Abstract
Background:
No clinical trial has directly compared nab-paclitaxel/gemcitabine (nab-P/G) with FOLFIRINOX (fluorouracil/leucovorin/oxaliplatin/irinotecan) in metastatic or advanced pancreatic cancer (mPC or aPC). We conducted a systematic review of real-world studies comparing these regimens in the first-line setting.
Methods:
Embase and MEDLINE databases through 22 January 2019, and Gastrointestinal Cancers Symposium 2019 abstracts were searched for real-world, retrospective studies comparing first-line nab-P/G versus FOLFIRINOX in mPC or aPC that met specific parameters. Studies with radiotherapy were excluded. Study quality was assessed using the Newcastle–Ottawa Scale.
Results:
Of 818 records initially identified, 35 were duplicates and 749 did not meet the eligibility criteria, mostly because they were either not comparative (n = 356) or not first line (n = 245). The remaining 34 studies (21 mPC; 13 aPC) assessed >6915 patients who received nab-P/G or FOLFIRINOX. In the studies identified, the median overall survival (OS) reached 14.4 and 15.9 months with nab-P/G and FOLFIRINOX, respectively, and median progression-free survival reached 8.5 and 11.7 months, respectively. Safety data were reported in 14 studies (2205 patients), including 8 single-institutional studies. In most single-institutional studies that reported safety data, rates were higher with FOLFIRINOX versus nab-P/G for grade 3/4 neutropenia (five of six studies) and febrile neutropenia (all three studies), while rates of grade 3/4 peripheral neuropathy were higher with nab-P/G in four of seven studies.
Conclusions:
Although FOLFIRINOX was associated with slightly longer median OS in more studies, the differences, when available, were not statistically significant. Therefore, a randomized, controlled trial is warranted. Toxicity profile differences represent key considerations for treatment decisions.
Keywords: FOLFIRINOX, nab-paclitaxel, pancreatic cancer, real-world evidence
Introduction
Pancreatic cancer is estimated to be the third leading cause of cancer-related mortality in the United States (US).1,2 As has been the case for many years, the projected number of deaths in 2019 (45,750) is expected to nearly equal the number of new cases (56,770).1,2 While the 5-year survival rate for all stages combined is approximately 9% (lowest among all cancers), more than half of all patients diagnosed with pancreatic cancer present with metastatic disease, which carries a 5-year survival rate of approximately 3%.1,2
Prior to recent treatment advances, single-agent gemcitabine was the standard of care for many years for patients with metastatic pancreatic cancer (mPC).3 Today, gemcitabine monotherapy remains a therapeutic option for patients with mPC who have poor performance status,4,5 but for patients with good performance status, it has been shown to be inferior compared with two newer chemotherapy combinations. In the PRODIGE/ACCORD trial of patients with mPC, treatment with FOLFIRINOX (fluorouracil, leucovorin, oxaliplatin, and irinotecan) resulted in a median overall survival (OS) of 11.1 months versus 6.8 months with gemcitabine alone [hazard ratio (HR), 0.57; 95% confidence interval (CI), 0.45–0.73; p < 0.001].6 The MPACT trial randomized patients with mPC to treatment with nab-paclitaxel plus gemcitabine (nab-P/G) or gemcitabine alone; the median OS with nab-P/G was 8.7 months versus 6.6 months with gemcitabine alone (HR, 0.72; 95% CI, 0.62–0.83; p < 0.001).7,8 Of note, the PRODIGE/ACCORD trial enrolled a higher percentage of patients with good performance status [Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 in 38.0%, 1 in 61.7%, and 2 in 0.3% of patients] compared with the MPACT trial [Karnofsky performance status of 100 (equivalent to ECOG PS of 0) in 16%, 90/80 (equivalent to ECOG PS of 1) in 76%, and 70/60 (equivalent to ECOG PS of 2) in 8% of patients].6,7,9 In a recent phase II study assessing nab-P/G specifically in patients with poor performance status (ECOG PS of 2), the median OS in patients with advanced disease who received treatment at the same dose and schedule as in the MPACT trial was 8.7 months.10
On the basis of these results, the current National Comprehensive Cancer Network and European Society for Medical Oncology guidelines recommend combination chemotherapy with nab-P/G or FOLFIRINOX as the preferred first-line treatments for patients with mPC who have good performance status.4,5 A recent study assessing the patterns and predictors of systemic therapy choices in mPC reported that the use of first-line gemcitabine monotherapy in the US decreased from 72% in 2006 to 16% in 2015. Conversely, there was a reciprocal increase in the use of either nab-P/G or FOLFIRINOX in this setting.11 This study also found that patients treated at community practices and by oncologists with lower volumes of patients with mPC were more likely to receive nab-P/G as a first-line treatment, while younger male patients were more likely to receive FOLFIRINOX.11 However, the rationale for choosing between nab-P/G and FOLFIRINOX remains unclear.
To date, there has been no head-to-head randomized, controlled trial comparing nab-P/G with FOLFIRINOX in patients with mPC. Therefore, a number of retrospective, nonrandomized studies from institutional or healthcare systems have compared nab-P/G and FOLFIRINOX in an attempt to elucidate differences in safety and effectiveness. However, cross-comparisons between trials are not ideal; for example, the numerically higher OS observed with FOLFIRINOX in PRODIGE/ACCORD6 versus nab-P/G in MPACT7 could lead physicians to believe that FOLFIRINOX has improved effectiveness. A recent systematic review of clinical trial data attempted to fill this gap.12 The study reported that several combination chemotherapies, including nab-P/G and FOLFIRINOX, were associated with significant improvement in survival compared with gemcitabine alone, but there were no significant differences between nab-P/G and FOLFIRINOX in terms of OS and progression-free survival (PFS).12 Furthermore, the available clinical trial data did not allow for a reliable assessment of differences in resource utilization, duration of treatment, or treatment costs. Given the lack of clinical trial data directly comparing the two regimens, there remains a need to assess the currently available real-world data to determine whether differences in outcomes exist between nab-P/G and FOLFIRINOX.
To address this need, we conducted a systematic review of real-world patient data comparing outcomes, including effectiveness, safety, duration of treatment, supportive care use, and resource utilization, with nab-P/G versus FOLFIRINOX as a first-line therapy in patients with advanced pancreatic cancer (aPC) which includes mPC. Our hope is that these results will facilitate more informed treatment decisions in this patient population.
Methods
Search strategy
The Embase and MEDLINE databases were searched through 22 January 2019, with no limit for the start date. In addition, the American Society of Clinical Oncology Meeting Library was searched for studies presented at the 2019 Gastrointestinal Cancers Symposium that were not yet indexed in the searched databases. Studies were included in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. The search was limited to publications in English, and the search terms, including nab-P/G and FOLFIRINOX, were designed to ensure full coverage of the relevant patient populations, interventions, study designs, and outcomes (Appendix A).
Eligibility criteria
Abstracts were separately screened by two independent reviewers and any discrepancies were resolved through discussion and consensus. Studies included were real-world, retrospective analyses of first-line therapy in patients with mPC or aPC that directly compared nab-P/G with FOLFIRINOX. Eligible studies were required to have data on effectiveness [OS, PFS, time to treatment failure (TTF), or overall response rate (ORR)], treatment duration, or resource utilization. Studies with radiotherapy and review articles were excluded, and duplicates were removed.
Data extraction and reporting
Selected studies were reviewed and data on populations, interventions, and outcomes were extracted from abstracts, posters, and full papers into a database. Data included region, treatment period, number of patients (total and in each arm), baseline and clinical characteristics (including ECOG PS), effectiveness and safety outcomes, treatment duration, cost of therapy, resource utilization, and second-line treatment, including any associated outcomes. The quality of the included studies was independently assessed by two reviewers using the Newcastle–Ottawa Scale (NOS).13 Any disagreements were resolved by a third reviewer. The NOS was developed to assess the quality of nonrandomized case-control and cohort studies based on three parameters, selection, comparability, and exposure/outcome, and assigns a maximum of 4, 2, and 3 stars, respectively, for these domains. A study was considered as high quality if the NOS score was ⩾7 stars.14,15 Because some studies did not report patient numbers, the values reported in this review are noted as being greater than the sum of the patient numbers reported in each particular category. In addition, the data reported varied by study (e.g. decimals/no decimals); we chose to represent all values as they appeared in the original reports for accuracy.
