Abstract
α-Fetoprotein is commonly used in the diagnosis of hepatocellular carcinoma. However, the diagnostic significance of α-fetoprotein has been questioned because a number of patients with hepatocellular carcinoma are α-fetoprotein negative. It is therefore necessary to develop novel noninvasive techniques for the early diagnosis of hepatocellular carcinoma, particularly when α-fetoprotein level is low or negative. The current study aimed to evaluate the diagnostic efficiency of hematological parameters to determine which can act as surrogate markers in α-fetoprotein–negative hepatocellular carcinoma. Therefore, a retrospective study was conducted on a training set recruited from Zhongnan Hospital of Wuhan University—including 171 α-fetoprotein–negative patients with hepatocellular carcinoma and 102 healthy individuals. The results show that mean values of mean platelet volume, red blood cell distribution width, mean platelet volume–PC ratio, neutrophils–lymphocytes ratio, and platelet count–lymphocytes ratio were significantly higher in patients with hepatocellular carcinoma in comparison to the healthy individuals. Most of these parameters showed moderate area under the curve in α-fetoprotein–negative patients with hepatocellular carcinoma, but their sensitivities or specificities were not satisfactory enough. So, we built a logistic regression model combining multiple hematological parameters. This model presented better diagnostic efficiency with area under the curve of 0.922, sensitivity of 83.0%, and specificity of 93.1%. In addition, the 4 validation sets from different hospitals were used to validate the model. They all showed good area under the curve with satisfactory sensitivities or specificities. These data indicate that the logistic regression model combining multiple hematological parameters has better diagnostic efficiency, and they might be helpful for the early diagnosis for α-fetoprotein–negative hepatocellular carcinoma.
Keywords: hematological parameters, AFP-negative HCC, diagnostic model
Introduction
Hepatocellular carcinoma (HCC) is the most common malignant cancer and the leading cause of cancer-associated deaths worldwide.1,2 The rapid increase of HCC cases is due to increased incidences of viral infections3 and metabolic diseases.4 The clinical course of HCC is mostly asymptomatic. In patients with HCC, focal changes in liver tissue are often incidentally detected in abdominal ultrasonography examination. The cancer is often too large and too advanced to be effectively treated,5 resulting in a poor 5-year survival rate.6 Nonetheless, the 5-year survival rate rises to over 70% if HCC is diagnosed at its early stage. α-fetoprotein (AFP) is commonly used in HCC diagnosis.1 However, its significance in HCC diagnosis is always questioned and debated. Elevated serum AFP is only observed in 60% to 70% of patients with HCC. When the cancer is less than 3 cm in diameter, merely 33% to 65% of patients with HCC have high serum AFP level.1,7 Furthermore, the nonspecific increase in serum AFP is observed in 11% to 47% of patients with liver cirrhosis.7 In addition, a substantial proportion of patients with HCC are AFP negative.8 Therefore, identification of novel biomarkers for early diagnosis of HCC might benefit patients with HCC, especially AFP-negative patients. Some biomarkers, such as glypican-3, Golgi protein-73, and micro-RNAs, are promising for screening early-stage HCC. However, their usage in clinical diagnosis is still limited, since their roles in HCC pathogenesis are not thoroughly understood.9 Accumulated evidence demonstrates that chronic inflammation and healing in the liver are closely related to HCC development. Chronic inflammation triggers persistent hepatic injury and concurrent regeneration, leading to the sequential development of fibrosis, cirrhosis, and eventually HCC.10 It is reported that more than 90% of HCC cases arise in the context of hepatic injury and inflammation.11 Preclinical and clinical studies have also pinpointed a plethora of inflammatory mediators and signaling pathways involved in HCC.12-15
Given the importance of inflammatory responses in HCC, we designed a survey and retrospectively evaluated the significance of hematological parameters in distinguishing AFP-negative patients with HCC from healthy individuals. In particular, we focused on white blood cell count (WBC), mean platelet volume (MPV), red blood cell distribution width (RDW), MPV/PC ratio, number of neutrophil/lymphocyte ratio (NLR), and platelet count/lymphocyte ratio (PLR). Corresponding receiver–operating characteristic (ROC) curves were generated to evaluate their diagnostic potentials. Finally, logistic regression prediction model was built for AFP-negative HCC and was validated in multiple patient sets from different hospitals.
