcid.org/0000-0002-5924-6238
Contributor Information
Francesca Romana Ponziani, Division of Internal Medicine, Gastroenterology and Hepatology, Fondazione Policlinico Agostino Gemelli IRCCS, Largo Agostino Gemelli 8, Rome, 00168, Italy.
Alberto Nicoletti, Internal Medicine, Gastroenterology and Hepatology, Fondazione Policlinico Agostino Gemelli IRCCS, Rome, Italy.
Antonio Gasbarrini, Internal Medicine, Gastroenterology and Hepatology, Fondazione Policlinico Agostino Gemelli IRCCS, Rome, Italy.
Maurizio Pompili, Internal Medicine, Gastroenterology and Hepatology, Fondazione Policlinico Agostino Gemelli IRCCS, Rome, Italy.
References
- 1. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 2. Nakagawa H, Maeda S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J Gastroenterol 2012; 18: 4071–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359: 1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okamoto M, Utsunomiya T, Wakiyama S, et al. Specific gene-expression profiles of noncancerous liver tissue predict the risk for multicentric occurrence of hepatocellular carcinoma in hepatitis C virus-positive patients. Ann Surg Oncol 2006; 13: 947–954. [DOI] [PubMed] [Google Scholar]
- 5. Hoshida Y, Villanueva A, Sangiovanni A, et al. Prognostic gene expression signature for patients with hepatitis C-related early-stage cirrhosis. Gastroenterology 2013; 144: 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abu Dayyeh BK, Yang M, Fuchs BC, et al. A functional polymorphism in the epidermal growth factor gene is associated with risk for hepatocellular carcinoma. Gastroenterology 2011; 141: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanabe KK, Lemoine A, Finkelstein DM, et al. Epidermal growth factor gene functional polymorphism and the risk of hepatocellular carcinoma in patients with cirrhosis. JAMA 2008; 299: 53–60. [DOI] [PubMed] [Google Scholar]
- 8. Tao Y, Ruan J, Yeh SH, et al. Rapid growth of a hepatocellular carcinoma and the driving mutations revealed by cell-population genetic analysis of whole-genome data. Proc Natl Acad Sci USA 2011; 108: 12042–12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D’Ambrosio R, Aghemo A, Rumi MG, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 2012; 56: 532–543. [DOI] [PubMed] [Google Scholar]
- 10. Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 2016; 63: 827–838. [DOI] [PubMed] [Google Scholar]
- 11. Viganò L, Conci S, Cescon M, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: a multicenter matched analysis with HCV-related HCC. J Hepatol 2015; 63: 93–101. [DOI] [PubMed] [Google Scholar]
- 12. Lynch SV, Pedersen O. The human intestinal microbiome in health and sisease. N Engl J Med 2016; 375: 2369–2379. [DOI] [PubMed] [Google Scholar]
- 13. Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol 2019; 16: 35–56. [DOI] [PubMed] [Google Scholar]
- 14. Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014; 60: 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ponziani FR, Gerardi V, Pecere S, et al. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J Gastroenterol 2015; 21: 12322–12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ponziani FR, Zocco MA, Cerrito L, et al. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol 2018; 12: 641–656. [DOI] [PubMed] [Google Scholar]
- 17. Hernandez-Gea V, Toffanin S, Friedman SL, et al. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013; 144: 512–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol 2017; 12: 153–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yin L, Wang Y, Guo X, et al. Comparison of gene expression in liver regeneration and hepatocellular carcinoma formation. Cancer Manag Res 2018; 10: 5691–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li T, Wan B, Huang J, et al. Comparison of gene expression in hepatocellular carcinoma, liver development, and liver regeneration. Mol Genet Genomics 2010; 283: 485–492. [DOI] [PubMed] [Google Scholar]
- 21. Coulon S, Heindryckx F, Geerts A, et al. Angiogenesis in chronic liver disease and its complications. Liver Int 2011; 31: 146–162. [DOI] [PubMed] [Google Scholar]
- 22. Zhu AX, Duda DG, Sahani DV, et al. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol 2011; 8: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med 2011; 365: 537–547. [DOI] [PubMed] [Google Scholar]
- 24. Wu XZ, Xie GR, Chen D. Hypoxia and hepatocellular carcinoma: the therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol 2007; 22: 1178–1182. [DOI] [PubMed] [Google Scholar]
- 25. Jung IH, Choi JH, Chung YY, et al. Predominant activation of JAK/STAT3 pathway by interleukin-6 is implicated in hepatocarcinogenesis. Neoplasia 2015; 17: 586–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Subramaniam A, Shanmugam MK, Perumal E, et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta 2013; 1835: 46–60. [DOI] [PubMed] [Google Scholar]
- 27. Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004; 431: 461–466. [DOI] [PubMed] [Google Scholar]