Results
Study selection
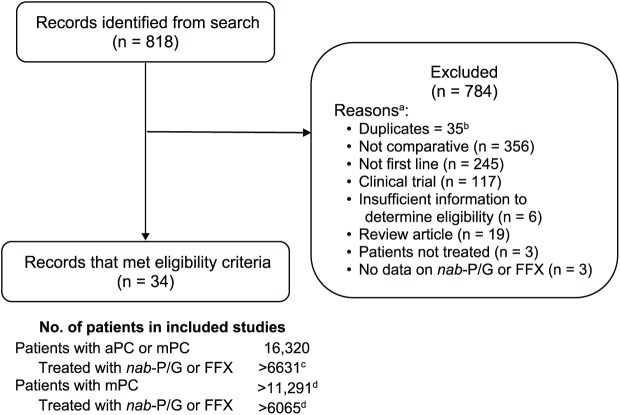
The initial search identified 818 records, of which 35 were duplicates; an additional 749 were excluded based on eligibility criteria, leaving 34 studies for further analysis (Figure 1).
Figure 1.
Study selection.
aPC, advanced pancreatic cancer; FFX, FOLFIRINOX; mPC, metastatic pancreatic cancer; nab-P/G, nab-paclitaxel/gemcitabine.
aStudies could be excluded for ⩾1 reason; once 1 reason was identified, no attempt was made to look for other potential reasons.
bIncludes abstracts for which full manuscripts were subsequently published, encore presentations (most recent presentation included), or abstracts that were presented with updated data later.
cThe symbol > indicates that some studies did not report the number of patients treated with nab-P/G or FFX and others evaluated additional treatment regimens.
dThe symbol > indicates that some studies did not report the number of patients with mPC.
Study and population characteristics
The 34 studies included patients treated between 2000 and 2018 in North America, Europe, and Asia (Table 1). Of these, 21 studies (62%) assessed only patients with mPC. Among 13 studies that assessed patients with aPC, 4 did not report the breakdown of the number of patients with aPC and mPC, and in the remaining 9 studies, most patients [1725 of 2205 (78%)] had mPC. Overall, 16,505 patients, including >11,476 (70%) with mPC, were assessed. Of these, >6915 patients, including >6349 (92%) with mPC, received nab-P/G or FOLFIRINOX. The numbers of patients treated with nab-P/G or FOLFIRINOX appear lower than the numbers of all assessed patients because some studies did not report the numbers separately for each regimen, while other studies evaluated additional regimens.
Table 1.
Study and population characteristics.
| Study | Publication type | Population |
Baseline characteristics |
Intervention, na |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region/ country |
Treatment period | N | Median age, years |
ECOG PS 0/1, % |
nab-P/G | FFX | ||||
| nab-P/G | FFX | nab-P/G | FFX | |||||||
| mPC studies | ||||||||||
| Beyer16 | Conference abstract | Europeb | 2012–2015 | 634 | NR | NR | NR | NR | 19 | 57 |
| Park17 | Conference abstract | NR | 2012–2014 | 27 | 66 | 55 | 89c | 18 | 9 | |
| Patel18 | Conference abstract | USA | 2013–2014 | 470 | 62 | 58 | NR | NR | 175 | 295 |
| Schmidt19 | Conference abstract | USA | 2011–2014 | 64 | 62c | NR | NR | 22d | 34d | |
| Abrams11 | Journal article | USA | 2005–2015 | 4011 | 68 | 61 | NR | NR | 543 | 609 |
| Braiteh20 | Journal article | USA | 2013–2014 | 248 | 69 | 62 | 82 | 82 | 122 | 80 |
| Caponnetto21 | Conference abstract | NR | NR | 43 | NR | NR | 100 | 100 | 20 | 23 |
| Kim22 | Journal article | USA | 2012–2016 | 345 | 64 (32% >70)e |
59 (12% >70)e |
NR | NR | 182 | 163 |
| Mañes-Sevilla23 | Journal article | Spain | 2012–2015 | 68 | 63e | 59e | 83 | 82 | 20d | 15d |
| McBride24 | Conference abstract | USA | 2013–2015 | 550 | 64e | 59e | NR | NR | 294 | 256 |
| Watanabe25 | Conference abstract | Japan | 2013–2015 | 135 | NR (12% >75) |
NR (4% >75) |
99 | 100 | 65 | 70 |
| Barrera26 | Conference abstract | Canada | 2010–2016 | 75 | 71 (68% >65) |
62 (43% >65) |
100 | 100 | 31 | 44 |
| Cartwright27 | Journal article | USA | 2013–2015 | 486 | 68 | 61 | 77 | 91 | 255 | 159 |
| Cho28 | Conference abstract | Korea | 2015-NR | 167 | 65f | 54f | NRg | NRg | 81 | 86 |
| Kang29 | Journal article | Korea | 2013–2016 | 308 | 62 (41% ⩾65) |
60 (31% ⩾65) |
97 | 99 | 149 | 159 |
| Kim30 | Journal article | USA | 2015 | 654 | 65e | 59e | 70 | 91 | 337 | 317 |
| Pacheco-Barcia31 | Conference abstract | NR | 2014–2018 | 46 | 63c | 52 | 96 | 25 | 21 | |
| Vivaldi32 | Conference abstract | NR | NR | 139 | 64c | NR | NR | 57 | 81 | |
| Barrera33 | Conference abstract | Canada | 2010–2018 | 203 | NR | NR | NR | NR | 60 | 66 |
| Henkel34 | Conference abstract | USA | 2000–2017 | 48 | NR | NR | 94 | 100 | 11 | 9 |
| Javed35 | Journal article | Europeh | 2012–2015 | 1030 | 64e | 60e | 68 | 83 | 81 | 204 |
| Subtotal (mPC studies) | 9751 | 2567 | 2757 | |||||||
| aPC studiesi | ||||||||||
| Cherniawsky36 | Conference abstract | Canada | 2014–2016 | 292 (205) | 55% ⩾65c | NR | NR | NR | NR | |
| Kasi37 | Conference abstract | USA | 2011–2016 | 154 (89) | 63 | 61 | 83 | 90 | 47 (33) | 107 (56) |
| Maeda38 | Conference abstract | Japan | 2014–2017 | 40 (11) | 71 | 64 | NR | NR | 9 (NR)d | 16 (NR)d |
| Muranaka39 | Journal article | Japan | 2013–2015 | 38 (18) | 67 | 63 | 100 | 100 | 22 (17) | 16 (1) |
| Papneja40 | Conference abstract | Canada | 2011–2016 | 119 (91) | 64 | 59 | 85 | 97 | 33 (21) | 86 (70) |
| Shahda41 | Conference abstract | USA | NR | 91 (NR) | NR | NR | NR | NR | NR | NR |
| Wang42 | Journal article | Canada | 2014–2016 | 225 (154) | 68 | 60 | 59 | 93 | 87 (66) | 92 (55) |
| Franco43 | Conference abstract | Spain | 2012–2016 | 136 (87) | 63c | 88c | 49 (NR) | 87 (NR) | ||
| Helen44 | Conference abstract | Canada | 2008–2016 | 3696 (NR) | 65c,e | NR | NR | NR | NR | |
| Kudlovich45 | Conference abstract | Canada | 2012–2016 | 87 (NR) | 75 | 69 | NR | NR | 21 (NR) | 52 (NR) |
| Tahara46 | Journal article | Japan | 2014–2017 | 27 (13) | 63 | 62 | 100 | 100 | 15 (7) | 12 (4) |
| Terashima47 | Journal article | Japan | 2009–2015 | 675 (NR) | NR | NR | NR | 87 | 20 (NR) | 47 (NR) |
| Hegewisch-Becker48 | Journal article | Germany | 2014–2017 | 1174 (1057) | 71 | 60 | 90 | 96 | 489 (439) | 284 (256) |
| Subtotal (aPC studies) | 6754 (>1725) |
>792 (>583) |
>799 (>442) |
|||||||
| Total | 16,505 (>11,476) |
>3359 (>3150) | >3556 (>3199) | |||||||
aPC, advanced pancreatic cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; FFX, FOLFIRINOX; mPC, metastatic pancreatic cancer; nab-P/G, nab-paclitaxel/gemcitabine; NR, not reported.
