Abstract
Background:
Efficacy of second-line systemic chemotherapy in recurrent gastric cancer with peritoneal metastasis (RGCPM) is limited. We assessed the feasibility, safety and possible efficacy of pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with RGCPM after ⩾1 line of palliative intravenous chemotherapy.
Methods:
In this open-label, single-arm, monocentric phase II ICH-GCP clinical trial, patients were scheduled for three courses of PIPAC with cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 (PIPAC C/D) every 6 weeks. Patients with bowel obstruction or extraperitoneal metastasis were ineligible. The primary endpoint was clinical benefit rate (CBR) by Response Evaluation Criteria in Solid Tumors based on clinical records. Secondary endpoints included overall survival (OS), median time to progression (TTP), peritoneal carcinomatosis index (PCI), histological regression and ascites volume. Safety and tolerability were assessed by Common Terminology Criteria for Adverse Events (CTCAE) version 4, quality of life (QoL) by EORTC-QLQ30 questionnaire.
Results:
A total of 25 patients were enrolled and available for the analysis of the primary endpoint. Of those 25 patients, 10 (40%) had a radiological complete, partial response or stable disease. Median OS [intention to treat (ITT)] was 6.7 months, median TTP was 2.7 months. Complete or major regression on histology were observed in 9/25 patients (36%, ITT) or 6/6 [100%, per protocol (PP)] patients. There were no suspected unexpected serious adverse reactions, no treatment-related deaths, no CTCAE grade 4 toxicity and three (12%) grade 3 toxicities. Changes in the QLQ-C30 scores during PIPAC C/D therapy were small and not significant.
Conclusions:
PIPAC C/D was well tolerated and active in patients with RGCPM. Survival was encouraging. Randomized controlled trials should now be designed in this indication.
Keywords: Cisplatin, doxorubicin, histological regression, gastric cancer, intraperitoneal chemotherapy, objective tumor response, peritoneal metastasis, pressurized intraperitoneal aerosol chemotherapy (PIPAC)
Introduction
Gastric cancer is the fifth most common cancer worldwide and associated with a poor prognosis and about 740,000 deaths per year.1,2 The 5-year overall survival is around 25% but varies greatly depending on the tumor stage (TNM) and histology. In particular, signet-ring histology is associated with dismal prognosis.3 Surgery with lymph node dissection is the primary treatment for medically fit patients with resectable tumors.4 Perioperative chemotherapy is recommended following curative (R0) resection. The recurrence rate is high after frontline multimodal therapy for 40–80% of the patients.5,6 The median survival of patients with unresectable gastric cancer treated with systemic chemotherapy is not greater than 12 months.7 Among such patients, the median survival of those with peritoneal metastasis (PM) was reported to be even worse at 6–10 months.8 Therefore, National Comprehensive Cancer Network Guidelines for Gastric Cancer encourages patients with gastric cancer to participate in well-designed clinical trials investigating novel therapeutic strategies to enable further advances.4
One avenue of research is to combine systemic and locoregional therapy. For example, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been shown to prolong life in patients with PM of gastric origin in good general condition with a limited extent of disease and less aggressive tumor biology9 with a cure rate of 11%.10 Unfortunately, this approach is restricted to a highly selected minority of patients and tumor recurrence is frequent after CRS and HIPEC in PM of gastric origin.9 Repeated CRS and HIPEC is not a rational option in these patients as potential benefits are abolished by high morbidity and mortality.11,12
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel palliative approach to treat PM for patients who are not eligible for CRS and HIPEC.13 Preclinical data suggested improved intraperitoneal distribution and higher tissue concentrations of chemotherapy agents in PIPAC compared with HIPEC.14–16 A cohort study in 24 patients with recurrent gastric cancer with PM (RGCPM) has suggested that application of intraperitoneal chemotherapy as a pressurized aerosol in RGCPM might be well tolerated and might induce objective regression in a significant proportion of patients; moreover, median survival was encouraging at 15.4 months.17 Another prospective study combining PIPAC with cisplatin and doxorubicin (PIPAC C/D) with combination palliative chemotherapy (XELOX) in 31 patients showed complete and partial pathological response in 60% and a median survival of 13 months.18 In a third cohort with 22 patients with RGCPM treated with combined palliative systemic chemotherapy (best choice) and PIPAC C/D, reported survival was 19.5 months.19 Overall, two systematic reviews have concluded that PIPAC is feasible and well tolerated. PIPAC does not deteriorate quality of life (QoL). Preliminary good response rates call for prospective analysis of oncological efficacy.20,21
The aim of this phase II ICH-GCP study is to investigate the efficacy and safety of PIPAC C/D in patients with RGCPM after at least one previous line of systemic palliative chemotherapy.
Materials and methods
Study design and patient eligibility
This is an open-label monocentric single-arm, phase II ICH-GCP clinical trial. Patients were scheduled for PIPAC C/D alone. Overall, three cycles of cisplatin 7.5 mg/m2 in 150 ml NaCl 0.9% and doxorubicin 1.5 mg/m2 body surface in 50 ml NaCl 0.9% was applied intraperitoneally at 6-week intervals. No CRS and no systemic chemotherapy were allowed by the protocol.
Institutional Review Board approval for this study was obtained from the Ethics Committee of the Ruhr University Bochum, Germany (reference number 4783-13FF; issued 14 October 2013). This study was approved by the German Federal Drug Agency (BfArM; reference number 4039261 issued on 30 October 2013). This study was registered with ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT01854255) and EudraCT (2013-002103-34).