Materials and Methods
Training Set
We retrospectively investigated 171 AFP-negative patients with HCC (serum AFP <20 µg/L1) at Zhongnan Hospital of Wuhan University from November 2016 to March 2018. Hepatocellular carcinoma (HCC) was diagnosed according to the guideline via histology or by 2 different imaging modalities without cirrhosis background. Staging was performed according to the tumor node metastasis (TNM) staging system. One hundred and seventy-one healthy individuals were randomly recruited from the Medical Examination Center when they were undergoing a routine physical examination in the same time period. The retrospective study was under approval of Medical Ethics Committee, Zhongnan Hospital of Wuhan University (201707), and written informed consent was obtained from all participants. Their demographic and blood test results were reviewed in the hospital medical database. The blood parameters of patients with HCC and healthy people were determined by a Beckman Coulter UniCel DxH800 (AY47639) hematology analyzer within 2 hours after venipuncture. The blood test was conducted before the administration of any treatments to avoid possible influences on blood parameters.
Logistic Regression Models
A formula for predicting AFP-negative HCC was developed based on the patients in the training set. Goodness of fit of the model was evaluated by the Hosmer-Lemeshow test, and the standard logistic regression formula is:
Regarding Logit(P) = ln[p/(1 − p)], “p” is the estimated probability of AFP-negative patients with HCC, “n” is the number of influence factors, “β” is the influence coefficient, “X” is the influence factor, and “β0” is a constant.
Validation Sets
Four external validation sets from 4 centers (Zhongnan Hospital of Wuhan University; Tongji Hospital, Tongji Medical College of Huazhong University Science and Technology; Union Hospital, Tongji Medical College of Huazhong Science and Technology University; and Renmin Hospital of Wuhan University) were used to assess the performance of the model including a total of 240 AFP-negative patients with HCC and 228 healthy controls. Ninety-one AFP-negative patients with HCC and 80 healthy controls, 46 AFP-negative patients with HCC and 45 healthy controls, 38 AFP-negative patients with HCC and 38 healthy controls, 65 AFP-negative patients with HCC and 65 healthy controls were recruited in these sets, respectively.
Statistical Analysis
Statistical analysis was performed using SPSS version 22.0 (SPSS, Chicago, Illinois) or Prism6 (GraphPad software, La Jolla, California). Data were presented as the mean (standard deviation [SD]). The Shapiro-Wilk test was used to check the normality of the distribution. Normally distributed numeric variables were evaluated by Student’s t test or 1-way analysis of variance. Non-normally distributed variables were analyzed by the Mann-Whitney U test or nonparametric test. A difference was considered statistically significant when P < .05. The area under the ROC curve was measured to evaluate the diagnostic value of each selected hematological parameter.
Results
Demographic Parameters of the Training Set
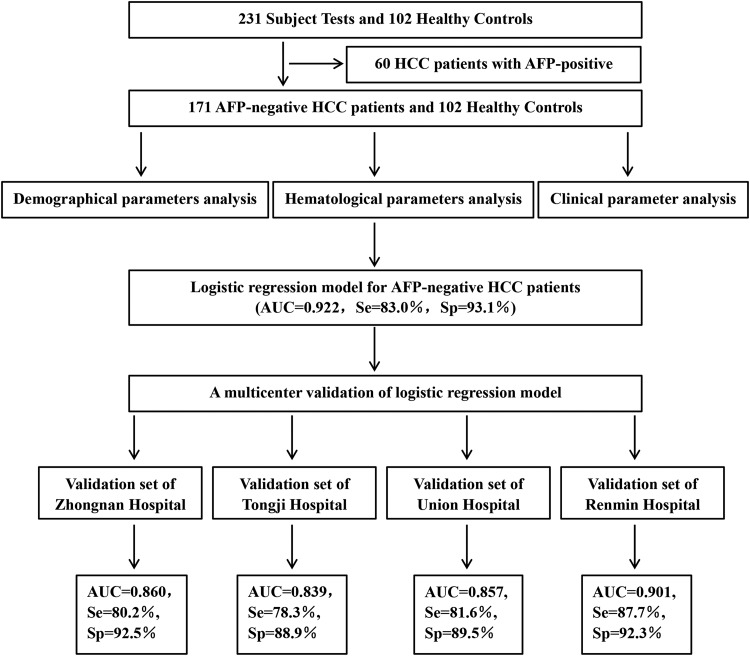
In all, 231 patients that met the requirements were enrolled in this study. The flowchart of the retrospective study is presented in Figure 1. After exclusion of 60 AFP-positive patients, 171 AFP-negative patients with HCC and 102 healthy individuals were recruited. The healthy individuals had no medical record of tumor and matched the patient group in age (P = .705), gender (P = .429), weight (P = .232), height (P = .112), smoking (P = 0.441), and drinking (P = .728; Supplementary Table S1).
