Abstract
Three mechanoresponsive polyurethane elastomers whose blue, green, and orange photoluminescence can be reversibly turned on by mechanical force were prepared and combined to create a blend that exhibits deformation-induced white photoluminescence. The three polyurethanes contain rotaxane-based supramolecular mechanoluminophores based on π-extended pyrene, anthracene, or 4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran (DCM) luminophores, respectively, and 1,4,5,8-naphthalenetetracarboxylic diimide as an electronically matched quencher. Each polymer shows instantly reversible, strain-dependent switching of its photoluminescence intensity when stretched and relaxed, as deformation leads to a spatial separation of the luminophore and quencher. The present study shows that the photoluminescence color can easily be tailored by variation of the luminophore and also by combining several mechanophores in one material and demonstrates that adaptability is a key advantage of supramolecular approaches to create mechanoresponsive polymers.
Short abstract
Properties of rotaxane-based mechanophores can be predictively tailored. This allowed creating polymers whose blue, green, orange, or white fluorescence can be reversibly switched by mechanical force.
Introduction
Mechanophores that change their photophysical properties upon activation by mechanical force are now widely studied, because embedding such moieties into polymers is one of the most promising ways to achieve mechanochromic polymers.1−3 Mechanochromic (luminescent) polymers can signal mechanical stresses and visualize material damage ahead of structural failure.4−23 Conventional mechanophores that are activated by the mechanically induced scission of covalent bonds suffer from several limitations, which are directly related to their general operating mechanism. First, the activation requires a relatively high activation energy, as the process involves the cleavage of covalent chemical bonds that are supposed to be stable in the absence of mechanical force.4,11,16,21,22 Second, the process is usually irreversible,5,7,10 or the return reaction requires activation energy or is slow,9,15−17 which results in limited reversibility. Third, the mechanical stimuli-induced cleavage reaction can typically also be caused by thermal treatment or light irradiation, which makes the process unspecific. To overcome the disadvantages of conventional mechanophores, we recently developed a supramolecular rotaxane-based mechanoluminophore that was composed of a cyclic compound containing a luminophore and a dumbbell-shaped molecule containing an electronically matched quencher and two stopper groups.24 A linear polyurethane containing the rotaxane-based supramolecular mechanoluminophore exhibited instantly reversible ON/OFF switching of its photoluminescence. The activation process does not involve any bond cleavage, but relies simply on the spatial separation of the luminophore and the quencher. Consequently, the activation energy is low;25 the process is fully and instantly reversible, specific to mechanical force, and cannot be triggered by light or heat. We note that mechanophores that do not require scission of covalent bonds for activation and change their photoluminescence properties are still limited.19,24,26
Here, we show another significant advantage of the supramolecular approach to mechanophores, i.e., that the optical signal produced can readily be tailored in a simple and rational manner without otherwise changing the mechanoresponse of the mechanophore in a given polymer, even though the molecular-level activation energies may be slightly different.27 Thus, blue-, green- and orange-light-emitting rotaxane-based supramolecular mechanoluminophores were created on the basis of our previous design24 by varying only the luminophore incorporated in the cyclic moiety (Figure 1). In the unactivated state, the luminophores are located close to the quencher, and therefore, their photoluminescence is suppressed, while upon application of mechanical force, they are drawn away from the center of the axle, and strong photoluminescence of each of the luminophores studied is turned on. Consequently, films of polyurethanes that contain the mechanophores individually show instantly reversible ON/OFF switching of the corresponding emission. As targeted, their mechanoresponsive characteristics were, with the exception of the emission color, identical. Blending the three polyurethanes allowed access to a white-light-emitting material that shows instantly reversible mechanically switchable emission.
Figure 1.
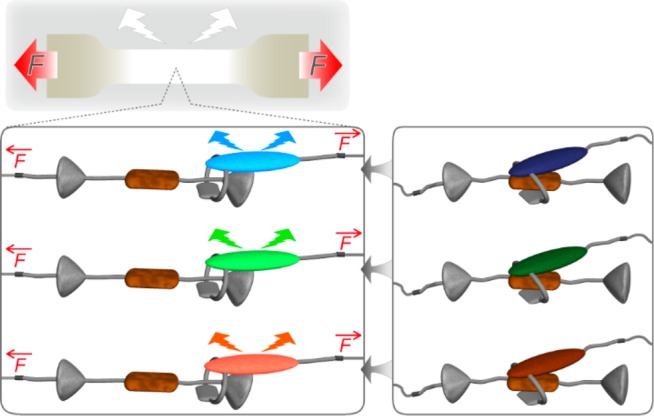
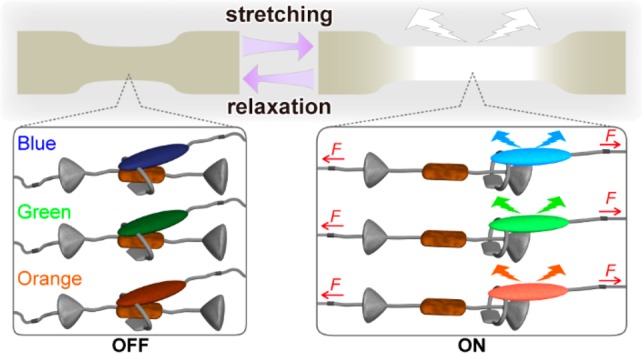
Schematic illustration of a white-light-emitting mechanoresponsive polyurethane blend involving three polymers with different rotaxane-based supramolecular mechanoluminophores. Right: idle state. Left: mechanically activated state. The rotaxanes contain cycles with attached fluorophores (blue, green, orange) and a matching quencher (brown) in the axle of a dumbbell-shaped molecule with two stoppers (gray). Red arrows indicate mechanical force.