Interventions other than nab-P/G or FFX, if any, are not shown.
United Kingdom, Germany, Italy, and Hungary.
Data not reported separately for each group.
first-line setting.
Mean age.
Not reported whether median or mean.
ECOG PS was better in the FFX group.
United Kingdom, Sweden, Germany, Italy, and Hungary.
aPC includes mPC. The number in parentheses is the number of patients with mPC.
In general, patients who received nab-P/G were older and had worse performance status than those who received FOLFIRINOX. Age was reported separately for the two groups in 21 studies, and the median/mean age of patients who received nab-P/G was numerically greater than those who received FOLFIRINOX (Table 1). In 11 of the 17 studies that reported ECOG PS separately for the two groups, a higher proportion of patients had good performance status (ECOG PS of 0 or 1) in the FOLFIRINOX cohort; 5 studies had equal proportions, and 1 had a higher proportion of patients with good performance status in the nab-P/G group (Table 1).
Of the 32 included studies, 23 (72%) were assessed by the NOS to be of high quality (⩾7 stars; Supplementary Table 1). The remaining nine studies were of moderate quality (seven studies with 6 stars; two studies with 5 stars), primarily due to comparability and outcome biases. The mean NOS score across all studies was 7.47. The most common comparability bias was the absence of study-controlled factors in addition to the compared patient population. The most common outcome bias was the lack of reporting of adequate follow up. Several studies did not receive an NOS star for selection and outcome parameters because they did not report a specific record or database used to ascertain exposures or assess outcomes.
Effectiveness outcomes
Overall, 31 studies (>5237 patients) reported an OS, PFS, or TTF (Table 2). Effectiveness outcomes generally overlapped between patients who received nab-P/G and those who received FOLFIRINOX. A total of 27 studies (>4173 patients) of various populations (aPC, mPC, ECOG PS 0/1) reported a total of 32 median OS values with FOLFIRINOX and 30 with nab-P/G (2 were reported as ‘not reached’; Table 2). Among the 30 direct comparisons, a numerically longer median OS was reported with FOLFIRINOX in 18 versus 9 with nab-P/G; 3 studies reported equal median OS values between the two groups. All reported median OS values for the two groups are shown in Figure 2. A statistical comparison (p value) of OS between the nab-P/G and FOLFIRINOX groups was reported in 13 studies (14 p values). No statistically significant difference in OS between the two groups (as judged by p > 0.05) was reported in 12 of the 14 comparisons; 1 study each reported significantly greater OS with nab-P/G (p = 0.002) and FOLFIRINOX (p = 0.02; Table 2). In two studies that also reported OS in patients with good performance status (ECOG PS 0 or 1), the median OS in patients with ECOG PS 0 or 1 treated with nab-P/G versus FOLFIRINOX were 12.1 versus 11.4 months and 14.1 versus 13.7 months, respectively.27,30
Table 2.
Effectiveness outcomes: OS, PFS, and TTF.
| Study |
n
|
Median 1L OS, mo |
Median 1L PFS, mo |
Median TTF, mo |
|||||
|---|---|---|---|---|---|---|---|---|---|
| nab-P/G | FFX | nab-P/G | FFX | p value | nab-P/G | FFX | nab-P/G | FFX | |
| mPC studies | |||||||||
| Beyer16 | 19 | 57 | 7 | 12 | NR | NR | NR | NR | NR |
| Park17 | 18 | 9 | 6.1 | 9.9 | NR | NR | NR | NR | NR |
| Braiteh20 | 122 | 80 | 8.6a | 8.6a | 0.53 | NR | NR | 3.4b | 3.8b |
| Caponnetto21 | 20 | 23 | NR | NR | NR | 6 | 5 | NR | NR |
| Kim22 | 182 | 163 | NR | NR | NR | NR | NR | 4.3b | 2.8b |
| Mañes-Sevilla23 | 20 | 15 | 9.2 | 11.4 | 0.41 | 5.4 | 7.1 | NR | NR |
| McBride24 | 294 | 256 | NR | NR | NR | NR | NR | 3.8b | 4b |
| Watanabe25c | 65 | 70 | 14.0 | 11.5 | NR | 6.5 | 5.7 | NR | NR |
| Barrera26 | 31 | 44 | 8.1 | 9.9 | 0.09 | 4.6 | 5.8 | NR | NR |
| Cartwright27 | 255 | 159 | 9.8d | 11.4d | 0.38 | NR | NR | 3.7e | 4.3e |
| Cho28 | 81 | 86 | 12.1 | 10.7 | 0.16 | 8.4 | 8.0 | NR | NR |
| Kang29 | 149 | 159f | 11.4 | 9.6 | 0.002 | 6.8 | 5.0 | NR | NR |
| Kim30 | 337 | 317 | 12.1g | 13.8g | 0.28 | NR | NR | NR | NR |
| Pacheco-Barcia31c | 25 | 21 | 7 | 14 | 0.02 | 4 | 8 | NR | NR |
| Vivaldi32c | 57 | 81 | 10.6 | 11.5 | NR | 6.2 | 6.4 | NR | NR |
| Barrera33 | 60 | 66 | NR | NR | NR | 5.5 | 5.1 | NR | NR |
| Henkel34 | 11 | 9 | 7.1 | 8.6 | NR | NR | NR | NR | NR |
| Javed35 | 81 | 204 | 7.9 | 9.9 | NR | NR | NR | NR | NR |
| aPC studiesh | |||||||||
| Cherniawsky36 | NR | NR | 10 | 11 | NR | 6.9 | 8.8 | NR | NR |
| Kasi37 | 47 | 107 | 10.8 | 15.9 | 0.17 | 5.7 | 11.7 | NR | NR |
| Maeda38 | 9 | 16 | 11.5 | 13.1 | NR | 6.1 | 6.3 | NR | NR |
| Muranaka39 | 22 | 16 | Not reached | 9.9 | 0.80 | 6.5 | 3.7 | NR | NR |
| Papneja40 | 33 | 86 | 9 | 9 | 0.88 | 4 | 6 | NR | NR |
| Shahda41 | NR | NR | 11.4–14.4i | 11.3–12.3i | NR | 4.6–6.1i | 5.3–9.4i | NR | NR |
| Wang42 | 87 | 92 | 10.5 (10.0) | 14.1 (9.4) | 0.09 | 8.5 (8.3) | 8.4 (6.6) | NR | NR |
| Franco43 | 49 | 87 | 13 | 13 | NR | NR | NR | NR | NR |
| Helen44 | NR | NR | NR (5.5) | NR (8.8) | NR | NR | NR | NR | NR |
| Kudlovich45 | 21 | 52 | 7.6 (NR) | 8.8 (NR) | NR | NR | NR | NR | NR |
| Tahara46 | 15 | 12 | Not reached (9.8) | 9.7 (9.7) | 0.44 (0.54) | Not reached | 8.8 | NR | NR |
| Terashima47 | 20 | 47 | 9.9 | 10.3 | NR | NR | NR | NR | NR |
| Hegewisch-Becker48 | 489 | 284 | 9.1 | 11.3 | NR | 5.6 | 6.3 | NR | NR |
| Total | 2619 | 2618 | |||||||
1L, first line; aPC, advanced pancreatic cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; FFX, FOLFIRINOX; mPC, metastatic pancreatic cancer; nab-P/G, nab-paclitaxel/gemcitabine; NR, not reported; OS, overall survival; PFS, progression-free survival; TTF, time to treatment failure.
Reported as database persistence, a proxy for OS.
Reported as time to discontinuation.
Modified FFX.
For patients with ECOG PS 0 or 1, median OS was 12.1 and 11.4 months in nab-P/G and FFX groups, respectively
(p = 0.68).
For patients with ECOG PS 0 or 1, median TTF was 4.2 and 4.3 months in nab-P/G and FFX groups, respectively.
59% of patients received modified FFX. The OS and PFS were reported to be statistically similar between FFX and mFFX groups.
