Abstract
In recent years, several drugs have been approved for the treatment of patients with metastatic cutaneous melanoma, completely reshaping the landscape of this aggressive disease. Immune therapy with cytotoxic T-lymphocyte antigen 4 and programmed cell death-1 inhibitors yielded significant and durable responses, achieving long-term disease control in up to 40% of the patients. BRAF inhibitors (BRAFi), in combination with MEK inhibitors, also resulted in improved overall survival compared with single-agent BRAFi in patients with BRAFV600-mutated metastatic melanoma. The optimized sequencing and duration of treatment, however, is yet to be found. In this article, we thoroughly review current data and discuss how to best sequence the various treatment modalities available at present, based on four distinct clinical presentations commonly seen in clinic. In addition, we review treatment options beyond checkpoint inhibitors and targeted therapy, which may be required by patients failing such effective treatments.
Keywords: anti-PD-1, BRAF inhibitor, combination immunotherapy, immunotherapy, melanoma, metastatic brain tumors, targeted therapy, treatment sequencing
Introduction
In recent years, we have witnessed great progress in the treatment of patients with metastatic melanoma. Most of the United States Food and Drug Administration (FDA) drug approvals have taken place in the past 7 years. Although metastatic melanoma still remains a frequently fatal disease, a large proportion of patients can now be anticipated to respond to therapy. Furthermore, a significant subset of patients can experience long-term remissions through the application of various treatment approaches.
The treatment modalities that are currently approved by the FDA, which henceforth will be used as the main regulatory agency for reference, or commonly used for the treatment of metastatic melanoma include: cytotoxic T lymphocyte antigen 4 (CTLA-4) inhibitor (ipilimumab); programmed cell death 1 (PD-1) inhibitors (nivolumab and pembrolizumab); BRAF inhibitors (BRAFi; vemurafenib, dabrafenib, and encorafenib); MEK inhibitors (MEKi; cobimetinib, trametinib and binimetinib); oncolytic virus [talimogene laherparepvec (T-VEC)]; high-dose interleukin-2 (HD IL-2); chemotherapy agents [nab-paclitaxel, dacarbazine (DTIC), temozolomide, fotemustine (not approved in the US; approved in Europe)]; combination chemotherapy with various regimens, such as carboplatin/paclitaxel and the CVD (cisplatin, vin-blastine, and DTIC) regimen; and biochemotherapy (CVD in combination with IL-2 and interferon-alfa).
The ideal treatment sequence for patients is not clearly established and remains a matter of great debate. In this review, we summarize the efficacy and toxicity results of the various treatment options and suggest algorithms that we typically use for the most common clinical scenarios of metastatic disease with current knowledge and available therapies.
Efficacy of the currently available treatment options
Chemotherapy
For many decades, chemotherapy was the only other treatment option apart from HD IL-2 for patients with metastatic melanoma. These agents typically do not yield significant tumor responses or improve survival outcomes.1–3 Most commonly used agents include dacarbazine and nab-paclitaxel. Other cytotoxic options with minor activity in patients with metastatic melanoma include fotemustine (not approved in the US), cisplatin and carboplatin, vinca alkaloids, and other nitrosoureas.4 Owing to its poor efficacy, chemotherapy use has greatly diminished over time, though some studies are evaluating synergistic effects with immunotherapy (ClinicalTrials.gov identifiers: NCT02617849 and NCT01721746).
Targeted therapy
BRAFi and MEKi
Two BRAFi, vemurafenib and dabrafenib, are currently available as single agents in patients whose tumors harbor a BRAF V600 mutation. Vemurafenib was approved by the FDA in 2011, whereas dabrafenib was approved in 2013. Both drugs showed significant activity and improved outcomes when compared with dacarbazine in randomized phase III trials.5,6 Trametinib and cobimetinib are MEK inhibitors approved for treatment of BRAF V600-mutant patients in combination with dabrafenib and vemurafenib, respectively. Trametinib is also approved as a single agent.7
Combination of BRAFi and MEKi
Four randomized phase III trials showed that the combination of BRAFi plus MEKi improved overall survival when compared with BRAFi alone. COMBI-d randomized 423 patients to either dabrafenib plus trametinib or to dabrafenib alone.8 The median progression-free survival (PFS) was 9.3 months in the dabrafenib–trametinib group and 8.8 months in the dabrafenib-only group [hazard ratio (HR): 0.75; p = 0.03]. The objective response rate (ORR) was 67% in the dabrafenib–trametinib group versus 51% for dabrafenib alone (p = 0.002). At 6 months, overall survival (OS) rates were 93% with dabrafenib–trametinib and 85% with dabrafenib alone (HR: 0.63; p = 0.02). Importantly, the rate of cutaneous toxicity was lower in the combination group than in the dabrafenib-only group (2% versus 9%), whereas pyrexia was more frequent (51% versus 28%) in the combination group. Similarly, the COMBI-v and COBRIM studies randomized patients to dabrafenib plus trametinib versus vemurafenib alone (COMBI-v) and vemurafenib plus cobimetinib versus vemurafenib alone (COBRIM), respectively.9,10 Vemurafenib/cobimetinib combo improved both PFS and OS compared with vemurafenib alone (HR: 0.58 for PFS and 0.70 for OS). Both trials confirmed the improved efficacy as well as reduced cutaneous toxicity (though liver enzyme elevation and pyrexia were higher) for the combinations, leading to approval of both BRAFi/MEKi combination therapies in the majority of countries worldwide. These combinations became the preferred BRAF-directed therapy in patients with BRAF-mutant metastatic melanoma over single-agent BRAFi, unless there is a contraindication to the combination. A third combination, consisting of encorafenib and binimetinib, also showed positive results over vemurafenib alone in the COLUMBUS phase III trial and received FDA approval.11 Interestingly, encorafenib was the first BRAFi that showed improved OS compared with another single-agent BRAFi, vemurafenib, posing the question of whether this combination may be more effective than the other two.12 Combined targeted therapy showed particularly good 5-year OS in patients with low clinical risk [fewer than that metastatic organ sites and normal baseline lactate dehydrogenase (LDH)], with 45–51% of patients alive.13
Immunotherapy
Anti-CTLA-4
Ipilimumab is a human monoclonal antibody that blocks the activity of CTLA-4, a downregulator of T-cell function, thus restoring T-cell activity for prolonged periods of time.14 It works primarily in the priming phase, in the lymph nodes, contributing to activation of T cells, though it also diminishes T-regulatory cells in the tumor microenvironment. Ipilimumab was approved by the FDA in 2011 for use in patients with advanced melanoma based on two randomized, phase III studies demonstrating survival superiority over chemotherapy alone and vaccine alone.15,16 A composite analysis of 12 clinical studies confirmed the potential long-term survival impact of ipilimumab.17 Most importantly, the survival curve reached a plateau of approximately 20%, which extended up to 10 years.17 Though currently not used alone as a first-line option, data consolidated a proof-of-concept of long-term survivorship achievable with immune-based therapies, already seen with interleukin-2 and cell therapies.