White-light-emitting crystals and solids,28−38 as well as “soft” materials39−41 such as supramolecular polymers,42−45 physical gels,40,46−52 organic–inorganic hybrid gels,53 micelles,54,55 vesicles,56−58 inclusion complexes,59,60 solvent-free liquid,61 particles,62−67 and DNA-based materials,68 have recently attracted considerable attention. Some of these materials are responsive and change their color from white to other colors in response to light irradiation,55 pH changes,56 addition of molecules,49 or mechanical stimuli.33,34,51 In these systems, the responsiveness originates from chemical changes of the luminophores and/or variations of the supramolecular structures, which in turn changes the relative contribution of the emission of different species contributing to broad-band emission. Interestingly, however, to the best of our knowledge, no white-light-emitting soft materials have been reported to exhibit a mechanically induced ON/OFF switching.
Results and Discussion
The cyclic building blocks containing the three different luminophores utilized in this study and the corresponding rotaxanes are depicted in Figure 2. The cyclic compounds 1, 2, and 3 feature 1,6-disubstituted pyrene,69−77 9,10-disubstituted anthracene,78−82 and a π-extended 4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran (DCM)28 groups as the emitter, respectively. The 9,10-bis(phenylethynyl)anthracene was used as the green emitter instead of 4,7-bis(phenylethynyl)-2,1,3-benzothiadiazole in our previous study because of the higher quantum efficiency. The three luminophores are well-studied and were selected on account of their ability to cover a broad range of the visible emission spectrum, and facile integration into a 1,5-disubstituted naphthalene crown ether, which is often used in neutral donor–acceptor rotaxanes.83−85 As in our recent study,24 an electron-poor 1,4,5,8-naphthalenetetracarboxylic diimide (NpI)84−87 was used as the matching quencher. The NpI also templated the rotaxane synthesis, which was achieved via 1,3-dipolar cycloaddition88 between alkyne and azide groups in precursors of the rodlike molecule in the presence of the cyclic luminophores (see the Supporting Information for details). The rotaxanes Rot-1, Rot-2, and Rot-3 thus produced feature two tetraphenylmethane units featuring three tert-butyl groups as stoppers that prevent the cyclic luminophores from sliding off. In each rotaxane, two hydroxy groups were introduced at the end of the luminophore and one of the stopper groups, enabling the covalent integration into polymer molecules. The rotaxane formation was confirmed by the quenching of the emitters’ photoluminescence (see below) and also by the observation of the expected shifts of the 1H NMR signals of the aromatic protons of the interlocked motifs (Figures S1–S3).
Figure 2.
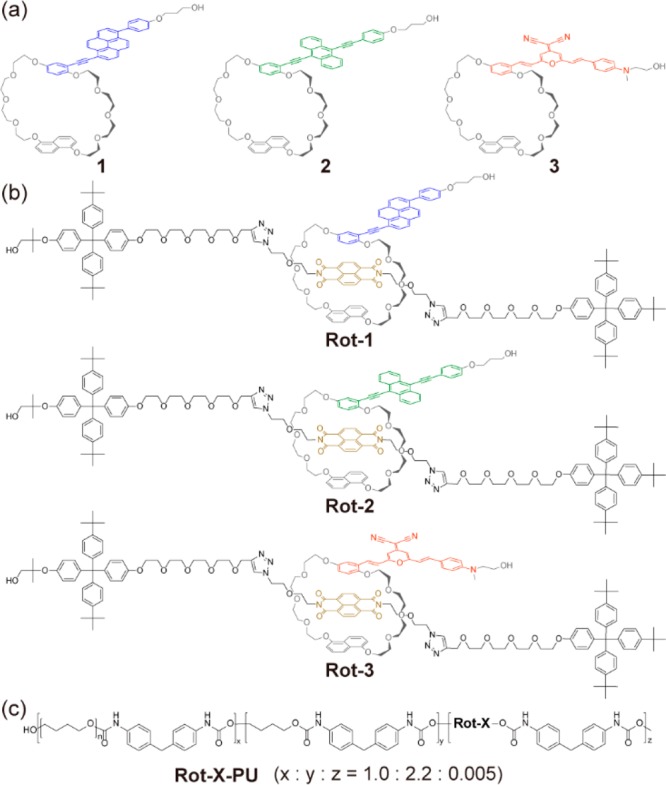
Molecular structures of (a) blue-, green-, and orange-emitting photoluminescent cyclic compounds 1, 2, and 3. (b) Rotaxane-based supramolecular mechanoluminophores Rot-1, Rot-2, and Rot-3. (c) Mechanoactive polyurethanes into which the supramolecular mechanoluminophores were individually incorporated. Rot-X represents Rot-1, Rot-2, or Rot-3.