For patients with ECOG PS 0 or 1, median OS was 14.1 and 13.7 months in nab-P/G and FFX groups, respectively.
aPC includes mPC. The number in parentheses is the number of patients with mPC.
Biomarker study observing homologous recombination deficiency low versus high in each treatment regimen with data presented here as a range.
Figure 2.
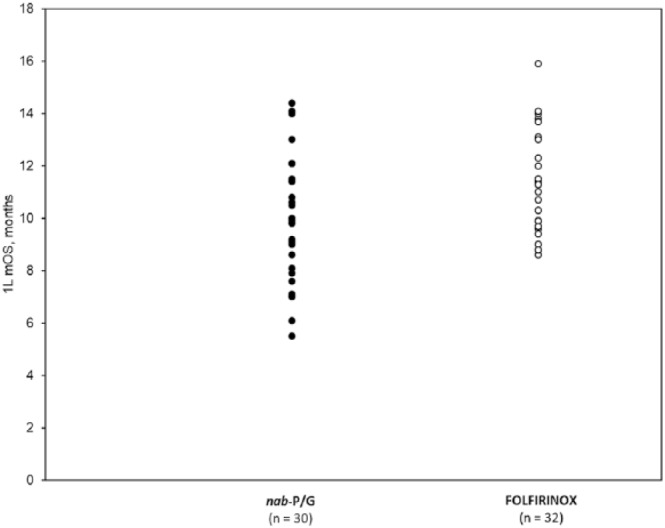
Overall survival in patients with advanced or metastatic pancreatic cancer receiving nab-P/G or FOLFIRINOX.
mOS, median overall survival; n, number of studies reporting mOS; nab-P/G, nab-paclitaxel/gemcitabine.
Among the 18 direct comparisons (10 in patients with mPC and 8 in those with aPC; >2388 patients), the median PFS was numerically longer with FOLFIRINOX in 10 studies versus 7 studies with nab-P/G; in 1 study, the median PFS for the nab-P/G group was not reached. The TTF or time to discontinuation was reported in four mPC studies (1511 patients), with numerically longer TTF in three studies with FOLFIRINOX versus one study with nab-P/G (Table 2).
Overall, six studies reported response data. In two studies that reported response data for patients with mPC, the ORRs were 34% and 39% with nab-P/G versus 34% (p = 0.88) and 27% (p = 0.02), respectively, with FOLFIRINOX.25,29 In four studies that reported response data for patients with aPC, the ORR was greater with nab-P/G or with FOLFIRINOX in two studies each.37,39,46,47 A total of six studies reported disease control rates (DCRs; three each in mPC and aPC). In five of these studies, the DCR was numerically greater with nab-P/G.25,29,37,39,46 Overall, one study (patients with mPC) reported numerically greater DCR with FOLFIRINOX.19
Treatment duration
A total of 10 studies reported the duration of treatment. Patients who received nab-P/G were treated for a median of 95 to 261 days versus 91 to 252 days with FOLFIRINOX.11,20,23,28,29,37,39,40,42,47
Second-line chemotherapy
A total of 10 studies (2184 patients) reported data on the proportion of patients who received second-line chemotherapy. In nine studies that reported data separately for the two regimens, 9–76% of patients treated with nab-P/G as first-line therapy received second-line therapy versus 9–94% of those treated with FOLFIRINOX as first-line therapy.11,17,19,26,29,39,40,42,46 Overall, one study reported that 44% of patients received second-line chemotherapy.33 Only two of these studies reported OS data in patients receiving second-line therapy. One study reported a median OS of 18 months in 6 patients treated with first-line nab-P/G and second-line FOLFIRINOX and 10.8 months in 20 patients treated with first-line FOLFIRINOX and second-line nab-P/G.19 The other study reported second-line median OS of 4.8 months in patients treated with first-line nab-P/G and second-line fluorouracil (alone or with oxaliplatin; 96% of patients) and 4.5 months in patients treated with first-line FOLFIRINOX and a second-line gemcitabine-based regimen (97% of patients).29
Safety outcomes
Safety outcomes were reported in 14 studies (2205 patients), including 8 single-institution studies. The incidence of all grade and grade 3/4 adverse events (AEs) is summarized in Table 3. Among the six single-institution studies to report grade 3/4 neutropenia, the rates were higher with FOLFIRINOX in five studies versus one study with nab-P/G. Among the seven single-institution studies to report grade 3/4 peripheral neuropathy, the rates were higher in one study with FOLFIRINOX versus four with nab-P/G; identical rates were reported in two studies. The incidence of grade 3/4 febrile neutropenia was higher with FOLFIRINOX in all three single-institution studies that reported this AE. In the two single-institution studies that reported discontinuation rates, a numerically greater percentage of patients treated with FOLFIRINOX discontinued due to AEs versus those treated with nab-P/G.
Table 3.
Safety outcomes.
| Study |
n
|
All grade (grade 3/4) AEs, FFX
versus nab-P/G, % |
Discontinuations due to AEs, FFX versus nab-P/G, % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| nab-P/G | FFX | Neutropenia | Peripheral neuropathy | Febrile neutropenia | Diarrhea | Fatigue / asthenia | Others | ||
| mPC studies | |||||||||
| Beyer16 | 19 | 57 | 88 versus 68 (NR) |
42 versus 41 (NR)a |
NR (NR) |
NR (NR) |
NR (NR) / NR (NR) |
• Rapid deterioration: 42 versus 31 (NR) | NR |
| Braiteh20 | 122 | 80 | 54 versus 50 (30 versus 28) |
NR (NR) |
2 versus 1 (3 versus 1) |
15 versus 9 (NR) |
11 versus 9 (NR) / NR (NR) | • Anemia: 66 versus 70 (6
versus 13) • Thrombocytopenia: 45 versus 43 (14 versus 11) • Nausea and vomiting: 29 versus 23 • Dehydration: 21 versus 14 |
Due to • Anemia: 8 versus 2 • Neutropenia: 6 versus 6 • Dehydration: 5 versus 3 • Nausea and vomiting: 0 versus 1 |
| Mañes-Sevilla23b | 20 | 15 | 5 versus 14 (NR) |
18 versus 15 (NR)c |
NR (NR) |
21 versus 4 (NR) |
NR (NR) / 11 versus 22 (NR) |
• AEs: 78 versus 70 (41
versus 35)d
• Vomiting: 23 versus 7 (NR) |
25 versus 17.5e |
| Watanabe25b,f | 65 | 70 | NR (47 versus 45) |
NR (4 versus 5) |
NR (9 versus 2) |
NR (1 versus 2) |
NR (NR) / NR (NR) |
• Anorexia: NR (13 versus 3) | NR |
| Barrera26b | 31 | 44 | NR (20 versus 13) |
NR (4 versus 7) |
NR (NR) |
NR (NR) |
NR (11 versus 26) / NR (NR) | • GI: 27 versus 10 | 11 versus 6.5 |
| Cho28b | 81 | 86 | NR (NR)g | NR (3.5 versus 18.5)d | NR (NR) |
NR (NR)g |
NR (NR) | NR | |
| Kang29b | 126 | 151 | 62 versus 67 (47 versus 37) | 13 versus 33 (3 versus 10) | 5 versus 0 (5 versus 0) | NR (NR) | 14 versus 10 (2 versus 2) / NR (NR) | • Anemia: 22 versus 81 (1
versus 6) • Thrombocytopenia: 19 versus 56 (3 versus 6) • Nausea: 22 versus 10 (17 versus 2) • Elevated transaminases: 9 versus 7 (0 versus 0) |
NR |
| Kim30 | 337 | 317 | 32 versus 33 (NR) |
NR (NR) |
16 versus 9 (NR) |
40 versus 21 (NR) |
44 versus 32 (NR) / NR (NR) | • Anemia: 45 versus 46
(NR) • Thrombocytopenia: 23 versus 27 (NR) • Stomatitis: 18 versus 4 (NR) • Abdominal pain: 18 versus 21 (NR) • Alopecia: 17 versus 16 (NR) • Decreased appetite: 30 versus 26 (NR) • Dehydration: 23 versus 15 (NR) • Mucositis: 18 versus 5 (NR) • Nausea and vomiting: 29 versus 18 (NR) |
NR |
| aPC studies h | |||||||||
| Kasi37b | 47 | 107 | NR (33 versus 17)d |
NR (6 versus 6)d |
NR (NR)d |
NR (5 versus 0)d |
NR (NR) / NR (NR) |
• Anemia: NR (14 versus 31)d
• Thrombocytopenia: NR (28 versus 6)d • Elevated transaminases: NR (4 versus 6)d • Elevated creatinine: (3 versus 4)d |
NR |
| Muranaka39b | 22 | 16 | 94 versus 100 (69 versus 55) |
31 versus 41 (0 versus 0) |
19 versus 9 (19 versus 9) |
44 versus 18 (0 versus 0) |
NR (NR) / NR (NR) |
• Anemia: 94 versus 86 (6
versus 18) • Nausea: 81 versus 23 (6 versus 0) • Anorexia: 81 versus 36 (6 versus 5) • Thrombocytopenia: 44 versus 55 (6 versus 14) • Alopecia: 19 versus 55 (NR) • Vomiting: 13 versus 5 (0 versus 0) |
NR |
| Papneja40 | 33 | 86 | 47 versus 58 (NR) |
63 versus 36 (NR)i |
NR (NR) |
52 versus 18 (NR) |