Patients were eligible if they had clinical confirmation of gastric cancer and PM; disease progression after at least one line of previous intravenous chemotherapy; blood and electrolyte counts (liver and renal function parameters within 10% of the normal range established in the laboratory of the study institution); tumor mass present on computed tomography (CT) scan in order to allow tumor response assessment with Response Evaluation Criteria in Solid Tumors (RECIST). All participants signed an informed consent form.
Patients were ineligible if they had extraperitoneal metastatic disease (with the exception of isolated malignant pleural effusion); bowel obstruction requiring nasogastric tubing or percutaneous endoscopic gastrostomy; chemotherapy or surgery within the last 4 weeks prior to enrolment; previous treatment with maximum cumulative doses of doxorubicin, daunorubicin, epirubicin, idarubicin or other anthracyclines and anthracenediones; history of allergic reaction to cisplatin or other platinum-containing compounds or doxorubicin; severe renal impairment, myelosuppression, severe hepatic impairment, severe myocardial insufficiency, recent myocardial infarction, severe arrhythmias; immunocompromised patients such as those with an immunosuppressive medication or a known disease of the immune system; involvement in the planning and conduct of the study; or pregnancy. PM was defined as synchronous or metachronous (before, respectively after gastric resection for primary gastric cancer). Progressive ascites was not an exclusion criterion.
The primary endpoint of the study was the clinical benefit rate (CBR), measured by an independent radiologist by RECIST (version 1.1). CBR is defined as the total number of patients with complete radiological response, partial response and stable disease. A baseline abdominal CT scan was required <4 weeks before first PIPAC C/D and a CT was repeated before each PIPAC cycle. Follow-up CT scans were planned at 3, 6, 9 and 12 months. According to the study protocol, a further CT scan was not performed in the case of clinical progressive disease (PD).
The secondary endpoints of the study were:
- the observed survival determined from the time point of the first PIPAC C/D until 1 year of follow up;
- the median TTP according to RECIST 1.122 after three cycles of PIPAC C/D;
- the peritoneal carcinomatosis index (PCI) assessed by laparoscopy before each PIPAC application;
- the degree of histological regression in multiple peritoneal biopsies taken at the beginning of each PIPAC C/D and assessed by independent pathological review (as documented in the clinical records: no vital tumor, major regression, minor regression, active tumor without sign of regression);
- the difference in ascites volume before the first, second, and third PIPAC C/D application (measured as the volume of liquid removed at the beginning of the procedure).
Patient-reported outcomes included QoL by questionnaire (QLQ-C30, German version) of the European Organization for Research and Treatment of Cancer (EORTC).23 QoL was assessed on the day of admission before each PIPAC C/D cycle and then every 8 weeks. Questionnaires were filled out by the patient without any interference of the medical team.
Safety and tolerability were assessed by the collection of adverse events, according to the Common Terminology Criteria for Adverse Events version 4.0,24 including physical examination results and laboratory assessments (chemistry and hematology). In an ancillary research project, additional biopsies were taken for determining mutational profiles of the PM sampled in this study, possibly associated with drug resistance.
The following examinations and parameters were measured at the time of enrolment: previous medical history, previous chemotherapy or radiotherapy, and previous surgery. In addition, the following examinations and parameters were measured before each PIPAC cycle: complete physical examination; vital signs; electrocardiogram; blood analysis [C-reactive protein (CRP), hematocrit, platelets, white blood cells count, bilirubin, albumin, total protein count, aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), sodium, potassium, creatinine, calcium, phosphate, magnesium, lactate dehydrogenase (LDH), creatinine kinase, carcinoembryonic antigen (CEA), cancer antigen 125, carbohydrate antigen 19-9] and urine analysis (microalbumin, creatinine, protein); and a pregnancy test. The day before every PIPAC C/D cycle and every day until discharge at the fourth postoperative day white blood count (WBC), CRP and serum creatinine were determined.
Operative procedure
The PIPAC procedure was performed as described elsewhere.25 A capnoperitoneum of a 12 mmHg CO2 was established followed by the insertion of two balloon safety trocars (Kii® 5 and 12 mmHg, Applied Medical, Duesseldorf, Germany). The PCI was documented based on lesion size and distribution.26 Tumor biopsies were taken at suspect lesion locations in all four quadrants as well as a local peritonectomy. This centimetric peritonectomy was performed in addition to millimetric peritoneal biopsies in order to reduce the number of false-negative peritoneal biopsies. Ascites was removed, and the total volume documented. A nebulizer (Capnopen®, Capnomed, Zimmern, Germany) was connected to an intravenous high-pressure injector (Medrad Arterion 7®, Bayer Healthcare, Berlin, Germany) and inserted into the abdomen. A pressurized aerosol containing cisplatin at a dose of 7.5 mg/m2 body surface in a solution of 150 ml NaCl 0.9% followed by doxorubicin at a dose of 1.5 mg/m2 body surface were applied by the nebulizer and angioinjector. Parameters of injection were set at a flow rate of 1.0 ml/sec and a maximum upstream pressure of 290 psi (=20 bar). After the application of both drugs, the steady-state was maintained for 30 min at a pressure of 12 mmHg and normothermia. Then the chemotherapy-containing aerosol was exsufflated via a closed line over two sequential microparticle filters into the air waste system of the hospital. At the end of the procedure, the trocars were removed and the procedure terminated. No abdominal drainage was applied. Occupational health safety aspects of PIPAC C/D have been described elsewhere27 and the procedure has been shown to be safe. PIPAC C/D was performed in an operating room equipped with an advanced ventilation system meeting the DIN-norm 1946-4, level 5; the injection was remote-controlled.