Absorption and photoluminescence measurements were carried out to characterize the optical properties of the three cyclic compounds and the corresponding rotaxanes (Figure 3). The absorption and emission spectra of chloroform solutions of both 1 and 2 show vibronic structures. The absorption band of 1 appears between 350 and 450 nm, whereas 2 absorbs between 400 and 500 nm. The emission spectrum of pyrene derivative 1 displays two emission peaks at 430 and 450 nm, while the anthracene derivative 2 shows two emission peaks at 497 and 526 nm and a shoulder around 560 nm. In chloroform solutions, both 1 and 2 exhibit high photoluminescence quantum yields of 0.94 and 0.91, respectively. The DCM derivative 3 displays a broad absorption band between 300 and 550 nm in a mixture of hexane and chloroform (3:2 v/v). In contrast to compounds 1 and 2, the photoluminescence spectrum of 3 is broad (λem,max = 605 nm), void of vibronic features, and the quantum yield is only 0.27. A superimposition of the photoluminescence spectra of 1–3 shows that they cover the entire visible wavelength region. Indeed, a mixed solution containing the three luminophores in a molar ratio of 1:6:10 in a hexane/chloroform mixture (3:2 v/v, Figure S4) exhibits white photoluminescence under excitation at 365 nm. Upon rotaxane formation, i.e., in Rot-1, Rot-2, and Rot-3, the photoluminescence of the three emitters is completely quenched, while the absorption spectra show slight red-shifts (Figure 3, dotted lines), which is indicative of electronic ground-state interactions between the luminophores and the NpI.
Figure 3.
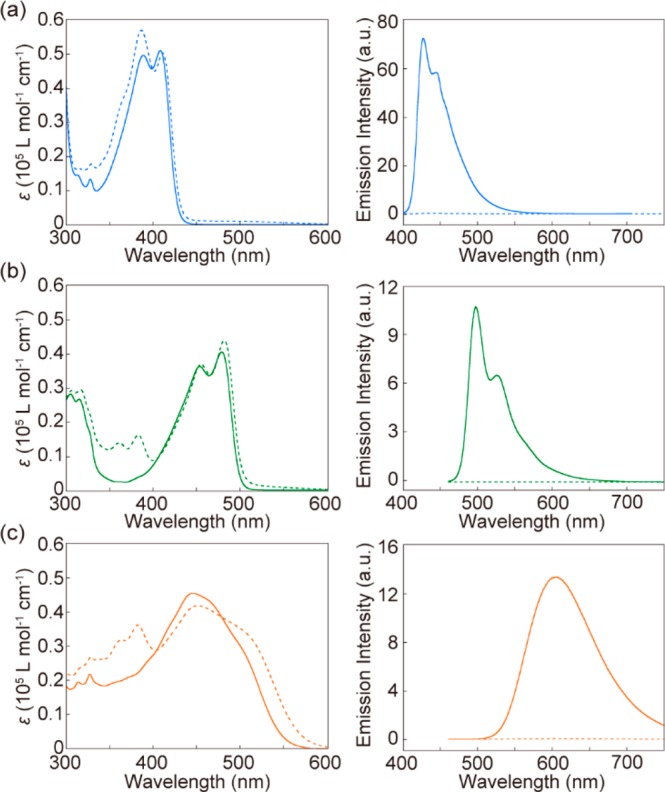
Absorption (left) and photoluminescence (right) spectra of (a) 1 (solid line) and Rot-1 (dotted line) in chloroform (1.0 × 10–5 M, λex = 380 nm), (b) 2 (solid line) and Rot-2 (dotted line) in chloroform (1.0 × 10–5 M, λex = 450 nm), and (c) 3 (solid line) and Rot-3 (dotted line) in hexane/chloroform (3:2 v/v, 1.0 × 10–5 M, λex = 450 nm).
After confirmation that the rotaxanes Rot-1, Rot-2, and Rot-3 show no photoluminescence in solution, these mechanophores were separately incorporated into linear, segmented polyurethanes (Rot-1-PU, Rot-2-PU, and Rot-3-PU) by way of polyaddition reactions that involved poly(tetrahydrofuran), 4,4′-methylenebis(phenylisocyanate), and 1,4-butanediol. On the basis of our earlier study, the rotaxane content was chosen to be ca. 0.45 wt %, and on account of this low concentration no clear peaks corresponding to the mechanophores can be observed in the polymers’ 1H NMR spectra (Figure S5). All polyurethanes were processed into thin films with a thickness of 80–100 μm by solution casting from THF solutions. Thermogravimetric analyses, differential scanning calorimetry, and dynamic mechanical analyses revealed that their thermomechanical properties are virtually identical to each other and also similar to those of previously reported polyurethanes24,89,90 of similar composition (Figures S6–S8). The elastic deformation behavior was confirmed by strain–stress curves obtained from the films (Figure S9 and Table S1).
As expected, polyurethane films of Rot-1-PU, Rot-2-PU, and Rot-3-PU show a significant increase in blue, green, and orange photoluminescence intensity upon uniaxial tensile deformation (Figure 4 and Figure S10, Movies S1–S3). The mechanically induced photoluminescence immediately disappeared when the mechanical stress was released, and this instantly reversible ON/OFF switching of the photoluminescence was repeatable without significant change over many cycles. The results show clearly that aside from the different photoluminescence colors the three rotaxanes behave similarly and that applied mechanical forces are transduced to the mechanophores where they induce the temporary separation of the luminophores and NpI moiety. We note that the fact that the mechanoresponse of the three polymers is comparable does not necessarily reflect that the molecular activation energies of the three rotaxanes are indeed the same, as mechanophores with different molecular response may result in very similar macroscopic responses when incorporated into polymers.22,91 However, the data unambiguously demonstrate that our molecular design strategy based on the shuttling function of rotaxane structures is robust and versatile.