66 versus 76 (NR) / NR (NR) | • Anemia: 55 versus 33
(NR) • Nausea: 53 versus 39 (NR) • Thrombocytopenia: 48 versus 57 (NR) • Infection: 37 versus 33 (NR) • DVT/PE: 34 versus 12 (NR) • Thromboembolism: 34 versus 9 (NR) • Vomiting: 26 versus 9 (NR) • Mucositis: 21 versus 3 (NR) • Rash: 6 versus 9 (NR) • Bleeding: 4 versus 6 (NR) |
NR |
| Wang42 | 87 | 92 | NR (NR) |
NR (NR) |
8 versus 1 (NR) |
NR (NR) |
NR (NR) / NR (NR) |
• Neutropenia leading to dose modification: 16
versus 15 (NR) • Neuropathy leading to dose modification: 21 versus 10 (NR) • Required ⩾2 dose modifications: 40 versus 13 (NR) |
28 versus 18.6 |
| Tahara46b | 15 | 12 | 92 versus 87 (58 versus 60) | 75 versus 73 (8 versus 7) | 0 versus 0 (NR) | 8 versus 20 (0 versus 0) | NR (NR) / NR (NR) | • Anemia: 58 versus 73 (25
versus 7) • Thrombocytopenia: 8 versus 20 (0 versus 7) • Interstitial pneumonia: 0 versus 27 (NR) • Thrombosis: 42 versus 7 • Liver disorder: 8 versus 13 |
NR |
| Terashima47 | 20 | 47 | 77 versus 65 (64 versus 45)d | NR (NR) | NR (NR) | 40 versus 5 (4 versus 0)d | 43 versus 50 (0 versus 0)d | • WBC decreased: 83 versus 75 (36
versus 30)d
• Anemia: 77 versus 65 (11 versus 25)d • Platelet count decreased: 68 versus 40 (8 versus 10)d • Fever: 17 versus 30 (0 versus 0)d • Vomiting: 17 versus 10 (2 versus 0)d • Anorexia: 68 versus 35 (8 versus 0)d • Pneumonitis: 2 versus 0 (2 versus 0)d |
NR |
| Total | 1025 | 1180 | |||||||
AE, adverse event; aPC, advanced pancreatic cancer; DVT, deep vein thrombosis; FFX, FOLFIRINOX; GI, gastrointestinal; mPC, metastatic pancreatic cancer; nab-P/G, nab-paclitaxel/gemcitabine; NR, not reported; PE, pulmonary embolism; WBC, white blood cell.
Reported as polyneuropathy.
Single-institution studies
Reported as sensory neuropathy.
Grade 3 or higher.
Reported as treatment suspension.
Used modified FFX.
Grade ⩾3 neutropenia and GI events were reported to be more common in the FFX group.
aPC includes mPC.
Reported as neuropathy.
Supportive care/resource utilization
A total of five studies (1986 patients) reported data on supportive care or resource utilization.18,20,22,25,27 In two studies (605 patients) that reported frequency of granulocyte colony-stimulating factor (G-CSF) use, fewer patients treated with nab-P/G received G-CSF (0% and 27%) versus those treated with FOLFIRINOX (21% and 55%, respectively).18,25 Overall, one study reported that patients treated with nab-P/G received fewer doses of G-CSF (2.02 per 100 days) and more doses of steroids (7.89 per 100 days) and erythropoiesis-stimulating agents (0.9 per 100 days) versus those treated with FOLFIRINOX (4.41, 5.79, and 0.13 doses per 100 days, respectively).20 A study (486 patients) reported that significantly fewer patients treated with nab-P/G received pegfilgrastim (13%) versus those treated with FOLFIRINOX (43%); the use of darbepoetin, antibiotics, pain medications, or medications for treating chemotherapy-induced nausea/vomiting was similar in the two groups.27 A study (345 patients) reported that hospitalization rates were significantly lower in patients treated with nab-P/G (24.7%) versus those treated with FOLFIRINOX (36.8%; p = 0.027), and hospital stays were significantly shorter with nab-P/G (1.7 days) versus FOLFIRINOX (3.6 days; p = 0.002).22
Cost of care
A total of four studies (1532 patients) reported data on costs associated with healthcare in patients treated with nab-P/G and FOLFIRINOX.18,22,24,28 Overall, two studies reported that the total monthly cost of care for patients treated with nab-P/G was US$16,628 and US$23,605 versus US$19,936 and US$26,575, respectively, for those treated with FOLFIRINOX.22,24 Monthly costs related to chemotherapy ranged from US$11,662 to US$12,103 for patients treated with nab-P/G and US$6384 to US$9564 for those treated with FOLFIRINOX, while those for supportive care ranged from US$1836 to US$2966 and US$3955 to US$8758, respectively.18,22,24 The majority of the supportive care cost was the cost of G-CSF, which ranged from US$917 to US$1005 and US$3214 to US$5459 per month, respectively.18,22,24 A study reported that the costs for patients treated with nab-P/G were lower for filgrastim (US$234), pegfilgrastim (US$759), and drug administration (US$1859) versus those treated with FOLFIRINOX (US$529, US$4860, and US$2969, respectively).24 Another study reported that the costs for erythropoietin, transfusions, and antiemetics were similar for the two regimens, but no individual values were reported.18 Another study that did not provide specific data noted that the cost of anticancer therapy was similar in the two groups, but the total cost of treatment was slightly lower in the nab-P/G group.28
Discussion
This systematic review reports data from 34 studies that assessed first-line treatment in a real-world setting of 16,505 patients, including at least 6349 patients with mPC who were treated with nab-P/G or FOLFIRINOX (see Table 1). Although the effectiveness of nab-P/G and FOLFIRINOX in patients with mPC or aPC varied among studies, most of the survival data tended to overlap (see Table 2 and Figure 2). Furthermore, in 12 of 14 statistical comparisons, the OS was not significantly different between patients treated with nab-P/G versus FOLFIRINOX. Overall, these data suggest that first-line nab-P/G and FOLFIRINOX have similar effectiveness in patients with aPC in the real-world setting.
In most studies reporting safety, the authors concluded that nab-P/G exhibited a more favorable safety profile than FOLFIRINOX. Some studies reported that patients treated with nab-P/G experienced fewer and less severe AEs, needed dose modifications less frequently, and discontinued less often because of AEs. In real-world studies, comparing safety data, especially nonlaboratory-based AEs, is problematic due to the lack of uniform definitions as used in randomized clinical trials; therefore, we focused on comparing grade 3/4 AEs reported for the two regimens within single-institutional studies. In most of these studies, the rates were higher with FOLFIRINOX versus nab-P/G for grade 3/4 neutropenia (five of six studies) and febrile neutropenia (three of three studies). Consistent with this, a numerically greater proportion of patients discontinued FOLFIRINOX treatment due to AEs in two studies. In contrast, the rates of grade 3/4 peripheral neuropathy were higher with nab-P/G versus FOLFIRINOX in most (four of seven) single-institutional studies. However, in the MPACT trial, grade ⩾3 neuropathy associated with nab-P/G was reported to improve to grade ⩽1 in a median of 29 days.7 Studies reporting supportive care, resource utilization, and cost data were limited but provided some insight into the differences between regimens, such as lower use of G-CSF with nab-P/G. Monthly costs of chemotherapy were higher with nab-P/G, but the overall monthly cost ranges were higher with FOLFIRINOX. Additional options, such as use of biosimilar G-CSF, may help reduce the overall cost associated with FOLFIRINOX treatment.