Anti-PD-1
Two anti-PD-1s agents are currently available for the treatment of patients with metastatic melanoma. Nivolumab is a human monoclonal IgG4 antibody that binds to PD-1 expressed on activated T cells, B cells, monocytes and natural killer cells, thereby inhibiting the interaction with its ligands, PD-L1 and PD-L2.18
Two large phase III trials confirmed nivolumab’s efficacy after accelerated approval based on an expansion cohort of a phase I trial.19 CheckMate-066 was a randomized trial that accrued 418 treatment-naïve, BRAF-wild-type patients with unresectable stage III or metastatic malignant melanoma.20 Patients were randomized in a 1:1 ratio to nivolumab at 3 mg/kg every 2 weeks or dacarbazine at 1000 mg/m2 every 3 weeks, with a primary endpoint of OS. Patients treated with nivolumab had a 58% reduced risk of death [HR: 0.42; 95% confidence interval (CI): 0.25–0.73; p < 0.001].
CheckMate-037 showed that patients previously treated with ipilimumab (and a BRAFi if patient had BRAF V600 mutation) may also benefit from nivolumab, compared with chemotherapy.21 The ORRs were 31.7% in the nivolumab arm and 10.6% in the chemotherapy arm. OS, however, was not statistically significantly longer, likely due to the 41% rate of crossover to anti-PD-1 after progression on chemotherapy, as well as the higher number of patients with central nervous system (CNS) metastases and high LDH in the nivolumab arm.2
Pembrolizumab is another fully human monoclonal IgG4 antibody that targets PD-1. Its accelerated approval was also based on an expansion cohort of a phase I trial.22 Its efficacy was confirmed through the pivotal phase III trial KEYNOTE-006, in which 834 patients were randomized to receive pembrolizumab at two different dosing schedules for up to 24 months, versus ipilimumab at 3 mg/kg for 4 cycles.23 Co-primary endpoints were OS and PFS. The study stopped accrual because of the early trend in improved OS for pembrolizumab, and OS analysis confirmed superiority of both pembrolizumab arms compared with ipilimumab (median OS: 32.7 months with pembrolizumab versus 15.9 months with ipilimumab; HR: 0.73; 95% CI: 0.61–0.89).24 The toxicity profile for pembrolizumab was similar to that seen with nivolumab, and superior compared with ipilimumab [grade 3–5 adverse events (AEs): 13.3% versus 19.9%].
Outcomes on anti-PD-1, similarly to targeted therapy, seem to be improved in treatment-naïve patients and in a low-risk population (normal baseline LDH, low tumor burden).25,26
Combination of anti-PD-1 and CTLA-4 blockade
To test safety and early efficacy of combined PD-1 and CTLA-4 blockade, a phase I dose escalation trial was launched and identified nivolumab at 1 mg/kg and ipilimumab at 3 mg/kg (Ipi/Nivo) as the dose to move forward for further testing.27 CheckMate-067 was a phase III trial that randomized patients to three arms: nivolumab or ipilimumab alone or the combination of both.28 The combination therapy demonstrated a significantly higher PFS (11.5 months; 95% CI: 8.9–16.7) compared with monotherapy with nivolumab (6.9 months; 95% CI: 4.3–9.5) or ipilimumab (2.9 months; 95% CI: 2.8–3.4). The HR for death or tumor progression was 0.42 (95% CI: 0.31–0.57) for Ipi/Nivo versus ipilimumab alone. The ORR was 57.6% in the cohort treated with the combination therapy, compared with 43.7% for nivolumab and 19% for ipilimumab.
Although the trial was not designed for comparison between the combination group versus nivolumab alone, the updated 4-year survival analysis showed a significant benefit of PFS favoring Ipi/Nivo (HR: 0.79; 95% CI: 0.65–0.97), but only numerically superior OS (4-year OS: 53% versus 46%; HR: 0.84; 95% CI: 0.67–1.05).29 In subgroup analysis, patients with BRAF mutations derived greater benefit from the combination versus nivolumab alone (4-year OS: 62% versus 50%; HR: 0.70 in BRAF-mutant patients; 49% versus 45%; HR: 0.92 for BRAF wild type). Tumor burden, LDH, and PD-L1 (cutoff of 5%) were not adequate discriminators. In addition, more patients were treatment-free at 4 years with Ipi/Nivo compared with nivolumab alone (71% versus 50%), which may warrant long-term cost effectiveness analysis of both regimens. Toxicity, as expected, was greater with combination: 59% of grade 3/4 treatment-related adverse events with Ipi/Nivo versus 22.4% with nivolumab alone. More patients also discontinued therapy due to AEs with the combination (30.4% versus 8%), but treatment-related deaths were low in both groups (0.6% and 0.3%, respectively).
Pembrolizumab in combination with ipilimumab was tested in a phase Ib trial, consisting of the standard 2 mg/kg dose of pembrolizumab and a reduced dose of ipilimumab (1 mg/kg), followed by pembrolizumab monotherapy.30 The responses were similar to the combination of ipilimumab and nivolumab [ORR: 61%; complete response (CR): 15%] and durable (median duration of response not reached). However, fewer grade 3 and 4 toxicities were observed (59% in ipilimumab/nivolumab versus 27% in reduced-dosed ipilimumab/pembrolizumab).30,31 Recently, Checkmate-511, a randomized phase III trial, tested two doses of Ipi/Nivo combination: standard dose of ipilimumab at 3 mg/kg and nivolumab at 1 mg/kg (Ipi3/Nivo1) and the inversed dose of nivolumab at 3 mg/kg and ipilimumab at 1 mg/kg (Ipi1/Nivo3).32 The primary endpoint was to assess the incidence of grade 3–5 AEs, and secondary endpoints included descriptive analysis only of response rate and survival outcomes. At 12 months, Ipi1/Nivo3 caused significantly less toxicity (grade 3–5 AEs: 33.9% versus 48.4% with standard dose; p = 0.0059). In the descriptive analysis, ORR (79.7% versus 81%), 12-month PFS (47.2% versus 46.4%) and 12-month OS (79.7% versus 81%) were similar. Only 2% of the patients, however, had brain metastasis in this trial.
