Abstract
Background:
Tumour markers in pleural fluid and their diagnostic value are subject to debate. Although there are several studies on this topic, standardized cut-off values do not exist. In this study we investigated the potential of a ratio of carcinoembryonic antigen (CEA) in pleural fluid and serum, serving as an individual marker for pleural cancer manifestation.
Methods:
A total of 201 consecutive patients with unclear pleural effusion were included in the study; 98 were diagnosed with malignant pleural effusion and 103 had an effusion due to other, benign reasons. CEA levels in pleural fluid and serum were measured.
Results:
By using receiver operating characteristics analysis, at the cut-off of 1.0, the CEA ratio showed a specificity of 92% and sensitivity of 85%, with a positive predictive value of 91% and a negative predictive value of 87%. These results are higher than in previous investigations on different pleural tumour markers and their combination.
Conclusions:
The CEA ratio is a useful tool in predicting pleural carcinosis. Elevated results in cytology-negative patients should lead to further investigations, such as repeated cytological examination or thoracoscopy.
Keywords: carcinoembryonic antigen, malignant pleural fluid, pleural carcinosis, thoracocentesis, tumour markers
Introduction
Malignant pleural effusion (MPE) is a common phenomenon in lung cancer and other cancer-related pleural diseases.1 However, a clear and fast diagnosis is often challenging. The presence of MPE is attributable to an advanced state of disease and a correct diagnosis is crucial for further therapeutic decisions. Furthermore, MPE influences the prognosis of the patients. The median survival with MPE has been reported as 3–12 months, depending on the site of the primary neoplasm.2
The initial diagnostic approaches include thoracocentesis with cytological and biochemical examination of the pleural fluid.2–4 Although thoracocentesis is the standard procedure and may be performed repeatedly, the sensitivity for the diagnosis of MPE is typically only 50–70%.5,6 Thoracoscopy increases diagnostic accuracy, and will exhibit a diagnosis in more than 90% of patients with pleural malignancy.7 However, this procedure may not be available at all facilities and some patients with poor performance status and/or comorbidities may not be suitable for this intervention. Therefore, additional examinations may be needed. The evaluation of different tumour markers in the pleural effusion and pleural cavity might be helpful in these decisions.
Among all tumour markers, the carcinoembryonic antigen (CEA) is the most examined and frequently used marker for pleural fluid. Although there have been numerous scientific evaluations, its significance remains controversial due to varying results. The high-quality papers among those published have shown high specificity, however sensitivity largely varies. Furthermore, variable cut-off values have been reported. A meta-analysis by Shi and colleagues showed an overall sensitivity of 0.54 with a specificity of 0.94.8 Several authors reported higher sensitivities when combining different tumour markers,9–13 and other authors reported that CEA has the highest diagnostic value when comparing different tumour markers in the pleural fluid.14–20 What all these studies have in common is that the analysis of tumour markers, and especially CEA, is recommended when MPE is suspected. Tumour markers had higher levels in malignant pleural fluid than in effusions due to benign conditions.
However, the overall composition, quantity of fluid and concentration of different molecules and proteins in the MPE largely varies among patients and cancer entities. Even in the same patient, recurring MPE might bring different results with every analysis. This might be a problem for the correct use of diagnostic tumour markers such as CEA in the pleural fluid. Up to now, there is no clear recommendation for a specific cut-off value, although there are several investigations with varying values ranging from 3 ng/ml to 50 ng/ml.6,9,10,15,21–28
In 1972, Light and colleagues first described a method for diagnostic separation of transudates and exudates within an individual patient.29 Inspired by Light’s criteria, we hypothesized that tumour markers in the pleural fluid should not be determined by a cut-off value, but rather evaluated using a ratio with respect to serum and pleural fluid levels of CEA. In 2004, Trape and colleagues reported promising results of simultaneous determination of tumour markers in pleural fluid and serum in a smaller cohort (22 patients with MPE).30 Korczynski and colleagues investigated CEA and other tumour markers and their ratio in 2009 in a similar small cohort of 36 patients with MPE, showing CEA as the most valuable tumour marker, especially when the ratio is calculated.31 They determined the best cut-off for the ratio to be 0.83.