In 2014, Gresham and colleagues performed a systematic review and network meta-analysis of randomized clinical trials of chemotherapy regimens in patients with aPC (9989 patients in 23 studies) that included nab-P/G and FOLFIRINOX.12 Our observation that there was no clear distinction in effectiveness between nab-P/G and FOLFIRINOX is consistent with their report of no significant difference in OS or PFS between the two regimens. In direct comparisons, FOLFIRINOX was associated with significantly higher odds of grade 3/4 neutropenia versus nab-P/G, whereas no statistically significant difference was noted between the two regimens in the odds ratios for grade 3/4 sensory neuropathy, fatigue, diarrhea, or febrile neutropenia.12
In the PRODIGE/ACCORD trial, the most common grade 3/4 AEs with FOLFIRINOX were neutropenia (46%), fatigue (24%), vomiting (15%), diarrhea (13%), thrombocytopenia (9%), sensory neuropathy (9%), anemia (8%), thromboembolism (7%), and febrile neutropenia (5%).6 In the MPACT study, the most common grade ⩾3 AEs with nab-P/G were neutropenia (38%), fatigue (17%), peripheral neuropathy (17%), thrombocytopenia (13%), anemia (13%), diarrhea (6%), and febrile neutropenia (3%).7 Post hoc analyses of the MPACT trial also demonstrated that nab-P/G dose reductions and dose delays are additional strategies that can help reduce toxicity without compromising efficacy, and that prolonged first-line treatment with nab-P/G until disease progression can improve survival rates.49,50 Similarly, various modifications in FOLFIRINOX components and doses are often experimented with in clinical practice in efforts to improve outcomes.
The recent results from the PRODIGE 24-ACCORD trial showed significantly longer disease-free survival and OS in patients receiving a modified FOLFIRINOX regimen (without bolus fluorouracil) compared with those receiving gemcitabine in the adjuvant setting.51 The most common grade 3/4 AEs reported with the modified FOLFIRINOX regimen were neutropenia (28%), diarrhea (19%), increased γ-glutamyltransferase levels (18%), paresthesia (13%), fatigue (11%), sensory peripheral neuropathy (9%), nausea (5%), and vomiting (5%).51 These results may support tolerability of modified FOLFIRINOX in the adjuvant setting and suggest the potential for use in patients with mPC. The National Comprehensive Cancer Network guidelines were revised recently to include modified FOLFIRINOX as a preferred category 1 recommendation for mPC.5
The results from the MPACT and PRODIGE trials brought meaningful promise to the treatment landscape for patients with mPC; therefore, the focus now is centered on determining the optimal sequence of these regimens. As a result of the phase III NAPOLI-1 trial, liposomal irinotecan plus fluorouracil and leucovorin is a National Comprehensive Cancer Network category 1 recommendation for patients who received first-line gemcitabine-based therapy.5,52,53 Further, several early-stage clinical trials have investigated the sequencing of nab-P and fluorouracil-based regimens. In GABRINOX, a phase I/II study that assessed treatment with nab-P/G followed by FOLFIRINOX in patients with mPC, a median OS of 17.8 months was reported.54 However, this regimen resulted in grade 3/4 neutropenia, thrombocytopenia, and diarrhea occurring at higher frequencies compared with nab-P/G or FOLFIRINOX alone.6,7 In SEENA-1, a phase II study of nab-P/G followed by either modified FOLFIRINOX (without bolus fluorouracil) or nab-P/G alternating with FOLFIRI (without oxaliplatin), a median OS of 12.3 months and a safety profile generally similar to that for nab-P/G or FOLFIRINOX alone were reported.6,7,55 A phase II study recently showed that using FOLFIRINOX in a stop-and-go fashion (4-month FOLFIRINOX followed by LV5FU2 maintenance) produced similar efficacy (PFS, 5.7 months; OS, 11.2 months) compared with 6-month FOLFIRINOX (PFS, 6.3 months; OS, 10.1 months).56 However, this strategy resulted in a greater proportion of patients with grade 3/4 neurotoxicity (19%) compared with standard FOLFIRINOX (10%). Finally, multiple algorithms have been proposed to guide treatment decisions in individual cases.57,58 Future efforts may shed additional light on appropriate sequencing regimens for personalized care.
Study limitations
The sample size of 34 studies may be considered relatively small, and this may impact the ability to draw strong conclusions from the data. In addition, in the absence of standardizing criteria used in randomized clinical trials, interpretation of certain outcomes (e.g. response data) is problematic with real-world evidence from multi-institutional studies. Furthermore, the studies varied in terms of population, treatment duration, study design, and details of specific results. Some of the differences in patient characteristics may also have affected the observed outcomes. For example, healthier/younger patients treated more frequently with FOLFIRINOX versus nab-P/G may have confounded the results in some studies.17,23,26,27,31,37,40 Finally, although data regarding cost have been presented, few studies report this type of information, and it is typically difficult to standardize or quantify.
In addition, studies of patients with aPC were included to maximize the inclusion of patients with mPC. The OS, a more reliable indicator of effectiveness than other measures, was reported in only approximately 50% of the patients treated with nab-P/G or FOLFIRINOX. To date, data from most of the included studies were not completely mature, and some studies did not report patient numbers, which may have added to the variability and lower quality of some studies caused by a reporting bias. Overall, the variability did not allow for performance of a robust meta-analysis with specific comparisons of effectiveness and safety outcomes.
Conclusion
To our knowledge, this is the first systematic review examining real-world outcomes with nab-P/G versus FOLFIRINOX as first-line therapy in patients with aPC or mPC. In the absence of a direct comparison in a head-to-head clinical trial of first-line therapy in patients with mPC, this systematic review may help highlight differences between the two regimens to assist with clinical decision making. While this report has limitations inherent to systematic reviews, it included a large number of patients, and a comparison of the real-world data with the published clinical trial data allowed for overarching conclusions with respect to outcomes. The variability of data in the current literature makes it difficult to directly compare the two standard first-line regimens for mPC. Although FOLFIRINOX was associated with slightly longer median OS in more studies, the differences, when available, were not statistically significant. Furthermore, FOLFIRINOX was associated with more treatment-related toxicities. Individual patient considerations, goals of care, and future molecular marker-driven clinical trials may improve patient–physician decision on selecting the best sequence of anticancer therapy for mPC.
Supplemental Material
Supplemental material, Supplementary_Table_1 for Real-world comparative effectiveness of nab-paclitaxel plus gemcitabine versus FOLFIRINOX in advanced pancreatic cancer: a systematic review by Elena Gabriela Chiorean, Winson Y. Cheung, Guido Giordano, George Kim and Salah-Eddin Al-Batran in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank Padmini Tandavakrishna, MS, for her assistance with the literature search. Writing assistance was provided by Narender Dhingra, MBBS, PhD, of MediTech Media, Ltd., and funded by Celgene Corporation. MediTech Media also provided editorial support. The authors are fully responsible for all content and editorial decisions for this manuscript.
Appendix
Appendix A.
