Abstract
Background:
Tumor environment has been recognized to affect cancer cell progression, such as tumor-associated macrophages. However, increasing evidences suggest that tumor cells are capable of regulating polarization of tumor-associated macrophages. In this study, we investigate the mechanism of how colon cancer cell impacts tumor-associated macrophages polarization.
Methods:
We employed flow cytometry to detect marker molecules on macrophage membrane, such as CD68, CD16, and CD204. In addition, we used enzyme-linked immunosorbent assay to examine the level of these cytokines (interleukin-6, interleukin-1β, interleukin-10, and Arginase-1) secreted by colon cancer cells into the culture medium. Western blot was utilized to probe downstream proteins of epidermal growth factor receptor (EGFR)/phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway.
Results:
We cocultured colon cancer cell lines (HCT8 or HCT116) with human myeloid leukemia mononuclear cells (THP-1) and found that interleukin-6 and interleukin-1β levels were reduced, and instead, interleukin-10 and Arginase-1 levels were elevated, suggesting that colon cancer cells contributed to M2 polarization of THP-1. Meanwhile, high level of various growth factors (transforming growth factor-β [TGF-β], epidermal growth factor [EGF], and hepatocyte growth factor [HGF]) was observed in the medium of THP-1 cocultured with colon cancer cells. Furthermore, the protein level of phosphorylated PI3K, AKT, and mTOR significantly increased in THP-1 cell cocultured with colon cancer cells compared to THP-1 group. Besides, we established that colon cancer cells exerted their stimulatory effect on M2 polarization of macrophage from monocyte THP-1 using EGFR antibody mAb225 and PI3K inhibitor LY294002.
Conclusion:
We provide evidence that EGF which are secreted by colon cancer cells play contributory role in M2 polarization of macrophages, which support the notion that tumor environment, including tumor-associated macrophages, can be targeted to develop effective strategies for treating cancer.
Keywords: macrophage, M2 polarization, colon cancer cell, EGF/EGFR, PI3K/AKT/mTOR pathway
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-associated death in China. Approximately 1.36 million patients are diagnosed with this malignancy each year.1,2 Besides of genetic and epigenetic factors, inflammatory microenvironment has been reported to contribute to development of cancers, including CRC.3 Tumor microenvironment consists of leukocytes, fibroblasts, and vascular endothelial cells, individually or combinatorially, affecting the growth, invasiveness, and metastasis of tumors.
Tumor-associated macrophages (TAMs) are one of the important immune cells in the tumor microenvironment. Generally speaking, macrophages are derived from monocytes differentiation in tissues, which can be classified into 2 forms: M1 and M2.4–6 The M1 type of macrophages, activated by cytokines such as interferon (IFN), then produce proinflammatory and immunostimulatory cytokines.7 Tumor-associated macrophages resemble the M2 macrophages (also termed alternatively activated macrophages), which are activated by Th2 cytokines, such as interleukin (IL)-4 and IL-10.8 In most cases with cancer, TAMs function to stimulate colon cancer cell growth, invasion, metastasis, and immune evasion.9 In contrast, colon tumor cells have the ability to promote macrophage polarization; however, it is a scarcity to identify the signal implicated in communication between tumor cells and TAMs.10
PI3K was identified as an oncogenic gene to transform normal cells into cancer cells.11,12 It has been established that PI3K exerts its effects via activating the downstream protein AKT and mTOR in a cascade manner.13,14 PI3K signaling is involved in a wide variety of cellular processes, including cell proliferation, survival, metabolism, and immunity.15 Interestingly, it has reported that Smad-PI3K-Akt-mTOR pathway mediates polarization of monocytes into M2 macrophage through enhancing anti-inflammatory cytokine expression.16
In this study, we sought to investigate whether colon cancer cells played the role in M2 polarization of macrophages from monocytes. The results revealed that colon cancer cells secreted EGF to bind EGFR of monocyte and then activate Smad-PI3K-Akt-mTOR pathway to contribute to polarization of monocytes into M2 macrophages. Our findings provide insights into the mechanism underlying colon cancer cells–induced M2 polarization of monocytes to macrophages and thereby developing the effective strategies for treating colon cancer by targeting TAMs.
Materials and Methods
Cell Lines and THP-1 Differentiation
The human colon cancer cell lines HCT8 and HCT116 and the human acute monocytic leukemia cell line THP-1 were obtained from ATCC, and the colon cancer cell lines were cultured in Dulbecco Modified Eagle Medium (DMEM; HyClone, Logan, Utah) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin (100 U/mL)/streptomycin (100 U/mL) at 37°C, 5% CO2. For THP-1 cell culture, we used DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin plus 0.05 mM 2-mercaptoethanol.
