Abstract
Drug–drug interactions are of significant concern in clinical practice in oncology, particularly in patients receiving Cyclin-dependent kinase (CDK) 4/6 inhibitors, which are typically exposed to long-term regimens. This article presents the highlights from the ‘First Workshop on Pharmacology and Management of CDK4/6 Inhibitors: Consensus about Concomitant Medications’. The article is structured into two modules. The educational module includes background information regarding drug metabolism, corrected QT (QTc) interval abnormalities, management of psychotropic drugs and a comprehensive review of selected adverse effects of palbociclib and ribociclib. The collaborative module presents the conclusions of the five working groups, each of which comprised five experts from different fields. From these conclusions positive lists of drugs for treating common comorbid conditions that can be safely administered concomitantly with palbociclib and/or ribociclib were developed.
Keywords: CDK inhibitors, palbociclib, ribociclib, breast cancer
Introduction
In the ‘First Workshop on Pharmacology and Management of Cyclin-dependent kinase (CDK) 4/6 inhibitors (CDK4/6i): Consensus about Concomitant Medications’, promoted by the Spanish Breast Cancer Cooperative Group SOLTI, medical oncologists specialized in breast cancer joined physicians specialized in pharmacology, cardiology, psychiatry, infectious diseases, palliative care or radiation oncology in an interdisciplinary discussion forum, at Spanish national level, to address different issues regarding patients treated with palbociclib and ribociclib. These issues included overall management of adverse events (AEs) of special interest, expert opinion about clinical situations for which evidence of treatment with these drugs is limited, and, above all, concomitant medications that may be safely administered. This workshop was held on 22 May 2018. This article brings together the issues that were addressed, the information compiled and the conclusions of this 1-day meeting.
Drug–drug interactions (DDIs) are a common issue in clinical practice, particularly in the oncology setting,1,2 but appear to be particularly relevant in the CDK4/6i scenario. Firstly, because the indication of palbociclib and ribociclib is expected to be massive in Spain and other countries where these drugs are marketed, given their high efficacy and overall good tolerability in patients with endocrine-sensitive, metastatic HER2-negative breast cancer. Secondly, and equally important, patients can receive CDK4/6i for long periods of time (median progression-free survival in first- and second-line therapy of about 2 years and 9 months, respectively);3–6 this prolonged treatment time favours the emergence of other clinical conditions that require concomitant drugs (e.g. infections, allergies, episodes of pain or depression). Thirdly, because there are slight differences between the pharmacological properties of palbociclib and ribociclib distinct recommendations have been made regarding DDIs and even in different trials for the same CDK4/6i. The workshop’s organizing committee believed that this has generated, and still does, some confusion among oncologists and can lead to attitudes that are either too permissive, with the subsequent risk of relevant DDIs or, on the contrary, too conservative, which could lead to undertreating the patient’s comorbidity.
All three facts seem to warrant comprehensive training for oncologists. In this context, the aim was to thoroughly review the potential for DDIs with CDK4/6i, including putative synergy in corrected QT (QTc) prolongation, in order to provide oncologists with a reason to prohibit or prescribe certain medications with caution. Above all, the aim was to elaborate ‘positive lists’ of medications indicated for the most common comorbid conditions that could be safely administered with palbociclib or ribociclib. We were aware that such ‘positive’ information was not included in any clinical trial protocol nor could it be provided by pharmaceutical companies.
Moreover, the aim was to delve into different issues regarding CDK4/6i’s toxicity, which are considered of special interest including neutropaenia, QTc prolongation and transaminitis/liver toxicity. Evidence regarding such toxicities were thoroughly reviewed and discussed at the workshop.
Issues regarding toxicity or management, for which scarce or no evidence exists, were addressed by means of a questionnaire aimed to gather the expert opinion of participants who had been invited because of their experience with CDK4/6i in clinical trials.
Finally, the aim was to elaborate and publish a consensus document with the evidence-based information compiled during the workshop, as it was considered that this could be extremely helpful in the daily practice of oncologists dealing with CDK4/6i. This consensus document is presented here. It covers all parts of the workshop with the exception of the clinicians’ preferences determined by the survey, as we considered that the results were based on a limited number of participants.
Methodology
Oncology participants were selected and individually invited to the workshop based on their recognized experience in managing CDK4/6i. Representatives of all Spanish regions and from two Spanish Breast Cancer Cooperative Groups (SOLTI and GEICAM) attended the meeting. Specialists from other disciplines were chosen because of their expertise in specific areas of interest related to patients with metastatic breast cancer (MBC) or CDK4/6i pharmacology or toxicity.
The workshop was organized in three parts.
Educational module: this included five lectures. Those regarding ‘Key Concepts on DDIs’, ‘QT Evaluation and Management’ and ‘Antidepressants’ were given by a hospital pharmacist specialist with wide experience in DDI assessment, a cardiologist specialized in congenital and acquired QT-interval syndromes, and a psychiatrist working in an oncology department for many years, respectively. These were followed by two talks given by two medical oncologists with wide experience in ribociclib and palbociclib treatments, who addressed neutropaenia, infections, QTc prolongation, liver toxicity and other CDK4/6i-related toxicities. Extensive and collective pre-work was undertaken by the organizers to ensure that a comprehensive review and rigorous information was presented at the workshop.
Individual 15-min questionnaires: each questionnaire comprised 24 questions. Besides demographic data, participants were asked about preferred therapeutic strategy (chemotherapy (CT) versus endocrine therapy (ET) + CDK4/6i versus CT followed by maintenance ET + CDK4/6i) in challenging clinical conditions such as inflammatory breast cancer, myelophthisis, peritoneal carcinomatosis or pulmonary lymphangitis. They were also asked about administration times of palbociclib/ribociclib regarding planned surgery and radiotherapy, reintroduction of CDK4/6i after recovered liver toxicity caused by one of them, and rare toxicities observed. The results of these questionnaires are not presented here.
Collaborative module: five working groups, each group made up of four or five experts from different fields, were formed. Each group received a template (previously prepared by three medical oncologists and three hospital pharmacist specialists) that included guidelines and references to elaborate and appropriate positive lists of medications for particular clinical condition(s), which were specific for them. In addition, each working group also received a PowerPoint presentation including: (a) a hypothetical clinical situation related to the group of medications assigned, which illustrated the risk for potential DDIs; (b) a template table to guide and unify the lists of medications presented across all the working groups. Then, each group explained the results obtained to the whole audience, which were discussed to reach a consensus.
The workshop was sponsored mainly by Pfizer and Novartis. Although representatives of both companies indicated their willingness to attend the workshop, this was not allowed in order to prevent any interference with the workshop dynamics. Instead, the workshop organizers provided the companies’ representatives with a draft of the manuscript so they could add insights regarding literature missing from the document. The two companies made no relevant comments or suggestions during the 48 h allowed for review and, therefore, no changes were made to the manuscript.
Educational module
Drug metabolic pathways and membrane transporters: how to interpret DDIs with palbociclib and ribociclib?
Main pharmacokinetic DDIs are secondary to alterations in the enzymatic metabolism, mainly at the cytochrome p450 level. Other DDIs result from inhibition or induction of membrane transporters, such as P-glycoprotein (P-gp) and organic anion transporters (OATs), which can be caused by some drugs. Several drugs are substrates for membrane transporters, which mean that they depend, at any step, on this active transport through the membranes to reach their site of action (process of absorption and distribution) or to be eliminated. These membrane transporters are proteins susceptible to being induced or inhibited by some drugs, which, as a consequence, may increase or decrease the concentration of the transporter substrates in the organism.7,8
Absorption
At this level, drug interaction can affect either the bioavailability of the drug, the amount of drug absorbed or the rate of absorption. The most frequent interactions affecting absorption are due to gastric pH modification. This occurs with proton pump inhibitors (PPIs), antacids or H2-receptor antagonists. Chelation with calcium, iron or ionic resins can lead to the formation of nonabsorbable compounds, reducing the bioavailability of concomitant drugs. Finally, modification of intestinal motility can also alter the absorption process.
The solubility of palbociclib is significantly reduced at pH values greater than 4. Co-administration of rabeprazole and palbociclib under fasting conditions decreased the maximum concentration (Cmax) and the area under the concentration curve (AUC) by 62% and 80%, respectively. However, when co-administered with food, Cmax and AUC decreased by 41% and 13%, respectively.9 Palbociclib therefore should be taken with food. It is expected that palbociclib absorption will not be affected by H2-receptor antagonists and local antacids.10
The highest solubility of ribociclib is at or below pH 4.5. DDIs are not expected with PPIs, H2-receptor antagonists or antacids.11 Ribociclib can be taken with or without food.12
Although palbociclib and ribociclib absorption does not seem to be affected by co-administration with antacids, the Summary of Product Characteristics (SPC) of most common antacids, such as magnesium hydroxide and amalgamate, recommends separating their intake for at least 2 h from other drugs.13,14
Finally, palbociclib and ribociclib can cause diarrhoea as a side effect, which may affect the absorption of other drugs.
Distribution
Distribution-related DDIs are not expected because palbociclib and ribociclib are not highly protein bound (palbociclib 85% and ribociclib 70%).10,12
Metabolism
Metabolism includes all the molecule reactions when partially or totally transformed in other substances.15
When a drug is metabolized by a specific CYP isoenzyme, it is known as a substrate for that CYP isoenzyme. Several drugs have the potential to inhibit or induce different CYP isoenzymes with variable intensity, which impacts on the metabolism of other drugs (Table S1, Supplementary material, File 1).
Taking into account the degree of dependence on a specific isoenzyme to be metabolized, substrates are classified as major (when they are mainly eliminated by that isoenzyme) or minor substrates (Table S1, Supplementary material, File 1). Moreover, the US Food and Drug Administration (FDA) distinguishes these other categories: sensitive substrates (SS), when a substrate demonstrates an increase in AUC of 5-fold or greater with strong index inhibitors; moderately sensitive substrates, when the increase in AUC is 2-fold or greater to less than 5-fold, and narrow therapeutic index (NTI) substrates, when a substrate has a narrow index between the minimum effective concentration and the minimum toxic concentration. Therefore, small variations of drugs with NTI can cause either a loss of efficacy or toxicity.
Palbociclib and ribociclib are major substrates for CYP3A4 so, hereinafter, only CYP3A4 will be specifically mentioned.
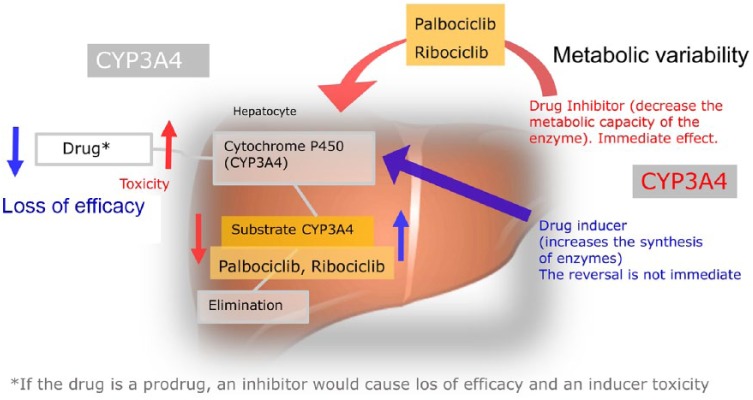
Palbociclib is a weak inhibitor of CYP3A4, but in the SPC,10 a dose reduction in NTI substrates is suggested when concomitantly administered with palbociclib, since their exposure could be increased. By contrast, ribociclib is a strong CYP3A4 inhibitor when administered at a 600 mg dose, and a moderate CYP3A4 inhibitor at a 400 mg dose.12 Therefore, due to its intrinsic CYP3A4 inhibitory properties, ribociclib may lead to increased serum concentrations of CYP3A4 substrates in a more extensive way (Figure 1).
Figure 1.
Drug mechanisms: drug interaction with palbociclib and ribociclib. If the drug is a prodrug, a CYP3A4 inhibitor will cause loss of efficacy and/or enhanced toxicity by the accumulated prodrug. The metabolism of palbociclib and ribociclib is described when they cross the hepatocyte membrane, mainly by passive diffusion, to be metabolized by the cytochrome P450 pathway, specifically by the CYP3A4 isoenzyme. The blue arrows indicate how an inducer drug acts on CYP3A4 and accelerates the production of CYP3A4 substrate (palbociclib and ribociclib) and as a consequence decreases the concentration drug in the blood, leading to a loss of efficacy. The red arrows indicate how a CYP3A4 inhibiting drug (palbociclib and ribociclib) decreases CYP3A4’s metabolic capacity and therefore a lesser amount of substrate will be formed to be eliminated and the drug would accumulate in the blood giving rise to toxicity.
On the other hand, co-administration of palbociclib or ribociclib with strong or moderate CYP3A4 inhibitors may increase the AUC of palbociclib or ribociclib and, consequently, their risk of toxicity. So, these CYP3A4 inhibitors should be avoided when possible. Co-administration of strong or moderate CYP3A4 inducers, on the contrary, may decrease exposure to palbociclib and ribociclib and lead to a lack of efficacy, so they also should be avoided. These recommendations are included in the SPC of both palbociclib and ribociclib.10,12 See Table 1 for recommendations and dose modification.
Table 1.
Dose modification of palbociclib or ribociclib in co-administration with drug inhibitors or inducers of CYP3A4.
| Strong inhibitor | Moderate inhibitor | Weak inhibitor | Strong inducer | Moderate inducer | Weak inducer | |
|---|---|---|---|---|---|---|
|
Palbociclib
125 mg once a day, 3 weeks on/ 1 week off |
Should be avoided. If unavoidable, reduce palbociclib dose to 75 mg.* | Risk of increased exposure. Monitor for possible increased toxicity. |
Low risk of increased exposure. | Should be avoided. Consider using an alternative with less potential to induce CYP3A4. |
Risk of decreased exposure and lack of efficacy. Monitor. |
Low risk of decreased exposure. |
|
Ribociclib
600 mg once a day, 3 weeks on/1 week off |
Should be avoided, if unavoidable, reduce ribociclib dose to 400 mg.* | Monitoring. | Low risk of increased exposure. | Should be avoided. Consider using an alternative with less potential to induce CYP3A4. |
Risk of decreased exposure and lack of efficacy. Monitor |
Low risk of decreased exposure. |
The reduction must be maintained during the treatment with the inhibitor and at least five half-lives of elimination after its withdrawal. A half-life of elimination is defined as the time required to eliminate 50% of the drug from the organism.
Membrane transporters
The role of membrane transporters, such as P-gp or breast cancer resistance protein (BCRP), in the development of drug resistance has been observed in several studies.16,17 The most studied membrane transporter, P-gp, is constitutively expressed in several normal human tissues, including the liver, kidney, small and large intestine, testes, adrenal gland and the placenta. It is also located in the endothelial cells of the blood-brain barrier (BBB). This particular tissue distribution indicates an important detoxification role for P-gp, and by extension, for many membrane transporters, by excreting xenobiotics and metabolites into urine, bile and the intestinal lumen.18 Some cancer cells induce the overexpression of this membrane efflux pump, facilitating a resistance to anticancer agents.16 In addition, some drugs can modulate P-gp function, either by enhancing (such as most antiepileptics) or inhibiting (such as verapamil or quinidine) its expression.18,19 When the membrane transporter is overexpressed, the drug substrate is carried out of the cells, for example, to the intestinal lumen, and consequently decreases its plasmatic concentration. By contrast, the inhibition of the efflux pump increases the plasmatic concentration of the drug substrate, with a subsequent enhanced risk of toxicity (Figure 2).
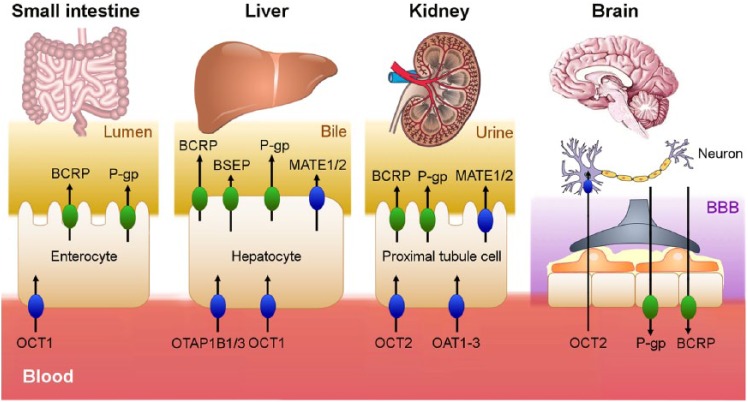
Figure 2.
The membrane transporters on which palbociclib (OCT1, P-gp, BCRP) and ribociclib (P-gp, BCRP, OATP1B1, OATP1B3, OCT1, OCT2, BSEP, MATE1) can act are illustrated. According to an in vitro study, palbociclib and ribociclib act as inhibitors of these transporters. As a result, a greater amount of drugs that are substrates for these transporters would accumulate in the blood causing the appearance of adverse effects. Those coloured in green refer to the ABC superfamily efflux pumps. Those coloured in blue refer to the SLC superfamily, which uptake the drug in the enterocyte, hepatocyte, proximal tubule cell and neuron.ABC, ATP-binding cassette; BBB, blood brain barrier; BCRP, breast cancer resistance protein; BSEP, bile salt export pump; MATE1, multidrug and toxin extrusion protein; OATP, organic anion-transporting polypeptide; OCT, organic cationic transporter; P-gp, P-glycoprotein; SLC, solute carrier.