Intention-to-treat and per-protocol population
The study was closed on 29 June 2017 after the inclusion of 25 patients because of the institutional change of the Principal Investigator (PI). The intention-to-treat (ITT) population was defined as all patients meeting the eligibility criteria (n = 25 patients). The per-protocol (PP) population was defined as all patients having received the allocated therapy of three PIPAC C/D cycles (n = 6 patients).
Statistical aspects
The sample size was calculated on the basis of a Simon two-stage design for a phase II study. Considering a reported response rate of 10–25% in the second-line therapy situation in gastric cancer,28 we regarded a proportion of patients with a CBR of 40% or more as proof of efficacy of PIPAC C/D in this patient population and of less than 20% as insufficient to continue the assessment. Assuming a risk of α = 0.05 (type I error) and β = 0.10 (type II error), we needed to include 24 patients for stage 1. Depending on the outcome events in stage 1, according to the Simon two-stage design, an additional 21 patients should be included (stage 2). Assuming a dropout rate of 10%, we intended to recruit at least 27 patients for stage 1. Descriptive statistics (means, standard deviations and ranges) are used for demographic data. The 95% confidence intervals were used to describe relative frequencies. Patients’ survival was modeled in a Kaplan–Meier survival curve. Changes in functional and symptoms scales of the EORTC-QLQ-C30 were described in profiles and statistical analyzed by sign test. SPSS software (version 24, IBM, Chicago, IL, USA) and SAS version 9.4 were used for statistical analysis.
Results
A total of 30 patients with synchronous or metachronous PM of gastric origin after at least one line of systemic chemotherapy were screened between 27 November 2013 and 6 April 2016, and 25 patients were enrolled. Overall, five patients were excluded because of bowel obstruction (n = 2), chemotherapy within 4 weeks before enrolment (n = 2) and a missing target lesion on CT scan (n = 1). The study was closed on 29 June 2017 after the inclusion of 25 patients because of the institutional change of the PI.
Patient, tumor, and treatment characteristics are summarized in Table 1. The age of the included patients was 55.1 ± 13.0 years with 10 males (40%) and 15 females (60%). The preoperative Karnofsky index was 81% ± 11%. Mean PCI was 15.3 ± 10.6. All patients had verified PM, confirmed by previous histology. A total of eight patients had a PCI ⩽ 12 (32%) and 17 patients had a PCI > 12 (68%). Most patients had a signet-ring cell tumor (n = 22; 88%) whereas three displayed an intestinal histology (12%). Mean ascites volume removed at time of first PIPAC C/D was 493 ml (min 0 ml, max 5500 ml). In 18 patients (72%), ascites volume was under 300 ml and 7 patients (28%) had more than 300 ml of ascites. All patients had received at least one line of chemotherapy before the first PIPAC C/D: 16 patients (64%) were in the second-line-situation, 5 patients (20%) were in the third-line-situation, 2 patients (8%) in the fourth-line-situation and 2 patients (8%) were in the fifth-line-situation. A total of 15 patients (60%) had previous gastrectomy and 3 patients had previous radiotherapy (12%). No patient received simultaneous systemic chemotherapy.
Table 1.
Patients, tumor and treatment characteristics.
| Value | % | |
|---|---|---|
| Number of patients | 25 | |
| Age | 55.1 ± 13.0 | |
| Sex (M:F) | 10 : 15 | 40% : 60% |
| Karnofksy index (%) | 81 ± 11 | |
| Peritoneal carcinomatosis index | ||
| mean | 15.3 ± 10.6 | |
| ⩽ 12 | 8 | 32% |
| >12 | 17 | 68% |
| Histology | ||
| - signet-ring | 22 | 88% |
| - intestinal | 3 | 12% |
| Ascites (ml) | ||
| mean | 493 ± 1192 | |
| ⩽300 | 18 | 72% |
| >300 | 7 | 28% |
| Previous chemotherapy lines | ||
| 1 | 25 | 100% |
| 2 | 16 | 64% |
| 3 | 5 | 20% |
| 4 | 2 | 8% |
| 5 | 2 | 8% |
| Previous surgery | ||
| Gastrectomy | 15 | 60% |
| Previous radiotherapy | 3 | 12% |
| Number of PIPAC cycles (n = 43) | ||
| 1 | 25 | 100% |
| 2 | 12 | 48% |
| 3 | 6 | 24% |
F, female; M, male; PIPAC, pressurized intraperitoneal aerosol chemotherapy.