To differentiate THP-1 monocytes into M1 macrophages, THP-1 cells were subject to treatment of 20 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, St. Louis, Missouri), 10 ng/mL LPS (Sigma-Aldrich), and 20 ng/mL IFN-γ (PeproTech, Offenbach, Germany) for 48 hours, in addition, to differentiate THP-1 monocytes into M2 macrophages, THP-1 cells were subject to treatment of 20 ng/mL PMA and 15 ng/mL IL-4 (PeproTech) for 48 hours, and then refreshed the culture medium with serum-free DMEM for another 24 hours. The medium or adherent THP-1 cells were collected for the following experiments.
Flow Cytometry
Cells (1 × 106 cells) were digested by trypsin and then washed with 1 × phosphate-buffered saline (PBS) twice; next, the cells were incubated with fluorescein isothiocyante (FITC)-antihuman CD68 (BD Biosciences, San Diego, California), PE-antihuman CD206 (BD Biosciences) or allophycocyanin (APC)-antihuman CD16 (BD Biosciences) for 30 minutes at 4°C in the dark. Cells were then washed with PBS buffer and subject to flow cytometry analyses.
Enzyme-Linked Immunosorbent Assay
Culture medium was collected for the measurement of cytokine concentration by IL-6 ELISA kit (Sino Biological, Beijing, China), IL-1β ELISA kit (Cusabio, Beijing, China), IL-10 ELISA kit (Sino biological, Beijing, China), Arginase-1 ELISA kit (Cusabio, Beijing, China), EGF ELISA kit (Abcam, Cambridge, Massachusetts), TGF-β ELISA kit (Abcam, Cambridge, Massachusetts), and HGF ELISA kit (Abcam, Cambridge, Massachusetts) according to manufacturer’s instructions. A series of cytokine concentrations (0, 5, 10, 15, 20 ng/mL) were used to plot the standard curve. The concentrations within the culture medium collected from different experimental groups were determined by applying the optical density value to the standard curve.
Western Blot
Cells were harvested and washed with 1× PBS buffer and then lysed by 1× sodium dodecyl sulfate (SDS) loading buffer. The lysates were boiled at 100°C for 5 minutes. The resultant was centrifuged at 10 000 rpm for 1 minute. Around 50 μg of total proteins was loaded onto SDS–polyacrylamide gel electrophoresis gel and resolved. After that, the proteins were transferred to polyvinylidene fluoride membrane at 300 mA for 2 to 3 hours. The membrane was blocked with 5% nonfat milk in 1× Tris Buffered saline Tween (TBST) for 1 hour at room temperature, and the membrane was then incubated with primary antibodies at 4°C overnight. The following day, the membrane was washed with 1× TBST for 3 times, 5 minutes each time. The membrane was incubated with secondary antibodies at room temperature for 1 hour. Finally, the membrane was incubated with electrochemiluminescence (ECL) solution and then exposed using Bio-Rad ChemiDoc Touch Imaging System. The following antibodies were used: anti-p-mTOR (Cell Signaling Technology, Danvers, Massachusetts), anti-mTOR (Cell Signaling Technology, Danvers, Massachusetts), anti-AKT (Cell Signaling Technology, Danvers, Massachusetts), anti-p-AKT (Cell Signaling Technology, Danvers, Massachusetts), anti-PI3K (Cell Signaling Technology, Danvers, Massachusetts), anti-p-PI3K (Cell Signaling Technology, Danvers, Massachusetts), anti-Smad1/5/8 (Cell Signaling Technology, Danvers, Massachusetts), anti-p-Smad1/5/8 (Cell Signaling Technology, Danvers, Massachusetts), anti-EGFR (Cell Signaling Technology, Danvers, Massachusetts), anti-p-EGFR (Cell Signaling Technology, Danvers, Massachusetts), anti-GAPDH (Proteintech, Chicago, Illinois).
Statistical Analysis
Each experiment was performed for 3 times, all values were presented as mean (SD), and comparisons of parameters were performed using the 2-tailed unpaired Student t test. *P < .05 was considered statistically significant.