- 28. Haybaeck J, Zeller N, Wolf MJ, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009; 16: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakagawa H, Umemura A, Taniguchi K, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 2014; 26: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tilg H, Wilmer A, Vogel W, et al. Serum levels of cytokines in chronic liver diseases. Gastroenterology 1992; 103: 264–274. [DOI] [PubMed] [Google Scholar]
- 32. Jiang R, Tan Z, Deng L, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology 2011; 54: 900–909. [DOI] [PubMed] [Google Scholar]
- 33. Maeda S, Kamata H, Luo JL, et al. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005; 121: 977–990. [DOI] [PubMed] [Google Scholar]
- 34. Sakurai T, Maeda S, Chang L, et al. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA 2006; 103: 10544–10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He G, Yu GY, Temkin V, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 2010; 17: 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luedde T, Beraza N, Kotsikoris V, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007; 11: 119–132. [DOI] [PubMed] [Google Scholar]
- 37. Kondylis V, Polykratis A, Ehlken H, et al. NEMO prevents steatohepatitis and hepatocellular carcinoma by inhibiting RIPK1 kinase activity-mediated hepatocyte apoptosis. Cancer Cell 2015; 28: 582–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep 2009; 10: 1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee H, Herrmann A, Deng JH, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell 2009; 15: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sakurai T, He G, Matsuzawa A, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell 2008; 14: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006; 10: 99–111. [DOI] [PubMed] [Google Scholar]
- 42. Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol 2006; 80: 1197–1213. [DOI] [PubMed] [Google Scholar]
- 43. Yoshiji H, Kuriyama S, Noguchi R, et al. Angiopoietin 2 displays a vascular endothelial growth factor dependent synergistic effect in hepatocellular carcinoma development in mice. Gut 2005; 54: 1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chia CS, Ban K, Ithnin H, et al. Expression of interleukin-18, interferon-gamma and interleukin-10 in hepatocellular carcinoma. Immunol Lett 2002; 84: 163–172. [DOI] [PubMed] [Google Scholar]
- 45. Beckebaum S, Zhang X, Chen X, et al. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res 2004; 10: 7260–7269. [DOI] [PubMed] [Google Scholar]
- 46. Zhou H, Huang H, Shi J, et al. Prognostic value of interleukin 2 and interleukin 15 in peritumoral hepatic tissues for patients with hepatitis B-related hepatocellular carcinoma after curative resection. Gut 2010; 59: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 47. Unitt E, Rushbrook SM, Marshall A, et al. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology 2005; 41: 722–730. [DOI] [PubMed] [Google Scholar]
- 48. Wan S, Kuo N, Kryczek I, et al. Myeloid cells in hepatocellular carcinoma. Hepatology 2015; 62: 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol 2017; 10: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cariani E, Pilli M, Zerbini A, et al. Immunological and molecular correlates of disease recurrence after liver resection for hepatocellular carcinoma. PLoS One 2012; 7: e32493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011; 128: 887–896. [DOI] [PubMed] [Google Scholar]
- 52. Wu K, Kryczek I, Chen L, et al. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res 2009; 69: 8067–8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol 2006; 27: 195–201. [DOI] [PubMed] [Google Scholar]
- 54. Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009; 206: 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Han Y, Chen Z, Yang Y, et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 2014; 59: 567–579. [DOI] [PubMed] [Google Scholar]
- 56. Kalathil S, Lugade AA, Miller A, et al. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res 2013; 73: 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mizukoshi E, Nakamoto Y, Arai K, et al. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology 2011; 53: 1206–1216. [DOI] [PubMed] [Google Scholar]
- 58. Kassel R, Cruise MW, Iezzoni JC, et al. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology 2009; 50: 1625–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nebbia G, Peppa D, Schurich A, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One 2012; 7: e47648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peng G, Li S, Wu W, et al. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol 2008; 45: 963–970. [DOI] [PubMed] [Google Scholar]
- 61. Nakamoto N, Cho H, Shaked A, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog 2009; 5: e1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology 2008; 134: 1927–1937, 1937.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moorman JP, Wang JM, Zhang Y, et al. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J Immunol 2012; 189: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 65. Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol 2009; 21: 317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Miao EA, Andersen-Nissen E, Warren SE, et al. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol 2007; 29: 275–288. [DOI] [PubMed] [Google Scholar]