T-VEC
T-VEC is a first-in-class oncolytic virus approved both by the FDA and the European Medicine Agency (EMA) for treatment of patients with metastatic or unresectable melanoma with injectable skin or nodal lesions. It is a modified herpes simplex virus (HSV) type 1, designed to selectively replicate in and lyse tumor cells while promoting regional and systemic antitumor immunity by recruiting and activating antigen-presenting cells with subsequent induction of tumor-specific T-cell responses.33 T-VEC is administered at 106 pfu/ml (to seroconvert HSV-seronegative patients), whereas subsequent T-VEC doses of 108 pfu/mL are administered 3 weeks after the first dose and then every 2 weeks.34 Injection into visceral lesions was not allowed. Its pivotal phase III OPTiM trial demonstrated an ORR rate of 26% in T-VEC treated patients [versus 5.7% in the control arm with (G-CSF)], with 10% of the total having complete remissions.34 Patients with skin and nodal disease only were found to have improved responses compared with patients with metastatic disease (stage IIIB/IIIC patients: ORR of 52%, as opposed to 26.7%, 6.3%, and 11.9% in M1a, M1b, and M1c disease, respectively). The majority of responses were durable, and median time to response was 4.2 months. T-VEC’s systemic immune response was demonstrated by shrinkage of uninjected visceral lesions by more than 50% in 15% of evaluable patients, as well as causing systemic immune-related AEs, such as vitiligo.34,35 Of note, pseudo-progression is a phenomenon frequently observed with this agent, occurring in more than 50% of cases.34,36
Toxicity leading to discontinuation of treatment occurred in only 4% of cases. More recently, T-VEC has been explored in combination both with ipilimumab (ORR of 39% versus 18% with ipilimumab alone) and with pembrolizumab (ORR: 62%; CR: 33%), demonstrating promising activity and acceptable safety profile.37,38 Although its efficacy combined with a favorable toxicity profile make it an attractive option for patients with loco-regional disease, its availability is currently restricted to use in Europe and the US.
HD IL-2
HD IL-2 was approved by the FDA in 1998 on the basis of the observation that it could produce durable complete remissions in approximately 5% of the patients.39 Although the ORR is only around 15%, the National Cancer Institute experience suggests that patients with disease primarily restricted to skin or lymph nodes have a much higher response rate, approaching 50%.40 HD IL-2 has modest effect on brain metastases, and, though it has been safely used, it is usually not pursued by most clinicians in this setting.41,42 HD IL-2 has a significant acute toxicity profile and can be administered only in centers with expertise with this treatment modality, and its use has greatly decreased after checkpoint inhibitors became available. However, it has shown antitumor activity in patients that failed checkpoint inhibitors (ORR of 21% following exposure to ipilimumab).43
Biochemotherapy
The combination of platinum-based chemotherapy (usually CVD) with IL-2 and interferon-alfa, was coined in the early 1990s as ‘biochemotherapy’. The only positive randomized trial, which compared biochemotherapy with chemotherapy (CVD alone), was conducted at MD Anderson Cancer Center, an institution with much experience with the use of IL-2-based regimens, with improved ORR (48%) and PFS (median of 4.9 months) over chemotherapy, without an OS improvement.44 Owing to its high toxicity, and need for continuous inpatient monitoring, biochemotherapy is now only rarely used in the treatment of patients with metastatic melanoma.
Cellular therapy
In vitro expansion of tumor infiltrating lymphocytes (TIL) followed by lymphodepletion with chemotherapy and TIL infusion has been used for over 30 years. Owing to its costs, manufacturing time, and individualized approach, its routine use has not yet been achieved. In the past decade, the expansion period of TILs has been reduced to 5–7 weeks, driving down costs. In addition, it became permissible to discuss this single-infusion treatment in the setting of increasingly more expensive treatment options. In anti-PD-1-naïve patients, ORR ranges from 40% to 60%, with long-term overall survival of 20–30% with TIL therapy.45–47 More recently, cohorts of patients refractory to anti-PD-1 still achieved a 30% response rate, with longer follow-up needed.48,49 Refinement of subsequent IL-2 administration and further reduction in costs and expansion time are still needed for this modality to be widely implemented, but it is already an important option to be considered, if available. Currently, only a few countries, such as Israel and the Netherlands, offer this treatment outside of clinical trials. T-cell receptor (TCR)-transduced T cells represent another cell therapy modality, with specific melanoma targets such as NY-ESO1 (also present in synovial sarcoma and liposarcoma) and MART-1.50,51 Such modality offers an advantage of targeting a known antigen, with reduced expansion time (around 2 weeks) and no requirement for tumor tissue. However, it is human leukocyte antigen (HLA) and antigen-restricted and responses are less durable, likely due to antigen loss and clone selection.
Systemic treatment of CNS metastases
The majority of the initial trials involving immunotherapy excluded patients with active melanoma brain metastases, despite melanoma bearing the highest propensity to invade the CNS among all tumors.52,53 Around 40–50% of patients develop clinically detected CNS metastases and up to 75% are found to have brain disease in autopsy series.54,55 The two main concerns regarding treating patients with CNS metastases with immunotherapy are the frequent requirement of peri-lesional edema control with systemic corticosteroids, and the poor brain penetration of large molecules, such as monoclonal antibodies, though the crossing of activated T cells could be enough to yield an anti-tumor effect.56,57 Recent efforts, however, have addressed specifically this subset of patients.
Ipilimumab
A phase II trial evaluated the activity of ipilimumab in patients with untreated brain metastases and showed an intracranial response rate of 16 % (n = 51) in patients not on corticosteroids.58 Only one CNS response was reported in a cohort of patients requiring corticosteroids at time of treatment initiation (N = 21; 5%). Patients on corticosteroids (exceeding prednisone 10 mg/day or equivalent) are generally thought to derive only minimal clinical benefit from ipilimumab therapy, although occasional responses have been noted. It remains unclear whether these patients do poorly because of immunosuppression or simply because of the worse prognosis associated with symptomatic CNS metastases.59
Anti-PD-1
Pembrolizumab was the first anti-PD-1 tested in a phase II trial specifically evaluating patients with brain metastases, both from melanoma and from lung cancer.60 Among the 18 patients with melanoma, the intracranial ORR was 22%. Of note, 4 of the 18 patients could not be evaluated for response but were included in the intention-to-treat analysis. Responses were concordant between the CNS and extracranial compartments.
Combination of ipilimumab and nivolumab
More recently, the combination of nivolumab and ipilimumab was tested in two separate phase II studies specifically addressing the activity in melanoma brain metastases. CheckMate-204 included previously untreated patients with at least one active brain metastasis measuring between 0.5 and 3.0 cm.61 Patients with neurologic symptoms or requiring corticosteroid were excluded. The intracranial ORR was 56% and the CR rate was 21%. Again, extracranial responses were concordant with those seen intracranially. The 6-month PFS was 67%, and median PFS has not been reached (95% CI: 7.5 months - NR). The ABC phase II study evaluated the combination of nivolumab plus ipilimumab versus nivolumab alone (N = 67) in a similar population (lesions of up to 4.0 cm).62 Patients with asymptomatic CNS disease showed an intracranial ORR of 44% for the combination versus 20% for nivolumab alone. The outcomes may have been slightly worse in the ABC trial owing to a higher number of patients with elevated LDH and higher disease burden (patients on average had a higher number of brain metastases). Taken together, these studies demonstrate that intracranial responses from immunotherapy can be profound and durable and that the combination strategy may lead to improved outcomes.