Figure 1.
The flowchart of the retrospective study.
Hematological Parameters Analysis of the Training Set
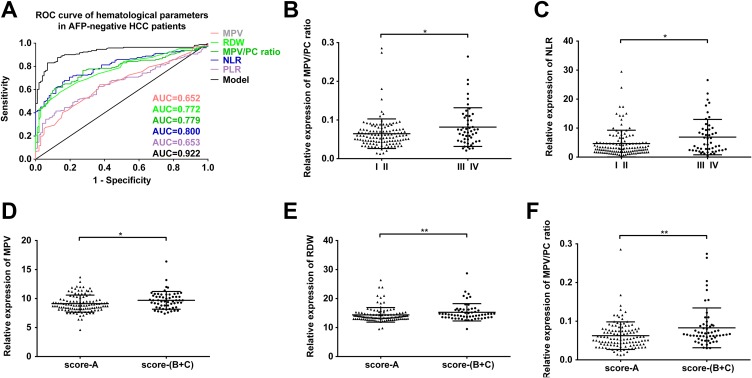
We focused on the levels of WBC, MPV, RDW, MPV/PC ratio, NLR, and PLR of these patients. As shown in Table 1, the values of MPV, RDW, MPV/PC ratio, NLR, and PLR were all significantly higher in AFP-negative patients with HCC as compared with healthy controls. No statistical difference in WBC was observed in between 2 groups. The ROC curve analysis showed that the area under the curve (AUC) of MPV, RDW, MPV/PC ratio, NLR, and PLR were 0.652, 0.772, 0.779, 0.800, and 0.653 in the patient group, respectively (Figure 2A). The detailed information of diagnostic performances in the training set is listed in Supplementary Table S2. In order to explain the significance of these parameters in AFP-negative HCC, we also proceeded the ROC with that 60 AFP-positive HCC, and the results (Table 1 and Supplementary Table S2) show that the AUC of MPV, RDW, NLR, PLR with AFP-positive HCC was lower.
Table 1.
Hematological Parameters Analysis of Training Set.a
| Parameters | AFP-Negative Patients With HCC | AFP-negative Patients With HCC | Healthy Controls | P1/P2 |
|---|---|---|---|---|
| n = 171 | n = 60 | n = 102 | ||
| WBC | 6.81 (3.50) | 6.33 (2.73) | 6.18 (1.26) | .931/.168 |
| MPV, fL | 9.31 (1.51) | 9.35 (1.59) | 8.59 (0.97) | <.0001/.006 |
| RDW, % | 14.67 (2.70) | 15.80 (3.73) | 13.07 (0.64) | <.0001/<.0001 |
| MPV–PC ratio | 0.069 (0.043) | 0.071 (0.033) | 0.041 (0.012) | <.0001/<.0001 |
| NLR | 5.32 (5.17) | 4.24 (4.43) | 1.83 (0.73) | <.0001/<.0001 |
| PLR | 168.97 (115.24) | 152.13 (93.98) | 114.99 (40.73) | <.0001/.007 |
Abbreviations: HCC, hepatocellular carcinoma; MPV, mean platelet volume; MPV/PC ratio, mean platelet volume to platelet count ratio; NLR, neutrophil to lymphocyte ratio; PC, platelet count; PLR, platelet to lymphocyte ratio; P1, P value of comparing AFP-negative HCC patients with Healthy controls; P2, P value of comparing AFP-positive HCC patients with Healthy controls; RDW, red blood cell distribution width; SD, Standard deviation; WBC, White blood cell.
a Hematological parameters are expressed as mean (SD);
Figure 2.