Membrane transporters are divided in two superfamilies: ATP-binding cassette (ABC), composed of efflux pumps and solute carrier (SLC), composed of uptake pumps.7
Available data from in vitro studies suggest that palbociclib passes through the membrane by passive diffusion, so it is not a substrate for membrane transporters in most tissues.16 However, palbociclib is actively thrown out of the cell by P-gp and BCRP at the BBB level,20,21 which would explain its poor brain penetration compared with an intact BBB. Ribociclib is a substrate for intestinal P-gp22 and probably slightly less affected by BBB membrane transporters.23,24
Based on in vitro data, palbociclib is predicted to have the potential to inhibit intestinal P-gp, BCRP and organic cationic transporter (OCT)1, while ribociclib can potentially inhibit P-gp, BCRP, organic anion-transporting polypeptide (OATP)1B1, OATP1B3, OCT1, OCT2, bile salt export pump (BSEP) and multidrug and toxin extrusion protein (MATE)1 activities. Consequently, palbociclib and ribociclib may increase the side effects of drugs, which are substrates for these transporters.
Palbociclib has a low potential to inhibit OATP1B1, OATP1B3, BSEP, OAT1, OAT3 and OCT2, so DDIs are not expected with the substrates for these transporters. Table S2 illustrates membrane transporters, their localization and examples of drugs that are substrates for each transporter (Table S2, Supplementary material, File 1).
Take-home messages
○ Ribociclib can be taken with or without food, and DDIs are not expected with PPIs. By contrast, palbociclib should be taken with food, and concomitant PPIs should be avoided.
○ Both palbociclib and ribociclib are substrates of the CYP3A4 enzymatic complex. As substrates for CYP3A4, the co-administration of palbociclib or ribociclib with strong or moderate CYP3A4 inhibitors or inducers may increase or decrease the AUC of palbociclib or ribociclib, respectively, leading either to a risk of increased toxicity or decreased efficacy.
○ Palbociclib is a weak inhibitor of CYP3A4, but a dose reduction of substrates with a NTI is suggested when taken concomitantly with palbociclib. Ribociclib is a moderate inhibitor at a dose of 400 mg/day and a strong inhibitor at a dose of 600 mg/day, so it may lead to increased serum concentrations of CYP3A4 substrates in a more extensive way.
○ Palbociclib has the potential to inhibit intestinal P-gp, BCRP and OCT1, while ribociclib can potentially inhibit P-gp, BCRP, OATP1B1, OATP1B3, OCT1, OCT2, BSEP and MATE1 activities. Consequently, palbociclib and ribociclib may increase the side effects of drugs, which are substrates for these membrane transporters.
QTc for oncologists: how to evaluate and manage it
The evaluation of the QTc interval, despite its apparent simplicity, has been a controversial issue among health professionals, whether or not they are cardiologists. In addition, some studies evaluating the consistency of its measurement between professionals are far from optimal. Less than 25% of nonexpert physicians seem able to correctly measure this interval.25
The QT, as its name denotes, is the interval between the beginning of the QRS complex and the end of the T wave on an electrocardiogram (ECG) (Figure 3), while QTc represents the QT corrected by the heart rate. The QT groups all the electrical activity of the ventricle, including depolarization and repolarization, and it basically represents an approach to the duration of the ventricular repolarization process, which is a consequence of a positive charge efflux from the cells (Ito, Ica, IKs and IKr currents).
Figure 3.
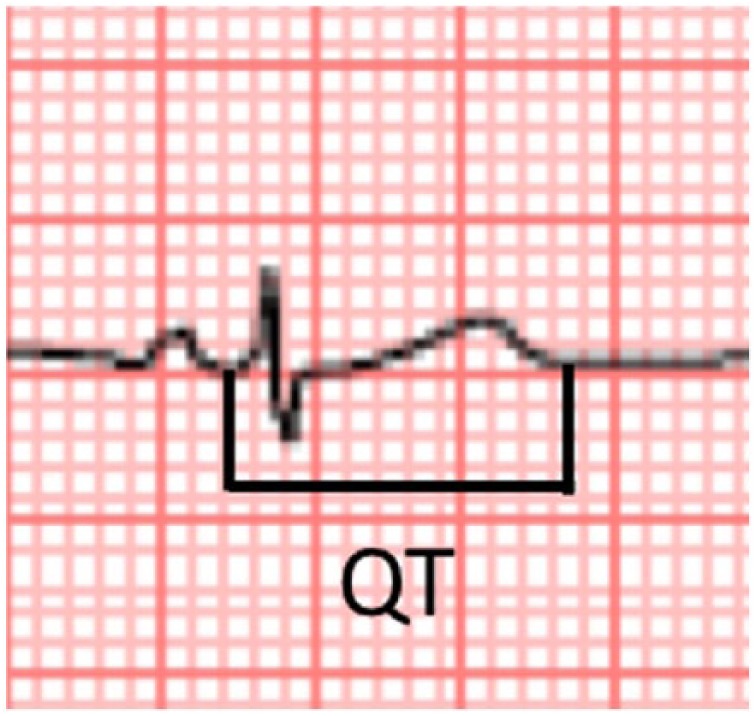
QT interval.
In spite of the simplicity of the concept, there usually are discrepancies regarding where to perform QT measurements. The main accepted strategy is to measure it on the ECG leads, where the waves are clearly seen. Often, due to the orientation of the ventricular vectors, lead II is the best for this purpose. Other leads could be used, aVR, aVF, V5, V6 and V4, in this order. By contrast, aVL is considered the worst lead to quantify the QT interval.26
Another source of controversy is the location of QT limits. Often, the end of the T wave is not clear, especially in the presence of U waves. A pragmatic method to solve this issue is the so-called ‘tangent method’. It basically traces a tangent line following the descending part of the T wave and measures the interval up to the point where this line crosses the isoelectric line (Figure 4).26,27
Figure 4.
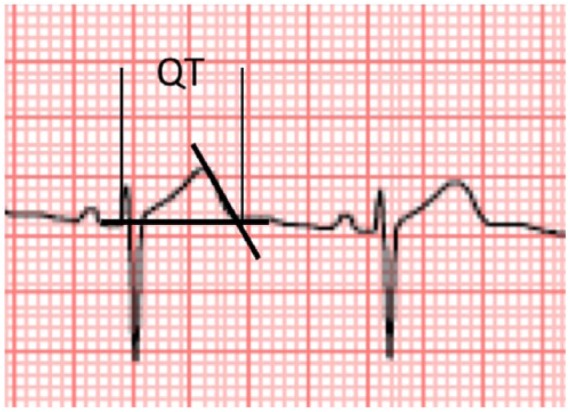
Tangent method to accurately measure the end of the T wave.
Moreover, repolarization duration, and therefore QT, is heavily influenced by multiple variables, heart rate being one of the most relevant. In physiological conditions, at increasing heart rates, the QT interval decreases due to shortening of the repolarization time (Figure 5). That is why we ultimately take QTc as a reference measure and use mathematical methods to correct QT duration according to heart rate. The most widely used method among cardiologists, and the most recommended for its simplicity and availability (e.g. apps, rules, etc.), is the Bazett formula (see Equation 1, Table S3, Supplementary material, File1). This method is far from optimal, since it ‘overcorrects; at high heart rates and ‘undercorrects’ at low heart rates.28 Most cancer clinical trials, however, require the Fridericia correction formula (see Equation 2, Table S3, Supplementary material, File1). Fridericia’s method, together with the Framingham correction (Equation 3, Table S3, Supplementary material, File1), showed in fact the best rate correction, and significantly improved a prediction of 30-day and 1-year mortality compared with Bazett’s method in a recent study.29 Bazett overestimated the number of patients with potentially dangerous QTc prolongation, which could lead to unnecessary safety measurements and withholding from the patient the first choice medication. There are online tools to calculate all three mentioned formulae.
Figure 5.
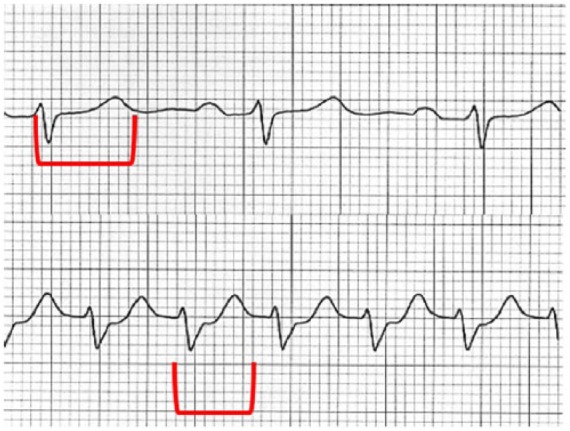
Heart rate influence over QTc. In the upper panel, a normal duration of QTc is noted. In the lower panel, heart rate is increased, evidencing a long QTc (when corrected) that in absolute terms has a similar duration when compared with the upper one.
QT and QTc are also influenced by QRS duration, as the QRS complex is actually included in the interval. For an abnormal QRS widening, a method should be used to correct (subtract) the contribution of this depolarization abnormality into the QTc value. The most widely used method, and the easiest approach, is to use the JT, that is, the interval between the J point (end of the QRS) and the end of the T wave. However, there are several complex mathematical methods that can be used to perform the correction.
So, why is QTc duration clinically important? The QTc interval is important because the prolongation of repolarization favours the development of polymorphic ventricular tachycardia or torsades de pointes (TdP) ECG, a condition associated with risk of arrhythmic death. Then, the physician’s efforts should be focused on identifying subjects with risk for developing this tragic event.
There are two main reasons why a QTc interval may be prolonged in a subject. The first one is an inherited condition, where at least one of the mechanisms controlling the repolarization process is abnormal. Several diseases sharing this type of abnormality are grouped under the name congenital long QT (LQT) syndrome. Hundreds of mutations in at least 12 genes have been associated with this condition. Most mutations are located in ion channels involved in the repolarization process. The three commonest subtypes (LQT1-3) involve genes KCNQ1, KCNH2 and SCN5A, respectively. The treatment and diagnosis of LQT syndrome are beyond the scope of this manuscript. The other reason why a QT interval can show an abnormal prolongation is the so-called acquired LQT. Multiple factors can influence QT duration and produce an abnormal duration of the interval. One of the main factors is the use of drugs able to bind KCNH2 and, consequently, cause an inhibition of the IKr current.30
In most cases, however, there is more than one factor influencing a drug-induced acquired QTc prolongation (e.g. ionic abnormalities, genetic background, etc.) and hence, the probability of developing TdP. Table 2 shows the factors that can increase this risk. Some are nonmodifiable variables, such as sex. The QTc interval is, on average, longer in women than in men so the QTc upper limit of normality for men is 450 ms and 460 ms for women. Most of the other known risk factors are actionable or may be minimized. In fact, the first approach to treat acquired LQT is to correct these possible coexisting factors, to determine the risk of developing a major arrhythmic event in comparison with the benefit provided by the likely culprit drug and to evaluate clinically the presence of symptoms associated with QTc prolongation (syncope). In general, with a QTc over 480 ms, the suspicious drug should be withdrawn, unless its clinical benefit surpasses the potential risks for arrhythmia. If a QTc is over 500 ms, it is mandatory to withdraw all possible QTc-prolonging agents. Patients with baseline QTc prolongation, significant QTc prolongation (500 ms), associated symptoms (syncope) or the presence of other factors that increase the risk of TdP (Table 2), regardless of the quantity of QTc prolongation, should be carefully evaluated by a cardiologist.
Table 2.
Factors related to TdP development.
| Female sex |
| Bradycardia |
| Hypokalaemia |
| Heart failure |
| Ventricular hypertrophy |
| Recent cardioversion from atrial fibrillation |
| High concentration of the drug with known, possible or conditional risk of TdP |
| Baseline QTc prolongation |
| Rapid rate of infusion of QTc-prolonging drug |
| Subclinical long QTc syndrome |
| Ion channel polymorphisms |
| Severe hypomagnesaemia |
Prevention is a key component in acquired LQT management, and implies an increased awareness among physicians regarding the potential of certain drugs to prolong QTc. Although the list of drugs with the potential for prolonging QTc interval increases daily, there are public resources available that can simplify their identification. The most widely used resource, which is also continuously updated, is the CredibleMeds database (https://crediblemeds.org).31 According to their risk of TdP development, drugs are categorized as known risk (KR), possible risk (PR) and conditional risk (CR). Definitions for each category are illustrated in Table 3. The preventive approach also includes identifying, by means of a clinical interview, the risk of suffering a congenital long QT syndrome. A family history of premature sudden death, family history of congenital LQT or recurrent syncope should suspect this condition. This background or the evidence for an acquired LQT development should prompt an evaluation by the cardiologist.
Table 3.
Categories of drugs according to their risk of TdP: the Arizona Center for Education and Research on Therapeutics (AZCERT) classification.29
| Category | Definition |
|---|---|
| Known risk of TdP | Drug causes frequent QTc prolongation. Risk of TdP is dose dependent. |
| Possible risk of TdP | Drug frequently prolongs QTc, but TdP rarely ensues. |
| Conditional risk of TdP | The risk of the drug causing QTc prolongation and TdP depends on the presence of essential cofactors such as hypokalaemia, promoting the adverse reaction (‘conditional’ risk). |
TdP, torsade de pointes.
Regarding CDK4/6i, and in spite of the fact that the risk of acquired LQT has been especially linked to ribociclib rather than palbociclib (see “Digging for neutropenia, QTc and infections: What actually matters?” section), the panel recommended the careful evaluation of all patients that will receive both palbociclib and ribociclib (baseline ECG and medical history) and avoid, if possible, concomitant QTc-prolonging drugs. Based on eligibility criteria of the trials performed, palbociclib should be avoided in patients with baseline prolongation greater than 480 ms, while ribociclib should be avoided with baseline QTc greater than 450 ms.
ECG controls should be especially performed in patients receiving ribociclib, and repeated at least 15 days after starting treatment during the first two cycles (see guidelines in “Digging for neutropenia, QTc and infections: What actually matters?” section). The panel also recommended repeating ECG in patients who started palbociclib with baseline grade 1 QTc (QTc between 450 ms and 480 ms) at least 15 days after the initiation of treatment. In addition, the ECG should also be repeated when QTc prolongation is suspected (e.g. syncope) or new concurrent conditions or drugs make it more likely to cause an adverse effect. Furthermore, careful monitoring should be performed in patients with risk of QTc prolongation (Table 3), including those affected with any diagnosed cardiac comorbidity.
Take-home messages
○ QTc prolongation should be suspected even with normal QTc on baseline ECG in patients with a history of recurrent syncope or a family history of LQT syndrome or sudden death. These aspects should be incorporated into the oncologist clinical interview.
○ Acquired QT prolongation, like the risk of TdP, is usually multifactorial. The correction of all contributing factors should always be taken into account.
○ Patients receiving drugs with the potential for prolonging QTc should be carefully evaluated at baseline and followed up according to the pharmacology of the drug. To decrease the risk, it is key to avoid the concomitant use of more than one potentially QTc-prolonging agent.
○ Once acquired LQT syndrome is diagnosed, a careful risk assessment should be performed, including clinical assessment, for the presence of associated symptoms (syncope). In general, the suspicious agent must be withdrawn if the patient is symptomatic, if QTc is more than 500 ms, or if other risk factors for TdP coexist. If QTc is between 480 ms and 500 ms, the suspected drug should be maintained only if the benefit surpasses the risk, and a cardiologist assessment and follow up are established.
Psychotropic drugs in the breast-cancer population: management of antidepressants in patients under CDK4/6i treatments
Prevalence of affective disorders in the breast-cancer population
MBC disease implies a serious threat to health and life. In addition to the psychological stress, there is growing evidence that biological stress caused by both the tumour and the treatment could directly affect brain function and facilitate the appearance of cognitive and affective symptoms.32–35
The prevalence of depressive symptoms among survivors of breast cancer (up to 66%) appears to be significantly higher in comparison to the general population, and persists longer (over 5 years) after diagnosis. By contrast, the prevalence of anxiety (up to 33%) does not seem to be significantly higher.36
Sleep disorders are present in 65% of women survivors of breast cancer versus 55% of a control group of the same age; its severity correlated with the presence of depressive symptoms, comorbidities, hot flashes and the residual effects of cancer.37
As for the metastatic disease (main focus of the present review), almost a quarter and a third of patients experience clinically relevant symptoms of depression and anxiety, respectively; both conditions are reported to be more common in patients with MBC treated with CT than in those receiving ET.38 However, an enhanced risk of depression has been noted in patients with MBC harbouring hormone-receptor positive (HR+) tumours; and for this HR+ population, a shorter median overall survival has been described in those women with depressive symptoms.39 Most importantly, in a prospective study including 125 patients with MBC, the improvement of depressive symptoms during the first year of intervention was associated with a subsequent longer survival.40
Treatment with antidepressants
Antidepressants are the drugs indicated for the treatment of depression, but they are also useful to treat anxiety and stress-related disorders by basically modulating the monoaminergic neurotransmission.
The pharmacological treatment of depression started in mid-20th century with the discovery of tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs). TCAs are mostly anticholinergic and have prominent side effects. MAOIs are also associated with a high risk of toxicity, DDIs and food interactions. Hence, both drug groups should be avoided in oncological patients, with the exception of amitriptyline, which is useful at very low doses to alleviate neuropathic pain.
Nowadays, new generations of antidepressants are available. They are equally effective, safer and better tolerated. Table S4 categorizes all these drugs according to their mechanism of action41 (Table S4, Supplementary material, File 1).
How to choose the right antidepressant
Selective serotonin reuptake inhibitors (SSRIs) represent the first-line pharmacological treatment for a depressive disorder, but other antidepressants can be chosen according to the accompanying symptoms (e.g. inhibition, anxiety, agitation, insomnia, anorexia etc.).