Figure 4.
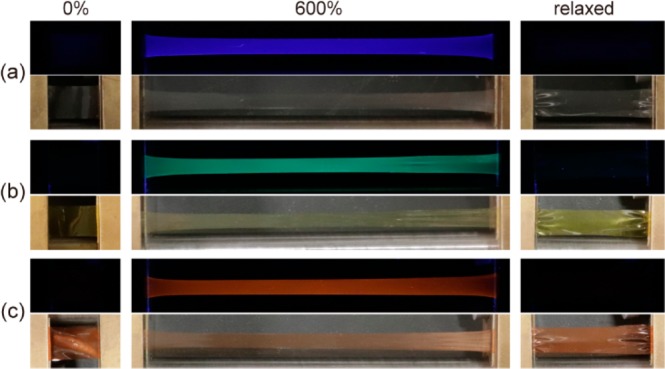
Photographs demonstrating the mechanoresponsive luminescence behavior of (a) Rot-1-PU, (b) Rot-2-PU, and (c) Rot-3-PU upon uniaxial deformation. The top row in each panel shows images taken under excitation with 365 nm UV light, while the bottom rows show images taken in room light. The applied strain λ = (L – L0)/L0 is shown above the columns of images.
We monitored the changes of the emission spectra during deformation of films of Rot-1-PU, Rot-2-PU, and Rot-3-PU to obtain more details about their mechanoresponsive luminescence behavior. As shown in Figure 5, all polyurethane films exhibit a gradual increase of the emission intensity when uniaxially stretched, and in each system the emission spectral shapes remain the same; only the intensity varies. The film of Rot-1-PU displays two emission peaks at 430 and 448 nm, which mirror the vibronic structure of the emission spectrum of 1 in chloroform. Because the concentration of the rotaxane-based mechanoluminophores in the solid polymers is approximately 1.5 × 10–3 M, self-absorption significantly affects the emission spectra, and the relative intensity of the peak observed at 432 nm is decreased. The same tendency was also observed for a Rot-2-PU film, which shows emission maxima at 495 and 521 nm. In the case of the orange-emissive Rot-3-PU, a broad emission with a maximum located at 600 nm is observed, also matching the spectral features of 3 in solution. It is noteworthy that, even in the absence of any mechanical stress, films of Rot-1-PU, Rot-2-PU, and Rot-3-PU show very faint photoluminescence (Figure 5), although almost complete quenching was observed in solution (Figure 3). The fact that the photoluminescence was also almost completely quenched in THF, the solvent used to process the films (Figure S11), indicates that the residual photoluminescence observed in the solid materials originates from a small fraction of the rotaxanes in which the emitter is positioned away from the quencher, either on account of kinetic trapping or because the limited molecular mobility makes the shuttling process slow.
Figure 5.
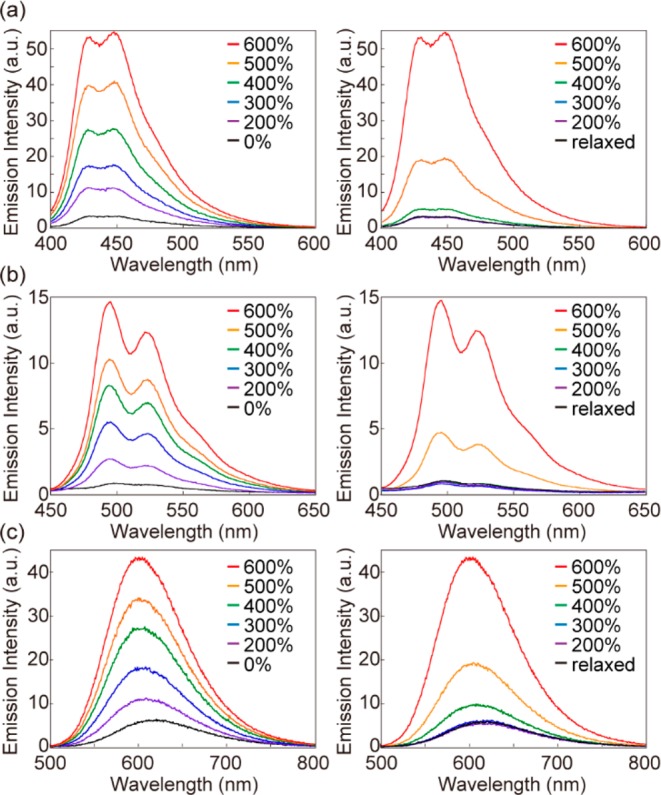
Change of the emission spectra of (a) Rot-1-PU (λex = 365 nm), (b) Rot-2-PU (λex = 380 nm), and (c) Rot-3-PU (λex = 365 nm) films upon uniaxial deformation. Shown are spectra collected upon gradually stretching the samples to the strain indicated (left) and subsequent relaxation from the maximum strain of 600% (right).
As shown in Figure 5, in all polymer films the emission intensity increases with the applied strain, and the behavior is reversible, although in the first elongation and relaxing cycle some hysteresis of the emission intensity is observed, which mirrors our earlier findings with another polyurethane-embedded rotaxane24 and is related to irreversible rearrangements of the hard phase at high strain. When cyclic tests were also performed beyond the first cycle, all polymers show reversible photoluminescence ON/OFF switching (Figure S12). After the cyclic tests, the samples were redissolved, and the fact that the emission spectra recorded mirror those of directly dissolved materials confirms that no dethreading of the cyclic luminophores occurred during processing or deformation (Figure S13). Overall, the three rotaxane-containing polyurethanes show a comparable correlation between applied strains and incremental and decremental variation of the emission intensities.