- 67. Salazar-Gonzalez RM, McSorley SJ. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Lett 2005; 101: 117–122. [DOI] [PubMed] [Google Scholar]
- 68. Luedde T, Schwabe RF. NF-κB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011; 8: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hatziapostolou M, Polytarchou C, Aggelidou E, et al. An HNF4α-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell 2011; 147: 1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 2000; 289: 1560–1563. [DOI] [PubMed] [Google Scholar]
- 71. Collier-Hyams LS, Sloane V, Batten BC, et al. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol 2005; 175: 4194–4198. [DOI] [PubMed] [Google Scholar]
- 72. Lee WJ. Bacterial-modulated signaling pathways in gut homeostasis. Sci Signal 2008; 1: pe24. [DOI] [PubMed] [Google Scholar]
- 73. Kumar A, Wu H, Collier-Hyams LS, et al. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J 2007; 26: 4457–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol 2006; 174: 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chiarugi P, Buricchi F. Protein tyrosine phosphorylation and reversible oxidation: two cross-talking posttranslation modifications. Antioxid Redox Signal 2007; 9: 1–24. [DOI] [PubMed] [Google Scholar]
- 76. Jones RM, Desai C, Darby TM, et al. Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep 2015; 12: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aviello G, Knaus UG. ROS in gastrointestinal inflammation: rescue or sabotage? Br J Pharmacol 2017; 174: 1704–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jones RM, Neish AS. Redox signaling mediated by the gut microbiota. Free Radic Biol Med 2017; 105: 41–47. [DOI] [PubMed] [Google Scholar]
- 79. Mardinoglu A, Shoaie S, Bergentall M, et al. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol 2015; 11: 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Amelio I, Cutruzzola F, Antonov A, et al. Serine and glycine metabolism in cancer. Trends Biochem Sci 2014; 39: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Han CY. Update on FXR biology: promising therapeutic target? Int J Mol Sci 2018; 19: pii: E2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang F, Huang X, Yi T, et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res 2007; 67: 863–867. [DOI] [PubMed] [Google Scholar]
- 83. Degirolamo C, Modica S, Vacca M, et al. Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology 2015; 61: 161–170. [DOI] [PubMed] [Google Scholar]
- 84. Bernstein H, Bernstein C, Payne CM, et al. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res 2005; 589: 47–65. [DOI] [PubMed] [Google Scholar]
- 85. Bernstein H, Bernstein C, Payne CM, et al. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol 2009; 15: 3329–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gadaleta RM, Oldenburg B, Willemsen EC, et al. Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-κB signaling in the intestine. Biochim Biophys Acta 2011; 1812: 851–858. [DOI] [PubMed] [Google Scholar]
- 87. Zhang HH, Mei MH, Fei R, et al. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J Viral Hepat 2010; 17(Suppl. 1): 34–43. [DOI] [PubMed] [Google Scholar]
- 88. Matsuzaki K, Murata M, Yoshida K, et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology 2007; 46: 48–57. [DOI] [PubMed] [Google Scholar]
- 89. Kobayashi K, Hernandez LD, Galán JE, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002; 110: 191–202. [DOI] [PubMed] [Google Scholar]
- 90. Liew FY, Xu D, Brint EK, et al. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 2005; 5: 446–458. [DOI] [PubMed] [Google Scholar]
- 91. Rauh MJ, Ho V, Pereira C, et al. SHIP represses the generation of alternatively activated macrophages. Immunity 2005; 23: 361–374. [DOI] [PubMed] [Google Scholar]
- 92. Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 2009; 30: 475–487. [DOI] [PubMed] [Google Scholar]
- 93. Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 2009; 50: 980–989. [DOI] [PubMed] [Google Scholar]
- 95. Liao R, Sun J, Wu H, et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res 2013; 32: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol 2013; 182: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yu LX, Yan HX, Liu Q, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology 2010; 52: 1322–1333. [DOI] [PubMed] [Google Scholar]
- 98. Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012; 21: 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang HL, Yu LX, Yang W, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol 2012; 57: 803–812. [DOI] [PubMed] [Google Scholar]
- 100. Li J, Sung CY, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA 2016; 113: E1306–E1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013; 499: 97–101. [DOI] [PubMed] [Google Scholar]