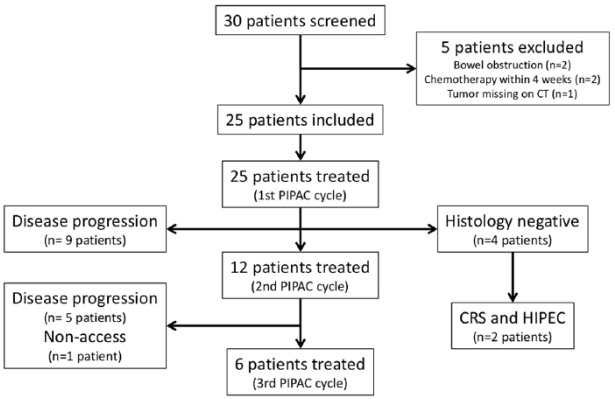
Patient flow is summarized in Figure 1. In total, 43 cycles of PIPAC C/D were performed. All patients (n = 25, ITT population) had at least one cycle of PIPAC C/D, 12 patients (48%) had two cycles and 6 patients (24%) three cycles of PIPAC C/D (PP population). A total of 25 patients underwent one cycle of PIPAC C/D. In spite of previously proven PM, intraoperative histology during the first cycle of PIPAC C/D was negative in four patients; these received no further PIPAC C/D, two of them had CRS and HIPEC later on. After 6 weeks, two patients withdrew their approval for study participation and seven patients showed clinically progressive disease; no second PIPAC cycle was performed in these nine patients. In one additional patient, abdominal access was not possible at PIPAC C/D cycle two (1/43, 2%) so that PIPAC C/D was technically feasible in 98% instances. After 6 weeks, at the time point of the third cycle of PIPAC C/D five patients had clinically progressive disease, so only six patients received the complete therapy protocol with three cycles of PIPAC C/D.
Figure 1.
Study flowchart.
CT, computed tomography; CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; PIPAC, pressurized intraperitoneal aerosol chemotherapy.
Primary outcome criteria
A CT scan was performed before each PIPAC cycle and then every 3 months to estimate radiological tumor response by RECIST version 1.1. In the ITT population, the CBR was 40% (95% confidence interval: 21–61%) or 10 patients (Supplementary Material 1). A complete radiological tumor regression (CR) was observed in a single patient after three cycles of PIPAC C/D alone (4% ITT; 16.7% PP; Figure 2). The patient was alive 31 months after the first PIPAC C/D. A partial response was seen in two patients ITT, resp. one PP (8% ITT, 16.7% PP), stable disease (SD) in seven patients ITT, resp. 4 PP (28% ITT, 66.7% PP) and three patients ITT, resp. 1 PP (12% ITT, 16.7% PP) displayed PD after PIPAC C/D treatment. According to the Simon design, a minimum of 6 patients out of 24 should have a clinical benefit in the first stage. Therefore, the study confirmed a CBR superior to 20%, which was chosen as the minimum rate for initiating stage 2 of the Simon design.
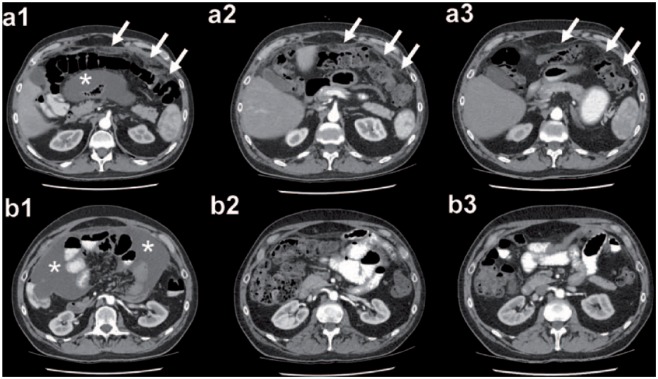
Figure 2.
Example of a complete radiological response according to RECIST version 1.1 in a patient with RGCPM after three cycles of PIPAC C/D. (a1/b1) PIPAC cycle 1: arrows highlight the thickening of the parietal peritoneal layer due to PM; star shows intraabdominal ascites. (a2/b2) PIPAC cycle 2: arrows mark the decreasing thickening of the parietal peritoneal layer; the ascites disappeared. (a3/b3) PIPAC cycle 3: arrows highlight the parietal peritoneal layer without any radiological signs of PM; no intraabdominal ascites can be seen. Patient was alive 31 months after PIPAC cycle 1.
C/D, cisplatin and doxorubicin; PIPAC, pressurized intraperitoneal aerosol chemotherapy; PM, peritoneal metastasis; RECIST, Response Evaluation Criteria in Solid Tumors; RGCPM, recurrent gastric cancer with peritoneal metastasis.
Secondary outcome criteria
Adverse events observed for eight patients (32% ITT) are summarized in Table 2. There were no treatment-related deaths (CTCAE grade 5) nor grade 4 toxicity. CTCAE grade 1 events occurred in all 25 patients (100%), grade 2 events in 5 patients (20%) and grade 3 events including abdominal pain (n = 1) and subileus (n = 2) were observed in 3 patients (12%).
Table 2.
Adverse events (according to CTCAE 4.0). Several AEs possible/patient.
| CTCAE grade | Number (patients) | Description |
|---|---|---|
| 5 | 0 | |
| 4 | 0 | |
| 3 | 3 | Subileus (n = 2), Abdominal pain (readmission, n = 1) |
| 2 | 5 | Hypoalbuminemia (n = 1), abdominal pain (n = 1), vomiting (n = 1), liver toxicity (ASAT/ALAT, n = 1), subcutaneous toxic emphysema (n = 1) |
| 1 | 25 | Abdominal pain, nausea |
AE, adverse event; ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase,
CTCAE, Common Terminology Criteria for Adverse Events version 4.0.
Of 25 patients, 12 received more than one cycle of PIPAC C/D and were eligible for histological tumor response assessment. Histological response after first PIPAC C/D could not be determined in 13 patients (48% ITT) who had no second procedure. Within the other 12 patients (48% ITT), a complete histological tumor regression was observed for one patient (4% ITT, 16.7% PP) without detection of any tumor in the multiple tissue biopsies and in the centimetric peritonectomy specimen. In eight further patients ITT, resp. 5 PP (32% ITT, 83.3% PP), a major histological response was documented. Overall, three patients in the ITT group had only a minor histological response or no response following PIPAC C/D therapy (12% ITT). Altogether, the histological response rate was 9/25 (36%, ITT) and 6/6 (100%, PP).