Finally, we blended the three mechanoresponsive polyurethanes and produced a mechanically responsive white-light-emitting elastomer. Through empirical mixing experiments, a weight ratio of 8:16:5 of Rot-1-PU:Rot-2-PU:Rot-3-PU was identified as the best composition to generate white light. It is noteworthy that, at the wavelength used here to excite the blend films (365 nm, see below), the molar extinction coefficient of compound 2 (ε = 0.24 × 104 L mol–1 cm–1) is much smaller than those of compounds 1 (ε = 2.7 × 104 L mol–1 cm–1) and 3 (ε = 2.0 × 104 L mol–1 cm–1) (Figure 3), which explains the large amount of Rot-2-PU that was necessary to achieve white emission. Clearly, the blending approach proved beneficial in the color optimization in contrast to the methods to prepare materials by chemically integrating all three rotaxanes into the same polymer.
Films of the blended material (Rot-Mix-PU) show, like the individual polymers, instantly, reversible photoluminescence switching behavior (Figure 6a and Figure S14, Movie S4). Under a strain of λ = 600%, bright white photoluminescence appears under excitation of 365 nm, while faint photoluminescence was observed for the as-prepared sample. As expected, the initial state recovers after the mechanical stress is released. The CIE coordinates for Rot-1-PU, Rot-2-PU, Rot-3-PU, and Rot-Mix-PU films under strain of λ = 600% were calculated to be (0.15, 0.08), (0.23, 0.54), (0.55, 0.43), and (0.30, 0.31), respectively (Figure 6b), and indicate that Rot-Mix-PU exhibits almost ideal white photoluminescence when uniaxially stretched.
Figure 6.
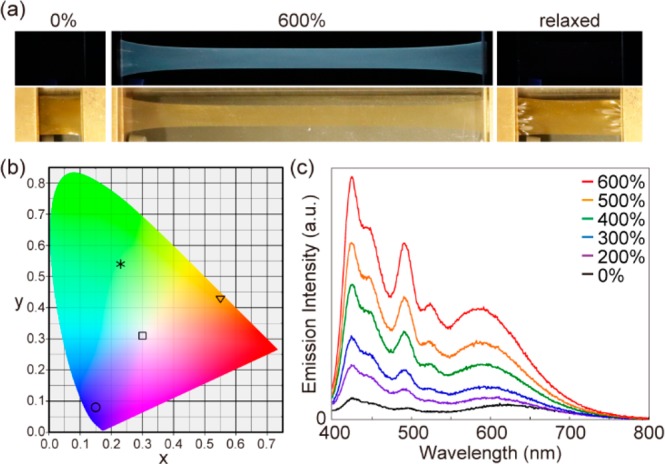
(a) Photographs demonstrating the mechanoresponsive luminescence behavior of Rot-Mix-PU (a physical blend of Rot-1-PU, Rot-2-PU, and Rot-3-PU in a weight ratio of 8:16:5) upon uniaxial deformation. The top images were taken under excitation with 365 nm UV light, while the bottom images were taken in room light. (b) CIE coordinates of Rot-1-PU (○), Rot-2-PU (∗), Rot-3-PU (▽), and Rot-Mix-PU (□) films under a strain of 600% (λex = 365 nm). (c) Change of the emission spectra of a Rot-Mix-PU (λex = 365 nm) film upon uniaxial deformation to the strain indicated.
The emission spectral changing behavior was monitored for Rot-Mix-PU upon tensile deformation (Figure 6c and Figure S15). As-prepared film shows faint photoluminescence in the absence of any mechanical stress. The intensities of the emission bands associated with the three luminophores clearly increase when the strain of the film is increased. The spectral shapes associated with the blue- and green-emitting mechanophores are slightly different from those observed in films of Rot-1-PU and Rot-2-PU, respectively, where self-absorption effects in the short-wavelength region that decrease the emission intensities of the peaks corresponding to the 0–0 transitions are more prominent. By contrast, the absorption of the Rot-Mix-PU film covers the wavelength for blue and green emission and shows all features of the emission peaks of the pyrene- and anthracene-based luminophores in solution. We also note that the ratio of the three polymers that afforded white emission in the solid state is different from the composition that afforded a white-emitting solution (Figure S4). Notably, the Rot-Mix-PU films require a much lower content of the red-light-emitting Rot-3-PU and a larger content of Rot-1-PU, which appears to indicate that a combination of self-absorption effect and energy transfer from Rot-1-PU to Rot-2-PU, and from Rot-2-PU to Rot-3-PU, occurs in the solid films. Indeed, while straining Rot-1-PU films to a strain of 600% leads to an increase of the emission intensity of ca. 1600% (λem = 430 nm), an increase of only ca. 1000% was observed for the Rot-Mix-PU film at 430 nm. On the other hand, Rot-Mix-PU shows almost the same contrast related to the emission band of Rot-3-PU (ca. 700% at 600 nm). As shown in Figure S16, the Rot-Mix-PU film also shows good reversibility of the emission intensity in all three regions upon cyclic testing. Moreover, after 20 cycles of stretching the sample to a strain of 600% and subsequent relaxation, the emission spectrum (Figure S17a), and the CIE coordinates of Rot-Mix-PU (Figure S17b), remained unchanged, and no changes could be observed when the sample was exposed to another 20 cycles of stretching to a strain of 800% and subsequent relaxation (Figure S18). These results suggest that all three rotaxanes are mechanically robust and do not dethread, even under repeated exposure of the blend to large strains.