- 102. Man SM. Inflammasomes in the gastrointestinal tract: infection, cancer and gut microbiota homeostasis. Nat Rev Gastroenterol Hepatol 2018; 15: 721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xie G, Wang X, Huang F, et al. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int J Cancer 2016; 139: 1764–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yamada S, Takashina Y, Watanabe M, et al. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget 2018; 9: 9925–9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ponziani F, Ojetti V, Tortora A, et al. The metabolic and toxicological considerations for mTOR inhibitors in the treatment of hepatocarcinoma. Expert Opin Drug Metab Toxicol 2011; 7: 1535–1546. [DOI] [PubMed] [Google Scholar]
- 106. Singh V, Yeoh BS, Chassaing B, et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 2018; 175: 679–694.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Donohoe DR, Collins LB, Wali A, et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell 2012; 48: 612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Belcheva A, Irrazabal T, Robertson SJ, et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014; 158: 288–299. [DOI] [PubMed] [Google Scholar]
- 109. Misikangas M, Tanayama H, Rajakangas J, et al. Inulin results in increased levels of beta-catenin and cyclin D1 as the adenomas increase in size from small to large in the Min/+ mouse. Br J Nutr 2008; 99: 963–970. [DOI] [PubMed] [Google Scholar]
- 110. Pajari AM, Rajakangas J, Päivärinta E, et al. Promotion of intestinal tumor formation by inulin is associated with an accumulation of cytosolic beta-catenin in Min mice. Int J Cancer 2003; 106: 653–660. [DOI] [PubMed] [Google Scholar]
- 111. Kaiko GE, Ryu SH, Koues OI, et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 2016; 165: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kim MH, Kang SG, Park JH, et al. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013; 145: 396–406.e1–10. [DOI] [PubMed] [Google Scholar]
- 113. Cai SY, Ouyang X, Chen Y, et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017; 2: e90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chi L, Xue J, Tu P, et al. Gut microbiome disruption altered the biotransformation and liver toxicity of arsenic in mice. Arch Toxicol. Epub ahead of print 24 October 2018. DOI: 10.1007/s00204-018-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Grąt M, Wronka KM, Krasnodębski M, et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transplant Proc 2016; 48: 1687–1691. [DOI] [PubMed] [Google Scholar]
- 116. Ponziani FR, Bhoori S, Castelli C, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. Epub ahead of print 10 July 2018. DOI: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 117. Ren Z, Li A, Jiang J, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2018: pii: gutjnl-2017-315084. DOI: 10.1136/gutjnl-2017-315084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Weber A, Boege Y, Reisinger F, et al. Chronic liver inflammation and hepatocellular carcinoma: persistence matters. Swiss Med Wkly 2011; 141: w13197. [DOI] [PubMed] [Google Scholar]
- 119. Wai PY, Kuo PC. Intersecting pathways in inflammation and cancer: hepatocellular carcinoma as a paradigm. World J Clin Oncol 2012; 3: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Szabo G, Lippai D. Molecular hepatic carcinogenesis: impact of inflammation. Dig Dis 2012; 30: 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Banales JM, Inarrairaegui M, Arbelaiz A, et al. Serum metabolites as diagnostic biomarkers for cholangiocarcinoma, hepatocellular carcinoma and primary sclerosing cholangitis. Hepatology. Epub ahead of print 16 October 2018. DOI: 10.1002/hep.30319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010; 156(Pt 11): 3216–3223. [DOI] [PubMed] [Google Scholar]
- 123. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA 2011; 108(Suppl. 1): 4554–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013; 152: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol 2017; 8: 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yu AQ, Li L. The potential role of probiotics in cancer prevention and treatment. Nutr Cancer 2016; 68: 535–544. [DOI] [PubMed] [Google Scholar]
- 127. Ponziani FR, Zocco MA, D’Aversa F, et al. Eubiotic properties of rifaximin: disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol 2017; 23: 4491–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gupta A, Cifu AS, Khanna S. Diagnosis and treatment of clostridium difficile infection. JAMA 2018; 320: 1031–1032. [DOI] [PubMed] [Google Scholar]
- 129. Taur Y, Coyte K, Schluter J, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med 2018; 10: pii: eaap9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhou D, Pan Q, Shen F, et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep 2017; 7: 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ferrere G, Wrzosek L, Cailleux F, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol 2017; 66: 806–815. [DOI] [PubMed] [Google Scholar]
- 132. Philips CA, Pande A, Shasthry SM, et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol 2017; 15: 600–602. [DOI] [PubMed] [Google Scholar]