Aside from depression, antidepressants may be indicated to treat specific symptoms such as insomnia (mirtazapine and trazodone), neuropathic pain (duloxetine and amitriptyline), hot flashes (venlafaxine and SSRIs), lack of appetite, nausea and/or vomiting (mirtazapine) and fatigue (bupropion and methylphenidate).41
Side effects and QTc prolongation
Second-generation antidepressants, albeit safe overall, may cause untoward effects such as hypersensitivity reactions, hepatotoxicity, gastrointestinal, cardiovascular or genitourinary symptoms, hyponatraemia, metabolic disorders or increased risk of bleeding; it is worth mentioning the risk of seizures related to bupropion, as well as the occurrence of withdrawal symptoms upon treatment discontinuation, most prominent with venlafaxine and paroxetine.42
A major concern is the effect of antidepressants on QTc-interval prolongation and the risk of TdP (see “QTc for oncologists: How to evaluate and manage it” section). Citalopram and escitalopram prolong QTc and have known risk of TdP (“QTc for oncologists: How to evaluate and manage it” section) at therapeutic doses, although the concern created by this issue has been a matter of debate,43,44 they are not recommended. Mirtazapine and venlafaxine are drugs with possible risk for TdP and must be administered with great caution with concurrent medical pathologies and polypharmacy. Other SSRIs, such as fluvoxamine, fluoxetine, paroxetine and sertraline, have been associated with a modest increase in the QTc interval,45 and have a conditional risk for TdP; they are the best option, provided that the conditions for their administration are taken into account (i.e. electrolyte imbalance, hyper- or hypothyroidism or concomitant use of QTc-prolonging drugs). At the moment, there is no evidence of TdP risk for duloxetine, desvenlafaxine, bupropion or for the newer antidepressants, vortioxetine and vilazodone.
DDIs with CDK4/6i
Antidepressants may present pharmacodynamic and pharmacokinetic interactions.
CDK4/6i palbociclib and ribociclib have been associated with QTc prolongation,46 so the concurrent use of antidepressants with TdP KR and PR should be avoided; antidepressants with TdP CR can be administered, provided that appropriate monitoring and control of other risk factors for TdP are established.
Palbociclib and ribociclib are metabolized mainly by CYP3A4, therefore, antidepressants with an inhibitory effect on CYP3A4 (i.e. nefazodone, currently not available in Spain, and fluvoxamine) should be avoided (see also “Drug metabolic pathways and membrane transporters: How to interpret drug-drug interactions with palbociclib and ribociclib?” section and collaborative module).
Ribociclib, as an inhibitor of CYP3A4,47 may increase the levels of antidepressants metabolized by CYP3A4, such as trazodone, mirtazapine, venlafaxine, reboxetine and vilazodone. Ribociclib is also an inhibitor of CYP1A2 and could affect the levels of amitriptyline, fluvoxamine, duloxetine and mirtazapine, but to a lesser extent.
Take-home messages
○ Depressive, sleeping and anxiety disorders are very common in patients with MBC and should be promptly recognized and treated. Published data suggest a worse overall survival for patients with luminal MBC suffering depression; importantly, an improvement in long-term outcomes has been observed for those patients with MBC experiencing a reduction of their depressive symptoms. DDIs with palbociclib or ribociclib exist for most antidepressants, either via CYP3A4 or mostly via QTc prolongation.
Digging for neutropaenia, QTc and infections: what actually matters?
For most oncologists, both palbociclib and ribociclib are considered to be well tolerated and very similar drugs in terms of clinical efficacy. However, slight differences in their side-effect profiles have been described. The aim of this section is to review these similarities and differences in detail, with the focus on three clinically relevant side effects, that is, neutropaenia, drug-induced QTc prolongation and infections. For this purpose, data have been compiled from multiple sources. Reports from the PALOMA and MONALEESA trials4–6,48,49 and data from conference abstracts, the ClinicalTrials.gov website, European Medicines Agency (EMA) evaluations50,51 and the Summary of Product Characteristics (SPC) of both drugs were comprehensively reviewed. At the time of the workshop, data from the most recent trial, MONALEESA-3, was only known from an abstract, but the complete results were released immediately afterwards,52 and therefore are also included here, together with the preliminary results of the CompLEEment study.50
Neutropaenia
The results from the PALOMA-2 trial showed that neutropaenia is the most common haematological (and nonhaematological) side effect associated with palbociclib. In that trial, all-grade neutropaenia and grade (G) 3 and 4 neutropaenia appeared in more than 80% and almost 70% of patients, respectively.3 About 20% of women experienced between three and five episodes of neutropaenia during treatment, and an additional 17% had more than six episodes.48 Median time from first dose to first onset of neutropaenia of any grade was 13 days, and median duration of G3/G4 neutropaenia was 15 days and 9 days, respectively.51 Due to this fact, almost 80% of patients needed temporary discontinuation of palbociclib, and in 33%, a dose reduction.3 Some differences between geographical areas were suggested.48 Nonetheless, it must be emphasized that only 1.5% incidence of febrile neutropaenia was observed, and just 1.5% of patients discontinued palbociclib due to neutropaenia.3 Similar results were observed in the PALOMA-3 trial in patients that experienced neutropaenic fever, and the rate of discontinuation due to neutropaenia was 2%.51 The recommendations of the palbociclib last data sheet included a close follow up of the G3 neutropaenia onset and duration during the first two cycles, and a dose reduction if G3/G4 neutropaenia is frequent or represents repetitive delays in day 1 of subsequent cycles. The majority of neutropaenia episodes were not complicated.6 Importantly and according to cumulative data from the PALOMA trials,3,6,48,53–55 most patients with neutropaenia will not have concomitant infections.
Ribociclib shows a similar incidence of G3/G4 neutropaenia (62%).5 However, by indirect comparison with the PALOMA trials, a lower incidence of neutropaenia episodes, dose interruption (50% in the first year, 18% afterwards) and incidence of G3/G4 neutropaenia beyond 6 months have been reported with ribociclib in pivotal trials.4,5,56,57 This is probably due to the restrictive G3 neutropaenia management in the MONALEESA-2 trial, also implemented in the SPC, which required a dose reduction of ribociclib in the presence of a second episode of G3 neutropaenia. Overall, 54% of women had a dose reduction of ribociclib (mostly just one) and this was mainly due to neutropaenia (24% of all patients in the ribociclib arm).50 Interruptions due to neutropaenia were reported in 42% of patients.5 Median time from first dose to first onset of neutropaenia of any grade was 16 days (29 days for G3/G4) and median duration of ⩾ G3 neutropaenia was 15 days.50 Median time to first dose reduction was 2.9 months.50 The rate of permanent ribociclib discontinuation was 0.9%.50 Again, most patients did not experience concomitant infections, and the incidence of febrile neutropaenia was very low (1.2%).4 Data from the MONALEESA-749 and MONALEESA-3 trials52 confirmed a similar incidence of G3/G4 neutropaenia (Table S5, Supplementary material, File 1) with low rates of febrile neutropaenia (1–2%).
Drug-induced QTc prolongation
A review of the QTc clinical significance and evaluation has been performed in “QTc for oncologists: How to evaluate and manage it” section, with a focus on the reported incidence of QTc with palbociclib and ribociclib.
Evaluation of QTc prolongation differed between palbociclib and ribociclib trials (Table 4). Palbociclib had been associated with QTc prolongation in dogs, but not in phase I trials.58 Therefore, the PALOMA trials permitted the inclusion of patients with basal QTc < 480 ms. By contrast, asymptomatic QTc prolongation was observed in phase I trials with ribociclib at doses ⩾ 600 mg.59 Therefore, closer monitoring and more strict inclusion criteria were established in the MONALEESA trials. All patients with basal QTc > 450 ms were excluded, and all ECGs were electronically reported and centrally evaluated.
Table 4.
| Participants | Evaluation | Timing | Results | |
|---|---|---|---|---|
| PALOMA-2 60,61 | Substudy ECG (n = 123) Basal QTc < 481 ms. |
Preferable automatic ECG lecture. Triplicate ECG. Centrally manual evaluation. |
Day 0: preD, 2, 4, 6 and 8 h later. C1D14: preD, 2, 4, 6 and 8 h later. |
No patients had a postbaseline absolute maximum QTc ⩾ 500 ms or a ΔQTc ⩾ 60 ms during the intensive QTc assessment period. Correlation between QTc and palbociclib concentration was weak. Aromatase inhibitors the upper bounds of one-sided 95% confidence interval of ΔQTc for all five QTc postbaseline time points were less than 20 ms, the postbaseline QTcs’ were to be considered noninferior to baseline and no clinically relevant effects of palbociclib in QTc were concluded |
| All population (n = 666) Basal QTc < 481 ms |
Preferable automatic ECG lecture. Triplicate ECG. Centrally manual evaluation. |
Day 0: C1D1, preD C1D14, preD C2D14, preD C4D1, preD C7D1, preD C10D1, preD |
QTc prolongation G2: palbociclib: 1.6%, placebo 0.9%. QTc prolongation G3: palbociclib: 0.2%, placebo 0%*. |
|
| MONALEESA-2 4,5 | All population (n = 668) Basal QTc < 451 ms |
Locally automatic ECG lecture. Triplicate ECG. Centrally manual evaluation. |
Day 0: C1D1, preD C1D14, preD C1D14, postD C2D1, preD C2D1, postD C3D1, preD C3D1, postD C4D1, preD C5D1, preD C6D1, preD C6D1, postD C7D1, preD C8D1, preD C9D1, preD C9D1, postD |
QTc prolongation G2: ribociclib: 3.6%, placebo 0.6%. QTc prolongation G3: palbociclib: 0.6%, placebo 0%.$ |
p values not provided.
Statistically significant differences for both G2 and G3 QTc prolongation.
Regarding palbociclib, a substudy aimed at evaluating QTc prolongation was performed in the context of the PALOMA-2 trial.62 ECG on day 0 and on C1D14 was centrally evaluated in 123 of the 660 patients included in the main study. No associations between palbociclib and prolongation of QTc were found. Both the EMA evaluation and the SPC of palbociclib described this absence of correlation. However, data from all 660 patients evaluated locally by the investigators showed an incidence of G2/G3 QTc prolongation (Fridericia correction) of 1.6% and 0.2% in the palbociclib arm versus 0.9% and 0% in the placebo arm, respectively.63
In the MONALEESA-2 trial, an increase in the incidence of QTc prolongation in the ribociclib arm compared with the placebo arm was described. The incidence of G2 and G3 prolongation was 3.6% and 0.6%, respectively, with ribociclib versus 0.6% and 0% with placebo. QTcF (Fredericia’s correction formula)-related AEs occurred within 30 days of initiation of treatment. Among the patients who had QTcF prolongation of > 480 ms, the median time to onset was 15 days and these changes were reversible with dose interruption and/or dose reduction. There were no reports of QTc prolongation after 18 months of treatment. There was one death in the ribociclib arm, which was attributed to G2 QTc prolongation; other risk factors for QTc prolongation were detected in this patient, such as G3 hypokalaemia and concomitant use of a prohibited drug with a known risk for QTc prolongation.4,5,51 Both the EMA evaluation and ribociclib SPC indicate that an ECG should be performed before initiating treatment and repeated at approximately C1D14 and C2D1, then as clinically indicated. Appropriate monitoring of serum electrolytes should be performed before the start of ribociclib treatment and at the beginning of each of the first six cycles. The use of ribociclib should be avoided in patients who already have, or who are, at significant risk of developing QTc prolongation. The concomitant use of drugs with risk of QTc prolongation should be checked at any time, including the use of medicinal or herbal products.64
In the more recently published MONALEESA-7 and MONALEESA-3 trials, the incidence of reported QTc prolongation (⩾ G2) was 7% and 3.9%, respectively. Grade 3 QTc prolongation was observed in 1% of MONALEESA-7 patients and 1.7% in the MONALEESA-3 population (0.4% in the placebo arm). Corresponding rates in their placebo arms are summarized in Table 5. The highest rate of G2 QTc in MONALEESA-7 may be due to the concomitant administration of tamoxifen, a drug with a well-known risk of QTcF potential prolongation: in the ribociclib group of the study, an increase of more than 60 ms from baseline in the QTcF interval was observed in 16% of patients receiving tamoxifen versus 7% receiving an NSAID (Nonsteroidal anti-inflammatory drug); in the placebo group this increase was observed in 7% of patients receiving tamoxifen versus 0% in patients receiving an NSAID. As for the last main study conducted, the CompLEEment phase IIIb trial, a low incidence of QTcF prolongation ⩾ 3 (0.5%) has been preliminarily observed among the 1008 patients analysed.65 This lower incidence in comparison with prior ribociclib trials might be due to an enhanced awareness of this toxicity among the investigators, leading to a better management of concomitant risk factors for QTc prolongation. However, this interpretation is flawed by the short follow up in this study and the lack of a control arm. Overall, the incidence of discontinuation due to QTc prolongation with ribociclib was less than 1% in all ribociclib trials.
Table 5.
Incidences of QTc prolongation in main ribociclib trials.
| QTc prolongation | |
|---|---|
| MONALEESA-25 | G2: ribociclib 3.6% versus placebo 0.6%$
G3: ribociclib 0.6% versus placebo 0%$ |
| MONALEESA-352 | ⩾ 2 : ribociclib: 5.6% versus placebo: 2.5%* |
| MONALEESA-749 | G2 : ribociclib 6% versus placebo 1%*
G3: ribociclib 1% versus placebo < 1%* |
| CompLEEment-165 | G2: ribociclib: not recorded G3: ribociclib: 0.5% |
p values not provided.
Statistically significant differences for both G2 and G3 QTc prolongation.
Take-home messages
○ Ribociclib has been associated with a higher incidence of G2/G3 QTc prolongation compared with placebo (3.6–6%/0.5–1.7% versus 0.6–1.7%/< 1%). A slightly increased incidence of QTc prolongation was seen in the palbociclib arm of the PALOMA-2 trial compared with the placebo arm (1.6%/0.2% versus 0.9/0%), which was not observed in a centrally reviewed PALOMA-2 substudy with ECGs (at baseline and day 14). This toxicity appears early after treatment initiation and reverses after drug interruption. The panel also found it reasonable to follow a conservative approach with palbociclib and recommended a basal ECG before treatment initiation with both CDK4/6i. Based on clinical trials eligibility criteria, ribociclib should not be started in patients with basal QTc > 450 ms and palbociclib with a QTc > 480 ms. Postbaseline QTc must be performed with ribociclib at C1D14 and C2D1, and then as clinically indicated, and whenever other risk factors are added (e.g. drugs with potential risk of QTc prolongation). The correction of all modifiable factors that can increase QTc (e.g. electrolyte imbalances) should be taken into account at any time during ribociclib treatment.
Infections
Overall, the introduction of CDK4/6i in breast-cancer trials has not been associated with a worryingly enhanced risk of infections. Overall, the combination of either palbociclib or ribociclib with ET was associated with only a slight increase of all-grade and G3/G4 infections with regard to et alone. Most common infections with palbociclib in the PALOMA-2 trial were upper and lower respiratory tract infections (14% and 13%, respectively) and urinary infections (12% all grades), almost all G1 or G2. With ribociclib, in the MONALEESA-2 trial,5 the most frequent infectious conditions were upper respiratory tract infections, urinary tract infections and viral infections. Table S6 illustrates the incidence and grade of reported infections in pivotal trials with palbociclib and ribociclib (Table S6, Supplementary material, File 1).
Take-home messages
○ Palbociclib and ribociclib show a similar incidence and pattern of haematological toxicity, with more than 60% of patients experiencing G3/G4 neutropaenia. A slightly fewer number of neutropaenic episodes and dose interruptions over 6 months appear to occur with ribociclib by indirect comparison with palbociclib data. This may be related to the different management of G3 neutropaenia, including early ribociclib dose reduction.
○ A minor increase in most commonG1/G2 infections with ribociclib and palbociclib is observed compared with placebo, which does not raise a major concern.
○ Rates of febrile neutropaenia are below 2% in all studies.
Reflecting on liver toxicity: incidence and management. Who is at risk?
For the workshop, a comprehensive review of liver enzyme abnormalities during treatment with ribociclib and palbociclib was performed. As in “Digging for neutropenia, QTc and infections: What actually matters?” section, the results from the main published studies,3–6,48,49,52,53,55–57,65–67 together with data available at clinicaltrials.gov, FDA/EMA documents and Pfizer/Novartis reports were considered.50,51,68–72 The US National Institutes of Health LiverTox database was also taken into account.70 Finally, a general PubMed/Google Scholar search was performed, including the terms <palbociclib>, <ribociclib>, <transaminitis>, <liver toxicity> and/or <liver failure>.
Ribociclib
Liver toxicity was not observed in phase 1 trials with ribociclib,59,73,74 but was closely monitored and strictly managed in phase III studies. Following the European Public Assessment Report, the management of transaminitis/liver toxicity during ribociclib treatment is summarized in Table 6, and mainly distinguishes aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) with or without concomitant elevation of bilirubin, the latter being the most clinically relevant.50 The frequency of all-grade AST and/or ALT toxicity was higher in the ribociclib arms than in the placebo arms in all MONALEESA trials, and ranged between 15% and 20% (see also Table S7, Supplementary material, File 1 for G3/G4 AEs).5,49,50,52 Liver toxicity was not described in the preliminary report of the CompLEEment trial, but the cut off for listing toxicities was above 15%.65 Rates of discontinuation due to liver toxicity (compared with the control arm) have been reported for the MONALEESA-2 and -7 trials, being very low in both studies (3% versus 0.6% in MONALEESA-2 and 3% versus 1.2% in MONALEESA-7).
Table 6.