In 2016, Tozzoli and colleagues evaluated 71 MPE (excluding mesothelioma) for CEA in pleural fluid and serum together with pleural cytology showing high sensitivity for nonsmall-cell lung cancer.28 Another publication from 2016 with a remarkable sample size of 130 patients investigated the differentiation of malignant and tuberculous pleural effusion by using CEA ratio.15
In a recent publication, Zhai and colleagues presented the results of two Chinese cohorts with a total of 119 MPE. Area under the curve (AUC) for CEA measurements in pleural fluid was slightly higher than for a CEA ratio of 1.1.32
The performance of other tumour markers such as CA 125, CA 15-3, CA 19-9, NSE, SCC and CYFRA 21-1 was investigated in most of the previously mentioned papers. However, CEA was consistently the best performing parameter either as an isolated value in pleural fluid (or as a ratio, if investigated).
Summarizing this, we decided to perform an analysis to determine the ratio of pleural fluid and serum levels of CEA in patients with MPE compared with patients with nonmalignant pleural fluid.
Materials and methods
Patients
In this prospective descriptive study we investigated and compared data from 201 consecutive patients over a period of 14 months. All patients were referred for investigation of pleural effusion. Per protocol, inclusion criteria involved age 18 years or older and written consent to participate. A diagnosis of malignant or nonmalignant effusion was made based on cytology and/or thoracoscopy and long-term follow up (at least 1 year). All aetiologies of pleural effusion were included in the analysis, except for malignant pleural mesothelioma, which was an exclusion criterion. Several studies have suggested that CEA is not a useful tool and apparently not frequently elevated when pleural effusion is caused by mesothelioma.33,34
All patients provided informed consent for examinations of their pleural fluid and further investigations including data analysis.
Effusions were considered malignant if malignant cells were found on cytological examination or in a biopsy specimen. Nonmalignant pleural effusion was diagnosed when cytology was negative for malignancy and no signs of a malignant disease were detected during follow up.
This study complied with the Declaration of Helsinki and was approved by the ethics committee of the county of Lower Austria (No. 384-2016).
Methods
Pleural fluid samples were extracted via thoracocentesis or thoracoscopy and were collected in dry tubes. The samples were brought to the laboratory immediately, centrifuged for 10 min at 1500 G, and stored frozen at −70°C until assayed.
Blood samples were obtained and CEA was assayed using electrochemiluminescence (Cobas6000, Roche, Mannheim, Germany) in both pleural fluid and blood. The manufacturer defines a cut-off value of 5.5 ng/ml in serum as the upper limit of normal.
Statistical analysis
Data were expressed as mean and standard deviation, median and range or as percentages. Statistical analysis was performed using statistical software (SPSS-PC, SPSS, Chicago, IL, USA).
Differences between the two groups were evaluated using the Mann–Whitney U test after testing for normal distribution using the Kolmogorov–Smirnov test. To compare different cut-offs for the main parameter of interest (CEA ratio pleural fluid/blood) a receiver operating characteristic (ROC) curve was constructed and the AUC was calculated. The threshold was selected based on the best diagnostic efficacy having achieved equilibrium between sensitivity and specificity by using Youden’s index. Sensitivity and specificity of serum and pleural fluid CEA were calculated by using the provider’s cut-off value for serum, although there is no validated cut-off value for CEA in pleural fluid. Positive predictive values (PPV) and negative predictive values (NPV) were calculated by using cross tables. All tests were two-sided and statistical significance was accepted for p values
< 0.05.
Results
Table 1 gives an overview of the baseline characteristics of the study population. A total of 103 pleural effusions were defined as benign pleural effusion (BPE) and 98 were defined as MPE, mainly associated with lung cancer (n = 83), and less often associated with pleural carcinosis secondary to other primary tumours (n = 15). The median age was equally distributed in both cohorts (69 years versus 71 years). The serum and pleural fluid levels of CEA and the ratio of serum and pleural fluid CEA were significantly higher in MPE.
Table 1.