Mean PCI seemed to be stable under PIPAC C/D therapy: PIPAC#1: 15.4 [95% confidence interval (CI): 11.0–19.8]; PIPAC#2: 11.1 (95% CI: 4.7–17.4); PIPAC#3: 13.3 (95% CI: 6.7–20.0). Mean ascites volume did not increase under PIPAC C/D therapy (PIPAC#1: 493 ml (95% CI: 0–985); PIPAC#2: 642 (95% CI: 0–1567); PIPAC #3: 83 ml (95% CI: 0–222 ml).
In the ITT analysis, median overall survival was 6.7 months (95% CI: 2.5–12.0; Figure 3). Overall, two patients were lost to follow up.
Figure 3.
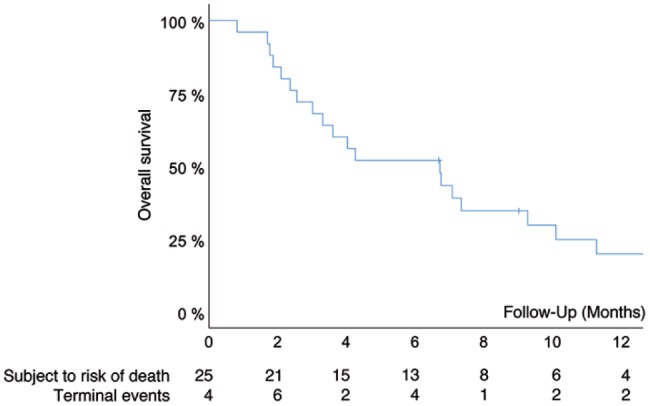
Overall survival of 25 patients with RGCPM treated with PIPAC C/D in the salvage situation. Median survival was 6.7 months (95% CI: 2.5–12.0).
x-axis, follow up (months since first PIPAC); y-axis, cumulative survival (Kaplan–Meier).
CI, confidence interval; PIPAC C/D, pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin; RGCPM, recurrent gastric cancer with peritoneal metastasis.
Functional and symptom scales (Table 3) of the EORTC-QLQ-C30 were analyzed for 14 patients with evaluation before and after the first PIPAC C/D treatment. No statistically significant changes were observed but deteriorations of more than 10 points were seen for pain and diarrhea while dyspnea symptoms were reduced.
Table 3.
Influence of PIPAC C/D on QoL assessed with EORTC-QLQ30 questionnaire. No statistically significant differences between baseline and after PIPAC therapy could be detected (n = 14, sign test for paired samples).
| EORTC-parameter | Baseline | After PIPAC#1 | p value |
|---|---|---|---|
| Physical functioning | 89.1 ± 9.65 | 87.6 ± 13.0 | 1.000 |
| Role functioning | 85.7 ± 14.4 | 84.5 ± 16.6 | 1.000 |
| Emotional functioning | 60.1 ± 36.4 | 61.9 ± 37.2 | 0.727 |
| Cognitive functioning | 73.8 ± 35.0 | 70.2 ± 33.4 | 1.000 |
| Social functioning | 54.8 ± 39.5 | 48.8 ± 39.5 | 0.549 |
| Global health | 54.8 ± 27.1 | 48.8 ± 22.8 | 0.227 |
| Fatigue | 44.4 ± 28.6 | 45.6 ± 28.7 | 1.000 |
| Nausea/vomiting | 7.1 ± 10.8 | 6.00 ± 10.6 | 1.000 |
| Pain | 25.0 ± 31.9 | 40.5 ± 33.8 | 0.388 |
| Dyspnea | 23.8 ± 27.5 | 11.9 ± 16.6 | 0.453 |
| Insomnia | 28.6 ± 34.2 | 38.1 ± 43.1 | 0.727 |
| Appetite loss | 28.6 ± 38.9 | 16.7 ± 25.3 | 0.625 |
| Constipation | 2.4 ± 8.9 | 11.9 ± 16.6 | 0.125 |
| Diarrhea | 9.5 ± 24.2 | 21.4 ± 31.0 | 0.063 |
| Financial difficulties | 11.9 ± 16.6 | 11.9 ± 28.1 | 1.000 |
EORTC, European Organization for Research and Treatment of Cancer; PIPAC C/D, pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin; QoL, quality of life.
Discussion
This study delivers the first controlled data on efficacy and safety of PIPAC C/D in recurrent gastric cancer with PM (RGCPM). PIPAC C/D might be an attractive therapeutic option in patients with RGCPM in the second-line situation and beyond since this study suggests that PIPAC C/D could be effective in the salvage situation even without additional palliative intravenous chemotherapy.
In the present prospective, open-label, ICH-GCP phase II trial, we investigated the efficacy of PIPAC C/D in RGCPM in the salvage situation. There is no study in the literature showing an objective tumor response (OTR) over 20% in RGCPM in the second-line situation. The hypothesis of this study was that PIPAC C/D could induce OTR in 40% patients, as assessed by RECIST on the basis of an abdominal CT scan. The results suggest that PIPAC C/D might be effective in treating RGCPM in the salvage situation with one complete radiological response, two objective responses and seven with SD (CBR 40%, 12/25, ITT). In a single patient with ascites and retraction of the mesentery, a complete radiological response was documented, and the patient was alive 31 months after the first PIPAC C/D.