Liver toxicity management with ribociclib according to EPAR.50 Main management differences compared with the MONALEESA-2 trial are presented in the footnote (a,b).
| Grade 1*
(> ULN – 3 × ULN) |
Grade 2*
(> 3–5 × ULN) |
Grade 3*
(> 5–20 × ULN) |
Grade 4*
(> 20 × ULN) |
|
|---|---|---|---|---|
| AST and/or ALT elevations from baseline**, without increase in total bilirubin above 2 × ULN | No dose adjustment is required. | Baseline grade < 2: dose interruption until recovery to ⩽ baseline grade: resume ribociclib at the same dose level. If grade 2 recurs, resume ribociclib at next lower dose level. |
Dose interruption of ribociclib until recovery to ⩽ baseline grade, then resume at next lower dose level. If grade 3 recurs, discontinue ribociclib. |
Discontinue ribociclib. |
| Baseline grade = 2: no dose interruption. |
||||
| Combined AST and/or ALT elevations with total bilirubin increase in the absence of cholestasis | If patients develop ALT and/or AST > 3 × ULN together with total bilirubin > 2 × ULN, irrespective of baseline grade, discontinue ribociclib. |
|||
*Grading according to Common Terminology Criteria for Adverse Events version 4.03
Baseline = prior to treatment initiation.
LFTs should be performed before initiating treatment with ribociclib. After initiating treatment, LFTs should be performed every 2 weeks during the first two cycles, at the beginning of each of the subsequent four cycles, and then as clinically indicated. If grade ⩾ 2 abnormalities are noted, more frequent monitoring is recommended.This guideline follows the management indicated in the MONALEESA trials with two exceptions:
no frequency for LFT monitoring is indicated after the re-introduction of ribociclib, when the drug was discontinued due to liver toxicity; by contrast, the MONALEESA-2 trial required LFTs to be performed twice a week for 2 weeks after resuming ribociclib; (b) there is no limited time period for liver toxicity recovery, beyond which it is not possible to re-start ribociclib; by contrast, all ribociclib trials limited that period to 28 days, after which ribociclib re-introductions were not allowed.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; LFT, liver function test; ULN, upper limit of normal.
Abnormalities in liver function tests (LFTs) associated with ribociclib were best characterized in the MONALEESA-2 trial. Here, G3/G4 liver toxicity was observed in 10.7% of women (37/344 patients). The different patterns of liver toxicity observed and their management were reviewed by the FDA and are summarized in Table S8 (Table S8, Supplementary material, File 1).72 The most frequent LFT abnormalities were transaminitis, accounting for 54% (28/37) of all G3/G4 toxicities. In addition, four patients had concomitant bilirubin elevation and met Hy’s law biochemical criteria, three of which related to ribociclib. Overall, only five liver abnormalities were reported as serious adverse events (SAEs). LFTs recovered to normal levels in all patients within 154 days after ribociclib discontinuation.50,65 No deaths attributable to liver toxicity were observed. Around 84% (31/37) of G3/G4 ALT/AST elevation events occurred within the first 6 months after treatment initiation. Median time to onset of this toxicity was 57 days, and median time to resolution (to normalization or grade ⩽ 2) was 24 days.50,65 No cases meeting Hy’s law criteria were reported in the MONALEESA-7 trial.68 Overall, taking into account all the population receiving ribociclib in pivotal trials, G3 liver toxicity rates appear to be relatively low, ranging between 5% and 10%. Moreover, only 1% of patients experienced life-threatening liver toxicity, and all liver abnormalities were reversible after ribociclib interruption.67,68 However, this toxicity raises a concern regarding the possibility of continuing ribociclib treatment, in particular because recovery may be slow and toxicity occurs early after treatment initiation. Since all studies required definitive drug discontinuation if recovery did not occur within 28 days of ribociclib interruption, most trial patients did not re-start ribociclib and, as a consequence, little evidence exists regarding the outcome of this strategy. Moreover, results from the few trial patients re-starting ribociclib because of faster liver enzyme recovery are probably not entirely applicable to other patients with later recoveries and who may re-start ribociclib outside the clinical trial setting.
Palbociclib
Overall, LFT abnormalities were not reported as AEs in the main palbociclib studies, but only those toxicities with frequencies above 5% or 10% were included.3,6,48,53,55,66 Nonetheless, comprehensive data regarding all toxicities, independently of their frequency, are available at clinicaltrial.gov. According to this database, no liver toxicity was observed in the phase I trial with palbociclib and letrozole.71 In the phase II randomized trial PALOMA-1, 7% and 1.20% of patients in the palbociclib arm who showed increases in AST and/or ALT experienced AEs and SAEs, respectively. These frequencies were slightly higher than in the control arm. Table S9 in Supplementary material, File 1 illustrates the frequency of LFT abnormalities in the PALOMA trials 1, 2 and 3, and in a recently published pooled analysis.75 Overall, G3/G4 AST and/or ALT elevation rates ranged between 1.2% and 3% with palbociclib versus 0 and 1% with placebo. No cases meeting Hy’s law criteria were observed in palbociclib-treated patients in all three PALOMA trials.75 The rate of discontinuation for liver toxicity was 0.2% versus 0.1% with palbociclib versus placebo in the PALOMA-2 trial and, to the authors’ knowledge, it has not been reported in the PALOMA-3 study. Regarding the management of LFT abnormalities with palbociclib, no specific recommendations for nonhaematological toxicities were made in phase III trials, nor has it been established by regulatory agencies. Due to the low frequency of clinically relevant liver toxicities with palbociclib, there is no mention of LFT monitoring by the FDA or in the EMA SPC.
Outside the clinical trial setting, two cases of pseudocirrhosis after treatment with palbociclib + letrozole have been recently communicated.76 The cases involved a 41-year-old Afro-American woman without prior liver disease and a 61-year-old White woman with possible prior pseudocirrhosis. Both patients experienced fatigue and ascites, and their LFTs showed a cholestatic pattern. The condition started after 3 months and 2 months of palbociclib + letrozole initiation, respectively, and uniformly resulted in liver failure and death. Causality was attributed to palbociclib, as no cases of pseudocirrhosis with letrozole had been previously reported. Drug-induced pseudocirrhosis is an entity nowadays increasingly recognized in patients with breast cancer receiving several antineoplastic drugs, including capecitabine, gemcitabine, trastuzumab and paclitaxel; furthermore, it is often associated with rapid regression of extensive liver metastases.77–79 In these two cases, no information regarding an objective response in the liver (if involved) was given, but the higher rate of objective responses described with the ET + CDK4/6i combination makes it reasonable for both cases to have a similar etiopathogenesis and points to the possibility of facing new cases in the future with any CDK4/6i.
Risk factors for CDK4/6i-related liver toxicity
No data regarding baseline characteristics of patients developing liver toxicity have been published. The incidence of transaminitis appears to be slightly inferior in the MONALEESA-7 trial (median age: 43 years)80 than in the MONALEESA-2 and -3 trials (median age: 61 years and 63 years, respectively), suggesting that an older age has a minor, if any, role in liver toxicity. All pivotal studies with palbociclib or ribociclib had an acceptable liver function as eligibility criteria. However, a screening for hepatitis virus B (HBV) or hepatitis virus C was not mandatory and, therefore, no data are available regarding liver toxicity in these patients.
At the workshop, the pre-existence of nonalcoholic fatty liver disease in some patients who afterwards developed liver toxicity was discussed. A review of the literature revealed that the role of nonalcoholic fatty liver disease as a risk factor for drug-induced hepatotoxicity is a matter of debate.81,82 The main argument against the putative role of fatty liver in drug-induced liver injury is the different incidences of both entities, the former being a common condition and the latter a highly infrequent event.82 However, according to a recent publication,83 patients with nonalcoholic fatty liver disease and obesity certainly have an increased risk of drug-induced liver injury. More data about predictive factors of CDK4/6i-related liver toxicity, if any, are required. In the meantime, it seems reasonable to monitor closely LFTs in obese women with fatty liver disease receiving CDK4/6i, and, more particularly, ribociclib.
Take-home messages
○ Ribociclib has been associated with an incidence of G3 liver toxicity ranging between 5% and 10%, so monitoring of liver function is required. Most liver abnormalities associated with ribociclib appear in the first 6 months of treatment and constitute isolated transaminitis, which reverts after treatment interruption. No deaths due to liver toxicity have been described in clinical trials and only 1.5% of patients (5/334) experienced concomitant rise in bilirubin levels and/or liver toxicity considered to be life threatening.
○ Due to slow recovery of liver function after interruption for liver toxicity, most women did not re-start treatment in the context of clinical trials, therefore, few data exist regarding the evolution of LFTs after ribociclib re-introduction. No factors predictive of liver toxicity with ribociclib have been identified so far, although some reports have suggested nonalcohol fatty liver combined with obesity is a potential risk factor for drug-induced liver toxicity.
○ Palbociclib is associated with very low rates of G3 transaminitis (1.2–3% with palbociclib versus 0–1% with placebo in randomized trials), with no cases meeting Hy’s law criteria reported in all three PALOMA studies. Based on these data, no specific monitoring of liver function is recommended.
○ Similarly to other antineoplastic drugs (e.g. capecitabine, trastuzumab and taxanes) able to induce rapid regression of extensive liver metastases, drug-induced pseudocirrhosis with CDK4/6i is also possible. Two cases with fatal outcomes related to palbociclib have been reported so far.
Revealing other toxicities: keeping alopecia in mind
The workshop also aimed to address other CDK4/6i toxicities for which specific management exists. Table 7 summarizes the incidence of these other AEs in pivotal studies according to the clinicaltrials.gov database.60,63,84 After a pre-work showing that for almost all these toxicities there was neither specific management nor new data regarding management, so no further mention was made at the meeting. By contrast, recent data regarding the management of endocrine-induced alopecia was published and therefore this issue was particularly addressed.
Table 7.
| PALOMA-260 |
PALOMA-363 |
MONALEESA-284 |
||||
|---|---|---|---|---|---|---|
| P + L | L | P + F | F | R + L | L | |
| Asthenia | 16.9 | 11.7 | 6.7 | 4.6 | 12.9 | 11.52 |
| Fatigue | 37.4 | 27.5 | 37.9 | 26.7 | 36.5 | 30.0 |
| Stomatitis | 15.3 | 5.9 | 11.6 | 2.3 | 12.3 | 6.7 |
| Rash | 13.7 | 9.9 | 17.8 | 11.7 | 17.1 | 7.9 |
| Pruritus | 8.8 | 3.60 | 5.51 | 5.81 | 13.5 | 5.8 |
| Tearing | 5.6 | 0.9 | < 5 | < 5 | 6.89 | 1.82 |
| Nausea | 37.4 | 27.5 | 28.9 | 25.6 | 51.5 | 28.5 |
| Vomiting | 15.5 | 16.7 | 14.5 | 11.6 | 29 | 15 |
| Decreased appetite | 14.9 | 9.0 | 12.8 | 7.6 | 18.2 | 15.1 |
| Dysgeusia | 10.1 | 4.9 | 10.1 | 5.0 | 9.28 | 5.76 |
| Gastric reflux | 6.0 | 3.15 | < 5 | < 5 | < 5 | < 5 |
| Diarrhoea | 26.1 | 19.4 | 19 | 14.4 | 35 | 22 |
| Constipation | 19.4 | 15.3 | 16.8 | 13.9 | 22.16 | 19.1 |
| Abdominal pain | 11.3 | 5.4 | 5.2 | 6 | 9 | 7.6 |
| Alopecia | 32.8 | 15.8 | 14.8 | 5.8 | 33.2 | 15.5 |
| Anaemia | 23.2 | 9.01 | 25.51 | 9.88 | 17.66 | 4.55 |
| Thrombocytopenia | 9.91 | 0.9 | 11.59 | 0 | 5.69 | 0.61 |
L, letrozole; P, palbociclib; R, ribociclib.
For decades, endocrine-related alopecia has elicited less interest than CT-induced alopecia, being frequently underreported in clinical trials. In a meta-analysis published in 2013, which included almost 20,000 patients with cancer from 35 studies, only 4.4% (95% confidence interval: 3.3–5.9%) of endocrine-related alopecia was described on average, being more frequent with tamoxifen and with combinations of Luteinizing hormone-releasing hormone (LHRH) analogues and tamoxifen/aromatase inhibitors.85 Recent communications, however, described a substantially higher frequency of this entity.86,87 In a survey-based study comprising 851 women with early breast cancer receiving AIs, 34% reported hair loss or hair thinning during their last month of therapy, hair changes that were not related to previous CT or age.88 Moreover, the frequency of endocrine-related alopecia appeared to increase with duration of treatment.86,87
Notably, a recent retrospective study aimed at characterizing endocrine-related alopecia in patients with breast cancer has been recently published.87 The study included 112 patients with early disease treated with AIs and tamoxifen with/without leuprolide. The severity of alopecia was grade 1 in 93% of patients, and the pattern was similar to androgenetic alopecia thought to be the result of unsynchronized miniaturization of hair follicles. Mean time to onset of hair loss from ET initiation was 16.8 months (range: 1–91 months). A total of 65 patients (58%) reported alopecia within the first 12 months of ET. A total of 52 women answered a specific quality-of-life questionnaire (Hairdex questionnaire) exploring functioning, symptoms, stigmatization, emotions and self-confidence. The questionnaire revealed that hair loss had the highest impact in the latter two domains. Importantly, 46 of the 112 patients were treated with topical minoxidil 5%, and 37 (80%) experienced a moderate or significant clinical improvement, as assessed by the investigator and a blinded reviewer. No AEs related to topical minoxidil were observed.
In palbociclib and ribociclib trials, all-grade alopecia was described in about one-third of patients in first-line trials,60,63 and in one out of six women in the second-third-line PALOMA-3 trial. These numbers are two or three-fold higher than alopecia rates observed with Et alone in the corresponding control arms (see Table 7). No studies addressing the characterization of alopecia related to CDK4/6i and ET have been published. The higher frequency of hair loss compared with Et alone may be related to the longer duration of the accompanying ET due to the improved progression-free survival with the combination, to a direct effect of CDK4/6i on the hair follicles by acting synergistically with ET toxicity, or probably to both. Of note, all-grade alopecia has been reported with abemaciclib in monotherapy in 9% of women (12/132 patients),89 suggesting that the CDK4/6i themselves may provoke hair loss. No data exist on the efficacy of topical minoxidil on patients treated with CDK4/6i. However, the mechanisms of action of minoxidil, albeit multiple, did not include any hormonal effect and seem to be basically related to its vasodilator properties on the scalp. Although systemic absorption has been reported,90 no DDIs were described after two decades of use, and only patients with heart disease are recommended to use with caution.91 Taking into account all data and the impact that hair loss has on patients’ quality of life, the panel agreed that it was reasonable to prescribe topical minoxidil 5% in patients treated with CDK4/6i. This consideration did not apply to alpha-reductase inhibitors, such as finasteride or dutasteride. These drugs, by blocking conversion of testosterone to dihydrotestosterone, increase testosterone levels, with the subsequent risk of also increasing oestrogen levels after conversion by the aromatase enzyme.
Take-home messages
○ There is no specific management for other toxicities reported with palbociclib or ribociclib, such as stomatitis, nausea/vomiting, rash, increased lacrimation, dysgeusia, diarrhoea, etc., with the exception of avoiding DDIs when treatment for these toxicities are needed. Alopecia is a frequent event with CDK4/6i treatment due to prolonged ET, direct toxicity on hair follicles or both. Recent data suggest that ET-related alopecia may improve with topical minoxidil 5%. No data exist on the efficacy of topical minoxidil on patients taking CDK4/6i but, considering the proven effect of alopecia on emotions and self-confidence of patients with breast cancer, the panel recomended its prescription, as the compound has no hormonal properties (unlike finasteride), and no DDIs are expected.
Collaborative module
This module addresses the risk of interaction between palbociclib/ribociclib and other drugs eventually used as concomitant or adjuvant therapies to CDK4/6i. It aims to provide a positive list of drugs that can be used safely in combination with palbociclib or ribociclib for most of the more common concomitant conditions suffered by patients with MBC. Furthermore, oncologists are provided with an explanation as why some drugs are prohibited (or should be used with caution), as well as a recommendation about regime adjustments.
In all sections, the following colour scheme has been used to grade the relative risk of each interaction: (a) green: low risk; orange: caution should be exercised; red: high risk.
Antibiotics, antivirals and antifungal medications: recommendations from the Specialists in Infectious Diseases
Patients with MBC often have some grade of immune suppression due to present or past CT, glucocorticoid treatment and, today, therapies with other drugs able to induce haematological toxicity, such as CDK 4/6i.
In pivotal trials, a slight increase in infection was observed when combining ET with palbociclib or ribociclib compared with ET + placebo. Only 3–6% of infections reported were considered G3/G4 (see also Table S6, Supplementary material, File 1).3–6,48,49,52 Most common infectious conditions were urinary tract infections and pneumonia, as reported in the general population.
According to this data, no special anti-infective prophylaxis is needed in patients with breast cancer taking CDK4/6i, otherwise considered immunocompetent. This population must follow the data sheet management guidelines, including blood tests and dose interruptions/adjustments when required. However, prophylactic management of infectious diseases must be considered in special circumstances.
HIV-positive patients. Patients who are known carriers of HIV were excluded from CDK4/6i trials, but there are no reasons to suspect that HIV patients will not benefit from these drugs or that they will have more severe toxicities provided that the HIV is under control with adequate treatment. In fact, a recent preclinical study has described a possible role for palbociclib in HIV treatment by blocking the HIV-1 reverse transcription,92 which, if confirmed, may provide an additional reason to introduce palbociclib in those patients sharing both conditions (breast cancer and HIV positivity). In all HIV+ patients for whom treatment with CDK4/6i is planned, close monitoring by the infectious disease specialist before and during treatment is mandatory. In patients with uncontrolled HIV infection, additional immune suppression using CDK4/6i could cause severe complications, and must be avoided until safety data supporting it has been obtained.