BRAFi and BRAFi/MEKi
BRAFi alone have also shown activity in patients with CNS metastases. In the multicenter phase II BREAK-MB study, 172 patients with asymptomatic brain metastases harboring a BRAF V600E or V600K mutation were treated with dabrafenib.63 In the 74 patients whose tumor contained a BRAF V600E mutation and whose brain metastases were treatment-naïve, objective responses were observed in 29 of 74 patients (39%). The response rate in those who had received prior local treatment was 31% (20 of 65). Objective responses in the CNS were observed in 5 of 33 patients (15%) with tumors containing a V600K mutation. The activity of vemurafenib in the CNS was also evaluated in a smaller phase II study.64 A total of 24 patients were treated, with 10 achieving a partial response (42% ORR), both intracranially and extracranially. Median PFS and OS, however, were short (3.9 and 5.2 months, respectively).
The combination of dabrafenib and trametinib was evaluated in the COMBI-MB study. In this phase II study, a total of 125 patients were included and the ORR was 55%, similar to extracranial responses. The median PFS, however, was around half of what was expected from the combination therapy patients with systemic disease only (5.6 months; 95% CI: 5.3–7.4).65
Chemotherapy
The activity of systemic chemotherapy in patients with brain metastases is very limited. A phase II study evaluated the efficacy of temozolomide in treatment-naïve patients with brain metastases showed a response rate of only 7%.66 The response rate with fotemustine was reported to be in the range of 10–20% in this patient population; however, responses to either agent were generally short-lived.67
Palliative care
Supportive care for metastatic melanoma patients should be started as early as possible, as it may impact on both quality of life and longer survival, according to studies conducted in different tumor types.68 Patients refractory to standard lines of therapy, who still represent up to half of the patients, often progress at a fast pace, with few effective options outside of clinical trials. Such patients may require specialized multidisciplinary attention to ensure that their symptoms are appropriately addressed, and advanced directives of care discussed.
Definition of clinical risk
In this section, we go through clinical scenarios in which a melanoma patient may present itself. For the purpose of discussion, we divided patients into high and low clinical risk disease, as follows.
Low clinical risk: low tumor burden (total volume <10 cm); number of metastatic sites (<3 organs); normal LDH; good performance status (0 or 1).
High clinical risk: high tumor burden (total volume of ⩾10 cm); number of metastatic sites (⩾3); LDH ⩾2× upper limit of normality; poor performance status (>1).
Clinical scenarios
In medicine, it is not possible to design precise algorithms. In this review, our goal is to focus on the most common clinical settings, while acknowledging that our discussion will not cover many gray areas. If available and feasible, enrollment in clinical trials should always be encouraged. We hereby review four distinct metastatic case presentations:
patient with low-risk disease and no CNS involvement;
patient with low-risk disease and CNS involvement;
patient with high-risk disease and no CNS involvement;
patient with high-risk disease and CNS involvement.
There are to date very limited data or recommendations on sequencing of therapies in patients with metastatic melanoma, particularly in patients with BRAF-mutated melanomas.69 One retrospective experience addressing this point reported on 93 patients with BRAF mutation-positive advanced melanoma who received vemurafenib or dabrafenib before (n = 45) or after (n = 48) treatment with ipilimumab 3 mg/kg.70 The median OS from first treatment was 9.9 and 14.5 months, respectively. Among patients treated with a BRAFi first, median survival from the end of BRAF inhibitor therapy was 1.2 months for those who did not complete ipilimumab treatment as per protocol, compared with 12.7 months for those who did (p < 0.001). This issue was further complicated (or facilitated) with the advent of anti-PD-1 therapy. CheckMate-064 is a phase II trial that addressed the matter by randomizing patients with metastatic melanoma to receive either induction with nivolumab for six doses followed by a planned switch of four doses of ipilimumab, or the reverse sequence.71 Both groups received nivolumab maintenance afterwards until progression or intolerable toxicity. Median OS was not reached in the nivolumab followed by ipilimumab arm (95% CI: 23.7–NR) versus 16.9 months [95% CI: 9.2–26.5 months; HR: 0.48 (95% CI: 0.29–0.80)] in the ipilimumab followed by nivolumab arm. One-year OS was 76% versus 54%, favoring nivolumab to be given first. The only study available regarding optimal sequence of BRAFi/MEKi and anti-PD-1 showed no clear winner, though there was a clear OS benefit for patients who did well in the first-line setting regardless of the first regimen received.72 In the following clinical discussions, the treatment approaches when there is no consensus or guidelines are based on authors’ opinions and clinical experience.
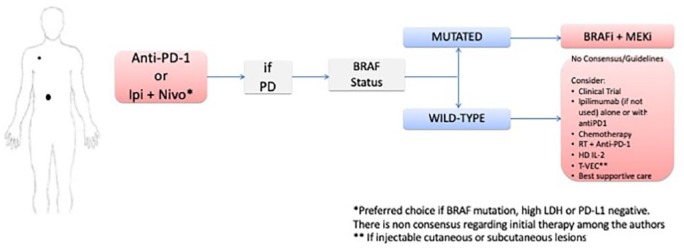
Clinical scenario 1: patients with low-risk disease and no evidence of CNS involvement
For patients with low-risk disease and no evidence of CNS involvement (Figure 1), both anti-PD-1 therapy alone and the combination of Ipi/Nivo as first-line options are adequate. Despite significant progress in this matter, there is no consensus on what the preferred approach for previously untreated patients is. The combination of immunotherapies was shown to be particularly beneficial over anti-PD-1 alone in patients harboring a BRAF mutation, with high LDH, and no PD-L1 expression.2 In patients without any of these characteristics, such as in this clinical scenario, anti-PD-1 monotherapy may be a solid treatment option, with less toxicity. This was mitigated by inverting Ipi/Nivo dosing schedules, with no apparent loss in efficacy, though CheckMate-511 was not powered for outcome analysis and not adopted in routine practice yet in most centers.32 Although the use of BRAFi/MEKi is also an adequate option in the first-line setting, as it improves OS and is also associated with long-term survival (5-year OS of 45% in patients with normal baseline LDH and 51% in patients with low tumor burden),13 the later use of immunotherapy may not be feasible in cases where resistance is associated with rapid disease progression. Though Ipi/Nivo combination may work fast, rescue with BRAFi/MEKi can happen in a matter of hours/days in a very symptomatic patient, as opposed to a few weeks with immunotherapy. Furthermore, this would preclude the patient from receiving a drug that may result in long-term, treatment-free survival. On the other hand, administering targeted therapy to a patient after exposure to anti-PD-1 may cause more toxicity and treatment interruptions.73 We favor immunotherapy with single-agent anti-PD-1 over targeted therapy in BRAF-mutated patients, justified by the potential for anti-PD-1 to produce long-term, off-therapy survival in approximately 30–40% of patients, as well as its favorable toxicity profile with single-agent anti-PD-1 relative to the BRAFi/MEKi combination and Ipi/Nivo. It is important to emphasize that there are no head-to-head prospective data comparing immunotherapy with BRAK/MEKi in patients who are treatment naive. A matching-adjusted indirect comparison of nivolumab/ipilimumab and BRAFi/MEKi showed a significant OS benefit of immunotherapy over targeted therapy after adjusting for baseline characteristics, though with its inherent retrospective biases.74 Two phase III studies (ClinicalTrials.gov identifiers: NCT02224781 and NCT02631447) evaluating the best treatment sequence (targeted therapy first with combination immunotherapy at disease progression versus the reverse sequence) are ongoing to address the sequencing of treatment question. Concomitant administration of radiation therapy to a metastatic site, either for palliative intent, treatment of oligometastatic or oligoprogressive disease, or for an abscopal effect with checkpoint blockade is an option that may improve outcomes based on retrospective cohorts.75–78 Prospective trials are ongoing to determine its role (ClinicalTrials.gov identifiers: NCT02407171, NCT03646617, and NCT03354962]. The most commonly used dose is 24 Gy, divided into three fractions.