Results
Differentiation of THP-1 Cells to Macrophages
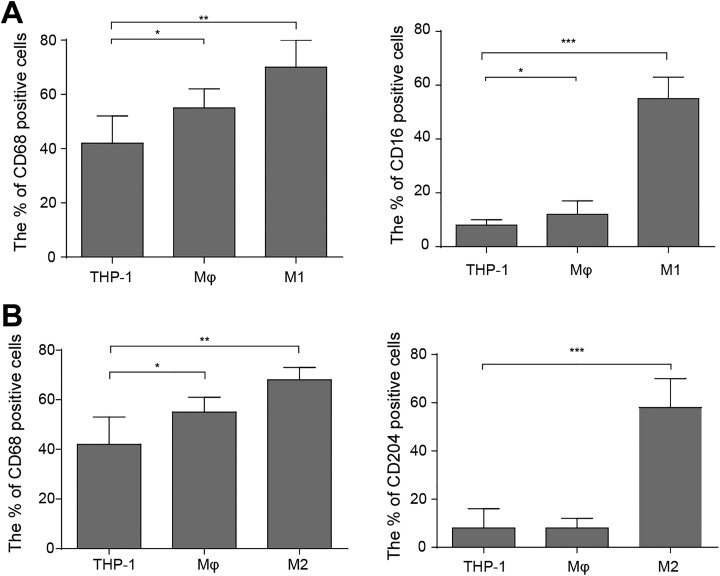
To investigate the role of colon cancer cells in polarization of macrophages, we first confirmed that M1 and M2 type of macrophages could be induced from human monocytes THP-1 by defined drugs. Macrophage marker CD68 was expressed in normal colon tissues and human colon carcinoma; CD204 was considered as a marker of M2 macrophage, while CD16 was a marker of M1 macrophage.17–20 Thus, we used PMA, LPS, and IFN treatment to induce M1 polarization and utilized PMA and IL-4 treatment to induce M2 polarization of THP-1 cell, respectively. The flow cytometry analyses showed that CD68 and CD16 levels were markedly upregulated in the cells upon treatment of PMA, LPS plus IFN, suggesting THP-1 cells were transformed into M1 type of macrophages (Figure 1A). On the other hand, CD68 and CD204 levels were higher in the cells upon treatment of PMA plus IL-4 than control, suggesting THP-1 cells were induced to M2 type of macrophage (Figure 1B). All these results confirmed that THP-1 cells could be induced to M1 or M2 polarization of macrophages.
Figure 1.
Differentiation of human myeloid leukemia mononuclear cells (THP-1) cell to macrophage. A, For M1 polarization, the THP-1 cells were treated with 20 ng/mL phorbol 12-myristate 13-acetate (PMA), 10 ng/mL lipopolysaccharide (LPS), and 20 ng/mL interferon-γ (IFN-γ) for 48 hours in total. Flow cytometry analyses and Statistics showing protein level of M1 macrophage-associated markers (CD68 and CD16). The mean (SD) in the graph presents the relative levels from 3 replications.*P < .05, **P < .01, ***P < .001. B, For M2 polarization, the THP-1 cells were treated 20 ng/mL with PMA and 15 ng/mL interleukin (IL)-4 for 48 hours in total. Flow cytometry analyses and Statistics showing protein level of M2 macrophage-associated markers (CD68 and CD204). The mean (SD) in the graph presents the relative levels from 3 replications.*P < .05, **P < .01, ***P < .001.
Colon Cancer Cells Promote M2 Polarization of THP-1 Cells
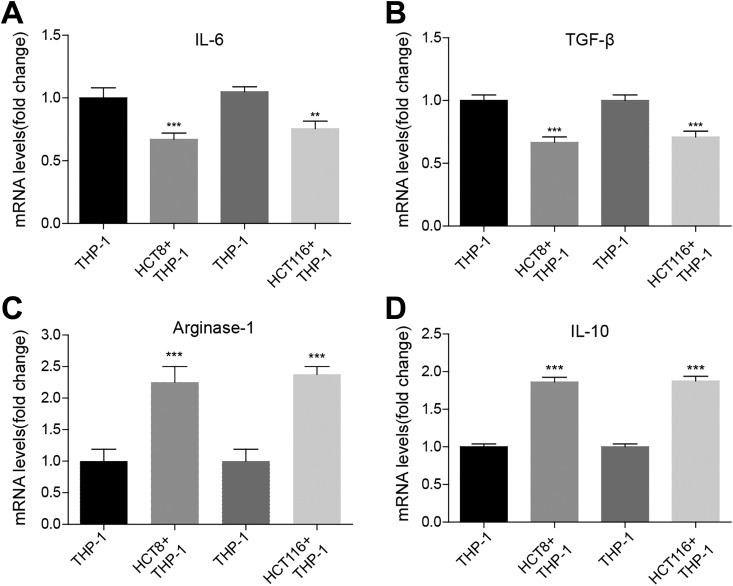
Next, in order to determine whether colon cancer cells had the effect on polarization of macrophages, we cocultured colon cancer cell lines HCT8 or HCT116, 2 well-studied colon cancer cell lines, where EGFR expression is relatively higher than other colon cell lines, with monocytes THP-1 and detected expression of macrophage type-specific markers by enzyme-linked immunosorbent assay (ELISA) in culture medium. We found that the level of M1-associated cytokines IL-6 and IL-1β decreased by approximately 25% in THP-1 cocultured with colon cancer cells (Figure 2A and B), whereas the level of M2-associated markers IL-10 and Arginase-1 increased by about 100% in THP-1 cocultured with colon cancer cells compared to THP-1 group (Figure 2C and D). Our findings suggested that colon cancer cells promoted M2 polarization of macrophage from monocytes.