Search terms and strategy.
| Coverage and strategy | Search terms |
|---|---|
| No. 1 (populations) | Pancreatic neoplasms OR pancreas cancer OR pancreas adenocarcinoma OR pancrea* n/3 adenocarcinoma OR pancrea* n/3 neoplasm |
| No. 2 (interventions) | Albumin bound paclitaxel OR nab-paclitaxel OR abraxane OR folfirinox |
| No. 3 (study design) | Observational OR registries OR retrospective* OR population OR prospective OR claims database OR electronic medical OR health records OR single center study |
| No. 4 (outcomes) | Treatment pathway OR overall survival OR progression-free survival OR disease-free survival OR disease progression OR ORR OR CR OR PR OR quality of life OR HRQOL OR QOL utility OR EQ-5D OR SF-6D OR functional assessment of cancer therapy OR FACT OR EORTC QLQ-C30 OR treatment and pathway OR sequence OR duration OR modification OR failure OR patient-reported outcome OR patient-reported outcome measures OR patient-reported outcomes OR health care utilization OR resource utilization OR hospitalization |
| Final search | Nos. 1 AND 2 AND 3 AND 4 |
Note: The asterisk was used as a wildcard symbol to capture the terms ‘pancreatic’ and ‘pancreas’, and ‘n/3’ limited the capturing to terms appearing within three words of ‘adenocarcinoma’ or ‘neoplasm’.
Footnotes
Funding: This work was supported by Celgene Corporation.
Conflict of interest statement: EGC has served in an advisory role for Pfizer, Novocure, Genentech/Roche, Celgene, AstraZeneca, Eisai, Five Prime, Vicus, Halozyme, Seattle Genetics, Ipsen, and Array; she has received travel, accommodations, expenses from AstraZeneca; and her institution has received research funding from Celgene, Incyte, Stemline Therapeutics, Ignyta, Merck, Lilly, and Boehringer Ingelheim. WYC declares no conflict of interest. GG has received honoraria from Celgene and Sanofi; consulting or advisory role for Celgene; and he has received travel, accommodations, expenses from Celgene. GK has been a consultant and speaker for Celgene and Ipsen. SEAB has been in a consulting or advisory role for Merck, Roche, Celgene, Lilly, Bristol-Myers Squibb, and SERVIER; he has been on the speakers’ bureau for Lilly, Roche, Celgene, and Nordic Bioscience; he has received funding from Celgene, Roche Pharma AG, Lilly, Novartis, Vifor Pharma, Medac, and Hospira.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Elena Gabriela Chiorean, Fred Hutchinson Cancer Research Center, University of Washington School of Medicine, 825 Eastlake Ave East, Seattle, WA 98109, USA.
Winson Y. Cheung, Alberta Health Services, Calgary, Canada
Guido Giordano, IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy.
George Kim, 21st Century Oncology, Jacksonville, FL, USA.
Salah-Eddin Al-Batran, Institute of Clinical Cancer Research, Krankenhaus Nordwest, University Cancer Center, Frankfurt, Germany.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society. Cancer facts and figures 2019, https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf (2019, accessed 14 February 2019).
- 3. Lau SC, Cheung WY. Evolving treatment landscape for early and advanced pancreatic cancer. World J Gastrointest Oncol 2017; 9: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ducreux M, Cuhna AS, Caramella Cet al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26(Suppl. 5): v56–v68. [DOI] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: pancreatic adenocarcinoma. Version 1.2019, https://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf (2018, accessed 11 January 2019).
- 6. Conroy T, Desseigne F, Ychou Met al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 7. Von Hoff DD, Ervin T, Arena FPet al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldstein D, El-Maraghi RH, Hammel Pet al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst 2015; 107: dju413. [DOI] [PubMed] [Google Scholar]
- 9. Panciroli C, Estival A, Lucente Get al. ECOG or Karnofsky performance status to assess functionality in glioblastoma patients among different observers. Mol Biomark Diagn 2017; S2: 032. [Google Scholar]
- 10. Macarulla T, Pazo-Cid R, Guillen-Ponce Cet al. Phase I/II trial to evaluate the efficacy and safety of nanoparticle albumin-bound paclitaxel in combination with gemcitabine in patients with pancreatic cancer and an ECOG performance status of 2. J Clin Oncol 2019; 37: 230–238. [DOI] [PubMed] [Google Scholar]
- 11. Abrams TA, Meyer G, Meyerhardt JAet al. Patterns of chemotherapy use in a US-based cohort of patients with metastatic pancreatic cancer. Oncologist 2017; 22: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gresham GK, Wells GA, Gill Set al. Chemotherapy regimens for advanced pancreatic cancer: a systematic review and network meta-analysis. BMC Cancer 2014; 14: 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells GA, Shea B, O’Connell Det al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2018, accessed 8 May 2018).
- 14. Li DY, Hao XY, Ma TMet al. The prognostic value of platelet-to-lymphocyte ratio in urological cancers: a meta-analysis. Sci Rep 2017; 7: 15387–017–15673–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castillo JJ, Dalia S, Pascual SK. Association between red blood cell transfusions and development of non-Hodgkin lymphoma: a meta-analysis of observational studies. Blood 2010; 116: 2897–2907. [DOI] [PubMed] [Google Scholar]
- 16. Beyer G, Javed MA, Le Net al. Real-life use of intensified chemotherapy for metastatic pancreatic cancer in Europe. United European Gastroenterol J 2016; 5: A376. [Google Scholar]
- 17. Park PYS, McAferty KV, Ahmadi Met al. Radiological markers of treatment responsiveness in patients (pts) with metastatic pancreatic ductal adenocarcinomas (mPDAC) receiving systemic chemotherapy. J Clin Oncol 2016; 34: 403. [Google Scholar]
- 18. Patel M, Parisi M, Hill JWet al. Utilization and cost of supportive care among patients receiving nab-paclitaxel + gemcitabine versus FOLFIRINOX for metastatic pancreatic cancer. Value Health 2016; 19: A143. [Google Scholar]
- 19. Schmidt SL, Durkal V, Pattali Jayavalsan Set al. Can the sequence of chemotherapy regimens influence outcome in patients with metastatic pancreatic adenocarcinoma (MPAC)? J Clin Oncol 2016; 34: 428. [Google Scholar]
- 20. Braiteh F, Patel MB, Parisi Met al. Comparative effectiveness and resource utilization of nab-paclitaxel plus gemcitabine vs FOLFIRINOX or gemcitabine for the first-line treatment of metastatic pancreatic adenocarcinoma in a US community setting. Cancer Manag Res 2017; 9: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caponnetto S, Gelibter A, Mosillo Cet al. Comparative effects of FOLFIRINOX and gemcitabine/nab-paclitaxel as first and second line chemotherapy for metastatic pancreatic cancer: single choice or sequence. Ann Oncol 2017; 28: D27. [Google Scholar]
- 22. Kim GP, Parisi MF, Patel MBet al. Comparison of treatment patterns, resource utilization, and cost of care in patients with metastatic pancreatic cancer treated with first-line nab-paclitaxel plus gemcitabine or FOLFIRINOX. Expert Rev Clin Pharmacol 2017; 10: 559–565. [DOI] [PubMed] [Google Scholar]
- 23. Manes Sevilla M, Gonzalez Haba E, Marzal Alfaro Met al. Effectiveness and safety with new treatments in pancreatic cancer. Eur J Clin Pharm 2017; 19: 233–238. [Google Scholar]
- 24. McBride A, Bonafede M, Cai Qet al. Healthcare costs and treatment patterns among metastatic pancreatic cancer (MPC) patients (pts) initiating first-line (1L) on nab-paclitaxel/gemcitabine (nab-P+G) or FOLFIRINOX (FFX). J Clin Oncol 2017; 35: 415. [Google Scholar]
- 25. Watanabe K, Hashimoto Y, Umemoto Ket al. Clinical outcome of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel as first line chemotherapy in metastatic pancreatic cancer. J Clin Oncol 2017; 35: 438. [Google Scholar]