Figure 1.
Clinical scenario 1: algorithm for the management of patients with low-risk disease and no CNS involvement.
BRAFi, BRAF inhibitor; CNS, central nervous system; HD IL-2, high-dose interleukin-2; Ipi + Nivo, ipilimumab + nivolumab; MEKi, MEK inhibitor; PD, progression of disease; RT, radiotherapy; SRS stereotactic radiosurgery.
It is important to state that when single-agent anti-PD-1 is preferred, both pembrolizumab and nivolumab are available options, with similar activity. As for BRAFi/MEKi, all three combinations, when available, are appropriate and with improved OS over vemurafenib.9,10,12 Encorafenib was the only BRAF inhibitor that was superior to vemurafenib alone, though since the combinations have not been compared head-to-head, it cannot be argued that one combo is superior to another.
In patients who fail to respond to anti-PD-1 therapy, the subsequent treatment decision should be based on the BRAF status of the tumor. If mutated, the patient should receive BRAFi/MEKi combination. If the tumor is not mutated, the patient could be considered for ipilimumab, if not previously used in combination with anti-PD-1. Ipilimumab can be considered for patients with BRAF-mutated tumors as well, as long as the disease remains mostly asymptomatic. Retrospective data demonstrate clinical activity with ipilimumab after progression on anti-PD-1, with an ORR of up to 22%.79–81 Ipilimumab was also tested in combination with pembrolizumab after failure of anti-PD-1 alone, showing an impressive ORR of 45% in 22 patients treated in a phase II trial.82 Alternative approaches after progression on ipilimumab include HD IL-2, biochemotherapy, or systemic chemotherapy with single-agent or combination chemotherapy, depending on the patient’s age and comorbidities. T-VEC is an option, where available, in patient with injectable cutaneous, subcutaneous or lymph node metastases. TIL therapy is an option worthy of consideration, but restricted to highly specialized centers. If feasible, TIL harvesting should occur prior to further treatments to improve its expansion. Clinical trials, whenever available, should be strongly considered. If performance status is rapidly declining, palliative care should be offered. The recommendations after failure of standard therapies are similar in the upcoming scenarios.
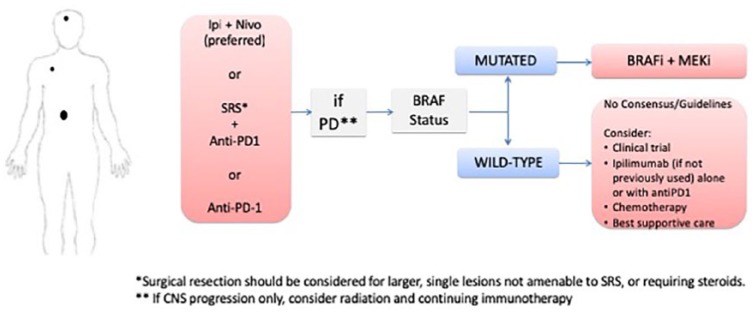
Clinical scenario 2: patients with low-risk disease and CNS involvement
In this setting, we favor treating patients with Ipi/Nivo as our first choice (Figure 2). This approach has yielded the best results for CNS disease, with an apparent long-term benefit.61,62 In addition, it may reduce the appearance of new brain metastases. Starting with an anti-PD-1 as monotherapy, with or without stereotactic radiosurgery (SRS), is also an option, with close monitoring in the CNS with magnetic resonance imaging of the brain every 6 weeks. In case of an increase in the CNS lesions during immunotherapy, but with systemic control, decreased LDH, and improved symptoms, we recommend waiting for the 12-week scan for further CNS management, as these lesions often present with early pseudo-progression.83 If the systemic disease is controlled, but CNS symptoms worsened, we recommend treating with SRS, if not done previously, and continue with systemic therapy. Regardless of treatment choice, it is vital that the radiation oncology team is included in the decision and be on standby in the case of loss of disease control. Concomitant treatment with immunotherapy and SRS is also an option. In retrospective series, concurrent administration of treatment was shown to improve outcomes, at the cost of an increased rate of radionecrosis (up to 27%), warranting caution when adopting this approach.84–88 The safety of the combination of radiation therapy with both immunotherapy and targeted therapy, however, has not been prospectively established, though clinical trials are ongoing (ClinicalTrials.gov identifiers: NCT02858869 and NCT03340129). For patients with a larger, symptomatic lesion, surgery should also be considered.
Figure 2.
Clinical scenario 2: algorithm for the management of patients with low-risk disease and brain metastases.
BRAFi, BRAF inhibitor; Ipi + Nivo, ipilimumab + nivolumab; MEKi, MEK inhibitor; PD, progression of disease; SRS, stereotactic radiosurgery.
If the patient develops systemic progressive disease to immunotherapy, either as sole therapy or in combination with radiotherapy, the subsequent treatment will depend on the BRAF status. If the tumor is BRAF-mutated, the patient should receive BRAFi plus MEKi. If the tumor is BRAF wild type, ipilimumab alone or in combination with anti-PD1 is favored, if the patient has not been exposed to ipilimumabe in the first line. Alternative treatments are systemic chemotherapy with temozolomide, fotemustine, CVD, nab-paclitaxel, or carboplatin/paclitaxel. In contrast, if systemic control is achieved with immunotherapy, but the CNS disease is progressing, treatment can be continued, and local control should be attempted with radiation, preferably SRS. It is known that SRS leads to similar intracranial control in up to three metastatic lesions compared with whole brain radiation, with less cognitive decline, as well as comparable intracranial control in up to 10 lesions versus 2–4 lesions.89,90
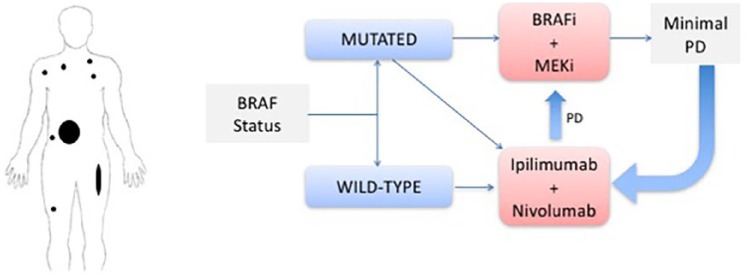
Clinical scenario 3: patients with high-risk disease and no CNS involvement
In patients with high-risk disease, particularly those in visceral crisis, and no CNS involvement (Figure 3), the BRAF status of the tumor is of critical importance, given the high response rates observed in patients treated with BRAFi/MEKi. Furthermore, the responses with tumor-targeted therapy are typically very rapid, resulting in prompt improvement of the patient’s clinical condition.