Figure 2.
Colon cancer cells promote M2 polarization of human myeloid leukemia mononuclear cells (THP-1) cell. A, Enzyme-linked immunosorbent assay (ELISA) assay showing the protein level of M1 macrophage-associated marker interleukin (IL)-6 in THP-1 alone or cocultured with colon cancer cell lines HCT8 or HCT116. The mean (SD) in the graph presents the relative levels from 3 replications. **P < .01, ***P < .001. B, ELISA assay showing the protein level of M1 macrophage-associated marker IL-1β in THP-1 alone or cocultured with colon cancer cell lines HCT8 or HCT116. The mean (SD) in the graph presents the relative levels from 3 replications. ***P < .001. The ELISA assay showing the protein level of M2 macrophage-associated marker Arginase-1 in THP-1 alone or cocultured with colon cancer cell lines HCT8 or HCT116. The mean (SD) in the graph presents the relative levels from 3 replications. ***P < .001. C, ELISA assay showing the protein level of M2 macrophage-associated marker IL-10 in THP-1 alone or cocultured with colon cancer cell lines HCT8 or HCT116. The mean (SD) in the graph presents the relative levels from 3 replications. ***P < .001.
Colon Cancer Cells Activate PI3K/AKT Pathway in THP-1 Cells
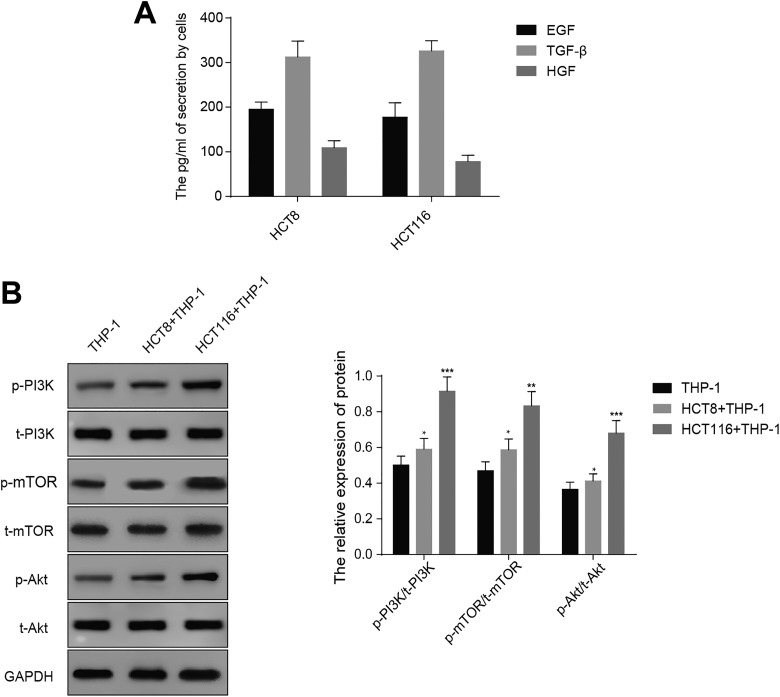
To explore the mechanism of how colon cancer cells contribute to M2 polarization of macrophages from monocytes, we hypothesized that cytokines secreted by colon cancer cells were responsible for this phenotype. We examined the level of previously reported cytokines (TGF-β, EGF, and HGF) secreted by colon cancer cells by ELISA approach. Table 1 displayed the levels of TGF-β, EGF, and HGF in HCT8 (195.3 [16.1] pg/mL, 312.4 [35.6] pg/mL, and 109.2 [15.7] pg/mL) and HCT116 (177.5 [32.2] pg/mL, 326.2 [22.6] pg/mL, and 78.1 [14.3] pg/mL), indicating the colon cancer cells have the ability to secrete these cytokines to medium (Figure 3A). In addition, some studies have reported that PI3K/AKT/mTOR pathway is involved in M2 polarization of macrophages from monocytes, and we examined whether this pathway was activated in THP-1 cells cocultured with HCT8 or HCT116.16 Western blot analysis exhibited significantly higher level of phosphorylated form of PI3K, AKT, and mTOR in THP-1 cells were cocultured with HCT8 or HCT116 cells relative to THP-1 cells alone (Figure 3B). These results revealed that colon cancer cells, more perhaps, induced M2 polarization of macrophages from monocytes through activation of PI3K/AKT/mTOR pathway and secretion of cytokines.