Baseline characteristics of the study population.
| All | MPE | BPE | p value | |
|---|---|---|---|---|
| Number of subjects (%) | 201 (100) | 98 (48.8) | 103 (51.2) | - |
| Sex: | ||||
| Female, n (%) | 74 (36.8) | 49 (66.2) | 25 (33.8) | - |
| Male, n (%) | 127 (63.2) | 49 (38.6) | 78 (61.4) | - |
| Age at thoracocentesis, years, median (range) | 69 (31–95) | 69 (38–90) | 71 (31–95) | NS |
| Serum – median CEA, ng/ml (range) |
2.55 (0.20–2314.00) | 6.90 (0.20–2314.00) | 1.60 (0.20–194.00) | < 0.001 |
| Pleural effusion – median CEA, ng/ml (range) | 2.55 (0.10–2805.00) | 35.0 (0.20–2805.00) | 0.90 (0.10–73.00) | < 0.001 |
| Ratio pleural CEA/serum, median (range) | 0.9 (0.1–413.8) | 2.6 (0.3–413.8) | 0.6 (0.1–5.0) | < 0.001 |
BPE, benign pleural effusion; CEA, carcinoembryonic antigen; MPE, malignant pleural effusion; NS, nonsignificant.
Table 2 describes the aetiology and histology of MPE. Lung adenocarcinoma (n = 68) and large-cell lung carcinoma (n = 2) showed the highest median values of CEA in pleural fluid among the different histological types of lung cancer, however the latter was much less represented. Among the MPE that was not associated with lung cancer, mammary carcinoma (n = 8) and one case of carcinoma of unknown primary showed the highest rates of pleural fluid CEA. In all MPE except for large-cell lung carcinoma (n = 2), ovarian carcinoma (n = 1) and salivary gland carcinoma (n = 1), median CEA in pleural fluid was higher compared with serum CEA. All patients with MPE were classified as stage IV (according to Union for International Cancer Control 8th edition).
Table 2.
Aetiology and diagnosis of the MPE (n = 98), median levels of CEA in serum and pleural fluid, and the median ratio of CEA in pleural fluid/serum.
| n (%) | CEA serum; ng/ml, median |
CEA pleural fluid; ng/ml, median |
Ratio pleural fluid/ serum, median |
|
|---|---|---|---|---|
| MPE associated with lung cancer, n (%) | ||||
| Adenocarcinoma | 62 (63.3) | 7.20 | 82.50 | 4.2 |
| Small-cell lung cancer | 13 (13.3) | 7.50 | 18.30 | 2.1 |
| Squamous-cell carcinoma | 6 (6.1) | 3.10 | 10.55 | 2.7 |
| Large-cell lung carcinoma | 2 (2.0) | 181.50 | 94.00 | 0.8 |
| MPE associated with other carcinomas, n (%) | ||||
| Mammary carcinoma | 8 (8.2) | 9.50 | 13.00 | 1.6 |
| Endometrial carcinoma | 2 (2.0) | 2.45 | 4.95 | 1.5 |
| Carcinoma of unknown primary | 1 (1.0) | 4.00 | 520.00 | 130.0 |
| Renal-cell carcinoma | 1 (1.0) | 1.60 | 1.80 | 1.1 |
| Ovarian carcinoma | 1 (1.0) | 0.60 | 0.40 | 0.7 |
| Salivary gland carcinoma | 1 (1.0) | 7.40 | 5.10 | 0.7 |
| Prostate carcinoma | 1 (1.0) | 3.00 | 5.90 | 2.0 |
CEA, carcinoembryonic antigen; MPE, malignant pleural effusion.
The final diagnosis of BPE is given in Table 3. The main reasons for BPE were congestive heart failure, parapneumonic effusion and pleural empyema. This is in line with other reports of the distribution of pleural effusions, when malignancy and trauma are excluded.35 Among the group of BPE were 11 cases of patients with lung malignancies, causing a pleural effusion that was not related to pleural carcinosis but to atelectasis caused by malign bronchial obstruction with effusion ex vacuo.
Table 3.
Aetiology and diagnosis of the pleural effusions without detection of malignancies (n = 103) and their median levels of CEA in serum and pleural fluid.