Conclusions
In conclusion, we developed the first white-light-emitting polymer that exhibits instantly reversible ON/OFF photoluminescence switching upon stretching and relaxation. The dynamic photophysical properties were achieved by incorporating blue-, green-, and orange-light-emitting supramolecular mechanophores based on interlocked rotaxane motifs. The present study highlights one of the advantages of rotaxane-based supramolecular mechanophores, i.e., that the photoluminescence color of such motifs can easily be tailored by replacing the luminophore and optionally combining several mechanophores in one material. While it might in principle be possible to create a multilayer assembly that emits white light under very specific conditions by stacking films of the three different polymers reported here, we note that, because of the different absorption spectra of the three layers, internal absorption effects (of both absorbed and emitted light) will have caused the emitted color to depend on the excitation direction and angle. The here-reported blends are symmetric, and off-white hues are only observed under very oblique angles (Movie S5). While it has yet to be investigated to what extent the molecular activation energies of the three rotaxanes are comparable, their response to macroscopic forces is the same in the soft polyurethanes investigated here, which bodes well for the chemical integration of multiple such mechanophores at strategically selected positions of the same polymer (e.g., backbone and cross-links) to monitor stress distributions at the molecular level in situ. In addition, the supramolecular mechanoluminophores studied here appear to be activated by very small mechanical forces, which renders them useful for mechanobiology experiments, which are currently ongoing.
Acknowledgments
We thank Ms. Miho Yamada for HRMS measurements. This work was performed under the International Cooperative Research Program of “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in “Network Joint Research Center for Materials and Devices”. This work was supported by Japan Science Technology Agency (JST), PRESTO (JPMJPR17P6), Advanced Technology Institute Research Grants 2017, and the Tanaka Rubber Science and Technology Award. Y.S. and M.P. acknowledge the Goho Life Sciences International Fund. M.P. acknowledges the Deanship of Scientific Research at King Khalid University for funding through Grant G.R.P-276-39. This work was also supported by the Swiss National Center of Competence in Research Bio-Inspired Materials.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.9b00173.
Experimental procedure and additional data (PDF)
Movie S1: mechanoresponsive luminescent behavior upon cyclic stretching of Rot-1-PU film (MP4)
Movie S2: mechanoresponsive luminescent behavior upon cyclic stretching of Rot-2-PU film (MP4)
Movie S3: mechanoresponsive luminescent behavior upon cyclic stretching of Rot-3-PU film (MP4)
Movie S4: mechanoresponsive luminescent behavior upon cyclic stretching a white-light-emitting polyurethane film made from the blend Rot-Mix-PU, showing reversible photoluminescence switching behavior (MP4)
Movie S5: mechanoresponsive luminescent behavior upon cyclic stretching a white-light-emitting polyurethane film made from the blend Rot-Mix-PU, with off-white hues only observed under very oblique angles (MP4)
The authors declare no competing financial interest.
Supplementary Material
References
- Caruso M. M.; Davis D. A.; Shen Q.; Odom S. A.; Sottos N. R.; White S. R.; Moore J. S. Mechanically-Induced Chemical Changes in Polymeric Materials. Chem. Rev. 2009, 109, 5755–5798. 10.1021/cr9001353. [DOI] [PubMed] [Google Scholar]
- Li J.; Nagamani C.; Moore J. S. Polymer Mechanochemistry: From Destructive to Productive. Acc. Chem. Res. 2015, 48, 2181–2190. 10.1021/acs.accounts.5b00184. [DOI] [PubMed] [Google Scholar]
- Calvino C.; Neumann L.; Weder C.; Schrettl S. Approaches to Polymeric Mechanochromic Materials. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 640–652. 10.1002/pola.28445. [DOI] [Google Scholar]