Table 1.
The Secretion Levels of TGF-β, EGF, HGF by Cells.
| Cells that secrete cytokines | TGF-β | EGF | HGF |
|---|---|---|---|
| HCT8, pg/mL | 195.3 (16.1) | 312.4 (35.6) | 109.2 (15.7) |
| HCT116, pg/mL | 177.5 (32.2) | 326.2 (22.6) | 78.1 (14.3) |
Abbreviations: EGF:epidermal growth factor; HGF:hepatocyte growth factor; TGF-β:transforming growth factor-β.
Figure 3.
Colon cancer cells activate PI3K/AKT pathway in THP-1 cell. A, Enzyme-linked immunosorbent assay (ELISA) assay and statistics of TGF-β1, HGF, and EGF level in medium of THP-1 cell cocultured with HCT8 or HCT116. The mean (SD) in the graph presents the relative levels from 3 replications. **P < .01. B, Western blot analyses and quantification showing the protein level of phosphorylated form of PI3K, AKT, and mTOR. GAPDH acts as internal control. The mean (SD) in the graph presents the relative levels from 3 replications. *P < .05, **P < .01, ***P < .001.
Colon Cancer Cells Promote M2 Polarization of Macrophages Via EGF/EGFR Signaling
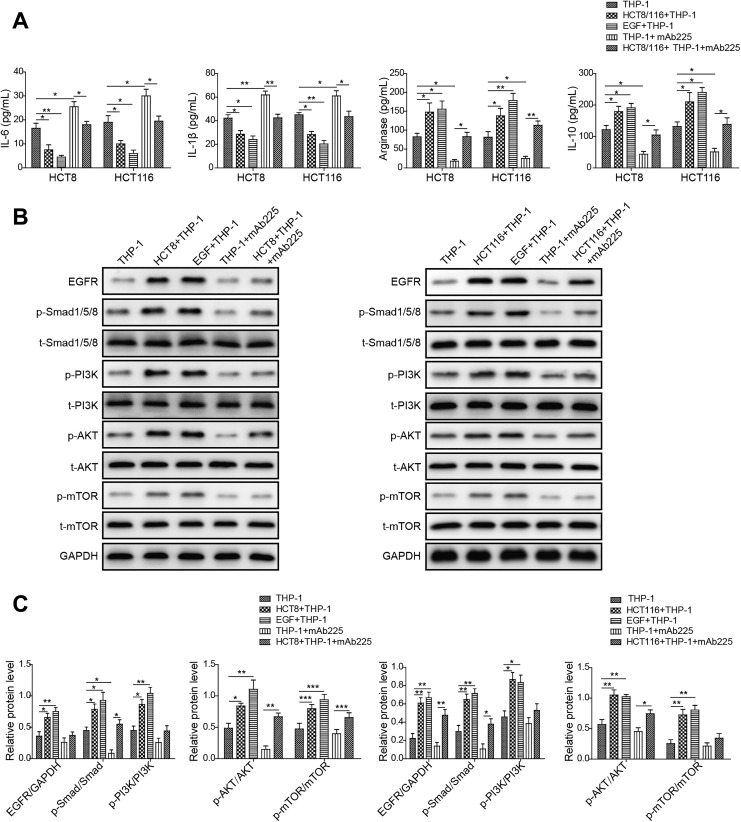
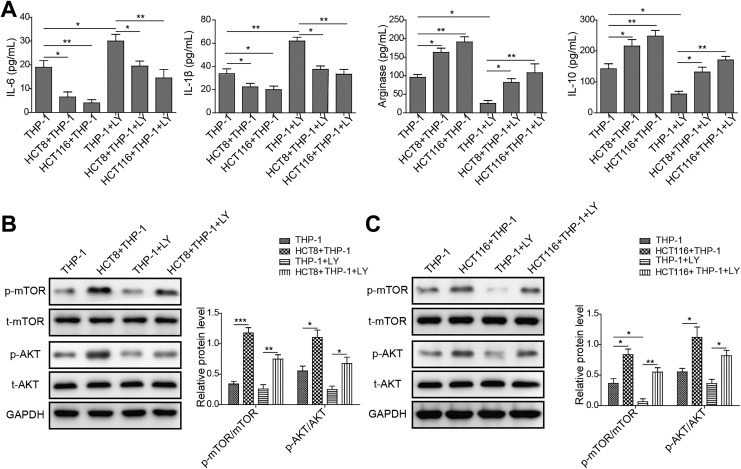
Because of high level of EGF, the reported cytokine involved in M2 polarization of macrophages, was detected in the medium of colon cancer cells cocultured with THP-1, we hypothesized that colon cancer cells might secrete EGF to exert the stimulatory role in M2 polarization of macrophages by binding EGFR.21 To address the question, we examined the effect of colon cancer cells, EGF, or colon cancer cells plus EGFR antibody mAb225 on M2 polarization of human monocytes THP-1 by detecting associated cytokines level. We observed that the level of M1-associated cytokines IL-6 and IL-1β decreased in THP-1 cocultured with colon cancer cells (HCT8 or HCT116) or treated with EGF, compared to THP-1 group. More importantly, we found that THP-1 cells treated with mAb225 (EGFR antibody) exhibited increased level of IL-6 and IL-1β relative to THP-1 cells. In addition, mAb225 could rescue the expression level of IL-6 and IL-1β in THP-1 cells cocultured with colon cancer cells comparable to THP-1 group. However, mAb225 added THP-1 cocultured with colon cancer cells (HCT8 or HCT116) prevented the rescue to some extent (Figure 4A). In contrast, the level of M2-associated markers IL-10 and Arginase-1 increased in THP-1 cocultured with colon cancer cells (HCT8 or HCT116) or treated with EGF, compared to THP-1 group. Besides, we found that THP-1cells treated with