Figure 3.
Clinical scenario 3: algorithm for the management of patients with high-risk disease and no CNS involvement.
BRAFi, BRAF inhibitor; CNS, central nervous system; Ipi, ipilimumab; MEKi, MEK inhibitor; PR, partial response.
Another option (if the patient is not in visceral crisis) is the combination of ipilimumab with nivolumab, which also results in high response rates (55–60%) with a median time to response of 2.76 months, though this may be overestimated based on the first scan dates.28 In addition, in some countries it may take a few days to weeks to receive the BRAF test results, so it is justifiable to start these patients on immunotherapy combination.
Patients treated with the combination of BRAFi/MEKi should be followed very closely with imaging studies in order to detect very early progression of disease. In this setting of a lower tumor burden, they should then be considered for immunotherapy in an attempt to achieve long-term survival. Again, we emphasize the need of prospective data comparing the treatment sequence before drawing any formal conclusions on which therapy is the best first-line choice.
In patients with high tumor burden and wild-type tumor, Ipi/Nivo is the treatment of choice.
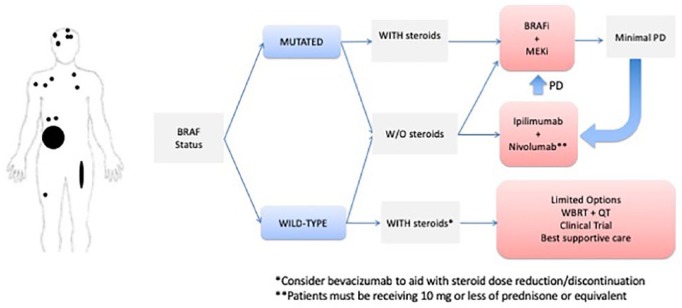
Clinical scenario 4: patients with high-risk disease and CNS involvement
In patients with high-risk disease and CNS metastases (Figure 4), the BRAF status of the tumor is also of paramount importance, as the use of the combination of BRAFi/MEKi results in high and rapid response rates both for systemic and CNS disease.65 Targeted therapy is unquestionably the best option if the patient requires high-dose steroids owing to increased cerebral peri-lesional edema. If the patient does not require steroids, both targeted therapy and Ipi/Nivo represent options. In this setting, we favor immunotherapy, as long-term disease control appears more likely than with targeted therapy.
Figure 4.
Clinical scenario 4: algorithm for the management of patients with high-risk disease and CNS involvement.
BRAFi, BRAF inhibitor; CNS, central nervous system; Ipi, ipilimumab; MEKi, MEK inhibitor; PR, partial response; QT, chemotherapy; WBRT, whole-brain radiotherapy.
For patients with BRAF wild-type tumors who are not on steroids, Ipi/Nivo is our preferred option. However, in this setting, if the patient requires steroids for symptomatic disease, the treatment options are limited and not very effective. Attempts to reduce peri-lesional edema with radiation and weaning off steroids should be taken. Whole-brain irradiation has an ORR of less than 20% (in more recent studies the response rate is <5%).91,92 Our experience suggests that a potentially useful strategy is administering bevacizumab for radiation-induced edema or necrosis in patients refractory to steroid use, not only to reduce its dose and enable immunotherapy, but because of its potential synergy with immunotherapy.93,94 The combination of anti-PD-L1 atezolizumab with bevacizumab is being explored in a phase II trial for patients with brain metastases for both asymptomatic patients and a small cohort of patients with minor symptoms or low-dose steroids (ClinicalTrials.gov identifier: NCT03175432).
As stated in the previous clinical scenario, patients responding to first-line targeted therapy should be followed very closely, as they should start immunotherapy at the earliest sign of disease progression, particularly if steroids are no longer required.
Duration of treatment
The optimal duration of therapy is yet to be found. An arbitrary duration of 2 years has been utilized by clinical trials, though this may be more than necessary for some patients, and not enough time for others. Recent findings of Keynote-006 showed that among 103 patients that completed the prespecified 2 years of pembrolizumab 85% of the patients remained progression-free at median follow up of 20 months after discontinuation.24 This number seems to be higher among patients reaching a complete response compared with patients with less than a CR. Importantly, only one patient of the eight who were eligible for rechallenge with pembrolizumab upon progression developed subsequent progressive disease so far. Among the eight patients who were rechallenged with anti-PD1 therapy, four had a new response and three had stable disease. Several other independent cohorts showed similar results both for anti-PD-1 alone and the combination with ipilimumab.95–97 Interestingly, another study found that the progression-free survival among patients with a partial response but a complete metabolic response by positron emission tomography computed tomography (PET-CT) was also extraordinarily high, compared with those without a complete metabolic response.98 Therefore, our recommendation is to treat patients until they reach a CR and discontinuing therapy after one subsequent confirmatory scan. If a PR is achieved, and the disease subsequently stabilizes, a PET-CT may be a useful aid to the decision to discontinue treatment sooner than 2 years.
For targeted therapy, it is unknown whether treatment discontinuation is safe after a CR is achieved, as the effect may be largely dependent on continuous kinase inhibition. A few retrospective case series reported on outcomes after elective discontinuation of therapy. All 11 patients in one cohort relapsed after discontinuation of single-agent BRAFi after a CR was achieved.99 In another series, half of 12 patients that discontinued BRAFi/MEKi combination after achieving a CR relapsed.100 We therefore do not recommend elective discontinuation of therapy if the patient has adequate tolerance to treatment.
Conclusion
Currently, several treatment options may be administered to metastatic melanoma, with long-term survival achieved in up to half of patients. Still, a substantial number of patients still develop resistance to immunotherapy and targeted therapy, requiring additional treatments. It is vital to understand the best sequencing in the different clinical presentations, ensuring that the maximal benefit will be extracted from the available choices.
Footnotes
Authors contributions: All authors made significant contributions to the conception, design, writing and review of the paper. All authors authorized the submission.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Antonio C. Buzaid has served on an advisory board for MSD, BMS, Roche, AstraZeneca, Novartis, Pfizer, Eisai, and Blau. Michael B. Atkins has served on an advisory board for BMS, MSD, Roche, Novartis, Pfizer, Array, Esai, and Exelixis. Gustavo Schvartsman has performed a consulting role for BMS, United Medical, and MSD. Isabella C. Glitza, Sanjiv S. Agarwala, and Patricia Taranto have no conflicts of interest to disclose.
Contributor Information
Gustavo Schvartsman, Centro de Oncologia e Hematologia – Hospital Israelita Albert Einstein, 627 Albert Einstein Avenue, São Paulo, SP 05653-120, Brazil.