- 26. Barrera I, Hamalova S, Ranger Jet al. FOLFIRINOX (FFX) versus gemcitabine with nab-paclitaxel (GNP) in the first line treatment (1LTx) of metastatic pancreatic cancer (mPC): a tertiary center experience. J Clin Oncol 2018; 36: 414.29236593 [Google Scholar]
- 27. Cartwright TH, Parisi M, Espirito JLet al. Clinical outcomes with first-line chemotherapy in a large retrospective study of patients with metastatic pancreatic cancer treated in a US community oncology setting. Drugs Real World Outcomes 2018; 5: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cho I, Kang H, Jo Jet al. FOLFIRINOX versus gemcitabine plus nab-paclitaxel for treatment of metastatic pancreatic cancer: a single-center cohort study. Ann Oncol 2018; 29: P-161. [Google Scholar]
- 29. Kang J, Hwang I, Yoo Cet al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs 2018; 36: 732–741. [DOI] [PubMed] [Google Scholar]
- 30. Kim S, Signorovitch JE, Yang Het al. Comparative effectiveness of nab-paclitaxel plus gemcitabine vs FOLFIRINOX in metastatic pancreatic cancer: a retrospective nationwide chart review in the United States. Adv Ther 2018; 35: 1564–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pacheco-Barcia V, France T, Zogopoulos Get al. Gemcitabine plus nab-paclitaxel versus modified FOLFIRINOX as first line chemotherapy in metastatic pancreatic cancer: a comparison of toxicity and survival. Ann Oncol 2018; 29: Abstract 164. [Google Scholar]
- 32. Vivaldi C, Cappelli C, Donati Fet al. Analysis of early tumor shrinkage and depth of response in metastatic pancreatic cancer patients treated with first-line modified FOLFIRINOX or gemcitabine + nab-paclitaxel. Ann Oncol 2018; 29: Abstract 159. [Google Scholar]
- 33. Barrera I, Ranger J, Roofigari Net al. Treatment sequencing in MPC, insights from a 3° care center. J Clin Oncol 2019; 37: Abstract 400. [Google Scholar]
- 34. Henkel E, Hernandez B, Michalek Jet al. Combination chemotherapy for pancreatic cancer in older adults: efficacy and safety analysis of patients at a majority-Hispanic NCI-designated cancer center. J Clin Oncol 2019; 37: Abstract 389. [Google Scholar]
- 35. Javed MA, Beyer G, Le Net al. Impact of intensified chemotherapy in metastatic pancreatic ductal adenocarcinoma (PDAC) in clinical routine in Europe. Pancreatology 2019; 19: 97–104. [DOI] [PubMed] [Google Scholar]
- 36. Cherniawsky HM, Wang Y, Ghosh Set al. Effect of advanced age, elevated bilirubin, and disease extent on outcomes of unresectable pancreatic cancer (UPC) patients receiving first-line chemotherapy: a population-based study. J Clin Oncol 2017; 35: 325.28095274 [Google Scholar]
- 37. Kasi A, Middinti A, Cao Aet al. FOLFIRINOX versus gemcitabine nab-paclitaxel for advanced pancreatic cancer: KU Cancer Center experience. J Clin Oncol 2017; 35: e15744. [Google Scholar]
- 38. Maeda O, Yokoyama Y, Yamaguchi Jet al. Real-world experience with FOLFIRINOX and gemcitabine plus nab-paclitaxel in the treatment of pancreatic cancer in Japan. Ann Oncol 2017; 28: 233P. [Google Scholar]
- 39. Muranaka T, Kuwatani M, Komatsu Yet al. Comparison of efficacy and toxicity of FOLFIRINOX and gemcitabine with nab-paclitaxel in unresectable pancreatic cancer. J Gastrointest Oncol 2017; 8: 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papneja N, Olson C, Chalchal Het al. Comparisons of outcomes of patients with advanced pancreatic cancer (APC) treated with FOLFIRINOX (FX) versus gemcitabine and nab-paclitaxel (GN): a population-based cohort study. Ann Oncol 2017; 28: 748P. [DOI] [PubMed] [Google Scholar]
- 41. Shahda S, Timms K, Ibrahim Aet al. Homologous recombination deficiency (HRD) in patients with pancreatic cancer (PC) and response to chemotherapy. J Clin Oncol 2017; 35: 317. [DOI] [PubMed] [Google Scholar]
- 42. Wang Y, Camateros P, Cheung WY. A real-world comparison of FOLFIRINOX, gemcitabine plus nab-paclitaxel, and gemcitabine in advanced pancreatic cancers. J Gastrointest Cancer 2017; 50: 62–68. [DOI] [PubMed] [Google Scholar]
- 43. Franco FF, C.Juan C, Valades JIMet al. Clinical outcomes of FOLFIRINOX and gemcitabine/nab-paclitaxel in the real world setting. J Clin Oncol 2018; 36: 440. [Google Scholar]
- 44. Helen G, Beca JM, Redmond-Misner Ret al. Comparative effectiveness and safety of the implementation of universal public funding of FOLFIRINOX (FFX) and gemcitabine (G) + nab-paclitaxel (GnP) in advanced pancreatic cancer (APC): a population-based study. J Clin Oncol 2018; 36: 375. [Google Scholar]
- 45. Kudlovich R, Zhang H, Kim Cet al. Treatment patterns, toxicity, and outcomes of elderly patients with advanced pancreatic cancer receiving first-line chemotherapy. J Clin Oncol 2018; 36: Abstract 71. [Google Scholar]
- 46. Tahara J, Shimizu K, Otsuka Net al. Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol 2018; 82: 245–250. [DOI] [PubMed] [Google Scholar]
- 47. Terashima T, Yamashita T, Sakai Aet al. Treatment patterns and outcomes of unresectable pancreatic cancer patients in real-life practice: a region-wide analysis. Jpn J Clin Oncol 2018; 48: 966–973. [DOI] [PubMed] [Google Scholar]
- 48. Hegewisch-Becker S, Aldaoud A, Wolf Tet al. Results from the prospective German TPK clinical cohort study: treatment algorithms and survival of 1,174 patients with locally advanced, inoperable, or metastatic pancreatic ductal adenocarcinoma. Int J Cancer 2019; 144: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scheithauer W, Ramanathan RK, Moore Met al. Dose modification and efficacy of nab-paclitaxel plus gemcitabine vs. gemcitabine for patients with metastatic pancreatic cancer: phase III MPACT trial. J Gastrointest Oncol 2016; 7: 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vogel A, Rommler-Zehrer J, Li JSet al. Efficacy and safety profile of nab-paclitaxel plus gemcitabine in patients with metastatic pancreatic cancer treated to disease progression: a subanalysis from a phase 3 trial (MPACT). BMC Cancer 2016; 16: 817–016–2798–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Conroy T, Hammel P, Hebbar Met al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018; 379: 2395–2406. [DOI] [PubMed] [Google Scholar]
- 52. Wang-Gillam A, Li CP, Bodoky Get al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 53. Wang-Gillam A, Hubner RA, Siveke JTet al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: final overall survival analysis and characteristics of long-term survivors. Eur J Cancer 2019; 108: 78–87. [DOI] [PubMed] [Google Scholar]
- 54. Assenat E, De La Fouchardiere C, Mollevi Cet al. Sequential treatment with nab-paclitaxel plus gemcitabine and FOLFIRINOX in metastatic pancreatic adenocarcinoma: GABRINOX phase II results. J Clin Oncol 2018; 36S: Abstract 4109. [Google Scholar]
- 55. Picozzi VJ, Leach JW, Seng JEet al. Initial gemcitabine/nab-paclitaxel (GA) followed by sequential (S) mFOLFIRINOX or alternating (A) mFOLFIRI in metastatic pancreatic cancer (mPC): the SEENA-1 study. J Clin Oncol 2017; 35: Abstract 359. [Google Scholar]
- 56. Dahan L, Phelip JM, Le Malicot Ket al. FOLFIRINOX until progression, FOLFIRINOX with maintenance treatment, or sequential treatment with gemcitabine and FOLFIRl.3 for first-line treatment of metastatic pancreatic cancer: a randomized phase II trial (PRODIGE 35-PANOPTIMOX). J Clin Oncol 2018; 36S: Abstract 4000. [Google Scholar]
- 57. Martin AM, Hidalgo M, Alvarez Ret al. From first line to sequential treatment in the management of metastatic pancreatic cancer. J Cancer 2018; 9: 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Uccello M, Moschetta M, Mak Get al. Towards an optimal treatment algorithm for metastatic pancreatic ductal adenocarcinoma (PDA). Curr Oncol 2018; 25: e90–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table_1 for Real-world comparative effectiveness of nab-paclitaxel plus gemcitabine versus FOLFIRINOX in advanced pancreatic cancer: a systematic review by Elena Gabriela Chiorean, Winson Y. Cheung, Guido Giordano, George Kim and Salah-Eddin Al-Batran in Therapeutic Advances in Medical Oncology