mAb225 displayed decreased level of IL-10 and Arginase-1 relative to THP-1 cells, and mAb225 could also rescue the expression level of IL-10 and Arginase-1; however, mAb225 added colon cancer cells (HCT8 or HCT116) impeded the rescue to some extent (Figure 4A). These data suggested that colon cancer cells promoted M2 polarization of macrophages from monocytes through EGF/EGFR activation.
Figure 4.
Colon cancer cells promote M2 polarization of macrophage via epidermal growth factor (EGF)/epidermal growth factor receptor (EGFR) signaling. A, Enzyme-linked immunosorbent assay (ELISA) assay showing the protein level of M2 macrophage-associated marker interleukin (IL)-6, IL-1β, Arginase-1, IL-10 in THP-1 alone, THP-1 cocultured with HCT8 or HCT116, THP-1 treated with EGF, THP-1 treated with mAb225, and THP-1 cocultured with HCT8 or HCT116 treated with mAb225. The mean (SD) in the graph presents the relative levels from 3 replications. *P < .05, **P < .01. B, Western blot analyses showing the protein level of phosphorylated form of EGFR, Smad1/5/8, phosphoinositide 3-kinase (PI3K), protein kinase B (AKT), and mammalian target of rapamycin (mTOR) in THP-1 alone, THP-1 cocultured with HCT8 or HCT116, THP-1 treated with EGF, THP-1 treated with mAb225, and THP-1 cocultured with HCT8 or HCT116 treated with mAb225. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) acts as internal control. C, Gray level analysis showing the protein level of phosphorylated form of EGFR, Smad1/5/8, PI3K, AKT, and mTOR in THP-1 alone, THP-1 cocultured with HCT8 or HCT116, THP-1 treated with EGF, THP-1 treated with mAb225, and THP-1 cocultured with HCT8 or HCT116 treated with mAb225. GAPDH acts as internal control. The mean (SD) in the graph presents the relative levels from 3 replications. *P < .05, **P < .01, ***P < .001.
Next, we determined whether downstream effectors of EGF/EGFR pathway were activated in THP-1 cells cocultured with colon cancer cells (HCT8 or HCT116) or treated with EGF, compared to THP-1 group. We observed that the level of phosphorylated PI3K/AKT/mTOR and Smads were increased in THP-1 cells cocultured with colon cancer cells (HCT8 or HCT116) or treated with EGF, whereas mAb225 abolished the activation of PI3K/AKT/mTOR pathway in THP-1 cells (Figure 4B and C). Together, these results indicated that colon cancer cell lines HCT8 and HCT116 secreted EGF to activate PI3K/AKT/mTOR pathway of THP-1 cell via binding to EGFR.