(A) The ROC curve analysis for the diagnostic value of MPV (AUC = 0.652, 95% CI = 0.587-0.717, P < .0001), RDW (AUC = 0.772, 95% CI = 0.718-0.826, P < .0001), MPV/PC ratio (AUC = 0.779, 95% CI = 0.724-0.834, P < .0001), NLR (AUC = 0.800, 95% CI = 0.749-0.852, P < .0001), PLR (AUC = 0.653, 95% CI = 0.589-0.717, P < .0001), and model (AUC = 0.922, 95% CI = 0.892-0.957, P < .0001) in 171 AFP-negative patients with HCC. (B) MPV–PC ratios of AFP-negative patients with HCC at different clinical stages (P = .034). (C) NLR of AFP-negative patients with HCC at different clinical stages (P = .024). (D) MPV of AFP-negative patients with HCC with different Child-Pugh scores (P = .013). (E) RDW values of AFP-negative patients with HCC with different Child-Pugh scores (P = .005). (F) MPV–PC ratios of AFP-negative patients with HCC at different Child-Pugh scores (P = .003). * P < 0.05, ** P < 0.01. AUC indicates area under the curve; HCC, hepatocellular carcinoma; MPV, mean platelet volume; NLR, neutrophils/lymphocytes ratio; RDW, red blood cell distribution width; PLR, platelet count/lymphocytes ratio; AFP, α-fetoprotein; ROC, receiver–operating characteristic.
The Correlation Between Hematological Parameters and Clinical Parameters
Chronic inflammation is associated with persistent hepatic injury and concurrent regeneration which leads to HCC. We analyzed hematological parameters and clinical parameters to find out their potential correlation in the progression and metastasis of AFP-negative HCC. We found that MPV–PC ratio and NLR were positively correlated with TNM stage (Figure 2B and C), while MPV, RDW, and MPV–PC ratio were significantly correlated with the Child-Pugh score (Figure 2D-F). However, these hematological parameters were not significantly correlated with gender, smoking, or drinking (Table 2).
Table 2.
Correlation between Hematological Parameters Level and Clinical Parameters.
| Parameters | Group | n | MPV | RDW | MPV/PC ratio | NLR | PLR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | P Value | Mean (SD) | P Value | Mean (SD) | P Value | Mean (SD) | P Value | Mean (SD) | P Value | |||
| Gender | Male | 125 | 9.25 (1.39) | .849 | 14.77 (2.66) | .188 | 0.068 (0.038) | .499 | 5.70 (5.48) | .057 | 172.88 (107.14) | .090 |
| Female | 46 | 9.48 (1.79) | 14.41 (2.81) | 0.071 (0.054) | 4.26 (4.08) | 158.32 (135.57) | ||||||
| Smoking | Negative | 120 | 9.31 (1.55) | .813 | 14.53 (2.62) | .495 | 0.066 (0.033) | .989 | 5.24 (4.93) | .830 | 172.50 (118.26) | .668 |
| Positive | 51 | 9.31 (1.42) | 14.99 (2.88) | 0.077 (0.058) | 5.49 (5.73) | 160.65 (108.49) | ||||||
| Drinking | Negative | 131 | 9.31 (1.54) | .933 | 14.67 (2.72) | .996 | 0.072 (0.046) | .244 | 5.39 (5.14) | .383 | 174.71 (125.46) | .776 |
| Positive | 40 | 9.31 (1.41) | 14.66 (2.65) | 0.061 (0.030) | 5.08 (5.30) | 150.16 (70.36) | ||||||
| Child-Pugh score | A | 113 | 9.29 (1.70) | .013a | 14.43 (2.71) | .005b | 0.065 (0.040) | .003b | 5.18 (5.22) | .392 | 169.13 (121.32) | .402 |
| B-C | 58 | 9.69 (1.53) | 15.25 (2.98) | 0.083 (0.051) | 5.54 (4.84) | 169.11 (97.93) | ||||||
| TNM stage | Ⅰ-Ⅱ | 121 | 9.21 (1.43) | .335 | 14.84 (3.00) | .637 | 0.064 (0.037) | .034a | 4.64 (4.53) | .024a | 157.87 (88.37) | .336 |
| Ⅲ-Ⅳ | 50 | 9.42 (1.69) | 14.45 (2.12) | 0.082 (0.050) | 6.89 (6.09) | 199.09 (164.05) | ||||||
Abbreviations: MPV, mean platelet volume; MPV/PC ratio, mean platelet volume to platelet count ratio; NLR, neutrophil to lymphocyte ratio; PC, platelet count; PLR, platelet to lymphocyte ratio; RDW, red blood cell distribution width; SD, Standard deviation; TNM, tumor node metastasis; WBC, White blood cell.