| n (%) | CEA serum; (median), ng/ml |
CEA pleural fluid; (median), ng/ml |
Ratio pleural fluid/serum (median) | |
|---|---|---|---|---|
| Congestive heart failure | 30 (29.1) | 1.60 | 0.50 | 0.4 |
| Parapneumonic effusion | 26 (25.2) | 1.80 | 1.20 | 0.7 |
| Empyema | 12 (11.6) | 1.40 | 0.90 | 0.7 |
| Effusion ex vacuo | 11 (10.8) | 3.20 | 2.60 | 0.9 |
| Post-thoracotomy syndrome | 4 (3.9) | 0.95 | 0.55 | 0.6 |
| Pleural fibrosis | 3 (2.9) | 0.80 | 0.50 | 0.5 |
| Chylothorax | 2 (1.9) | 0.85 | 0.50 | 0.7 |
| Pleural tuberculosis | 2 (1.9) | 0.70 | 0.50 | 0.7 |
| Polyserositis | 2 (1.9) | 2.20 | 1.00 | 0.5 |
| Pulmonary embolism | 2 (1.9) | 0.85 | 0.60 | 0.7 |
| Rheumatoid arthritis | 2 (1.9) | 1.05 | 0.85 | 0.8 |
| Sjögren’s syndrome | 1 (1.0) | 0.80 | 0.20 | 0.3 |
| Ascites | 1 (1.0) | 3.20 | 0.70 | 0.2 |
| Sarcoidosis | 1 (1.0) | 2.00 | 1.90 | 0.9 |
| Grover’s disease | 1 (1.0) | 0.60 | 0.20 | 0.3 |
| Asbestosis | 1 (1.0) | 2.20 | 2.00 | 0.9 |
| Postoperative effusion (spine surgery) | 1 (1.0) | 1.60 | 1.30 | 0.8 |
| Unknown aetiology | 1 (1.0) | 0.80 | 1.30 | 1.6 |
CEA, carcinoembryonic antigen.
Median serum CEA was consistently higher than pleural CEA in BPE, accept for one case of pleural effusion with unknown aetiology. A total of 6 out of the 11 cases of pleural effusion ex vacuo secondary to atelectasis (without a proof of malignancy by thoracocentesis) did not reach the end of the 1-year follow up due to earlier cancer-related death.
The isolated diagnostic performance (sensitivity, specificity, PPV and NPV) of CEA in serum (cut-off 5.5 ng/ml, as suggested by the provider) and pleural fluid (no validated cut-off available) was as follows: 0.54, 0.89, 0.83 and 0.67 for serum and 0.74, 0.92, 0.90 and 0.79 for pleural fluid, respectively.
The performance of CEA pleural fluid/serum ratio at different thresholds (Table 4) was analysed by ROC curve (AUC = 0.903) as seen in Figure 1. The highest diagnostic performance was reached at a threshold of 1.0 with a specificity of 92%, sensitivity of 85%, PPV of 91% and NPV of 87%. A ratio of 1.0 was assumed to be the best cut-off to distinguish BPE and MPE.
Table 4.
Point estimates for pleural fluid/serum ratio of CEA.
| Cut-off value | Specificity | Sensitivity | PPV | NPV | LHR+ | LHR– | J |
|---|---|---|---|---|---|---|---|
| 0.5 | 0.45 | 0.93 | 0.61 | 0.87 | 1.69 | 0.16 | 0.38 |
| 0.8 | 0.70 | 0.87 | 0.73 | 0.85 | 2.90 | 0.19 | 0.57 |
| 1.0 | 0.92 | 0.85 | 0.91 | 0.87 | 10.63 | 0.16 | 0.77 |
| 1.2 | 0.94 | 0.78 | 0.92 | 0.82 | 13.00 | 0.23 | 0.72 |
| 1.5 | 0.95 | 0.66 | 0.92 | 0.75 | 13.20 | 0.36 | 0.61 |
CEA, carcinoembryonic antigen; J, Youden’s index; LHR+, positive likelihood ratio; LHR−, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Figure 1.
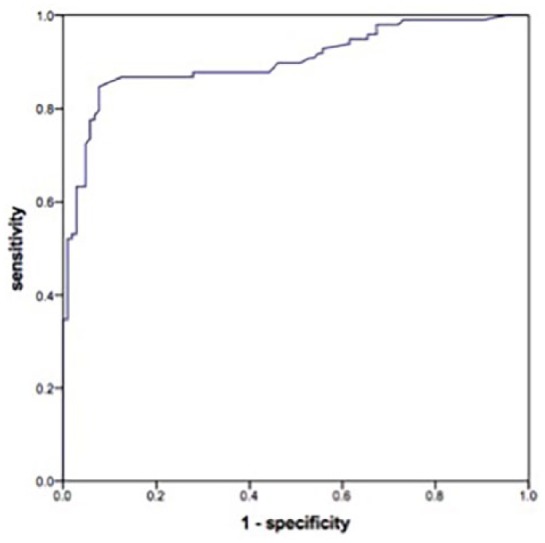
ROC curve for pleural fluid/serum ratio of CEA (AUC 0.903; 95% confidence interval 0.858–0.947; p < 0.001).