Glucocorticoid treatment. Patients treated with glucocorticoids were not specifically excluded from pivotal trials with CDK4/6i, but in general this therapy was limited to low doses and/or a short period of time. Outside the clinical trial setting, patients receive long or more intensive glucocorticoid courses, so a higher level of immune suppression with concomitant CDK4/6i is anticipated. In this context, it is especially relevant to introduce chemoprophylaxis for Pneumocystis jirovecii. According to National Comprehensive Cancer Network (NCCN) guidelines, patients receiving over 20 mg of prednisone/day for more than 4 weeks (or an equivalent dose of other corticoids) should begin trimethoprim-sulfamethoxazole (TMP/SMX) at prophylactic doses (800/160 mg twice a week, 800/160 mg daily or 400/80 mg daily).93 TMP/SMX must be used with caution, particularly with ribociclib, when therapeutic doses are used, because concomitant CDK4/6i can increase bioavailability of TMP/SMX, therefore increasing the risk of medullar toxicity. However, at a prophylactic dosage, no DDIs are expected. Prophylactic TMP/SMX should also be considered in patients with prolonged lymphopenia, irrespective of treatment with corticoids, since they are also at risk of presenting with P. jirovecii pneumonia.
Chronic carriers of HBV. There are no specific safety data for HBV carriers treated with palbociclib or ribociclib. In patients with breast cancer who are receiving CT, the prophylaxis of HBV reactivation has shown efficacy.94 Although the introduction of lamivudine has been demonstrated to be useful in preventing HBV reactivation in this setting,95 better results have been recently obtained with tenofovir and entacavir.96,97 Based on these data, the authors recommended initiating prophylaxis treatment in chronic carrier patients of HBV infection (positive HBsAg or HBV DNA) when it is planned to treat them with CDK4/6i. This recommendation also takes into account that no DDIs between tenofovir, entacavir or lamivudine and CDK 4/6i are expected.
According to the workshop’s objectives, the panel elaborated a list of anti-infective agents categorized by risk of pharmacokinetic interactions and potential synergy for QTc prolongation (Table 8).
Table 8.
Anti-infective agents categorized according to their potential risk for DDIs and QTc prolongation in combination with CDK4/6i.
| Antimicrobial therapy | Drug | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdP risk | Comments |
|---|---|---|---|---|---|---|---|
| Antibiotics | |||||||
| β-lactams | – | – | – | – | Not known | Low risk of interaction with palbociclib and ribociclib. SAFE OPTIONS |
|
| Prophylactic (low dose) trimethoprim-sulfamethoxazole | – | – | – | – | Not known | ||
| Tetracyclines: Doxycycline1 Minocycline1 |
– – |
– – |
– – |
– – |
Not known Not known |
||
| Fosfomycin1 | – | – | – | – | Not known | ||
| Linezolid1 | – | – | – | – | Not known | ||
| Clindamycin | – | – | – | – | Not known | ||
| Glycopeptides: Teicoplanin1 Vancomycin1 Dalbavancin |
– – – |
– – – |
– – – |
– – – |
Not known Not known Not known |
||
| Aminoglucosides Amikacin1 Gentamicin1 |
– – |
– – |
– – |
– – |
Not known Not known |
||
| Daptomycin1 | – | – | – | – | Not known | ||
| Trimethoprim/ sulfamethoxazole |
Major (trimetho-prim) | – | – | – | Not known | Caution should be exercised | |
| Macrolides: Azithromycin |
Minor |
– |
– |
– |
known |
||
| Fluoroquinolones: Levofloxacin Moxifloxacin Norfloxacin Ofloxacin |
– – – – |
– – – – |
– – – – |
– – – – |
Known Known Possible Possible |
||
| Metronidazole | – | – | – | – | Conditional | ||
| Macrolides: Erythromycin Clarithromycin |
MajorMajor |
ModerateStrong |
– – |
gp-P– |
KnownKnown |
High risk of DDIs. Should be avoided |
|
| Rifampicin1 | – | – | Strong | OATP1B1, gp-P | Not known | ||
| Fluoroquinolones: Ciprofloxacin |
– |
Moderate-Weak |
– | OAT3, gp-P | Known | ||
| Antiherpetic | |||||||
| Acyclovir1
Famciclovir Valacyclovir |
– – – |
– – – |
– – – |
– – – |
Not known Not known Not known |
Low risk of interaction with palbociclib and ribociclib. SAFE OPTIONS |
|
| Brivudine Ganciclovir Valganciclovir |
– – – |
– – – |
– – – |
– – – |
Not knownNot known Not known |
||
| Flu | |||||||
| Oseltamivir1 | – | – | – | – | Not known | Low risk of interaction with palbociclib and ribociclib. SAFE OPTIONS |
|
| HIV, hepatitis | |||||||
| Nucleoside analog reverse transcriptase inhibitors (lamivudine, abacavir, tenofivir)Integrase inhibitors (dolutegravir, raltegravir) | –Minor (dolutegravir) | – – |
– – |
gp-P (tenofivir)gp-P (dolutegravir) | Not knownNot known | Low risk of interaction with palbociclib and ribociclib. SAFE OPTIONS |
|
| Non-nucleoside reverse transcriptase inhibitors: Nevirapine |
Major |
– |
Weak |
Not known |
Caution should be exercised | ||
| Protease inhibitors for HIV and HCV (atazanavir, darunavir, lopinavir, indinavir (usually administered in combination with ritonavir) | Major | Strong | – | gp-P (darunavir, ritonavir) | Not known | High risk; consider antiretroviral class switch | |
| Non-nucleoside reverse transcriptase inhibitors: Efavirenz |
Major |
– |
Moderate |
– |
Not known |
||
| Antifungal | |||||||
| Amphotericin B | – | – | – | – | Conditional3 | Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS | |
| Echinocandins: Caspofungin1 Anidulafungin Micafungin1 |
– Minor – |
– – – |
– – – |
– – – |
Not known Not known Not known |
||
| Amphotericin B | – | – | – | – | Conditional3 | Caution should be exercised3 | |
| FluconazoleI traconazole Isavuconazole1 Posaconazole Voriconazole |
–Major Major – Minor |
Moderate Strong Moderate Strong Strong |
– – – – – |
– – – – – |
known Conditional – Conditional Conditional |
High risk of DDIs. Should be avoided |
|
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk of DDIs. Plasmatic concentrations of the CYP3A4 substrate could be increased (or decreased) when used concomitantly with a CYP3A4 inhibitor (or inducer) moderate or strong. TdP,Torsades de pointes; gp-P, P-glycoprotein; OATP1B1, organic anion transporter polypeptide; OAT3, organic anion transporter.
Not classified risk QT. According to the source consulted (www.crediblemeds.org), the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories.
Ribociclib can increase concentration of trimethoprim, with bone marrow toxicity.
Amphotericin B with conditional TdP risk, caution should be exercised in combination with Ribociclib.
Drugs for the prevention and treatment of digestive symptoms and antihistamine drugs
Digestive toxicity is commonly associated with palbociclib or ribociclib treatment (see tables in educational module, “Reflecting on liver toxicity: Incidence and management. Who is at risk?” section and Supplementary material, File 1). In the PALOMA-23 and PALOMA-3 trials,6 a similar proportion of all-grade nausea (35% and 32%, respectively), diarrhoea (26% and 25%, respectively), constipation (19% in both studies) and vomiting (15% and 17%, respectively) were described in the palbociclib arm. Moreover, 9% and 4% of women had dyspepsia in these studies. All these digestive toxicities were more frequent than in the corresponding control arm. However, almost all of them were reported as G1 or G2, and most did not require medical treatment. With ribociclib, and regarding all-grade toxicity, nausea (51%, 45% and 31%, respectively), diarrhoea (35%, 29% and 19%, respectively), vomiting (29%, 26% and 18%, respectively) and constipation (25%, 15% and 16%, respectively) were described in the MONALEESA-3 trial.4,49,52 Almost all were G1/G2 toxicities, and some required pharmacological intervention.
Both the palbociclib and ribociclib studies3,4,6,49,52 described an overall 17% of all-grade rash incidence, 14–17.8% (palbociclib) and 13–18% (ribociclib), most of them G1/G2, and sometimes requiring treatment with antihistamine drugs. All-grade pruritus has also been frequently reported in the MONALEESA trials, with incidences ranging between 9% and 19%, mostly considered to be G1/G2.
In this section, potential DDIs between palbociclib and ribociclib and common drugs used for treating digestive symptoms (i.e. antiemetics, prokinetics, antacids, gastric mucosal protective drugs, antidiarrhoeals and laxatives), together with antihistamine drugs have been reviewed (Table 9). As in previous sections, the drugs considered safe options are listed for all indications.
Table 9.
Categorization of drugs according to their risk of DDIs and QTc prolongation when used concomitantly with ribociclib or palbociclib.
| Drug | CYP3A4 substrate |
CYP3A4 inhibitor |
CYP3A4 inducer |
Membrane transporter substrate | TdP risk | Comments | |
|---|---|---|---|---|---|---|---|
| Antiemetics | |||||||
| Metoclopramide Olanzapine Palonosetron Granisetron |
– – Minor Minor |
– – – – |
– – – – |
– – – – |
Conditional Conditional Possible Possible |
Low risk of interaction with palbociclib SAFE OPTIONS |
|
| Metoclopramide Olanzapine Palonosetron Granisetron |
– – Minor Minor |
– – – – |
– – – – |
– – – – |
Conditional Conditional Possible Possible |
Caution should be exercised in combination with ribociclib | |
| Rolapitant Fosaprepitant Dexamethasone1 |
Major Major Major |
– Weak – |
– – Weak |
– – gp-P |
Not known Not known Not known |
Caution should be exercised in combination with ribociclib or palbociclib | |
| Aprepitant Netupitant Ondansetron Domperidone |
Major Major Major Major |
Moderate Moderate – – |
– – – – |
– – gp-P – |
Not known Not known Known Known |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
|
| Antacids and gastric mucosal protective | |||||||
| Ranitidine Famotidine2 Aluminum hydroxide (separate administration of palbociclib or ribociclib from the administration of any antacids by at least 2 hours) Bismuth subsalicylate Zinc Acexamate Sucralfate |
– – – – – – |
– – – – – – |
– – – – – – |
gp-P, OCT2 OCT2 – – – – |
Not known Conditional2 Not Known Not known Not known Not known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
| Esomeprazole Omeprazole Pantoprazole |
Minor Minor Minor |
– – – |
– – – |
– – – |
Conditional Conditional Conditional |
Low risk of interaction with palbociclib SAFE OPTIONS |
|
| Esomeprazole Omeprazole Pantoprazole |
Minor Minor Minor |
– – – |
– – – |
– – – |
Conditional Conditional Conditional |
Caution should be exercised in combination with ribociclib | |
| Lansoprazole Rabeprazole |
Major Major |
– – |
– – |
– – |
Conditional Conditional |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
|
| Antidiarrheals | |||||||
| Racecadotril Loperamipe2 |
– – |
– – |
– – |
– gp-P |
– Conditional2 |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
| Prokinetics and laxative | |||||||
| Lactulose Macrogol Magnesium Hydroxide Scopolamine -butylbromide |
– – – – |
– – – – |
– – – – |
– – – – |
Not known Not known Not known Not known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
| Cinitapride Naloxegol3 |
Major Major |
– – |
– – |
– gp-P |
Not known Not Known |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
|
| Antihistamines | |||||||
| Dexchlorpheniramine Cetirizine4 Loratadine4 Desloratadine4 Fexofenadine4 |
– Minor Minor Minor – |
– – – – – |
– – – – – |
– gp-p gpP gpP gpP |
Not known Not known4 Not known4 Not known4 Not known4 |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
| Diphenhydramine Promethazine |
– – |
– – |
– – |
OATP1B1/3 – |
Conditional Possible |
Low risk of interaction with palbociclib SAFE OPTIONS |
|
| Diphenhydramine Promethazine |
– – |
– – |
– – |
OATP1B1/3 – |
Conditional Possible |
Caution should be exercised in combination with ribociclib | |
| Ebastine Rupatadine4 |
Major Major |
– – |
– – |
– – |
Not known Not known4 |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
|
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk of DDIs. Plasmatic concentrations of the CYP3A4 substrate could be increased (or decreased) when used concomitantly with a CYP3A4 inhibitor (or inducer) moderate or strong. TdP,Torsades de pointes; gp-P, P-glycoprotein; OATP1B1, organic anion transporter polypeptide; OCT2, organic cation transporter.
According to the source consulted (www.crediblemeds.org), the drug is under active review for possible risk of QT prolongation and torsades de pointes.
Drug with conditional TdP risk, caution should be exercised in combination with Ribociclib.
If naloxegol cannot be changed when starting treatment with palbociclib or ribociclib, consider reducing the dose and closely monitoring for side effects.
Not classified risk QT. According to the source consulted (www.crediblemeds.org), the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories.
Drugs for endocrine and cardiovascular disorders
In this section, potential DDIs between available CDK4/6i and some of the most common drugs used in the treatment of dyslipidaemia, diabetes and cardiovascular disorders, including antiplatelet drugs and anticoagulants, were examined.
Regarding antihypertensive drugs (Table 10) and compounds used to treat congestive heart failure, most palbociclib and ribociclib DDIs occur via CYP3A4, with increased risk for ribociclib due to its pharmacokinetic properties (see “Drug metabolic pathways and membrane transporters: How to interpret drug-drug interactions with palbociclib and ribociclib?” section in the educational module). Angiotensin II receptor antagonists (excepting losartan), angiotensin-converting-enzyme inhibitors, beta blockers (excepting bisoprolol), potassium-sparing diuretics (excepting eplenorone) and torsemide are considered safe options. Other commonly used loop diuretics should be administered with caution due to their conditional risk of TdP. Calcium-channel blockers are major substrates for CYP3A4 and, at the same time, moderate inhibitors, hence, clinically relevant DDIs with palbociclib, and particularly with ribociclib, are expected and, subsequently, concomitant administration should be avoided. Losartan and eplenorone, as well as other major substrates for CYP3A4, are not recommended. Bisoprolol should also be avoided since, besides being a major substrate for CYP3A4, it has an NTI; its potential risk for TdP is being investigated.31
Table 10.
Drugs for treatment of hypertension and congestive heart failure: Categorization according to their risk of DDIs and QTc prolongation when used concomitantly with ribociclib and palbociclib.
| Antihypertensives | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdP risk | Comments |
|---|---|---|---|---|---|---|
|
ARA-II antagonists Candesartan Eprosartan Irbesartan Olmesartan Telmisartan Valsartan |
– – – – – – |
– – – – – – |
– – – – – – |
– – – – – – |
Not Known Not Known Not Known Not Known Not Known Not Known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
ACE inhibitors
Enalapril Captopril Fosinopril Ramipril Quinapril |
– – – – – |
– – – – – |
– – – – – |
– – – – – |
Not known Not Known Not Known Not Known Not Known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
Beta blockers
Atenolol Metoprolol Nadolol Pindolol Labetalol Propranolol Carvedilol |
– – – – – Minor Minor |
– – – – – – – |
– – – – – – – |
– – – – – – – |
Not Known Not Known Not Known Not Known Not Known Not Known Not Known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
Calcium channel blockers: Dihydropyridines Clevidipine |
– |
– |
– |
– |
Not Known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
Diuretics: Potassium sparing diuretics: Amiloride Triamterene Spironolactone |
– – – |
– – – |
– – – |
– – – |
Not known Not known Not known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
Diuretics: Loop diuretics Torsemide Furosemide Hydrochlorothiazide Indapamide |
– – – – |
– – – – |
– – – – |
OATP1B1 OAT3 – – |
Conditional Conditional Conditional Conditional |
Low risk of interaction with palbociclib
SAFE OPTIONS |
|
Diuretics: Loop diuretics Torsemide Furosemide Hydrochlorothiazide Indapamide |
– – – – |
– – – – |
– – – – |
OATP1B1 OAT3 – – |
Conditional Conditional Conditional Conditional |
Caution should be exercised in combination with ribociclib |
|
ARA- II antagonists: Losartan |
Major, NTI |
– |
– |
– |
Not known |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
|
Potassium sparing diuretics: Eplerenone |
Major, sensitive |
– | – | – | Not known | High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
|
Beta blockers: Bisopropol1 |
Major, NTI |
– |
– |
– |
Not known |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
|
Calcium channel blockers: Dihydropyridines Amlodipine Barnidipine Felodipine Lacidipine Lercadipine Manidipine Nifedipinde Nimodipino Nisoldipine Nitrendipine Nicardipine Non dihydropyridines: Verapamil Diltiazem |
Major Major Major Major Major Major Major Major Major Major Major Major Major |
– – – – – – Moderate – – – Moderate Moderate – |
– – – – – – – – – – – – – |
– – – – – – – – – – – gp-P gp-P |
Not Known Not Known Not Known Not Known Not Known Not Known Not Known Not Known Not Known Not Known Possible Not known Not Known |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk of DDIs. Plasmatic concentrations of the CYP3A4 substrate could be increased (or decreased) when used concomitantly with a CYP3A4 inhibitor (or inducer) moderate or strong. ARA-II: angiotensin II receptor blockers; ACE inhibitor: angiotensin-converting enzyme inhibitors NTI: narrow therapeutic index. TdP,Torsades de pointes; gp-P, P-glycoprotein; OATP1B1, organic anion transporter polypeptide; OAT3, organic anion transporter.
Not classified risk QT. According to the source consulted (www.crediblemeds.org), the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories.
Regarding antidiabetics drugs (Table 11) some of the DDIs described with palbociclib and ribociclib result from competition for the membrane transporters. Therefore, biguanides (metformin) and some sodium-glucose cotransporter 2 (SGLT2) inhibitors (e.g. canagliflozin, capaglifozin) must be administered with caution if concomitantly used with these CDK4/6i; in these cases, glycaemia should be carefully monitored. Meglitinides and repaglinide, also SGLT2 inhibitors, should be avoided, as well as saxagliptin and linagliptin (dipeptidyl peptidase 4 inhibitors [DPP-4i]). The latter may also interact with palbociclib and ribociclib via membrane transporters but they are, first and foremost, major substrates of CYP3A4. By contrast, sulfonylureas, alpha-glucosidase inhibitors, glucagon-like peptide-1 receptor agonists and other DPP-4i, namely vildagliptin, alogliptin and sitagliptin, can be safely administered.