- Davis D. A.; Hamilton A.; Yang J.; Cremar L. D.; Van Gough D.; Potisek S. L.; Ong M. T.; Braun P. V.; Martinez T. J.; White S. R.; Moore J. S.; Sottos N. R. Force-Induced Activation of Covalent Bonds in Mechanoresponsive Polymeric Materials. Nature 2009, 459, 68–72. 10.1038/nature07970. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Spiering A. J. H.; Karthikeyan S.; Peters G. W. M.; Meijer E. W.; Sijbesma R. P. Mechanically Induced Chemiluminescence from Polymers Incorporating a 1,2-Dioxetane Unit in the Main Chain. Nat. Chem. 2012, 4, 559–562. 10.1038/nchem.1358. [DOI] [PubMed] [Google Scholar]
- Gossweiler G. R.; Hewage G. B.; Soriano G.; Wang Q.; Welshofer G. W.; Zhao X.; Craig S. L. Mechanochemical Activation of Covalent Bonds in Polymers with Full and Repeatable Macroscopic Shape Recovery. ACS Macro Lett. 2014, 3, 216–219. 10.1021/mz500031q. [DOI] [PubMed] [Google Scholar]
- Ducrot E.; Chen Y.; Bulters M.; Sijbesma R. P.; Creton C. Toughening Elastomers with Sacrificial Bonds and Watching Them Break. Science 2014, 344, 186–189. 10.1126/science.1248494. [DOI] [PubMed] [Google Scholar]
- Li J.; Shiraki T.; Hu B.; Wright R. A. E.; Zhao B.; Moore J. S. Mechanophore Activation at Heterointerfaces. J. Am. Chem. Soc. 2014, 136, 15925–15928. 10.1021/ja509949d. [DOI] [PubMed] [Google Scholar]
- Imato K.; Kanehara T.; Ohishi T.; Nishihara M.; Yajima H.; Ito M.; Takahara A.; Otsuka H. Mechanochromic Dynamic Covalent Elastomers: Quantitative Stress Evaluation and Autonomous Recovery. ACS Macro Lett. 2015, 4, 1307–1311. 10.1021/acsmacrolett.5b00717. [DOI] [PubMed] [Google Scholar]
- Kean Z. S.; Gossweiler G. R.; Kouznetsova T. B.; Hewage G. B.; Craig S. L. A Coumarin Dimer Probe of Mechanochemical Scission Efficiency in the Sonochemical Activation of Chain-Centered Mechanophore Polymers. Chem. Commun. 2015, 51, 9157–9160. 10.1039/C5CC01836F. [DOI] [PubMed] [Google Scholar]
- Gossweiler G. R.; Kouznetsova T. B.; Craig S. L. Force-Rate Characterization of Two Spiropyran-Based Molecular Force Probes. J. Am. Chem. Soc. 2015, 137, 6148–6151. 10.1021/jacs.5b02492. [DOI] [PubMed] [Google Scholar]
- Verstraeten F.; Göstl R.; Sijbesma R. P. Stress-Induced Colouration and Crosslinking of Polymeric Materials by Mechanochemical Formation of Triphenylimidazolyl Radicals. Chem. Commun. 2016, 52, 8608–8611. 10.1039/C6CC04312G. [DOI] [PubMed] [Google Scholar]
- Li H.; Göstl R.; Delgove M.; Sweeck J.; Zhang Q.; Sijbesma R. P.; Heuts J. P. A. Promoting Mechanochemistry of Covalent Bonds by Noncovalent Micellar Aggregation. ACS Macro Lett. 2016, 5, 995–998. 10.1021/acsmacrolett.6b00579. [DOI] [PubMed] [Google Scholar]
- Göstl R.; Sijbesma R. P. π-Extended Anthracenes as Sensitive Probes for Mechanical Stress. Chem. Sci. 2016, 7, 370–375. 10.1039/C5SC03297K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imato K.; Kanehara T.; Nojima S.; Ohishi T.; Higaki Y.; Takahara A.; Otsuka H. Repeatable Mechanochemical Activation of Dynamic Covalent Bonds in Thermoplastic Elastomers. Chem. Commun. 2016, 52, 10482–10485. 10.1039/C6CC04767J. [DOI] [PubMed] [Google Scholar]
- Robb M. J.; Kim T. A.; Halmes A. J.; White S. R.; Sottos N. R.; Moore J. S. Regioisomer-Specific Mechanochromism of Naphthopyran in Polymeric Materials. J. Am. Chem. Soc. 2016, 138, 12328–12331. 10.1021/jacs.6b07610. [DOI] [PubMed] [Google Scholar]
- Wang T.; Zhang N.; Dai J.; Li Z.; Bai W.; Bai R. Novel Reversible Mechanochromic Elastomer with High Sensitivity: Bond Scission and Bending-Induced Multicolor Switching. ACS Appl. Mater. Interfaces 2017, 9, 11874–11881. 10.1021/acsami.7b00176. [DOI] [PubMed] [Google Scholar]
- Kida J.; Imato K.; Goseki R.; Aoki D.; Morimoto M.; Otsuka H. The Photoregulation of a Mechanochemical Polymer Scission. Nat. Commun. 2018, 9, 3504. 10.1038/s41467-018-05996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filonenko G. A.; Lugger J. A. M.; Liu C.; van Heeswijk E. P. A.; Hendrix M. M. R. M.; Weber M.; Müller C.; Hensen E. J. M.; Sijbesma R. P.; Pidko E. A. Tracking Local Mechanical Impact in Heterogeneous Polymers with Direct Optical Imaging. Angew. Chem., Int. Ed. 2018, 57, 16385–16390. 10.1002/anie.201809108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karman M.; Verde-Sesto E.; Weder C. Mechanochemical Activation of Polymer-Embedded Photoluminescent Benzoxazole Moieties. ACS Macro Lett. 2018, 7, 1028–1033. 10.1021/acsmacrolett.8b00520. [DOI] [PubMed] [Google Scholar]