Discussion
MPE is an important aetiology of pleural effusions with the necessity for a correct diagnosis due to its impact on further therapeutic strategies. Thoracocentesis with cytological examination is the most often used diagnostic tool, but sensitivity is low, ranging from only 50% to 70%.5,6 Therefore, additional tools like tumour markers might be useful to decide if further invasive diagnostic techniques, for example, thoracoscopy, need to be applied. In this study, we demonstrated that the ratio of CEA in pleural fluid and serum is a useful individual parameter and has a high value for the above-mentioned decisions.
We were able to detect a specificity of 92% and a sensitivity of 85% for a CEA ratio of 1.0, which is the best eligible cut-off value according to ROC curve and J-index. Sensitivity and specificity are in line with the results of Trape and colleagues,30 Korcynzski and colleagues,31 Zhai and colleagues32 and Tozzoli and colleagues,28 and they are considerably higher than the overall sensitivity of 54% of previous pleural fluid CEA studies reported in the meta-analysis by Shi and colleagues8 and Nguyen and colleagues,36 or combinations of various tumour markers.13
Our study has some limitations. One limitation is that, although we took a 1-year follow up on cytology-negative patients, 6 out of 11 patients, assumed to have BPE secondary to their cancer disease with negative effusion cytology (marked as ‘effusion ex vacuo’), did not reach the end of the follow up because of cancer-related death. Therefore, it is not possible retrospectively to rule out pleural cancer involvement in these patients. On the other hand, in this subgroup the median pleural fluid CEA level was lower than median serum CEA levels, thus having a ratio < 1. Additionally 5 out of the 6 patients had a transudate according to Light’s criteria, which makes a MPE even more unlikely. Thoracoscopy was either not indicated (in cases of already proven advanced-stage disease) or was not possible to perform (death, low performance score) in these patients. However, to preserve consecutiveness we counted them (without positive pleural cytology but known cancer) as having a BPE. On the other hand, performing thoracoscopy in a study without creating a benefit for the patient (e.g. when metastatic disease is already diagnosed and talcum pleurodesis is not indicated and/or possible) seems to be problematic from an ethical viewpoint.
The potential of the CEA ratio clearly lies in those patients who would have a different treatment strategy pending the involvement of the pleura in their cancer disease. In other patients, with unknown aetiology of the effusion and additional findings indicating a possible underlying malign disease (e.g. exudate or suspect lymph nodes), the CEA ratio might be beneficial to indicate more concrete diagnostic steps such as a thoracoscopy.
Our study shows that a negative CEA ratio has an acceptable NPV and might be helpful in indicating, if < 1, further investigation for other nonmalignant aetiologies of unknown exudative pleural effusions.
In this study, the measurement of CEA in pleural fluid was not externally controlled, for example, by assigning another laboratory unit, which could be seen as a limitation.
Another limitation of this study which should be mentioned is that we did not evaluate the effusion/serum ratio of other tumour markers, particularly those known to be useful in lung cancer (e.g. CYFRA 21-1 or NSE). We did not investigate any combinations of various tumour markers that might increase sensitivity and specificity. However, the results of the few preceding studies showed that the CEA value in pleural fluid as well as the CEA ratio has the best AUC of all investigated tumour markers.15,30,31 We suggest further analysis to investigate the ratio of other tumour markers, which will probably confirm the use of CEA as the most effective tumour marker in pleural effusion, at least for lung cancer.
Conclusion
Simultaneous determination of CEA in pleural fluid and blood is of high value in the diagnosis of MPE, with exception of malignant pleura mesothelioma.
In cases of suspicious MPE with a negative cytology, particularly in the absence of a visible tumour and/or borderline suitability for invasive procedures such as thoracoscopy, the determination of the ratio of CEA in the pleural fluid and blood serum may be helpful as a complementary tool for the differential diagnosis of pleural effusion.