Patricia Taranto, Department of Medical Oncology, Hospital Israelita Albert Einstein, São Paulo, SP, Brazil.
Isabella C. Glitza, Department of Melanoma Medical Oncology, The University of Texas, MD Anderson Cancer Center, Houston, TX, USA
Sanjiv S. Agarwala, Department of Hematology and Oncology, and Temple University, Easton, PA, USA
Michael B. Atkins, Department of Oncology, Georgetown University School of Medicine, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA
Antonio C. Buzaid, Department of Medical Oncology, Hospital Israelita Albert Einstein, São Paulo, SP, Brazila and Department of Medical Oncology, A Beneficência Portuguesa de São Paulo - BP, São Paulo, SP, Brazil
References
- 1. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in checkmate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2017: Jco2016718023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Middleton MR, Grob J, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000; 18: 158–158. [DOI] [PubMed] [Google Scholar]
- 4. Kottschade LA, Suman VJ, Amatruda T, 3rd, et al. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group Study, N057E(1). Cancer 2011; 117: 1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF V600E and BRAF V600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014; 15: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hauschild A, Grob J-J, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380: 358–365. [DOI] [PubMed] [Google Scholar]
- 7. Flaherty KT, Robert C, Hersey P, et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N Engl J Med 2012; 367: 107–114. [DOI] [PubMed] [Google Scholar]
- 8. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014; 371: 1877–1888. [DOI] [PubMed] [Google Scholar]
- 9. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372: 30–39. [DOI] [PubMed] [Google Scholar]
- 10. Ascierto PA, McArthur GA, Dreno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016; 17: 1248–1260. [DOI] [PubMed] [Google Scholar]
- 11. Dummer R, Ascierto P, Gogas H, et al. 1215OResults of COLUMBUS Part 2: a phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) versus ENCO in BRAF-mutant melanoma. Ann Oncol 2017; 28. [Google Scholar]
- 12. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018; 19: 1315–1327. [DOI] [PubMed] [Google Scholar]
- 13. Long GV, Eroglu Z, Infante J, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol 2018; 36: 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melero I, Hervas-Stubbs S, Glennie M, et al. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 2007; 7: 95. [DOI] [PubMed] [Google Scholar]
- 15. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 2010: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- 17. Schadendorf D, Hodi F, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in metastatic or locally advanced, unresectable melanoma. J Clin Oncol 2015; 33: 1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014; 2: 846–856. [DOI] [PubMed] [Google Scholar]
- 19. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32: 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 21. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–384. [DOI] [PubMed] [Google Scholar]
- 22. Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 23. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 24. Long GV, Schachter J, Ribas A, et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. J Clin Oncol 2018; 36: 9503–9503. [Google Scholar]
- 25. Nakamura Y, Kitano S, Takahashi A, et al. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget 2016; 7: 77404–77415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019. DOI: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hodi F, Chiarion-Sileni V, Gonzalez R, et al. LBA44 Overall survival at 4 years of follow-up in a phase III trial of nivolumab plus ipilimumab combination therapy in advanced melanoma (CheckMate 067). Ann Oncol 2018; 29: mdy424. 054. [Google Scholar]
- 30. Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol 2017; 18: 1202–1210. [DOI] [PubMed] [Google Scholar]
- 31. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377: 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lebbé C, Meyer N, Mortier L, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV checkMate 511 trial. 2019. J Clin Oncol 2019; 37: 867–875. doi: 10.1200/JCO.18.01998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006; 12: 6737–6747. [DOI] [PubMed] [Google Scholar]
- 34. Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015; 33: 2780–2788. [DOI] [PubMed] [Google Scholar]
- 35. Byrne KT, Turk MJ. New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget 2011; 2: 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang CJ, Tai KF, Roffler S, et al. The immunization site of cytokine-secreting tumor cell vaccines influences the trafficking of tumor-specific T lymphocytes and antitumor efficacy against regional tumors. J Immunol 2004; 173: 6025–6032. [DOI] [PubMed] [Google Scholar]
- 37. Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. Epub ahead of print 5 October 2017. DOI: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017; 170: 1109–1119.e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999; 17: 2105–2105. [DOI] [PubMed] [Google Scholar]
- 40. Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol 2001; 19: 3477–3482. [DOI] [PubMed] [Google Scholar]
- 41. Guirguis LM, Yang JC, White DE, et al. Safety and efficacy of high-dose interleukin-2 therapy in patients with brain metastases. J Immunother 2002; 25: 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Powell S, Dudek AZ. Single-institution outcome of high-dose interleukin-2 (HD IL-2) therapy for metastatic melanoma and analysis of favorable response in brain metastases. Anticancer Res 2009; 29: 4189–4193. [PubMed] [Google Scholar]
- 43. Buchbinder EI, Gunturi A, Perritt J, et al. A retrospective analysis of high-dose interleukin-2 (HD IL-2) following Ipilimumab in metastatic melanoma. J Immunother Cancer 2016; 4: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eton O, Legha SS, Bedikian AY, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol 2002; 20: 2045–2052. [DOI] [PubMed] [Google Scholar]
- 45. Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17: 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Besser MJ, Shapira-Frommer R, Itzhaki O, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res 2013; 19: 4792–4800. [DOI] [PubMed] [Google Scholar]
- 47. Goff SL, Dudley ME, Citrin DE, et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol 2016; 34: 2389–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sarnaik A, Curti BD, Davar D, et al. A phase 2, multicenter study to assess the efficacy and safety of autologous tumor-infiltrating lymphocytes (LN-144) for the treatment of patients with metastatic melanoma. J Clin Oncol 2018; 36: 9595–9595. doi: 10.1200/JCO.2018.36.15_suppl.9503 [Google Scholar]
- 49. Forget MA, Haymaker C, Hess KR, et al. Prospective analysis of adoptive TIL therapy in patients with metastatic melanoma: response, impact of anti-CTLA4, and biomarkers to predict clinical outcome. Clin Cancer Res 2018; 24: 4416–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chodon T, Comin-Anduix B, Chmielowski B, et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res 2014; 20: 2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Robbins PF, Kassim SH, Tran TL, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 2015; 21: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nayak L, Lee EQ, Wen PY. Epidemiology of Brain Metastases. Curr Oncol Rep 2012; 14: 48–54. [DOI] [PubMed] [Google Scholar]
- 53. Cohen JV, Tawbi H, Margolin KA, et al. Melanoma central nervous system metastases: current approaches, challenges, and opportunities. Pigment Cell Melanoma Res 2016; 29: 627–642. doi: 10.1111/pcmr.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de la Monte SM, Moore GW, Hutchins GM. Patterned distribution of metastases from malignant melanoma in humans. Cancer Res 1983; 43: 3427–3433. [PubMed] [Google Scholar]