However, the study was closed after stage 1 for organizational reasons. Therefore, it was not possible, to reach a statistically valid confirmation of a CBR of ⩾40%, which was aimed as the clinically relevant rate for further studies. Nevertheless, these results deliver the rationale for further clinical studies evaluating PIPAC C/D in RGCPM, in particular for controlled studies comparing PIPAC C/D versus systemic chemotherapy in the second-line situation and beyond, or for studies comparing systemic chemotherapy alone versus a combination of systemic chemotherapy with (low-dose) PIPAC C/D.
Moreover, this study had also several exploratory endpoints. First, a high rate of objective histological regression (36% ITT, 100% PP) was observed. This confirms previous data obtained in recurrent ovarian cancer: objective tumor regression on histology was observed in 26/34 (76%) patients in the PP analysis.29 Another phase II trial evaluating PIPAC C/D and PIPAC with oxaliplatin in various histologies also reported an OTR in 67% of 35 patients.30 The sensitivity of a CT scan is low (below 30%) for peritoneal lesions with a diameter <10 mm.31 To address this methodological challenge, this trial evaluated tumor response with laparoscopy (PCI) and histology. In this study, mean PCI remained stable under PIPAC C/D therapy. We acknowledge the clinical significance of PCI in PM patients, in particular for its prognostic value (survival) and predictive value (results of CRS and HIPEC). However, we found PCI to be difficult to compare between repeated PIPAC applications, since it is not possible to distinguish visually between vital PM and avital scarring. Moreover, the mesothelial-mesenchymal transition of the peritoneum and associated fibrosis are central features of carcinogenesis of PM, but also of wound healing.32 Therefore, we do not trust PCI as a response criteria for PIPAC therapy and prefer the objective histological assessment by an independent pathologist.33 In the future, this approach combining radiology, laparoscopy and histological regression grading might overcome current limitations of imaging in assessment of therapy response in PM.
Second, PIPAC C/D was well tolerated with no mortality and no CTCAE grade 4 adverse events. In four patients (16%), CTCAE grade 3 events were observed: two patients had to stay longer in the hospital or to be readmitted because of abdominal pain or because of subileus. This is in accordance with a recent systematic review on 1197 patients with PM of various primary tumors in 22 studies: adverse events CTCAE grades 1, 2, 3, 4, and 5 were observed in 537 (45%), 167 (14%), 83 (7%), 10 (0.8%), and 19 (1.6%) cases, respectively.34
Overall median mean survival was 6.7 months, which appears promising since most patients were heavily pretreated, had extensive PM and the vast majority of tumors showed signet-ring histology. Since there was no control group and no randomization, survival data cannot be compared with other available therapeutic options. Moreover, heterogeneity of the patients (second to fifth-line situation) makes interpretation of results more difficult. However, the preferred inclusion of patients in the late-stage, salvage situation including critically ill patients appeared ethically justified since this study was the first evaluating efficacy and tolerability of PIPAC C/D in gastric cancer.
The combination of PIPAC with systemic treatment would be the approach aiming to improve survival in these patients. Against this framework, it has to be noted that, to our knowledge and according to a recent systematic literature review,35 no randomized controlled trial has so far, evaluated the effect of systemic palliative chemotherapy in RGCPM beyond the second-line situation. In the second-line situation, survival figures between 4.0 and 7.7 months have been reported in four randomized controlled trials, irrespectively of the presence of PM.36–39 In contrast, there is strong evidence for targeted agents in this situation. In the RAINBOW trial, overall survival with ramucirumab plus paclitaxel was significantly longer than with placebo plus paclitaxel [median 9.6 months (95% CI 8.5–10.8) versus 7.4 months (95% CI 6.3–8.4)]. However, in this trial only 45% patients had documented PM.40 When the data from the RAINBOW and REGARD trial were pooled, the presence of PM (41.6% patients) was the most significant factor of worse prognosis, with independent significance [p < 0.001, hazard ratio (HR) 1.62].
Out of the four patients with no histological proof of PM at the first PIPAC, two received systemic chemotherapy later on, and two were then treated with CRS and HIPEC. Both patients recurred within a short time after CRS and HIPEC, giving an indirect confirmation of the presence of malignant peritoneal disease, and questioning the indication for CRS and HIPEC in this situation. This early recurrence might be explained by aggressive tumor biology, since both tumors showed signet-ring histology.41
In the present trial, PIPAC C/D was well tolerated and the patients had relatively long therapy-free intervals (6 weeks) between PIPAC cycles. No statistically significant changes in QoL were observed. These patient-related aspects appear important or even determinant in the palliative situation and confirm several reports published previously.33 The good tolerability of PIPAC C/D is also a novelty compared with other intraperitoneal chemotherapy regimens, which are limited by a high local toxicity.42 Thus, in future comparative trials, a further progression-free survival advantage offered by second or third-line systemic chemotherapy in addition to PIPAC C/D should be balanced against potential additional side effects and impairment of QoL.
However, whereas the results of this study are promising their interpretation must remain cautious. It can be objected that only six patients received the full treatment protocol: however, since expected median survival of patients with RGCPM in the second-line situation without systemic chemotherapy is 2.5 months29 and since 3 months are needed to apply three PIPAC cycles, it had to be expected that many patients pass away during the course of the study and are no longer eligible for further therapy.