Acknowledgments
Some results of this study (i.e. baseline characteristics, CEA ratio ROC curve) were presented at the ATS International Congress 2018 in San Diego, USA, during a RAPiD poster discussion session on 22 May 2018.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
ORCID iD: Klaus Hackner  https://orcid.org/0000-0002-6378-5840
https://orcid.org/0000-0002-6378-5840
Contributor Information
Klaus Hackner, Department of Pneumology, University Hospital Krems, Mitterweg and Karl Landsteiner University of Health Sciences 10, 3500 Krems, Austria.
Peter Errhalt, Department of Pneumology, University Hospital Krems, Austria, and Karl Landsteiner University of Health Sciences, Krems, Austria.
Sabin Handzhiev, Department of Pneumology, University Hospital Krems, Austria, and Karl Landsteiner University of Health Sciences, Krems, Austria.
References
- 1. Heffner JE. Management of the patient with a malignant pleural effusion. Semin Respir Crit Care Med 2010; 31: 723–733. [DOI] [PubMed] [Google Scholar]
- 2. Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(Suppl. 2): ii32–ii40. [DOI] [PubMed] [Google Scholar]
- 3. Fenton KN, Richardson JD. Diagnosis and management of malignant pleural effusions. Am J Surg 1995; 170: 69–74. [DOI] [PubMed] [Google Scholar]
- 4. American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000; 162: 1987–2001. [DOI] [PubMed] [Google Scholar]
- 5. Nance KV, Shermer RW, Askin FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol 1991; 4: 320–324. [PubMed] [Google Scholar]
- 6. Antonangelo L, Sales RK, Cora AP, et al. Pleural fluid tumour markers in malignant pleural effusion with inconclusive cytologic results. Curr Oncol 2015; 22: e336–e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edmondstone WM. Investigation of pleural effusion: comparison between fibreoptic thoracoscopy, needle biopsy and cytology. Respir Med 1990; 84: 23–26. [DOI] [PubMed] [Google Scholar]
- 8. Shi HZ, Liang QL, Jiang J, et al. Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: a meta-analysis. Respirology 2008; 13: 518–527. [DOI] [PubMed] [Google Scholar]
- 9. Porcel JM, Vives M, Esquerda A, et al. Use of a panel of tumor markers (carcinoembryonic antigen, cancer antigen 125, carbohydrate antigen 15–3, and cytokeratin 19 fragments) in pleural fluid for the differential diagnosis of benign and malignant effusions. Chest 2004; 126: 1757–1763. [DOI] [PubMed] [Google Scholar]
- 10. Lee JH, Chang JH. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, neuron-specific enolase, and cytokeratin 19 fragments in patients with effusions from primary lung cancer. Chest 2005; 128: 2298–2303. [DOI] [PubMed] [Google Scholar]
- 11. Liang QL, Shi HZ, Qin XJ, et al. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax 2008; 63: 35–41. [DOI] [PubMed] [Google Scholar]
- 12. Villena V, Lopez-Encuentra A, Echave-Sustaeta J, et al. Diagnostic value of CA 72–4, carcinoembryonic antigen, CA 15–3, and CA 19–9 assay in pleural fluid. A study of 207 patients. Cancer 1996; 78: 736–740. [DOI] [PubMed] [Google Scholar]
- 13. Yang Y, Liu YL, Shi HZ. Diagnostic accuracy of combinations of tumor markers for malignant pleural effusion: an updated meta-analysis. Respiration 2017; 94: 62–69. [DOI] [PubMed] [Google Scholar]
- 14. Menard O, Dousset B, Jacob C, et al. Improvement of the diagnosis of the cause of pleural effusion in patients with lung cancer by simultaneous quantification of carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE) pleural levels. Euro J Cancer 1993; 29A: 1806–1809. [DOI] [PubMed] [Google Scholar]
- 15. Gu Y, Zhai K, Shi HZ. Clinical value of tumor markers for determining cause of pleural effusion. Chin Med J 2016; 129: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hackbarth JS, Murata K, Reilly WM, et al. Performance of CEA and CA19–9 in identifying pleural effusions caused by specific malignancies. Clin Biochem 2010; 43: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 17. Hernandez L, Espasa A, Fernandez C, et al. CEA and CA 549 in serum and pleural fluid of patients with pleural effusion. Lung Cancer 2002; 36: 83–89. [DOI] [PubMed] [Google Scholar]