Table 11.
Drugs for treatment of diabetes: Categorization according to their risk of DDIs and QTc prolongation when used concomitantly with ribociclib and palbociclib.
| Antidiabetics | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdP risk | Comments |
|---|---|---|---|---|---|---|
|
Sulfonylureas: Glicazide Glibenclamide Glisentide Glipizide Gliquidone |
– – – – – |
– – – – – |
– – – – – |
OATP1B1 OATP1B3 – OATP1B3 – |
Not known Not known Not known Not known Not known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
Alfa glycosidase inhibitors: Ascarbose Miglitol |
– – |
– – |
– – |
Not known Not known |
||
|
GLP-1: Albiglutide Dulaglutide Exenatide Liraglutide lixisenatide |
– – – – – |
– – – – – |
– – – – – |
– – – – – |
Not known Not known Not known Not known Not known |
|
|
DPP-4 inhibitor: Vildagliptin Alogliptin Sitagliptin |
– Minor – |
– – – |
– – – |
– – gp-P |
Not known Not known Not known |
|
|
SGLT2 inhibitor: Canagliflozin Dapagliflozin |
Minor – |
– – |
– – |
gp-P gp-P |
Not known Not known |
|
|
Biguanides: Metformin |
– |
– |
– |
OCT2, OCT1, MATE 1/2 |
Not known |
Caution should be exercised in combination with ribociclib or palbociclib |
|
Meglitinides: Repaglinide |
Major |
– |
– |
OATP1B1/1B3 |
Not known |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
|
DPP-4 inhibitor: Saxagliptin Linagliptin |
Major Major |
– – |
– – |
gp-P gp-P |
Not known Not known |
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk of DDIs. Plasmatic concentrations of the CYP3A4 substrate could be increased (or decreased) when used concomitantly with a CYP3A4 inhibitor (or inducer) moderate or strong. GLP-1: Glucagonlike-peptide 1; SGLT2: Sodium -glucosecotransporter 2; DPP-4: dipeptidylpeptidase IV; TdP,Torsades de pointes; gp-P, P-glycoprotein; OATP1B1, organic anion transporter polypeptide; OCT2, OCT1 organic cation transporter 1 and 2; MATE 1/2, multidrug and toxin extrusion 1 and 2
Palbociclib and ribociclib can interact with some statins via time-dependent inhibition of CYP3A4, in particular with simvastatin and atorvastatin (Table 12). Therefore, side effects and response to both drugs should be closely monitored if concomitantly used. One case of fatal rhabdomyolysis after initiation of palbociclib-fulvestrant initiation in a woman receiving atorvastatin has been reported.31 Safer alternatives are pitavastatin, rosuvastatin or pravastatin.
Table 12.
Lipid-lowering drugs categorized according to their risk of DDIs and QTc prolongation when used concomitantly with ribociclib and palbociclib.
| Lipid-lowering | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdP risk | Comments |
|---|---|---|---|---|---|---|
|
Statins: Pitavastatin1 |
– |
– |
– |
OATP1B1/1B3 |
Not known |
Low risk of interaction with palbociclib and ribociclib. SAFE OPTIONS |
|
Fibrates: Fenofibrate1 Gemfibrozil |
– Minor |
– – |
– – |
– – |
Not known Not known |
|
|
Statins: Fluvastatin |
Minor |
– |
– |
OATP1B1/1B3 |
Not known |
Caution should be exercised in combination with ribociclib or palbociclib |
| Pravastatin1 | Minor | – | – | OATP1B1/1B3, OAT1/OAT3 | Not Known | |
| Rosuvastatin1 | Minor | – | – | OATP1B1/1B3, BCRP | Not known | |
|
Statins: Simvastatin1 |
Major/ sensitive |
– |
– |
OATP1B1/1B3 |
Not known |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
| Atorvastatin | Major/mode-rate sensitive | – | – | OATP1B1/1B3, gp-P |
Not known |
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk of DDIs. Plasmatic concentrations of the CYP3A4 substrate could be increased (or decreased) when used concomitantly with a CYP3A4 inhibitor (or inducer) moderate or strong. TdP,Torsades de pointes; gp-P, P-glycoprotein; OATP1B1/3, organic anion transporter polypeptide; OAT1/3, organic anion transporter 1 and 3; OCT2/1 organic cation transporter 1 and 2 ; BCRP, breast cancer resistance protein.
According to the source consulted (www.crediblemeds.org), the drug is under active review for possible risk of QT prolongation and torsades de pointes.
Finally, most antiplatelet and anticoagulant drugs do not have relevant DDIs with palbociclib and ribociclib (Table 13). The few DDIs described are metabolic in nature, via CYP3A4 metabolism or membrane transporters. Exceptionally, cilostazol, an antiplatelet drug, has known TdP risk, hence it is not recommended and should be particularly avoided with ribociclib.
Table 13.
Antithrombotic drugs categorized according to their risk of DDIs and QTc prolongation when used concomitantly with ribociclib and palbociclib.
| Drug | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdP risk | Comments | |
|---|---|---|---|---|---|---|---|
| Antiplatelet | |||||||
| Acetylsalicylic acid | – | – | – | – | Not Known | Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
| Clopidogrel1 | Minor | – | – | – | Not Known | ||
| Epoprostenol sodium | – | – | – | – | Not Known | ||
| Eptifibatide | – | – | – | – | Not Known | ||
| Iloprost | – | – | – | – | Not Known | ||
| Tirofiban | – | – | – | – | Not Known | ||
| Triflusal | – | – | – | – | Not Known | ||
| Dipyridamole | – | – | – | – | Not Known | ||
| Abciximab | – | – | – | – | Not Known | ||
| Selexipag | Minor | – | – | OATP1B1, OATP1B3, P-gp, BCRP | Not Known | Caution should be exercised in combination with ribociclib or palbociclib | |
| Ticlopidine | Major | – | – | – | Not Known | High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib | |
| Prasugrel | Major3 | – | – | – | Not Known | ||
| Ticagrelor2 | Major Sensitive /NTI |
weak | – | P-gp | Not Known | ||
| Cilostazol | Major | weak | – | – | Known | ||
| Anticoagulants | |||||||
| Heparin and low molecular weight heparin | – | – | – | – | Not Known | Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
|
| Acenocoumarol1 | – | – | – | – | Not Known | ||
| Warfarin2 | Minor | – | – | – | Not Known | ||
| Argatroban | – | – | – | – | Not Known | ||
| Bivalirudin | – | – | – | – | Not Known | ||
| Dabigatran2 | – | – | – | P-gp | Not Known | Caution should be exercised in combination with ribociclib or palbociclib | |
| Edoxaban | Minor | – | – | P-gp | Not Known | ||
| Apixaban | Major | – | – | P-gp, BCRP | Not Known | High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
|
| Rivaroxaban | Major4 | – | – | P-gp, BCRP | Not Known | ||
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk of DDIs. Plasmatic concentrations of the CYP3A4 substrate could be increased (or decreased) when used concomitantly with a CYP3A4 inhibitor (or inducer) moderate or strong. NTI: narrow therapeutic index. TdP,Torsades de pointes; gp-P, P-glycoprotein; OATP1B1/3, organic anion transporter polypeptide; BCRP, breast cancer resistance protein.
Not classified risk QT. According to the source consulted (www.crediblemeds.org), the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories.
According to the source consulted (www.crediblemeds.org), the drug is under active review for possible risk of QT prolongation and torsades de pointes.
Prasugrel is a pro-drug which is converted to the active metabolite primarily by CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19.
Rivaroxaban is moderate sensitive substrate [98].
Analgesics and opioids
Pain control represents one of the greatest challenges in patients with cancer. It is one of the most common symptoms associated with cancer, affecting up to 64% of patients with advanced disease.99 According to the International Association for the Study of Pain, pain prevalence in breast cancer varies from 40% to 89%.100 When prescribing analgesics, side effects and DDIs with other medications must be taken into account. Therapies for pain must be integrated into the patient’s oncological management.101
According to the PALOMA-1 trial pain assessment, the addition of palbociclib to the letrozole treatment had no impact on pain control, although results were not adjusted for the concomitant use of opioids and other analgesic medications.102 In the MONALEESA-2 study, a trend was observed favouring ribociclib + letrozole for pain reduction.103
Taking into account a potential DDI with palbociclib and ribociclib, the metabolism profile and QTc-prolongation risk of the most frequently used drugs for pain control were reviewed and classified by drug interaction risk potential (see Tables 14–16 for non-opioid analgesics, opioid analgesics and adjuvants/anticonvulsants, respectively).
Table 14.
Non-opioid analgesics categorized according to their risk for DDIs and QTc prolongation when used concomitantly with ribociclib or palbociclib.
| Non-opioid analgesics | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdP risk[31] | Comments |
|---|---|---|---|---|---|---|
| AAS1
Metamizole Aceclofenac Indomethacin Ketorolac Piroxicam1 Tenoxicam Dexketoprofen Flurbiprofen Ibuprofen1,2 Sulindac Naproxen1,3 MephenamicacidAcetaminophen2 Diclofenac Meloxicam Celecoxib2 Etoricoxib |
– – – – – – – – – – – – –Minor Minor Minor Minor minor |
– – – – – – – – – – – – – – – – – – |
– – – – – – – – – – – – – – – – – – |
– – – – – – – – – – – – – – – – – – |
Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
| Parecoxib | Major | – | – | – | Not known | Caution should be exercised in combination with ribociclib or palbociclib |
| Ergotamine Dihydro-ergotamine |
Major, NTI | – – |
– – |
– – |
Not known Not known |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk: Plasmatic concentrations of the substrate could be increased when used concomitantly with a CYP3A4 inhibitor, such as palbociclib (weak) or ribociclib (moderate-potent). Risk of increased toxicity. NTI: narrow therapeutic index
It can inhibit MRP-2 and MRP-4 renal transporters. Interaction of non-steroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport [104].
Not classified risk QT. According to the source consulted (www.crediblemeds.org), the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories.
According to the source consulted (www.crediblemeds.org), the drug is under active review for possible risk of QT prolongation and torsades de pointes.
Table 15.
Opioid analgesics categorized according to their risk for DDIs and QTc prolongation when used concomitantly with ribociclib or palbociclib.
| Opioid analgesics | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4Inducer | Membrane transporter substrate | TdP risk | Comments |
|---|---|---|---|---|---|---|
| Morphine Hydromorphone TapentadolCodeine |
– – –Minor |
– – – – |
– – – – |
P-gp*
– – – |
Not known Not known Not knownNot known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
| Tramadol Buprenorphine Oxycodone |
Major Major Major |
– – – |
– – – |
– – – |
Possible Possible Not known |
Caution: risk of increased toxicity of the substrate |
| Methadone Fentanyl‡ |
Major Major NTI |
– – |
– – |
– – |
Known risk$
Not known |
High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk: Plasmatic concentrations of the substrate could be increased when used concomitantly with a CYP3A4 inhibitor, such as palbociclib (weak) or ribociclib (moderate-potent). Risk of increased toxicity. NTI: narrow therapeutic index.
In vitro data suggest that palbociclib and ribociclib may inhibit P-glycoprotein (P-gp) and that inhibition may increase exposition to substrates, but there is no in vivo evidence of this interaction.
QTc interval prolongation risk of the CYP3A4 substrate can be increased as a consequence of the metabolic inhibition (substrate accumulation).
If fentanyl cannot be changed when starting treatment with palbociclib or ribociclib, consider reducing the dose and closely monitoring for side effects.
Table 16.
Adjuvants and anticonvulsants categorized according to their risk for DDIs and QTc prolongation when used concomitantly with ribociclib or palbociclib.
| Adjuvants and anticonvulsants | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer |
Membrane transporter substrate | TdP risk | Comments |
|---|---|---|---|---|---|---|
| Pregabalin1
Gabapentin1 Levetiracetam1 Lamotrigine1 Topiramate1 Baclofen1 Clonidine1 Octreotide1 Alendronate1 Zoledronate Denosumab |
– – – – – – – – – – – |
– – – – – – – – – – – |
– – – – – – – – – – – |
– – – – – – – – – – – |
Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known Not known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
| Lacosamide1
Valproic acid1 Prednisone2 Methylprednisolone2 Hydrocortisone Dextromethorphan |
Minor Minor Minor Minor Minor Minor |
– – – – – – |
– – – – – – |
– – – – P-gp – |
Not known Not known Not known Not known Not known Not known |
|
| Dexamethasone | Major | – | Weak 3 | P-gp | Not known | Caution should be exercised in combination with ribociclib or palbociclib |
| Zonisamide | Major | – | – | – | Not known | |
| Oxcarbazepine | – | – | Weak | – | Not known | |
| Ketamine | Major | – | – | – | Not known | |
| Carbamazepine | Major | – | Strong | – | Not known | High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
| Phenobarbital | – | – | Strong | – | Not known | |
| Phenytoin | Minor | – | Strong | – | Not known |
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk of DDIs. Plasmatic concentrations of the CYP3A4 substrate (palbociclib or ribociclib) could be decreased when used concomitantly with a CYP3A4 inducer moderate or strong. TdP,Torsades de pointes; gp-P, P-glycoprotein.
Not classified risk QT. According to the source consulted (www.crediblemeds.org), the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories.
According to the source consulted (www.crediblemeds.org), the drug is under active review for possible risk of QT prolongation and torsades de pointes
Dexamethasone is a CYP3A4 inducer weak-moderate. Risk of decreasing levels of palbociclib or ribociclib by metabolism induction
Antidepressants and antipsychotics
The risk of concomitant treatments with palbociclib/ribociclib and antidepressants, antipsychotic, and anxiolytic/hypnotic drugs is summarized in Tables 17–19, respectively.
Table 17.
Antidepressants categorized according to their safety as concomitant treatment with palbociclib and ribociclib.
| Antidepressants | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdP risk | Comments |
|---|---|---|---|---|---|---|
| Duloxetine1 | – | – | – | – | Not known | Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
| Desvenlafaxine Vortioxetine2 |
Minor Minor |
– – |
– – |
– – |
Not known Not known |
|
| Paroxetine1 | – | – | – | – | Conditional | Caution should be exercised in combination with ribociclib or palbociclib |
| Sertraline1
Fluoxetine1,3 |
Minor Minor |
– – |
– – |
– – |
Conditional Conditional |
|
| Trazodone | Major | – | – | – | Conditional | High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
| Mirtazapine Venlafaxine |
Major Major |
– – |
– – |
– – |
Possible Possible |
|
| Citalopram Escitalopram |
Major, NTI Major, NTI |
– – |
– – |
– – |
Known Known |
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk: Plasmatic concentrations of the substrate could be increased when used concomitantly with a CYP3A4 inhibitor, such as palbociclib (weak) or ribociclib (moderate-potent). Risk of increased toxicity. NTI, narrow therapeutic index; SPC, Summary of Product Characteristics; TdP, torsades de pointes.
Fluoxetine and paroxetine are strong CYP2D6 inhibitors; duloxetine is moderate CYP2D6 inhibitors; and sertraline is a weak CYP2D6 inhibitor. Concomitant use with tamoxifen should be avoided.
Controversial data. The Summary of Product Characteristics[105] and other authors [106] categorize Vortioxetine as a major substrate of CYP2D6 and a minor substrate of CYP3A4. For Uptodate® [107], vortioxetine is a major substrate for CYP2D6 and CYP3A4.
Fluoxetine has a longer half-life.
Table 18.
Antipsychotic drugs categorized according to their safety as concomitant treatment with palbociclib and ribociclib.
| Antipsychotic | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdP risk | Comments |
|---|---|---|---|---|---|---|
| Amisulpride1
Olanzapine1 |
– – |
– – |
– – |
– – |
Conditional Conditional |
Low risk of interaction with palbociclib
SAFE OPTIONS |
| Amisulpride1
Olanzapine1 |
– – |
– – |
– – |
– – |
Conditional Conditional |
Caution should be exercised in combination with ribociclib |
| Paliperidone | – | – | – | – | Possible | Caution should be exercised in combination with ribociclib or palbociclib |
| Risperidone Asenapine Perphenazine Clozapine |
Minor Minor Minor Minor |
– – – – |
– – – – |
– – – – |
Possible Possible Possible Possible |
|
| Quetiapine2 | Major NTI | – | – | – | Conditional | |
| Sulpiride | – | – | – | – | Known | High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib |
| Chlorpromazine levomepromazine |
Minor Minor |
– – |
– – |
– – |
Known Known |
|
| Ziprasidone | Minor | Moderate | – | – | Conditional | |
| Aripiprazole | Major | – | – | – | Possible | |
| Haloperidol | Major | – | – | – | Known | |
| Pimozide | Major NTI | – | – | – | Conditional |
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk: Plasmatic concentrations of the substrate could be increased when used concomitantly with a CYP3A4 inhibitor, such as palbociclib (weak) or ribociclib (moderate-potent). Risk of increased toxicity. NTI, narrow therapeutic index; TdP, torsades de pointes.
Amisulpride and olanzapine are associated with Conditional Risk of TdP, but this risk is dose-dependent. Both drugs are probably safe at a therapeutic dose.
Quetiapine is mainly prescribed for elderly patients at low doses as anxiolytic.
Table 19.
Anxiolytics and hypnotics categorized according to their safety as concomitant treatment with palbociclib and ribociclib.
| Anxiolytics and hypnotics | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdPrisk | Comments |
|---|---|---|---|---|---|---|
| Lorazepam1
Lormetazepam1 Clotiazepam1 |
– – – |
– – – |
– – – |
– – – |
Not known Not known Not known |
Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
| Bromazepam1 | Minor | – | – | – | Not known | |
| Clobazam | Minor | – | Weak | – | Not known | |
| Diazepam1
Clorazepate1 Clonazepam1 Midazolam1 Flurazepam1 |
Major Major Major Major Major |
– – – – – |
– – – – – |
– – – – – |
Not known Not known Not known Not known Not known |
Caution should be exercised in combination with ribociclib or palbociclib |
| Alprazolam1 | Major | Weak | – | – | Not known | |
| Zolpidem1
Zopiclone1 |
Major Major |
– – |
– – |
– – |
Not known Not known |
Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. TdP, Torsades de pointes.