On the basis of the results of this trial, of the PIPAC-GA2 trial18 and of a registry study,19 further evaluation of PIPAC C/D in gastric cancer with PM is warranted. A randomized multicentric phase II protocol in France comparing palliative systemic chemotherapy with palliative systemic chemotherapy combined with PIPAC C/D will soon include the first patient.43 The hypothesis of this study is that additional PIPAC C/D will offer prolonged survival and preserved QoL. Another prospective, open, randomized multicenter phase III clinical study in Germany will evaluate the effects of PIPAC C/D combined with systemic chemotherapy (mFOLFOX6) versus intravenous systemic chemotherapy alone on patients with metastatic upper gastrointestinal tumors with peritoneal seeding. The primary outcome is progression-free survival.44 Results of these studies are expected in 2022.
Conclusion
PIPAC C/D is well tolerated and active in patients with PM of gastric origin in the salvage situation. Survival is encouraging. Randomized controlled trials should now be designed for this indication.
Supplemental Material
Supplemental material, Suppl_material_1 for Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: a phase II study by Florian Struller, Philipp Horvath, Wiebke Solass, Frank-Jürgen Weinreich, Dirk Strumberg, Marios K. Kokkalis, Imma Fischer, Christoph Meisner, Alfred Königsrainer and Marc A. Reymond in Therapeutic Advances in Medical Oncology
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Marc André Reymond is a holder of patents related to PIPAC C/D technology and a shareholder of Capnomed GmbH, Zimmern, Germany. The other authors have no potential conflict of interests.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Florian Struller, Department of General and Transplant Surgery, Tübingen, University Hospital, Hoppe-Seyler-Strasse 3, 72076 Tübingen, Germany.
Philipp Horvath, Department of General and Transplant Surgery, University Hospital Tübingen, Germany.
Wiebke Solass, Department of Pathology, University Hospital Tübingen, Germany.
Frank-Jürgen Weinreich, Department of General and Transplant Surgery, University Hospital Tübingen, Germany.
Dirk Strumberg, Department of Medical Oncology, Marien Hospital, Ruhr University Bochum, Germany.
Marios K. Kokkalis, Department of General and Transplant Surgery, University Hospital Tübingen, Germany
Imma Fischer, Institute for Clinical Epidemiology and Applied Biometrics, University Hospital Tübingen, Germany.
Christoph Meisner, Institute for Clinical Epidemiology and Applied Biometrics, University Hospital Tübingen, Germany.
Alfred Königsrainer, Department of General and Transplant Surgery, University Hospital Tübingen, Germany.
Marc A. Reymond, Department of General and Transplant Surgery, University Hospital Tübingen, Germany National Center for Pleura and Peritoneum, University Hospital, Tübingen, Germany
References
- 1. Chan WL, Yuen KK, Siu SW, et al. Third-line systemic treatment versus best supportive care for advanced/metastatic gastric cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2017; 116: 68–81. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 3. Heger U, Blank S, Wiecha C, et al. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann Surg Oncol 2014; 21: 1739–1748. [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network. Guidelines version 5. https://www.nccn.org/patients/guidelines/content/PDF/stomach.pdf (2017, accessed 15 January 2018).
- 5. Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol 2006; 12: 3237–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gunderson LL. Gastric cancer—patterns of relapse after surgical resection. Semin Radiat Oncol 2002; 12: 150–161. [DOI] [PubMed] [Google Scholar]
- 7. Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe PM after failure of prior combination using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013; 31: 4438–4444. [DOI] [PubMed] [Google Scholar]
- 8. Kono K, Yong WP, Okayama H, et al. Intraperitoneal chemotherapy for gastric cancer with peritoneal disease: experience from Singapore and Japan. Gastric Cancer 2017; 20(Suppl. 1): 122–127. [DOI] [PubMed] [Google Scholar]
- 9. Boerner T, Graichen A, Jeiter T, et al. CRS-HIPEC prolongs survival but is not curative for patients with peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol 2016; 23: 3972–3977. [DOI] [PubMed] [Google Scholar]
- 10. Chia CS, You B, Decullier E, et al. ; BIG RENAPE Group. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol 2016; 23: 1971–1979. [DOI] [PubMed] [Google Scholar]
- 11. Gill RS, Al-Adra DP, Nagendran J, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol 2011; 104: 692–698. [DOI] [PubMed] [Google Scholar]
- 12. Wu Z, Li Z, Ji J. Morbidity and mortality of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in advanced gastric cancer. Transl Gastroenterol Hepatol 2016; 1: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reymond MA, Hu B, Garcia A, et al. Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg Endosc 2000; 14: 51–55. [DOI] [PubMed] [Google Scholar]
- 14. Solaß W, Hetzel A, Nadiradze G, et al. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 2012; 26: 1849–1855. [DOI] [PubMed] [Google Scholar]
- 15. Solass W, Herbette A, Schwarz T, et al. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg Endosc 2012; 26: 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eveno C, Haidara A, Ali I, et al. Experimental pharmacokinetics evaluation of chemotherapy delivery by PIPAC for colon cancer: first evidence for efficacy. Pleura Peritoneum 2017; 2: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nadiradze G, Giger-Pabst U, Zieren J, et al. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric PM. J Gastrointest Surg 2016; 20: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khomyakov V, Ryabov A, Ivanov A, et al. Bidirectional chemotherapy in gastric cancer with PM combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and Doxorubicin administered as a pressurized aerosol: an open-label, Phase-2 study (PIPAC-GA2). Pleura Peritoneum 2016; 1: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alyami M, Bonnot PE, Villeneuve L, et al. ; BIG-RENAPE Working Group. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for nonresectable peritoneal carcinomatosis from gastric cancer. J Clin Oncol 2018; 36(Suppl. 4S): abstract 149. [Google Scholar]