- 18. Sharma SK, Bhat S, Chandel V, et al. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, and cytokeratin 19 fragments in patients with effusion from nonsmall cell lung cancer. J Carcinog 2015; 14: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shitrit D, Zingerman B, Shitrit AB, et al. Diagnostic value of CYFRA 21–1, CEA, CA 19–9, CA 15–3, and CA 125 assays in pleural effusions: analysis of 116 cases and review of the literature. Oncologist 2005; 10: 501–507. [DOI] [PubMed] [Google Scholar]
- 20. Topolcan O, Holubec L, Polivkova V, et al. Tumor markers in pleural effusions. Anticancer Res 2007; 27: 1921–1924. [PubMed] [Google Scholar]
- 21. Ogushi F, Fukuoka M, Takada M, et al. Carcinoembryonic antigen (CEA) levels in pleural effusions and sera of lung cancer patients. Jpn J Clin Oncol 1984; 14: 321–327. [PubMed] [Google Scholar]
- 22. Radjenovic-Petkovic T, Pejcic T, Nastasijevic-Borovac D, et al. Diagnostic value of CEA in pleural fluid for differential diagnosis of benign and malign pleural effusion. Med Arh 2009; 63: 141–142. [PubMed] [Google Scholar]
- 23. Garcia-Pachon E, Padilla-Navas I, Dosda MD, et al. Elevated level of carcinoembryonic antigen in nonmalignant pleural effusions. Chest 1997; 111: 643–647. [DOI] [PubMed] [Google Scholar]
- 24. Romero S, Fernandez C, Arriero JM, et al. CEA, CA 15–3 and CYFRA 21–1 in serum and pleural fluid of patients with pleural effusions. Eur Respir J 1996; 9: 17–23. [DOI] [PubMed] [Google Scholar]
- 25. San Jose ME, Alvarez D, Valdes L, et al. Utility of tumour markers in the diagnosis of neoplastic pleural effusion. Clin Chim Acta 1997; 265: 193–205. [DOI] [PubMed] [Google Scholar]
- 26. Salama G, Miedouge M, Rouzaud P, et al. Evaluation of pleural CYFRA 21–1 and carcinoembryonic antigen in the diagnosis of malignant pleural effusions. Br J Cancer 1998; 77: 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miedouge M, Rouzaud P, Salama G, et al. Evaluation of seven tumour markers in pleural fluid for the diagnosis of malignant effusions. Br J Cancer 1999; 81: 1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tozzoli R, Basso SM, D’Aurizio F, et al. Evaluation of predictive value of pleural CEA in patients with pleural effusions and histological findings: a prospective study and literature review. Clin Biochem 2016; 49: 1227–1231. [DOI] [PubMed] [Google Scholar]
- 29. Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507–513. [DOI] [PubMed] [Google Scholar]
- 30. Trape J, Molina R, Sant F. Clinical evaluation of the simultaneous determination of tumor markers in fluid and serum and their ratio in the differential diagnosis of serous effusions. Tumour Biol 2004; 25: 276–281. [DOI] [PubMed] [Google Scholar]
- 31. Korczynski P, Krenke R, Safianowska A, et al. Diagnostic utility of pleural fluid and serum markers in differentiation between malignant and non-malignant pleural effusions. Eur J Med Res 2009; 14(Suppl. 4): 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhai K, Wang W, Wang Y, et al. Diagnostic accuracy of tumor markers for malignant pleural effusion: a derivation and validation study. J Thorac Dis 2017; 9: 5220–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whitaker D, Shilkin KB, Stuckey M, et al. Pleural fluid CEA levels in the diagnosis of malignant mesothelioma. Pathology 1986; 18: 328–329. [DOI] [PubMed] [Google Scholar]
- 34. Mezger J, Calavrezos A, Drings P, et al. Value of serum and effusion fluid CEA levels for distinguishing between diffuse malignant mesothelioma and carcinomatous pleural metastases. Lung 1994; 172: 183–184. [DOI] [PubMed] [Google Scholar]
- 35. Bintcliffe OJ, Lee GY, Rahman NM, et al. The management of benign non-infective pleural effusions. Eur Res Rev 2016; 25: 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen AH, Miller EJ, Wichman CS, et al. Diagnostic value of tumor antigens in malignant pleural effusion: a meta-analysis. Transl Res 2015; 166: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]