Not classified risk QT. According to the source consulted (www.crediblemeds.org), the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories.
Complementary alternative medicine
A large and not well-known proportion of patients with breast cancer use alternative and complementary medicine. There are reports of prevalence of use in up to 87% of patients.108,109
Some alternative and complementary preparations have shown efficacy in preclinical studies, but have not demonstrated relevant anticancer effects in the clinical setting. However, patients actually use these preparations and some are reluctant to abandon them when beginning CDK4/6i therapy.
Overall, there is scarce information about potential DDIs with these complementary compounds. There are huge amounts of these formulations, so a comprehensive review of all of them was not possible. The most used alternative preparations in our immediate environment were addressed at the workshop,. The most relevant conclusions are shown in Table 20.
Table 20.
Complementary and alternative medicine including herbal products.
| CAM | CYP3A4 Substrate |
CYP3A4 Inhibitor |
CYP3A4 Inducer | Membrane transporter substrate | TdP risk | Comments |
|---|---|---|---|---|---|---|
| Aloe Vera | – | Yes | – | – | Not known | Low risk of interaction with palbociclib and ribociclib SAFE OPTIONS |
| Graviola1 or Guanabana (Annona Muricata) | – | – | – | – | Not known | |
| Homeopathy | – | – | – | – | Not known | |
| Insulin Potentiation Therapy | – | – | – | – | Not known | |
| Vitamin C | – | – | – | – | Not known | |
| Scorpion Venom | – | – | – | – | Not known | |
| Mate | – | – | – | – | Not known | |
| Maitake/Shiitake | – | – | – | – | Not known | |
| Selenium | – | – | – | – | Not known | |
| Ginkgo Biloba1 | – | Possible, not well known | – | – | Not known | Use with caution |
| Ginseng | – | Yes, moderate | – | – | Not known | Use with caution |
| Curcuma | – | Yes, moderate at high doses | – | – | Not known | Use with caution, low dose can be safe |
| Green tea extract | – | Yes, mild to strong, depending on source | – | – | Not known | Some brands can produce strong inhibition and should be avoided [110] |
| Reishi | – | Yes, depending on dose | – | – | Not known | Use with caution [111] |
| Rooibos | – | – | Yes | – | Not known | Use with caution [112] |
| Ukrain | – | – | – | – | Not known | Use with caution, additional hematological side effects [113] |
| Cat’s Claw | – | Yes; moderate-strong | – | – | Not known | High risk of DDIs. Should be avoided the combination with ribociclib or palbociclib. |
| Cimifuga racemosa2 | – | Possible, not well known | – | – | Not known | |
| Grapefruit juice Pomegranate |
– | Yes, moderate | – | – | Not known | |
| St. John’s wort (Hypericum perforatum | – | – | Yes, moderate | – | Not known |
CAM, complementary and alternative medicines. Green: Low risk of interaction with palbociclib and ribociclib, SAFE OPTIONS. Orange: Caution should be exercised. Red: High risk: Plasmatic concentrations of the substrate (palbociclib or ribociclib) could be increased when used concomitantly with a CYP3A4 inhibitor or decreased when used concomitantly with a CYP3A4 inducer. TdP,Torsades de pointes; gp-P, P-glycoprotein
Graviola, ginkgo biloba, inhibit P-gp and may affect the metabolism of substrate drugs
Do not use; also, possible estrogenic effect.
An important consideration is that there are many different formulations, doses, schemes and routes of administration for these drugs or foods, making it impossible, in many cases, to set forth general recommendations by type of product. For example, green tea extract can inhibit CYP3A4 in a range that varies from 5.6% to 89.9%. In this case, taking a green tea infusion some days will not have any relevant risk for CDK4/6i-treated patients, but those treated with Natrol™ 500 mg,108 will be at high risk of enhanced CDK4/6i toxicity. Treatments affected by this important consideration are accompanied with the statement ‘Use with caution’ in Table 20, and patients should be advised to avoid them. If, despite this recommendation, a patient still wants to take it, a careful evaluation of the dosage is needed before prescribing palbociclib or ribociclib.
Preparations in green in Table 20 do not show an important risk of drug interaction, but are not necessarily safe or a good option for cancer treatment. Being classified as a safe preparation only implies a low risk of interaction.
Conclusion
The Spanish SOLTI-promoted workshop entitled ‘CDK4/6 Inhibitors in Breast Cancer: Consensus Workshop on the Management of Concomitant Medication’ gathered professionals from different specialties to address key questions related to the management of palbociclib and ribociclib, the two CDK4/6 inhibitors approved in Spain. The issues raised in this 1-day meeting included a comprehensive review of some selected toxicities and the mechanisms underlying potential DDIs. The ultimate goal was to elaborate ‘positive lists’ of medications that could be safely administered concomitantly with palbociclib and ribociclib.
As a result, lists of safe drugs or groups of drugs are provided, These positive lists comprise a total of 273 drugs belonging to 18 families, and include anti-infective agents, psychotropic drugs, analgesics, anticonvulsants and drugs to treat endocrine and cardiovascular disorders. In addition, when possible, the reason why a substantial number of concomitant medications must be used with caution or are totally prohibited in combination with palbociclib and ribociclib is clearly established. A safety evaluation for the most requested complementary and alternative medicines (18 compounds) is also attempted, in spite of the scarcity of evidence for this setting. The panel felt that all this information will be extremely useful in the oncologist’s daily practice and hope that it will result in a safer management of patients with luminal MBC.
Supplemental Material
Supplemental material, InhibCDK-Supplementary_Material_v2_01012019 for Palbociclib and ribociclib in breast cancer: consensus workshop on the management of concomitant medication by Meritxell Bellet, Faten Ahmad, Rafael Villanueva, Carolina Valdivia, Julián Palomino-Doza, Ada Ruiz, Xavier Gonzàlez, Encarna Adrover, Analía Azaro, Maria Valls-Margarit, Josep Lluís Parra, Juan Aguilar, Maria Vidal, Anastasi Martín, Joaquíín Gavilá, Santiago Escrivá-de-Romaní, Antonia Perelló, Cristina Hernando, Ainhara Lahuerta, Pilar Zamora, Victoria Reyes, María Alcalde, Helena Masanas, Pamela Céliz, Isabel Ruíz, Miguel Gil, Miguel Àngel Seguí and Lorena de la Peña in Therapeutic Advances in Medical Oncology
Footnotes
Funding: The project received funding from Novartis, Pfizer, Grünenthal, Esteve and Kyowa Hakko Kirin; none of the funding bodies participated in the workshop discussion. The authors would like to thank i2e3 research institute for providing editorial assistance on behalf of SOLTI.
Conflict of interest statement: MB has received grants for attending meetings/conferences from Pfizer, Roche and Glaxo, fees as a scientific consultant from Novartis, Pfizer and Lilly and has participated in sponsored speakers bureau or educational activities with Novartis and Pfizer. RV has participated in sponsored speakers bureau or educational activities with Roche and Novartis. CV has received grants for attending meetings/conferences from Roche, Amgen, Janssen and Daiichi Sankyo. XG has received grants for attending meetings/conferences from Roche, fees as a scientific consultant from BMS and has participated in sponsored speakers bureau or educational activities with Roche. EA has received grants for attending meetings/conferences from Roche, Pfizer and Celgene and has participated in sponsored speakers bureau or educational activities with Roche, Novartis, Celgene, AstraZeneca and Glaxo. MV has participated in sponsored speakers bureau or educational activities with Roche and Celgene. JG has received fees as a scientific consultant from Roche and has participated in sponsored speakers bureau or educational activities with Pfizer, AstraZeneca, Novartis and Roche. SER has received grants for attending meetings/conferences from Roche and Daiichi Sankyo, fees as a scientific consultant from Roche and Laboratoires Pierre Fabre and has participated in sponsored speakers bureau or educational activities with Roche. AP has participated in sponsored speakers bureau or educational activities with Novartis and Roche. AL has participated in sponsored speakers bureau or educational activities with Roche, BMS, Eisai, Novartis and AstraZeneca. PZ has received grants for attending meetings/conferences from Roche and has participated in sponsored speakers bureau or educational activities with Roche. IR has received fees as a scientific consultant from Gilead, Pfizer and Merck Sharp & Dohme and has participated in sponsored speakers bureau or educational activities with Astellas, Celgene, Gilead, Merck Sharp & Dohme and Pfizer. MG has received fees as a scientific consultant from Pfizer and has participated in sponsored speakers bureau or educational activities with Novartis, Pfizer and Roche. MAS has received fees as a scientific consultant from Pfizer and has participated in sponsored speakers bureau or educational activities with Novartis, Pfizer and Roche.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Xavier Gonzàlez  https://orcid.org/0000-0002-5797-7037
https://orcid.org/0000-0002-5797-7037
Juan Aguilar  https://orcid.org/0000-0002-9838-1950
https://orcid.org/0000-0002-9838-1950
Contributor Information
Meritxell Bellet, Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology, Passeig Vall d’Hebron 119–129, Barcelona, Spain.
Faten Ahmad, Vall d’Hebron Research Institute, Vall d’Hebron University Hospital, Barcelona, Spain.
Rafael Villanueva, Institut Català d’Oncologia, Hospital Moisès Broggi, Barcelona, Spain.
Carolina Valdivia, Vall d’Hebron University Hospital, Barcelona, Spain.
Julián Palomino-Doza, Hereditary Cardiopathies Unit, Hospital Universitario 12 de Octubre, Madrid, Spain.
Ada Ruiz, Institut de Neuropsiquiatria i Addiccions, Hospital del Mar, and Hospital del Mar Medical Research Institute, Barcelona, Spain.
Xavier Gonzàlez, Instituto Oncológico Dr Rosell, Hospital General De Catalunya, SOLTI, Barcelona, Spain.
Encarna Adrover, Servicio de Oncología Médica, Complejo Hospital Universitario Albacete, Albacete, Spain.
Analía Azaro, Medical Oncology Department, Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology. Barcelona, Spain.
Maria Valls-Margarit, SOLTI, Barcelona, Spain.
Josep Lluís Parra, SOLTI, Barcelona, Spain.
Juan Aguilar, Medical Oncology Department and Infectious Diseases Department, Vall d’Hebron University Hospital, Barcelona, Spain.
Maria Vidal, Hospital Clínic Barcelona and Translational Genomics and Targeted Therapeutics in Solid Tumors Group, Institut d’Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain.
Anastasi Martín, Unitat de Cures Palliatives, Vall d’Hebron University Hospital, Barcelona, Spain.
Joaquín Gavilá, Fundacion Instituto Valenciano De Oncologia, Valencia, Spain.
Santiago Escrivá-de-Romaní, Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology, Barcelona, Spain.
Antonia Perelló, Hospital Universitario Son Espases, Palma de Mallorca, Spain.
Cristina Hernando, Hospital Clínico Universitario de Valencia, INCLIVA Institut d’Investigació Sanitària and Centro de Investigación Biomédica en Red Cáncer, Valencia, Spain.
Ainhara Lahuerta, Fundación Onkologikoa, Donostia – San Sebastián, Spain.
Pilar Zamora, Servicio de Oncologia Médica, Hospital Universitario La Paz, Madrid, Spain.
Victoria Reyes, Radiation Oncology Department, Vall d’Hebron University Hospital, Barcelona, Spain.
María Alcalde, Vall d’Hebron Research Institute, Vall d’Hebron University Hospital, Barcelona, Spain.
Helena Masanas, SOLTI, Barcelona, Spain.
Pamela Céliz, SOLTI, Barcelona, Spain.
Isabel Ruíz, Vall d’Hebron Research Institute, Vall d’Hebron University Hospital, Barcelona, Spain.
Miguel Gil, Institut Català d’Oncologia, IDIBELL Institut d’Investigació Biomédica de Bellvitge, L’Hospitalet, Barcelona, Spain.
Miguel Àngel Seguí, Hospital Universitari, Parc Tauli Sabadell, Barcelona, Spain.
Lorena de la Peña, SOLTI, Barcelona, Spain.
References
- 1. van Leeuwen RWF, Brundel DHS, Neef C, et al. Prevalence of potential drug-drug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer 2013; 108: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan Q, Ismail M, Khan S. Frequency, characteristics and risk factors of QT interval prolonging drugs and drug-drug interactions in cancer patients: a multicenter study. BMC Pharmacol Toxicol 2017; 18: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 4. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 5. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol Off J Eur Soc Med Oncol 2018; 29: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 6. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phas. Lancet Oncol 2016; 17: 425–439. [DOI] [PubMed] [Google Scholar]
- 7. Giacomini KM, Huang S, Tweedie DJ, et al. Membrane transporters in drug development. Nature 2012; 9: 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tannenbaum C, Sheehan NL. Understanding and preventing drug–drug and drug–gene interactions. Expert Rev Clin Pharmacol 2014; 7: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun W, Klamerus KJ, Yuhas LM, et al. Impact of acid-reducing agents on the pharmacokinetics of palbociclib, a weak base with pH-dependent solubility, with different food intake conditions. Clin Pharmacol Drug Dev 2017; 6: 614–626. [DOI] [PubMed] [Google Scholar]
- 10. Ibrance (palbociclib). Summary of product characteristics, https://www.ema.europa.eu/documents/product-information/ibrance-epar-product-information_en.pdf (2016, accessed 1 June 2018).
- 11. Samant TS, Dhuria S, Lu Y, et al. Ribociclib bioavailability is not affected by gastric pH changes or food intake: in silico and clinical evaluations. Clin Pharmacol Ther 2018; 104: 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kisqali (ribociclib). Summary of product characteristics, https://www.ema.europa.eu/documents/product-information/kisqali-epar-product-information_en.pdf (accessed 1 June 2018).
- 13. Almax summary of product characteristics, https://cima.aemps.es/cima/pdfs/es/ft/55397/FT_55397.html.pdf (2013, accessed 1 June 2018).
- 14. Magnesia cinfa summary of product characteristics, https://cima.aemps.es/cima/pdfs/es/ft/34551/FT_34551.html.pdf (2013, accessed 1 June 2018).
- 15. Flórez J. Farmacología Humana. 4th ed. Editorial Masson, 2004, 1440 p. [Google Scholar]
- 16. Binkhathlan Z, Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr Cancer Drug Targets 2013; 13: 326–346. [DOI] [PubMed] [Google Scholar]
- 17. Center for Drug Evaluation and Research. US Food and Drug Administration. Drug development and drug interactions: table of substrates, inhibitors and inducers. Center for Drug Evaluation and Research, https://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm080499.htm (2017, accessed 1 June 2018). [Google Scholar]
- 18. Lü Y, Yan Y, Wang X-F. Antiepileptic drug-induced multidrug resistance P-glycoprotein overexpression in astrocytes cultured from rat brains. Chin Med J (Engl) 2004; 117: 1682–1686. [PubMed] [Google Scholar]
- 19. Amin ML. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights 2013; 7: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raub TJ, Wishart GN, Kulanthaivel P, et al. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos 2015; 43: 1360–1371. [DOI] [PubMed] [Google Scholar]
- 21. de Gooijer MC, Zhang P, Thota N, et al. P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Invest New Drugs 2015; 33: 1012–1019. [DOI] [PubMed] [Google Scholar]
- 22. Patel Y, Davis A, Kala A, et al. CNS penetration of the CDK4/6 inhibitor ribociclib (LEE011) in non-tumor bearing mice and mice bearing orthotopic pediatric brain tumors. Neuro-Oncol 2016; 18: 152. [Google Scholar]
- 23. Sorf A, Hofman J, Kučera R, et al. Ribociclib shows potential for pharmacokinetic drug-drug interactions being a substrate of ABCB1 and potent inhibitor of ABCB1, ABCG2 and CYP450 isoforms in vitro. Biochem Pharmacol 2018; 154: 10–17. [DOI] [PubMed] [Google Scholar]
- 24. Kala A, Patel YT, Davis A, et al. Development and validation of LC-MS/MS methods for the measurement of ribociclib, a CDK4/6 inhibitor, in mouse plasma and Ringer’s solution and its application to a cerebral microdialysis study. J Chromatogr B Analyt Technol Biomed Life Sci 2017; 1057: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Viskin S, Rosovski U, Sands AJ, et al. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Hear Rhythm 2005; 2: 569–574. [DOI] [PubMed] [Google Scholar]
- 26. Postema PG, Wilde AAM. The measurement of the QT interval. Curr Cardiol Rev 2014; 10: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rautaharju PM, Surawicz B, Gettes LS, et al. ; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119: e241–e250. [DOI] [PubMed] [Google Scholar]
- 28. Luo S, Michler K, Johnston P, et al. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol 2004; 37(Suppl.): 81–90. [DOI] [PubMed] [Google Scholar]
- 29. Vandenberk B, Vandael E, Robyns T, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc 2016; 5: e003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Noord C, Eijgelsheim M, Stricker BHC. Drug- and non-6al in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol 2011; 29: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woosley RL, Heise CW, Romero K. CredibleMeds. QTdrugs list. AZ: AZCERT, Inc, https://crediblemeds.org/ (accessed 1 June 2018). [Google Scholar]
- 32. Pyter LM, Cochrane SF, Ouwenga RL, et al. Mammary tumors induce select cognitive impairments. Brain Behav Immun 2010; 24: 903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pyter LM, Pineros V, Galang JA, et al. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc Natl Acad Sci U S A 2009; 106: 9069–9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ren X, St Clair DK, Butterfield DA. Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol Res 2017; 117: 267–273. [DOI] [PubMed] [Google Scholar]
- 35. Kovalchuk A, Ilnytskyy Y, Rodriguez-Juarez R, et al. Chemo brain or tumor brain – that is the question: the presence of extracranial tumors profoundly affects molecular processes in the prefrontal cortex of TumorGraft mice. Aging (Albany NY) 2017; 9: 1660–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maass SWMC, Roorda C, Berendsen AJ, et al. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: a systematic review. Maturitas 2015; 82: 100–108. [DOI] [PubMed] [Google Scholar]
- 37. Otte JL, Carpenter JS, Russell KM, et al. Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. J Pain Symptom Manage 2010; 39: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shin JA, El-Jawahri A, Parkes A, et al. Quality of life, mood, and prognostic understanding in patients with metastatic breast cancer. J Palliat Med 2016; 19: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo X, Xu J, Ying E, et al. Correlation between hormone receptor status and depressive symptoms in patients with metastatic breast cancer. Oncotarget 2017; 8: 50774–50781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giese-Davis J, Collie K, Rancourt KMS, et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol 2011; 29: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grassi L, Nanni MG, Rodin G, et al. The use of antidepressants in oncology: a review and practical tips for oncologists. Ann Oncol Off J Eur Soc Med Oncol 2018; 29: 101–111. [DOI] [PubMed] [Google Scholar]
- 42. Carvalho AF, Sharma MS, Brunoni AR, et al. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom 2016; 85: 270–288. [DOI] [PubMed] [Google Scholar]
- 43. Zivin K, Pfeiffer PN, Bohnert ASB, et al. Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40 mg. Am J Psychiatry 2013; 170: 642–650. [DOI] [PubMed] [Google Scholar]
- 44. Álvarez E, Vieira S, Garcia-Moll X. Citalopram, escitalopram and prolonged QT: warning or alarm? Rev Psiquiatr y Salud Ment 2014; 7: 147–150. [DOI] [PubMed] [Google Scholar]
- 45. Beach SR, Kostis WJ, Celano CM, et al. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J Clin Psychiatry 2014; 75: e441–e449. [DOI] [PubMed] [Google Scholar]
- 46. Coppola C, Rienzo A, Piscopo G, et al. Management of QT prolongation induced by anti-cancer drugs: target therapy and old agents. Different algorithms for different drugs. Cancer Treat Rev 2018; 63: 135–143. [DOI] [PubMed] [Google Scholar]
- 47. Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat 2017; 166: 41–54. [DOI] [PubMed] [Google Scholar]
- 48. Dieras V, Harbeck N, Joy A, et al. PALOMA-2: neutropenia (NP) patterns in patients (Pts) with estrogen receptor2positive (ER1)/human epidermal growth factor receptor 22negative (HER2-) first-line advanced breast cancer (ABC) receiving palbociclib 1 letrozole (P1L). Ann Oncol 2017; 28(Suppl. 5): v74–v108. [Google Scholar]
- 49. Tripathy D, Im S-A, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018; 19: 904–915. [DOI] [PubMed] [Google Scholar]
- 50. Committee for Medicinal Products for Human Use. European Public Assessment Report: Ribociclib. EMA/CHMP/506968/2017. https://www.ema.europa.eu/documents/assessment-report/kisqali-epar-public-assessment-report_en.pdf (2017, accessed 1 June 2018).