PI3K/AKT/mTOR Pathway Is Essential for Colon Cancer Cells–Stimulated M2 Polarization of Macrophages
Finally, we attempted to confirm the essential role of PI3K/AKT/mTOR pathway in M2 polarization of macrophages from monocytes using pharmaceutical inhibitor LY294002 of PI3K. The ELISA result showed that level of M1-associated cytokines IL-6 and IL-1β decreased in THP-1 cocultured with colon cancer cells (HCT8 or HCT116) compared to THP-1 group, which was abrogated using PI3K inhibitor LY294002 (Figure 5A). Nevertheless, the level of M2-associated markers IL-10 and Arginase-1 increased in THP-1 cocultured with colon cancer cells (HCT8 or HCT116) relative to THP-1 group, in parallel, the increase in M2-associated markers (IL-10 and Arginase-1) level was blocked by LY294002 treatment (Figure 5A). To validate, we probed whether LY294002 efficiently inhibited activation of downstream effectors. Expectedly, we found that the protein levels of phosphorylated AKT and mTOR, in large part, declined upon LY294002 treatment (Figure 5B and C). Our findings indicated that PI3K/AKT/mTOR pathway was critical for M2 polarization of macrophages from monocytes.
Figure 5.
phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway is essential for colon cancer cells-stimulated M2 polarization of macrophage. A, Enzyme-linked immunosorbent assay (ELISA) assay showing the protein level of M2 macrophage-associated marker interleukin (IL)-6, IL-1β, Arginase-1, IL-10 in human myeloid leukemia mononuclear cells (THP-1) alone, THP-1 cocultured with HCT8 or HCT116, THP-1 treated with LY294002, and THP-1 cocultured with HCT8 or HCT116 treated with LY294002. The mean (SD) in the graph presents the relative levels from 3 replications. *P < .05, **P < .01. B, Western blot analyses showing the protein level of phosphorylated form of AKT and mTOR in THP-1 alone, THP-1 cocultured with HCT8 or HCT116, THP-1 treated with LY294002, and THP-1 cocultured with HCT8 or HCT116 treated with LY294002. GAPDH acts as internal control. C, Gray-level analysis showing the protein level of phosphorylated form of AKT and mTOR in THP-1 alone, THP-1 cocultured with HCT8 or HCT116, THP-1 treated with LY294002, and THP-1 cocultured with HCT8 or HCT116 treated with LY294002. GAPDH acts as internal control. The mean (SD) in the graph presents the relative levels from 3 replications. *P < .05, **P < .01, ***P < .001.
Discussion
Here, we first illuminate the molecular mechanism of colon cancer cells promoting polarization of monocytes into M2 macrophages. We found that colon cancer cells secreted EGF to the medium and then activated EGFR/PI3K/AKT/mTOR pathway in THP-1 cells. The activated pathway, in turn, increased the expression of M2 macrophage-associated cytokines, which was consistent with previous reports. To confirm, we determined the effect of colon cancer cells on M2 polarization of macrophages by pharmaceutical inhibition.
A number of studies have shown that TAMs have the positive role in cancer cell phenotypes, such as cell proliferation, invasiveness, migration, and metastasis.22,23 However, cancer cells also have the roles in regulating polarization of monocytes to macrophages.4,24 More recently, colon cancer cells have been found to prevent M1- to M2-macrophage polarization by secreting IGF-1. In this study, the authors confirmed the critical role of EGFR signaling in colon cancer cells.25 Our findings uncovered the detailed mechanism by which colon cancer cells led to M2 polarization of macrophages from monocytes. Thus, colon cancer cells and TAMs reciprocally affect and enhance malignancy of colon cancer.
It is a longstanding question of how cancer cells impact their neighboring cells. Recently, Colegio et al showed that lung tumor–derived lactic acid induced M2 polarization of macrophages, which was mediated by HIF-1.24 In this study, they found that lung cancer cells secreted lactic acid–containing vesicles to influence monocytes through HIF-1 signaling. In addition, TAM polarization has also been demonstrated to be modulated by miR-145 secreted by CRC cells.4 These findings prompt us to hypothesize that other regulators secreted by colon cancer cells may have a critical role in M2 polarization of macrophage. Thus, it will be of significance to identify the potential factors responsible for this phenotype.