a P < .05.
b P < .01.
The Logistic Regression Model for AFP-Negative HCC
Each of the abovementioned factor with a significant difference in the univariate analysis was used in the multivariate model. Mean platelet volume, RDW, MPV–PC ratio, NLR, and PLR were considered independent variables (Table 1) and were included in the multivariate logistic regression model. In all, 171 AFP-negative patients with HCC and 102 healthy controls in the training set were used to build the model. The final logistic regression model for predicting AFP-negative patients with HCC was:
Logit (P) = 13.733 + 0.217(MPV) − 0.692(RDW) − 79.166(MPV/PC ratio) − 0.707(NLR) − 0.008 (PLR), the performance of the model was good with AUC of 0.922 (Figure 2A), and the estimated probability at sensitivity and specificity maximum sum are at a cutoff probability of 0.358, which means if the estimated probability was <.358, it was classified into the AFP-negative HCC patient group. On the contrary, those with a probability of ≥.358 would be classified into the negative group.
Multicenter Validation of the Logistic Regression Model
The validity of the logistic regression model was assessed in 4 external validation sets from 4 centers. A total of 240 AFP-negative patients with HCC (n = 91, 46, 38, and 65 from 4 centers, respectively) and 228 healthy controls (n = 80, 45, 38 and 65 from 4 centers, respectively) were recruited.
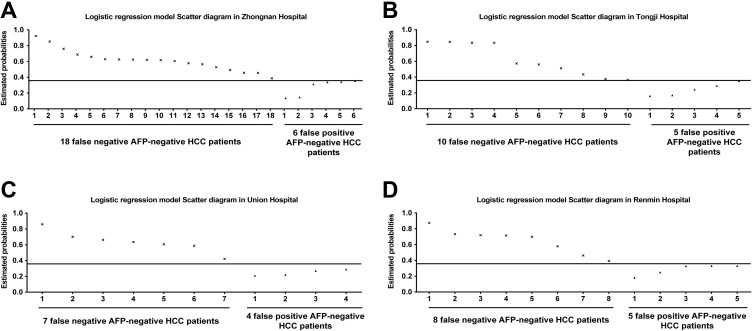
The estimated probability of 240 AFP-negative patients with HCC and 228 healthy controls were calculated using the formula Logit (P). In the cohort of Zhongnan Hospital of Wuhan University, the probabilities of 73 (out of 91) AFP-negative patients with HCC were <0.358, and the probabilities of 74 (out of 80) healthy controls were more than 0.358 (Figure 3A). The sensitivity and specificity of the model for AFP-negative HCC was 80.2% and 92.5%, respectively, with the AUC of 0.860. In the cohort of Tongji Hospital, the probabilities of 36 (out of 46) AFP-negative patients with HCC were <0.358, and the probabilities of 40 (out of 45) healthy controls were more than 0.358 (Figure 3B). The sensitivity and specificity of the model for AFP-negative HCC was 78.3% and 88.9%, respectively, with the AUC of 0.839. In the cohort of Union Hospital, the probabilities of 31 (out of 38) AFP-negative HCC patients were <.358, and the probabilities 34 (out of 38) healthy controls were more than .358 (Figure 3C). The sensitivity and specificity of the model for AFP-negative HCC was 81.6% and 89.5%, respectively, with the AUC of 0.857. In the cohort of Renmin Hospital of Wuhan University, the probabilities 57 (out of 65) AFP-negative patients with HCC were <.358, and the probabilities 60 (out of 65) healthy controls were more than 358 (Figure 3D). The sensitivity and specificity of the model for HCC was 87.7% and 92.3%, respectively, with the AUC of 0.901. The diagnostic performances of logistic regression models are shown in Table 3.