- 133. Philips CA, Phadke N, Ganesan K, et al. Healthy donor faecal transplant for corticosteroid non-responsive severe alcoholic hepatitis. BMJ Case Rep 2017; 2017: pii: bcr-2017-222310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ren YD, Ye ZS, Yang LZ, et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology 2017; 65: 1765–1768. [DOI] [PubMed] [Google Scholar]
- 135. Bajaj JS, Kakiyama G, Savidge T, et al. Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology 2018; 68: 1549–1558. [DOI] [PubMed] [Google Scholar]
- 136. Wang WW, Zhang Y, Huang XB, et al. Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J Gastroenterol 2017; 23: 6983–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kao D, Roach B, Park H, et al. Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology 2016; 63: 339–340. [DOI] [PubMed] [Google Scholar]
- 138. Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017; 66: 1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018; 555: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int 2018; 38: 2018–2027. [DOI] [PubMed] [Google Scholar]
- 141. Zhou YY, Zhu GQ, Liu T, et al. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci Rep 2016; 6: 33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. de Oliveira S, Houseright RA, Graves AL, et al. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. Epub ahead of print 18 December 2018. DOI: 10.1016/j.jhep.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ma W, Chen J, Meng Y, et al. Metformin alters gut microbiota of healthy mice: implication for its potential role in gut microbiota homeostasis. Front Microbiol 2018; 9: 1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015; 528: 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 2017; 23: 850–858. [DOI] [PubMed] [Google Scholar]
- 146. de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, et al. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 2017; 40: 54–62. [DOI] [PubMed] [Google Scholar]
- 147. Lee M, Chung GE, Lee JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology 2017; 66: 1556–1569. [DOI] [PubMed] [Google Scholar]
- 148. Simon TG, Ma Y, Ludvigsson JF, et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol 2018; 4: 1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sitia G, Iannacone M, Guidotti LG. Anti-platelet therapy in the prevention of hepatitis B virus-associated hepatocellular carcinoma. J Hepatol 2013; 59: 1135–1138. [DOI] [PubMed] [Google Scholar]
- 150. Xue L, Huang Z, Zhou X, et al. The possible effects of mesalazine on the intestinal microbiota. Aliment Pharmacol Ther 2012; 36: 813–814. [DOI] [PubMed] [Google Scholar]
- 151. Sun B, Li L, Zhou X. Comparative analysis of the gut microbiota in distinct statin response patients in East China. J Microbiol 2018; 56: 886–892. [DOI] [PubMed] [Google Scholar]
- 152. Khan TJ, Ahmed YM, Zamzami MA, et al. Atorvastatin treatment modulates the gut microbiota of the hypercholesterolemic patients. OMICS 2018; 22: 154–163. [DOI] [PubMed] [Google Scholar]
- 153. Nolan JA, Skuse P, Govindarajan K, et al. The influence of rosuvastatin on the gastrointestinal microbiota and host gene expression profiles. Am J Physiol Gastrointest Liver Physiol 2017; 312: G488–G497. [DOI] [PubMed] [Google Scholar]
- 154. Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 2005; 4: 977–987. [DOI] [PubMed] [Google Scholar]
- 155. Côté-Daigneault J, Mehandru S, Ungaro R, et al. Potential immunomodulatory effects of statins in inflammatory bowel disease. Inflamm Bowel Dis 2016; 22: 724–732. [DOI] [PubMed] [Google Scholar]
- 156. Bahrami A, Parsamanesh N, Atkin SL, et al. Effect of statins on toll-like receptors: a new insight to pleiotropic effects. Pharmacol Res 2018; 135: 230–238. [DOI] [PubMed] [Google Scholar]
- 157. Kim G, Jang SY, Nam CM, et al. Statin use and the risk of hepatocellular carcinoma in patients at high risk: a nationwide nested case-control study. J Hepatol 2018; 68: 476–484. [DOI] [PubMed] [Google Scholar]
- 158. Chang FM, Wang YP, Lang HC, et al. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: a population-based study. Hepatology 2017; 66: 896–907. [DOI] [PubMed] [Google Scholar]
- 159. Simon TG, Bonilla H, Yan P, et al. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology 2016; 64: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hsiang JC, Wong GL, Tse YK, et al. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: a propensity score landmark analysis. J Hepatol 2015; 63: 1190–1197. [DOI] [PubMed] [Google Scholar]
- 161. Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 2013; 144: 323–332. [DOI] [PubMed] [Google Scholar]
- 162. Yokohama K, Fukunishi S, Ii M, et al. Rosuvastatin as a potential preventive drug for the development of hepatocellular carcinoma associated with non-alcoholic fatty liver disease in mice. Int J Mol Med 2016; 38: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 163. Zitvogel L, Galluzzi L, Viaud S, et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med 2015; 7: 271ps1. [DOI] [PMC free article] [PubMed] [Google Scholar]