Traditionally, cancer treatment approaches are surgical resection, chemotherapy, and radiotherapy. Recently, with the discovery of more targets, targeted therapy has been an important method for treating cancer. EGFR is overexpressed in 60% to 80% of colon cancers, leading to development of EGFR-targeted drugs.26 Our study offers another mechanism that can be utilized to develop strategies for treating colon cancer, for example, the development of drugs for disrupting the M2 polarization-induced signaling or reprograming M2 macrophages into M1.
Overall, our data reveal a novel mechanism by which colon cancer cells regulate M2 polarization of macrophages, providing a rationale for targeting macrophage polarization to treating colon cancer in combination with traditional therapies.
Acknowledgments
The authors would like to give our sincere gratitude to the reviewers for their constructive comments.
Abbreviations
- CRC
colorectal cancer
- DMEM
Dulbecco Modified Eagle Medium
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- IFN
interferon
- PMA
phorbol 12-myristate 13-acetate
- PBS
phosphate-buffered saline
- TAM
tumor-associated macrophage
- SDS
sodium dodecyl sulfate.
Authors’ Note: Our study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Junwen Yang, PhD  https://orcid.org/0000-0002-4740-1181
https://orcid.org/0000-0002-4740-1181
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 2. Norton SE, Dunn ET, McCall JL, Munro F, Kemp RA. Gut macrophage phenotype is dependent on the tumor microenvironment in colorectal cancer. Clin Transl Immunology. 2016;5(4):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 4. Shinohara H, Kuranaga Y, Kumazaki M, et al. Regulated polarization of tumor-associated macrophages by mir-145 via colorectal cancer-derived extracellular vesicles. J Immunol. 2017;199(4):1505–1515. [DOI] [PubMed] [Google Scholar]
- 5. Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10(6):453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33(7):1478–1483. [DOI] [PubMed] [Google Scholar]
- 7. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. [DOI] [PubMed] [Google Scholar]
- 9. Kang JC, Chen JS, Lee CH, Chang JJ, Shieh YS. Intratumoral macrophage counts correlate with tumor progression in colorectal cancer. J Surg Oncol. 2010;102(3):242–248. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Sime W, Juhas M, Sjolander A. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur J Cancer. 2013;49(15):3320–3334. [DOI] [PubMed] [Google Scholar]
- 11. Kaplan DR, Whitman M, Schaffhausen B, et al. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987;50(7):1021–1029. [DOI] [PubMed] [Google Scholar]
- 12. Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315(6016):239–242. [DOI] [PubMed] [Google Scholar]
- 13. Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8(3):187–198. [DOI] [PubMed] [Google Scholar]
- 14. Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193–204. [DOI] [PubMed] [Google Scholar]
- 15. Araki N, Hatae T, Furukawa A, Swanson JA. Phosphoinositide-3-kinase-independent contractile activities associated with Fcgamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci. 2003;116(pt 2):247–257. [DOI] [PubMed] [Google Scholar]
- 16. Rocher C, Singla DK. SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS One. 2013;8(12):e84009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang M, Liu J, Shao J, et al. Cathepsin S-mediated autophagic flux in tumor-associated macrophages accelerate tumor development by promoting M2 polarization. Mol Cancer. 2014;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee H, Roshanravan H, Wang Y, et al. ApoL1 renal risk variants induce aberrant THP-1 monocyte differentiation and increase eicosanoid production via enhanced expression of cyclooxygenase-2. Am J Physiol Renal Physiol. 2018;315(1):F140–F150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawachi A, Yoshida H, Kitano S, Ino Y, Kato T, Hiraoka N. Tumor-associated CD204+ M2 macrophages are unfavorable prognostic indicators in uterine cervical adenocarcinoma. Cancer Sci. 2018;109(3):863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang Q, Pan D, Yang Y, et al. Luteolin regulates macrophage polarization via the PI3K/Akt pathway to inhibit the apoptosis stimulated by angiotensin II. Curr Pharm Biotechnol. 2018. doi:10.2174/1389201019666180629143251. [DOI] [PubMed] [Google Scholar]
- 21. Wei J, Besner GE. M1 to M2 macrophage polarization in heparin-binding epidermal growth factor-like growth factor therapy for necrotizing enterocolitis. J Surg Res. 2015;197(1):126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. [DOI] [PubMed] [Google Scholar]
- 23. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, Chen L, Ma K, et al. Polarization of macrophages in the tumor microenvironment is influenced by EGFR signaling within colon cancer cells. Oncotarget. 2016;7(46):75366–75378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen RB. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer. 2003;2(4):246–251. [DOI] [PubMed] [Google Scholar]