Figure 3.
The scatter diagrams of the logistic regression model in 4 external validation sets. (A) Eighteen false-negative AFP-negative patients with HCC and 6 false-positive AFP-negative patients with HCC in the cohort of Zhongnan Hospital. (B) Ten false-negative AFP-negative HCC patients and 5 false-positive AFP-negative patients with HCC in the cohort of Tongji Hospital. (C) Seven false-negative AFP-negative patients with HCC and 4 false-positive AFP-negative patients with HCC in the cohort of Union Hospital. (D) Eight false-negative AFP-negative patients with HCC and 5 false-positive AFP-negative patients with HCC in the cohort of Renmin Hospital. HCC indicates Hepatocellular carcinoma; AFP, α-fetoprotein.
Table 3.
Diagnostic Performances of Logistic Regression Model in Training Set and Validation Sets.
| Group | AFP-Negative Patients With HCC vs Healthy controls | ||||
|---|---|---|---|---|---|
| AUC | 95% CI | P Value | Se (%) | Sp (%) | |
| Training set | |||||
| Zhongnan Hospital | 0.922 | 0.892-0.957 | <.0001 | 83.0 | 93.1 |
| Validation sets | |||||
| Zhongnan Hospital | 0.860 | 0.801-0.919 | <.0001 | 80.2 | 92.5 |
| Tongji Hospital | 0.839 | 0.752-0.926 | <.0001 | 78.3 | 88.9 |
| Xiehe Hospital | 0.857 | 0.766-0.949 | <.0001 | 81.6 | 89.5 |
| Renmin Hospital | 0.901 | 0.841-0.960 | <.0001 | 87.7 | 92.3 |
Abbreviations: AFP, α-fetoprotein; AUC, area under the curve; CI, confidence interval; HCC, hepatocellular carcinoma; Se, sensitivity; Sp, specificity
Discussion
The HCC diagnosis remains difficult, especially in the early stage. If early diagnosis is successful, the 5-year survival rate of patients with HCC will be substantially enhanced.9 The AFP has been widely used as a biomarker for HCC surveillance over the past 2 decades.16 However, studies have indicated that the diagnostic accuracy of AFP is limited in HCC detection.17 Furthermore, a substantial group of patients with HCC is AFP negative.1 Therefore, identification of novel biomarkers for the diagnosis of early-stage HCC is of great importance for patients, particularly for those AFP-negative patients.
Numerous epidemiological and clinical studies have provided convincing evidence that chronic inflammation leads to carcinogenesis.18 Hepatocellular carcinoma is an inflammation-related cancer8,19 and is complicated by the coexistence of inflammation. Therefore, we evaluated the diagnostic efficiency of hematological parameters that have long been considered markers of systemic inflammatory response20,21 in the blood test on AFP-negative patients with HCC. Our results indicated that MPV, RDW, MPV–PC ratio, NLR, and PLR were significantly higher in patients and were useful for distinguishing AFP-negative patients with HCC from healthy individuals. Among them, the elevation in NLR in patients could be the consequence of increased neutrophil counts and decreased lymphocyte counts. Upregulation of peripheral neutrophils is thought to reflect an intrinsically aggressive nature of tumor cells because it is induced by cytokines produced by tumor cells.22 Neutrophils promote tumor growth and metastasis by remodeling the extracellular matrix, and they release reactants to inhibit the function of cytotoxic lymphocytes.23 On the other hand, downregulation of lymphocytes could affect HCC growth. In our research, NLR was the most effective indicator (AUC = 0.800) for the diagnosis of AFP-negative HCC than MPV (AUC = 0.652), RDW(AUC = 0.772), MPV–PC ratio(AUC = 0.779), and PLR (AUC = 0.653), but its sensitivity(67.8%) was unsatisfactory. In addition, NLR was highly correlated with the TNM stage but without significant correlation with the Child-Pugh score. This is because the Child-Pugh score is a sum of 5 variables. Interestingly, the AUC of PLR was relatively low (0.653) but with the highest specificity (90.2%). However, PLR had no correlation with the Child-Pugh score or TNM stage in our study. Hence, it might be better to combine multiple hematological parameters to detect AFP-negative HCC.