- 55. Patel J, Didolkar MS, Pickren J, et al. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg 1978; 135: 807–810. [DOI] [PubMed] [Google Scholar]
- 56. Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol 2012; 33: 579–589. [DOI] [PubMed] [Google Scholar]
- 57. Kluger HM, Zito CR, Barr ML, et al. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res 2015; 21: 3052–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012; 13: 459–465. [DOI] [PubMed] [Google Scholar]
- 59. Soffietti R, Ruda R, Mutani R. Management of brain metastases. J Neurol 2002; 249: 1357–1369. [DOI] [PubMed] [Google Scholar]
- 60. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016; 17: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tawbi HA, Forsyth PA, Algazi A, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med 2018; 379: 722–730. doi: 10.1056/NEJMoa1805453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018; 19: 672–681. doi: 10.1016/S1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- 63. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 64. Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer 2014; 50: 611–621. [DOI] [PubMed] [Google Scholar]
- 65. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 2017; 18: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clinical Oncol 2004; 22: 2101–2107. [DOI] [PubMed] [Google Scholar]
- 67. Jacquillat C, Khayat D, Banzet P, et al. Chemotherapy by fotemustine in cerebral metastases of disseminated malignant melanoma. Cancer Chemother Pharmacol 1990; 25: 263–266. [DOI] [PubMed] [Google Scholar]
- 68. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363: 733–742. [DOI] [PubMed] [Google Scholar]
- 69. Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol 2013; 14: e60–e69. [DOI] [PubMed] [Google Scholar]
- 70. Ascierto PA, Simeone E, Sileni VC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest 2014; 32: 144–149. [DOI] [PubMed] [Google Scholar]
- 71. Weber JS, Gibney G, Sullivan RJ, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 2016; 17: 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Johnson DB, Pectasides E, Feld E, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-PD-1 before and after BRAF inhibition. J Immunother 2017; 40: 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Saab KR, Mooradian MJ, Wang DY, et al. Tolerance and efficacy of BRAF plus MEK inhibition in patients with melanoma who previously have received programmed cell death protein 1-based therapy. Cancer. Epub ahead of print 6 December 2018. DOI: 10.1002/cncr.31889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Atkins MB, McDermott D, Tarhini A, et al. Matching-adjusted indirect comparison of nivolumab + ipilimumab and BRAF + MEK inhibitors for the treatment of BRAF-mutant treatment-naive advanced melanoma. Proc Annu Meet Am Assoc Cancer Res 2018; 59. [Google Scholar]
- 75. Gabani P, Robinson CG, Ansstas G, et al. Use of extracranial radiation therapy in metastatic melanoma patients receiving immunotherapy. Radiother Oncol 2018; 127: 310–317. [DOI] [PubMed] [Google Scholar]
- 76. Aboudaram A, Modesto A, Chaltiel L, et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: a safe and effective combination. Melanoma Res 2017; 27: 485–491. [DOI] [PubMed] [Google Scholar]
- 77. Kropp LM, De Los Santos JF, McKee SB, et al. Radiotherapy to control limited melanoma progression following ipilimumab. J Immunother 2016; 39: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Maity A, Mick R, Huang AC, et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br J Cancer 018; 119: 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zimmer L, Apuri S, Eroglu Z, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer 2017; 75: 47–55. [DOI] [PubMed] [Google Scholar]
- 80. Aya F, Gaba L, Victoria I, et al. Ipilimumab after progression on anti-PD-1 treatment in advanced melanoma. Future Oncol 2016; 12: 2683–2688. [DOI] [PubMed] [Google Scholar]
- 81. Bowyer S, Prithviraj P, Lorigan P, et al. Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer 2016; 114: 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Olson D, Luke JJ, Hallmeyer S, et al. Phase II trial of pembrolizumab (pembro) plus 1 mg/kg ipilimumab (ipi) immediately following progression on anti-PD-1 Ab in melanoma (mel). J Clin Oncol 2018; 36: 9514–9514. [Google Scholar]
- 83. Cohen JV, Alomari AK, Vortmeyer AO, et al. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res 2016; 4: 179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Patel KR, Chowdhary M, Switchenko JM, et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res 2016; 26: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ahmed KA, Abuodeh YA, Echevarria MI, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol 2016; 27: 2288–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Qian JM, Yu JB, Kluger HM, et al. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016; 122: 3051–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fang P, Jiang W, Allen P, et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neuro Oncol 2017; 133: 595–602. [DOI] [PubMed] [Google Scholar]
- 88. Pauline T, Clara A, Bastien O, et al. Impact of radiotherapy administered simultaneously with systemic treatment in patients with melanoma brain metastases within MelBase, a French multicentric prospective cohort. Eur J Cancer 2019; 112: 38–46. [DOI] [PubMed] [Google Scholar]
- 89. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 2016; 316: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014; 15: 387–395. [DOI] [PubMed] [Google Scholar]
- 91. Ellerhorst J, Strom E, Nardone E, et al. Whole brain irradiation for patients with metastatic melanoma: a review of 87 cases. Int J Radiat Oncol Biol Phys 2001; 49: 93–97. [DOI] [PubMed] [Google Scholar]
- 92. Stevens G, Firth I, Coates A. Cerebral metastases from malignant melanoma. Radiother Oncol 1992; 23: 185–191. [DOI] [PubMed] [Google Scholar]
- 93. Glitza IC, Guha-Thakurta N, D’Souza NM, et al. Bevacizumab as an effective treatment for radiation necrosis after radiotherapy for melanoma brain metastases. Melanoma Res 2017; 27: 580–584. [DOI] [PubMed] [Google Scholar]
- 94. Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014; 2: 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Schvartsman G, Ma J, Bassett RL, et al. Outcomes of metastatic melanoma (MM) patients (pts) after discontinuation of anti-Programmed-Death 1 (PD1) therapy without disease progression. J Clin Oncol 2018; 36: 9549–9549. [Google Scholar]
- 96. Betof Warner A, Shoushtari AN, Houghton S, et al. Characterization of complete responders to combination nivolumab (nivo) and ipilimumab (ipi) in patients (pts) with advanced, unresectable melanoma. J Clin Oncol 2018; 36: 9552–9552. [Google Scholar]
- 97. Christiansen SA, Swoboda D, Gardner K, et al. Off treatment survival (OTS) in patients (pts) with advanced melanoma after anti-PD1 therapy. J Clin Oncol 2018; 36: 9554–9554. [Google Scholar]
- 98. Tan AC, Emmett L, Lo S, et al. Utility of 1-year FDG-PET (PET) to determine outcomes from anti-PD-1 (PD1) based therapy in patients (pts) with metastatic melanoma (MM). J Clin Oncol 2018; 36: 9517–9517. [Google Scholar]
- 99. Desvignes C, Abi Rached H, Templier C, et al. BRAF inhibitor discontinuation and rechallenge in advanced melanoma patients with a complete initial treatment response. Melanoma Res 2017; 27: 281–287. [DOI] [PubMed] [Google Scholar]
- 100. Carlino MS, Vanella V, Girgis C, et al. Cessation of targeted therapy after a complete response in BRAF-mutant advanced melanoma: a case series. Br J Cancer 2016; 115: 1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]