- 51. Committee for Medicinal Products for Human Use. European Public Assessment Report: Palbociclib. EMA/652627/2016. https://www.ema.europa.eu/documents/assessment-report/ibrance-epar-public-assessment-report_en.pdf (2016, accessed 1 June 2018).
- 52. Slamon DJ, Neven P, Chia S, et al. Ribociclib (RIB) + fulvestrant (FUL) in postmenopausal women with hormone receptor-positive (HR+), HER2-negative (HER2 –) advanced breast cancer (ABC): Results from MONALEESA-3. J Clin Oncol 2018; 36(Suppl.): 2. [Google Scholar]
- 53. Cristofanilli M, DeMichele A, Giorgetti C, et al. Predictors of prolonged benefit from palbociclib (PAL) plus fulvestrant (F) in women with endocrine-resistant hormone receptor–positive/human epidermal growth factor receptor 2–negative (HR+/HER2–) advanced breast cancer (ABC) in PALOMA-3. Am Soc Clin Oncol 2018; 104:21–31. [DOI] [PubMed] [Google Scholar]
- 54. Harbeck N, Dieras V, Finn R, et al. Abstract P5-19-01: impact of palbociclib plus letrozole on patient-reported general health status compared with letrozole alone in ER+/HER2-advanced/metastatic breast cancer. In 2017 San Antonio Breast Cancer Symposium, San Antonio, Texas, 5–9 December 2017, Abstract P5-19-01. AACR. [Google Scholar]
- 55. Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist 2016; 21: 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Janni W, Alba E, Bachelot T, et al. First-line ribociclib plus letrozole in postmenopausal women with HR+, HER2- advanced breast cancer: tumor response and pain reduction in the phase 3 MONALEESA-2 trial. Breast Cancer Res Treat 2018; 169: 469–479. [DOI] [PubMed] [Google Scholar]
- 57. O’Shaughnessy J, Petrakova K, Sonke GS, et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2- advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat 2018; 168: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zheng J, Amantea M, Wang D. Abstract P5-19-16: effect of palbociclib concentration on heart rate-corrected QT interval in patients with cancer. Philadelphia, PA: AACR, 2015. [Google Scholar]
- 59. Infante JR, Cassier PA, Gerecitano JF, et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res 2016; 22: 5696–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. A study of palbociclib (PD-0332991) + letrozole vs. letrozole for 1st line treatment of postmenopausal women with ER+/HER2- advanced breast cancer (PALOMA-2). Clinical Trials.gov, https://clinicaltrials.gov/ct2/show/results/NCT01740427?term=PALOMA+2&rank=1&view=results (2018, accessed 1 June 2018).
- 61. Durairaj C, Ruiz-Garcia A, Gauthier ER, et al. Palbociclib has no clinically relevant effect on the QTc interval in patients with advanced breast cancer. Anticancer Drugs 2018; 29: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ruiz A, Gauthier E, Durairaj C, et al. Abstract P4-22-10: evaluation of the effects of palbociclib (PAL)+ letrozole (LET) on QTc. Philadelphia, PA: AACR, 2017. [Google Scholar]
- 63. Palbociclib (PD-0332991). Combined with fulvestrant in hormone receptor+ HER2-negative metastatic breast cancer after endocrine failure (PALOMA-3). Clinical Trials.gov, https://clinicaltrials.gov/ct2/show/results/NCT01942135?term=PALOMA+3&rank=1 (2018, accessed 1 June 2018).
- 64. DeLaurentiis M, Neven P, Jerusalem GHM, et al. Ribociclib (RIBO) + letrozole (LET) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC) with no prior endocrine therapy (ET) for ABC: preliminary results from the p. J Clin Oncol 2018; 36(15 Suppl.): 1056. [Google Scholar]
- 65. DeLaurentiis M, Neven P, Jerusalem GHM, et al. Ribociclib+letrozole in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC) with no prior endocrine therapy for ABC: preliminary results from the phase 3b CompLEEment-1 trial. J Clin Oncol 2018; 36(15 Suppl.): 1056. [Google Scholar]
- 66. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 67. Janni W, Burris HA, Blackwell KL, et al. First-line ribociclib plus letrozole for postmenopausal women with hormone receptor-positive (HR+), HER2-negative (HER2-) advanced breast cancer (ABC): MONALEESA-2 safety results. J Clin Oncol 2017; 35(15 Suppl.): 1047. [Google Scholar]
- 68. KISQALI® (ribociclib). Highlights of prescribing information. Novartis, https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kisqali.pdf (2018, accessed 1 June 2018).
- 69. IBRANCE® (palbociclib). Highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207103s000lbl.pdf (2015, accessed 1 June 2018).
- 70. LIVERTOX®. Search Livertox Database, https://livertox.nih.gov/ (2016, accessed 1 June 2018).
- 71. Study of letrozole with or without palbociclib (PD-0332991) for the first-line treatment of hormone-receptor positive advanced breast cancer – study results. ClinicalTrials.go. Clinical Trials.gov, https://clinicaltrials.gov/ct2/show/results/NCT00721409?term=NCT00721409&rank=1&view=results#outcome1 (2018, accessed 1 June 2018).
- 72. Center for Drug Evaluation and Research. NDA/BLA multi-disciplinary review and evaluation NDA 209092 KISQALI (ribociclib), https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209092Orig1s000MultidisciplineR.pdf (2017, accessed 1 June 2018).
- 73. Geoerger B, Bourdeaut F, DuBois SG, et al. A phase I study of the CDK4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumors. Clin Cancer Res 2017; 23: 2433–2441. [DOI] [PubMed] [Google Scholar]
- 74. Doi T, Hewes B, Kakizume T, et al. Phase I study of single-agent ribociclib in Japanese patients with advanced solid tumors. Cancer Sci 2018; 109: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Diéras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2-advanced breast cancer. JNCI J Natl Cancer Inst 2018; 18 July. doi: 10.1093/jnci/djy109. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vuppalanchi R, Saxena R, Storniolo AMV, et al. Pseudocirrhosis and liver failure in patients with metastatic breast cancer after treatment with palbociclib. Hepatology 2017; 65: 1762–1764. [DOI] [PubMed] [Google Scholar]
- 77. Jüngst C, Krämer J, Schneider G, et al. Subacute liver failure by pseudocirrhotic metastatic breast cancer infiltration. Ann Hepatol 12: 834–836. [PubMed] [Google Scholar]
- 78. Jeong WK, Choi S-Y, Kim J. Pseudocirrhosis as a complication after chemotherapy for hepatic metastasis from breast cancer. Clin Mol Hepatol 2013; 19: 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Adike A, Karlin N, Menias C, et al. Pseudocirrhosis: a case series and literature review. Case Rep Gastroenterol 2016; 10: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Broderick JM. MONALEESA-7 data shows ribociclib active in premenopausal breast cancer subgroups. Targeted Oncol, https://www.targetedonc.com/news/monaleesa7-data-shows-ribociclib-active-in-premenopausal-breast-cancer-subgroups (2018, accessed 1 June 2018).
- 81. Fromenty B, Begriche K, Moreau C, et al. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J Clin Transl Res 2017; 3(Suppl. 1): 212–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Teschke R, Danan G. Drug-induced liver injury: is chronic liver disease a risk factor and a clinical issue? Expert Opin Drug Metab Toxicol 2017; 13: 425–438. [DOI] [PubMed] [Google Scholar]
- 83. Fromenty B. Drug-induced liver injury in obesity. J Hepatol 2013; 58: 824–826. [DOI] [PubMed] [Google Scholar]
- 84. MONALEESA-2. Study of efficacy and safety of LEE011 in postmenopausal women with advanced breast cancer. Clinical Trials.gov, https://clinicaltrials.gov/ct2/show/results/NCT01958021?term=MONALEESA+2&rank=1 (2018, accessed 1 June 2018).
- 85. Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist 2013; 18: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Trüeb RM. Minoxidil for endocrine therapy–induced alopecia in women with breast cancer – Saint Agatha’s Blessing? JAMA Dermatol 2018; 154: 656. [DOI] [PubMed] [Google Scholar]
- 87. Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol 2018; 154: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gallicchio L, Calhoun C, Helzlsouer KJ. Aromatase inhibitor therapy and hair loss among breast cancer survivors. Breast Cancer Res Treat 2013; 142: 435–443. [DOI] [PubMed] [Google Scholar]
- 89. Verzenio (abemaciclib). CDK4 & CDK6 inhibitor, https://www.verzenio.com/hcp (accessed 1 June 2018).
- 90. Eller MG, Szpunar GJ, Della-Coletta AA. Absorption of minoxidil after topical application: effect of frequency and site of application. Clin Pharmacol Ther 1989; 45: 396–402. [DOI] [PubMed] [Google Scholar]
- 91. Drug interactions checker – for drugs, food & alcohol. https://www.drugs.com/drug_interactions.html (accessed 1 June 2018).
- 92. Pauls E, Badia R, Torres-Torronteras J, et al. Palbociclib, a selective inhibitor of cyclin-dependent kinase4/6, blocks HIV-1 reverse transcription through the control of sterile α motif and HD domain-containing protein-1 (SAMHD1) activity. AIDS 2014; 28: 2213–2222. [DOI] [PubMed] [Google Scholar]
- 93. National Comprehensive Cancer Network. Prevention and treatment of cancer-related infections. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf (2018, accessed 1 June 2018).
- 94. Liu Z, Jiang L, Liang G, et al. Hepatitis B virus reactivation in breast cancer patients undergoing chemotherapy: a review and meta-analysis of prophylaxis management. J Viral Hepat 2017; 24: 561–572. [DOI] [PubMed] [Google Scholar]
- 95. Lee HJ, Kim DY, Keam B, et al. Lamivudine prophylaxis for hepatitis B virus carrier patients with breast cancer during adjuvant chemotherapy. Breast Cancer 2014; 21: 387–393. [DOI] [PubMed] [Google Scholar]
- 96. Kuo M-T, Tseng P-L, Chou Y-P, et al. Role of hepatitis B surface antigen in hepatitis B virus relapse after entecavir or tenofovir prophylaxis in patients undergoing cancer chemotherapy. J Gastroenterol Hepatol 2018; 33: 1766–1772. [DOI] [PubMed] [Google Scholar]
- 97. Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA 2014; 312: 2521–2530. [DOI] [PubMed] [Google Scholar]
- 98. Center for Drug Evaluation and Research. U S Food and Drug Administration, https://www.fda.gov/ (2018, accessed 1 June 2018).
- 99. Adult Cancer Pain. NCCN clinical practice guidelines in oncology. National Comprehensive Cancer Network, https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf (2018, accessed 1 June 2018). [DOI] [PubMed]
- 100. International Association for the Study of Pain. Epidemiology of cancer pain, https://s3.amazonaws.com/rdcms-iasp/files/production/public/Content/ContentFolders/GlobalYearAgainstPain2/CancerPainFactSheets/Epidemiology_Final.pdf (2008, accessed 1 June 2018).
- 101. Edmond Charlton J. Cancer pain. In: Core curriculum for professional education in pain. Washington DC: IASP Press, 2005. [Google Scholar]
- 102. Bell T, Crown JP, Lang I, et al. Impact of palbociclib plus letrozole on pain severity and pain interference with daily activities in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer as first-line treatment. Curr Med Res Opin 2016; 32: 959–965. [DOI] [PubMed] [Google Scholar]
- 103. Verma S, O’Shaughnessy J, Burris HA, et al. Health-related quality of life (HRQoL) of postmenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC) treated with ribociclib + letrozole: Results from MONALEESA-2. J Clin Oncol 2017; 35(15 Suppl.): 1020. [Google Scholar]
- 104. El-Sheikh AAK, van den Heuvel JJMW, Koenderink JB, et al. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther 2007; 320: 229–235. [DOI] [PubMed] [Google Scholar]
- 105. Brintellix tablets 5, 10 and 20 mg – summary of product characteristics (SmPC) - (eMC). The electronic Medicines Compendium (eMC), https://www.medicines.org.uk/emc/product/7121/smpc (2017, accessed 31 July 2018). [Google Scholar]
- 106. Chen G, Højer A-M, Areberg J, et al. Vortioxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet 2018; 57: 673–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. UpToDate Online. Waltham, MA, https://www.uptodate.com (2018, accessed 1 June 2018). [Google Scholar]
- 108. Ashikaga T, Bosompra K, O’Brien P, et al. Use of complimentary and alternative medicine by breast cancer patients: prevalence, patterns and communication with physicians. Support Care Cancer 2002; 10: 542–548. [DOI] [PubMed] [Google Scholar]
- 109. Greenlee H, Neugut AI, Falci L, et al. Association between complementary and alternative medicine use and breast cancer chemotherapy initiation. JAMA Oncol 2016; 2: 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wanwimolruk S, Wong K, Wanwimolruk P. Variable inhibitory effect of different brands of commercial herbal supplements on human cytochrome P-450 CYP3A4. Drug Metabol Drug Interact 2009; 24: 17–35. [DOI] [PubMed] [Google Scholar]
- 111. Wang X, Zhao X, Li D, et al. Effects of ganoderma lucidum polysaccharide on CYP2E1, CYP1A2 and CYP3A activities in BCG-immune hepatic injury in rats. Biol Pharm Bull 2007; 30: 1702–1706. [DOI] [PubMed] [Google Scholar]
- 112. Matsuda K, Nishimura Y, Kurata N, et al. Effects of continuous ingestion of herbal teas on intestinal CYP3A in the rat. J Pharmacol Sci 2007; 103: 214–221. [DOI] [PubMed] [Google Scholar]
- 113. Ernst E, Schmidt K. Ukrain – a new cancer cure? A systematic review of randomised clinical trials. BMC Cancer 2005; 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, InhibCDK-Supplementary_Material_v2_01012019 for Palbociclib and ribociclib in breast cancer: consensus workshop on the management of concomitant medication by Meritxell Bellet, Faten Ahmad, Rafael Villanueva, Carolina Valdivia, Julián Palomino-Doza, Ada Ruiz, Xavier Gonzàlez, Encarna Adrover, Analía Azaro, Maria Valls-Margarit, Josep Lluís Parra, Juan Aguilar, Maria Vidal, Anastasi Martín, Joaquíín Gavilá, Santiago Escrivá-de-Romaní, Antonia Perelló, Cristina Hernando, Ainhara Lahuerta, Pilar Zamora, Victoria Reyes, María Alcalde, Helena Masanas, Pamela Céliz, Isabel Ruíz, Miguel Gil, Miguel Àngel Seguí and Lorena de la Peña in Therapeutic Advances in Medical Oncology