We built a logistic regression model for AFP-negative HCC which combines multiple hematological parameters including MPV, RDW, MPV–PC ratio, NLR, and PLR. It presented better diagnostic efficiency (AUC = 0.922, sensitivity = 83.0%, specificity = 93.1%) than any single hematological parameter. Of course, the model that combined different parameters including AFP with des-γ-carboxy prothrombin (DCP) or AFP-L3 has been reported.24,25 However, comparing to the literature, we found that our model has better AUC (0.922) than the model of combing AFP and DCP (AUC = 0.910),25 and the specificity (93.1%) of our model was also better that the GALAD model combing AFP, AFP-L3, and DCP (89.7% with UK, 89.1% with Japan, and 88.2% with Germany).24 Furthermore, we validated this model in 4 validation sets. In order to avoid selection bias, we recruited patients and healthy controls from 4 different hospitals to establish different validation sets. This model showed good diagnostic efficiency in all validation sets (AUC = 0.860 with Zhongnan Hospital, AUC = 0.839 with Tongji Hospital, AUC = 0.857 with Union Hospital, and AUC = 0.901 with Renmin Hospital), indicating that this model is able to predict AFP-negative HCC. Previous studies have reported the significance of single hematological parameters in HCC detection. Kurt et al26 showed that MPV could be a potential or adjunctive HCC marker in patients with chronic liver diseases. Cho et al27 and Kinoshita et al28 reported that MPV–PC ratio and NLR are good for HCC diagnosis. However, the significance of combinatory hematological parameters in the diagnosis of AFP-negative HCC is not well studied. In our study, the logistic regression model showed a better predictive ability than any single hematological parameter in the diagnosis of AFP-negative HCC. It could be a better potential or adjunctive marker of AFP-negative HCC.
In conclusion, the model that combines multiple hematological parameters (MPV, RDW, MPV/PC ratio, NLR, and PLR) might improve the diagnosis of AFP-negative HCC. However, our results need to be verified by further clinical investigations and follow-ups.
Supplemental Material
Supplemental Material, Table_S1_and_S2 for A Logistic Regression Model for Noninvasive Prediction of AFP-Negative Hepatocellular Carcinoma by Chang-Liang Luo, Yuan Rong, Hao Chen, Wu-Wen Zhang, Long Wu, Diao Wei, Xiu-Qi Wei, Lie-Jun Mei and Fu-Bing Wang in Technology in Cancer Research & Treatment
Acknowledgments
The authors would like to thank Department of Laboratory Medicine, Zhongnan Hospital of Wuhan University for providing laboratory facilities.
Abbreviations
- AFP
α-fetoprotein
- AUC
area under the curve
- HCC
hepatocellular carcinoma
- MPV
mean platelet volume
- NLR
neutrophil/lymphocyte ratio
- RDW
red blood cell distribution width
- PLR
platelet count/lymphocyte ratio
- ROC
receiver–operating characteristic
- TNM
tumor node metastasis
- WBC
white blood cell
Authors’ Note: Chang-Liang Luo and Yuan Rong contributed equally to this work. The retrospectively study was under approval of Medical Ethics Committee, Zhongnan Hospital of Wuhan University (201707). Written informed consent was obtained from all participants.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by Science and Technology Innovation Fostering Foundation of Zhongnan Hospital of Wuhan University (cxpy20160025,), and National Natural Science Foundation of China (No. 81672114).
ORCID iD: Fu-Bing Wang, PhD  https://orcid.org/0000-0002-5971-2622
https://orcid.org/0000-0002-5971-2622
Supplemental Material: Supplemental material for this article is available online.