- 20. Grass F, Vuagniaux A, Teixeira-Farinha H, et al. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg 2017; 104: 669–678. [DOI] [PubMed] [Google Scholar]
- 21. Tempfer C, Giger-Pabst U, Hilal Z, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal carcinomatosis: systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Arch Gynecol Obstet 2018; 298: 243–257. DOI: 10.1007/s00404-018-4784-7. [DOI] [PubMed] [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 23. van der Kloot WA, Kobayashi K, Yamaoka K. Summarizing the fifteen scales of the EORTC QLQ-C30 questionnaire by five aggregate scales with two underlying dimensions: a literature review and an empirical study. J Psychosoc Oncol 2014; 32: 413–430. [DOI] [PubMed] [Google Scholar]
- 24. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0; published: May 28, 2009 (v4.03: 14 June 2010). National Institutes of Health and National Cancer Institute, 2010. [Google Scholar]
- 25. Solass W, Kerb R, Murdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014; 21: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996; 82: 359–374. [DOI] [PubMed] [Google Scholar]
- 27. Ametsbichler P, Böhlandt A, Nowak D, et al. Occupational exposure to cisplatin/oxaliplatin during Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)? Eur J Surg Oncol 2018; 44: 1793–1799. [DOI] [PubMed] [Google Scholar]
- 28. Chan WL, Yuen KK, Siu SW, et al. Third-line systemic treatment versus best supportive care for advanced/metastatic gastric cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2017; 116: 68–81. [DOI] [PubMed] [Google Scholar]
- 29. Tempfer CB, Winnekendonk G, Solass W, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: a phase 2 study. Gynecol Oncol 2015; 137: 223–228. [DOI] [PubMed] [Google Scholar]
- 30. Graversen M, Detlefsen S, Bjerregaard JK, et al. Prospective, single-center implementation and response evaluation of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for PM. Ther Adv Med Oncol 2018; 10: 1758835918777036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duhr CD, Kenn W, Kickuth R, et al. Optimizing of preoperative computed tomography for diagnosis in patients with peritoneal carcinomatosis. World J Surg Oncol 2011; 9: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson RB. Hypoxia, cytokines and stromal recruitment: parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura Peritoneum 2018; 3: 20180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Solass W, Sempoux C, Carr NJ, et al. Peritoneal sampling and histological assessment of therapeutic responsein PM: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum 2016; 1: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tempfer C, Giger-Pabst U, Hilal Z, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal carcinomatosis: systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Arch Gynecol Obstet 2018; 298: 243–257. [DOI] [PubMed] [Google Scholar]
- 35. Dahdaleh FS, Turaga KK. Evolving treatment strategies and outcomes in advanced gastric cancer with PM. Surg Oncol Clin N Am 2018; 27: 519–537. [DOI] [PubMed] [Google Scholar]
- 36. Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer–a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011; 47: 2306–2314. [DOI] [PubMed] [Google Scholar]
- 37. Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012; 30: 1513–1518. [DOI] [PubMed] [Google Scholar]
- 38. Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014; 15: 78–86. [DOI] [PubMed] [Google Scholar]
- 39. Nishina T, Boku N, Gotoh M, et al. Randomized phase II study of second-line chemotherapy with the best available 5-fluorouracil regimen versus weekly administration of paclitaxel in far advanced gastric cancer with severe peritoneal metastases refractory to 5-fluorouracil-containing regimens (JCOG0407). Gastric Cancer 2016; 19: 902–910. [DOI] [PubMed] [Google Scholar]
- 40. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014; 15: 1224–1235. [DOI] [PubMed] [Google Scholar]
- 41. Bonnot PE, Piessen G, Mercier F, et al. Is cytoreductive surgery and hyperthermic intraperitonealchemotherapy reasonable treatment for gastric signet-ring cell adenocarcinoma and linitis plastica with PM? CYTO-CHIP study—Ancillary results. J Clin Oncol 2018; 36: 4073–4073. [Google Scholar]
- 42. Hubner M, Grass F, Teixeira-Farinha H, et al. Pressurized IntraPeritoneal Aerosol Chemotherapy - Practical aspects. Eur J Surg Oncol 2017; 43: 1102–1109. [DOI] [PubMed] [Google Scholar]
- 43. Eveno C, Jouvin I, Pocard M. PIPAC EstoK 01: Pressurized Intraperitoneal Aerosol Chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric PM: a randomized and multicenter phase II study. Pleura Peritoneum 2018; 3: 20180116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goetze TO, Al-Batran SE, Pabst U, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in combination with standard of care chemotherapy in primarily untreated chemo naïve upper GI adenocarcinomas with peritoneal seeding - a phase II/III trial of the AIO / CAOGI / ACO. Pleura Peritoneum 2018; 3: 20180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Suppl_material_1 for Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: a phase II study by Florian Struller, Philipp Horvath, Wiebke Solass, Frank-Jürgen Weinreich, Dirk Strumberg, Marios K. Kokkalis, Imma Fischer, Christoph Meisner, Alfred Königsrainer and Marc A. Reymond in Therapeutic Advances in Medical Oncology