References
- 1. She S, Xiang Y, Yang M, et al. C-reactive protein is a biomarker of AFP-negative HBV-related hepatocellular carcinoma. Int J Oncol. 2015;47(2):543–554. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Asia-Pacific Working Party on Prevention of Hepatocellular Carcinoma. Prevention of hepatocellular carcinoma in the Asia-Pacific region: consensus statements. J Gastroenterol Hepatol. 2010;25(4):657–663. [DOI] [PubMed] [Google Scholar]
- 4. Lee S, Mardinoglu A, Zhang C, Lee D, Nielsen J. Dysregulated signaling hubs of liver lipid metabolism reveal hepatocellular carcinoma pathogenesis. Nucleic Acids Res. 2016;44(12):5529–5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2010;16(4):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 7. Gines P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7(1):122–128. [DOI] [PubMed] [Google Scholar]
- 8. Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277(1-2):103–108. [DOI] [PubMed] [Google Scholar]
- 9. Forner A, Bruix J. Biomarkers for early diagnosis of hepatocellular carcinoma. Lancet Oncol. 2012;13(8):750–751. [DOI] [PubMed] [Google Scholar]
- 10. Kwon OS, Choi SH, Kim JH. Inflammation and Hepatic Fibrosis, Then Hepatocellular Carcinoma [in Korean]. Korean J Gastroenterol. 2015;66(6):320–324. [DOI] [PubMed] [Google Scholar]
- 11. Nakagawa H, Maeda S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J Gastroenterol. 2012;18(31):4071–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. [DOI] [PubMed] [Google Scholar]
- 13. Weber A, Boege Y, Reisinger F, Heikenwalder M. Chronic liver inflammation and hepatocellular carcinoma: persistence matters. Swiss Med Wkly. 2011;141:w13197. [DOI] [PubMed] [Google Scholar]
- 14. Szabo G, Lippai D. Molecular hepatic carcinogenesis: impact of inflammation. Dig Dis. 2012;30(3):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wai PY, Kuo PC. Intersecting pathways in inflammation and cancer: hepatocellular carcinoma as a paradigm. World J Clin Oncol. 2012;3(2):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song PP, Xia JF, Inagaki Y, et al. Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol. 2016;22(1):262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song P, Tobe RG, Inagaki Y, et al. The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011. Liver Int. 2012;32(7):1053–1063. [DOI] [PubMed] [Google Scholar]
- 18. Demaria S, Pikarsky E, Karin M, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33(4):335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401–435. [DOI] [PubMed] [Google Scholar]
- 20. Yildirim Cetin G, Gul O, Kesici-Metin F, Gokalp I, Sayarlioglu M. Evaluation of the mean platelet volume and red cell distribution width in FMF: are they related to subclinical inflammation or not? Int J Chronic Dis. 2014;2014:127426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bozan N, Alpayci M, Aslan M, et al. Mean platelet volume, red cell distribution width, platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios in patients with ankylosing spondylitis and their relationships with high-frequency hearing thresholds. Eur Arch Otorhinolaryngol. 2016;273(11):3663–3672. [DOI] [PubMed] [Google Scholar]
- 22. Lee Y, Kim SH, Han JY, Kim HT, Yun T, Lee JS. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol. 2012;138(12):2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kemal Y, Yucel I, Ekiz K, et al. Elevated serum neutrophil to lymphocyte and platelet to lymphocyte ratios could be useful in lung cancer diagnosis. Asian Pac J Cancer Prev. 2014;15(6):2651–2654. [DOI] [PubMed] [Google Scholar]
- 24. Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol. 2016;14(6):875–886.e876. [DOI] [PubMed] [Google Scholar]
- 25. Ertle JM, Heider D, Wichert M, et al. A combination of alpha-fetoprotein and des-gamma-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87(2):121–131. [DOI] [PubMed] [Google Scholar]
- 26. Kurt M, Onal IK, Sayilir AY, et al. The role of mean platelet volume in the diagnosis of hepatocellular carcinoma in patients with chronic liver disease. Hepatogastroenterology. 2012;59(117):1580–1582. [DOI] [PubMed] [Google Scholar]
- 27. Cho SY, Yang JJ, You E, et al. Mean platelet volume/platelet count ratio in hepatocellular carcinoma. Platelets. 2013;24(5):375–377. [DOI] [PubMed] [Google Scholar]
- 28. Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Table_S1_and_S2 for A Logistic Regression Model for Noninvasive Prediction of AFP-Negative Hepatocellular Carcinoma by Chang-Liang Luo, Yuan Rong, Hao Chen, Wu-Wen Zhang, Long Wu, Diao Wei, Xiu-Qi Wei, Lie-Jun Mei and Fu-Bing Wang in Technology in Cancer Research & Treatment