- Barbee M. H.; Kouznetsova T.; Barrett S. L.; Gossweiler G. R.; Lin Y.; Rastogi S. K.; Brittain W. J.; Craig S. L. Substituent Effects and Mechanism in a Mechanochemical Reaction. J. Am. Chem. Soc. 2018, 140, 12746–12750. 10.1021/jacs.8b09263. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Barbee M. H.; Chang C.-C.; Craig S. L. Regiochemical Effects on Mechanophore Activation in Bulk Materials. J. Am. Chem. Soc. 2018, 140, 15969–15975. 10.1021/jacs.8b10376. [DOI] [PubMed] [Google Scholar]
- Sung J.; Robb M. J.; White S. R.; Moore J. S.; Sottos N. R. Interfacial Mechanophore Activation Using Laser-Induced Stress Waves. J. Am. Chem. Soc. 2018, 140, 5000–5003. 10.1021/jacs.8b01427. [DOI] [PubMed] [Google Scholar]
- Sagara Y.; Karman M.; Verde-Sesto E.; Matsuo K.; Kim Y.; Tamaoki N.; Weder C. Rotaxanes as Mechanochromic Fluorescent Force Transducers in Polymers. J. Am. Chem. Soc. 2018, 140, 1584–1587. 10.1021/jacs.7b12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; De Bo G. Impact of a Mechanical Bond on the Activation of a Mechanophore. J. Am. Chem. Soc. 2018, 140, 12724–12727. 10.1021/jacs.8b08590. [DOI] [PubMed] [Google Scholar]
- Yamakado T.; Otsubo K.; Osuka A.; Saito S. Compression of a Flapping Mechanophore Accompanied by Thermal Void Collapse in a Crystalline Phase. J. Am. Chem. Soc. 2018, 140, 6245–6248. 10.1021/jacs.8b03833. [DOI] [PubMed] [Google Scholar]
- Fahrenbach A. C.; Bruns C. J.; Cao D.; Stoddart J. F. Ground-State Thermodynamics of Bistable Redox-Active Donor–Acceptor Mechanically Interlocked Molecules. Acc. Chem. Res. 2012, 45, 1581–1592. 10.1021/ar3000629. [DOI] [PubMed] [Google Scholar]
- Park S.; Kwon J. E.; Kim S. H.; Seo J.; Chung K.; Park S.-Y.; Jang D.-J.; Medina B. M.; Gierschner J.; Park S. Y. A White-Light-Emitting Molecule: Frustrated Energy Transfer between Constituent Emitting Centers. J. Am. Chem. Soc. 2009, 131, 14043–14049. 10.1021/ja902533f. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yan J.; Zhou Y.; Pei J. Surface Modification of Self-Assembled One-Dimensional Organic Structures: White-Light Emission and Beyond. J. Am. Chem. Soc. 2010, 132, 15872–15874. 10.1021/ja106354m. [DOI] [PubMed] [Google Scholar]
- Lei Y.-L.; Jin Y.; Zhou D.-Y.; Gu W.; Shi X.-B.; Liao L.-S.; Lee S.-T. White-Light Emitting Microtubes of Mixed Organic Charge-Transfer Complexes. Adv. Mater. 2012, 24, 5345–5351. 10.1002/adma.201201493. [DOI] [PubMed] [Google Scholar]
- Jin X.-H.; Chen C.; Ren C.-X.; Cai L.-X.; Zhang J. Bright White-Light Emission from a Novel Donor–Acceptor Organic Molecule in the Solid State via Intermolecular Charge Transfer. Chem. Commun. 2014, 50, 15878–15881. 10.1039/C4CC07063A. [DOI] [PubMed] [Google Scholar]
- Mao Z.; Yang Z.; Mu Y.; Zhang Y.; Wang Y.-F.; Chi Z.; Lo C.-C.; Liu S.; Lien A.; Xu J. Linearly Tunable Emission Colors Obtained from a Fluorescent–Phosphorescent Dual-Emission Compound by Mechanical Stimuli. Angew. Chem., Int. Ed. 2015, 54, 6270–6273. 10.1002/anie.201500426. [DOI] [PubMed] [Google Scholar]
- Xu B.; Mu Y.; Mao Z.; Xie Z.; Wu H.; Zhang Y.; Jin C.; Chi Z.; Liu S.; Xu J.; Wu Y.-C.; Lu P.-Y.; Lien A.; Bryce M. R. Achieving Remarkable Mechanochromism and White-light Emission with Thermally Activated Delayed Fluorescence through the Molecular Heredity Principle. Chem. Sci. 2016, 7, 2201–2206. 10.1039/C5SC04155D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z.; Zhao W.; Lam J. W. Y.; Peng Q.; Ma H.; Liang G.; Shuai Z.; Tang B. Z. White Light Emission from a Single Organic Molecule with Dual Phosphorescence at Room Temperature. Nat. Commun. 2017, 8, 416. 10.1038/s41467-017-00362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y.; Sheng T.; Zhu X.; Zhuo C.; Su S.; Li H.; Hu S.; Zhu Q.-L.; Wu X. Introduction of Red-Green-Blue Fluorescent Dyes into a Metal–Organic Framework for Tunable White Light Emission. Adv. Mater. 2017, 29, 1700778. 10.1002/adma.201700778. [DOI] [PubMed] [Google Scholar]
- Li Z. Z.; Liang F.; Zhuo M. P.; Shi Y. L.; Wang X. D.; Liao L. S. White-Emissive Self-Assembled Organic Microcrystals. Small 2017, 13, 1604110. 10.1002/smll.201604110. [DOI] [PubMed] [Google Scholar]
- Li J.-A.; Zhou J.; Mao Z.; Xie Z.; Yang Z.; Xu B.; Liu C.; Chen X.; Ren D.; Pan H.; Shi G.; Zhang Y.; Chi Z. Transient and Persistent Room-Temperature Mechanoluminescence from a White-Light-Emitting AIEgen with Tricolor Emission Switching Triggered by Light. Angew. Chem., Int. Ed. 2018, 57, 6449–6453. 10.1002/anie.201800762. [DOI] [PubMed] [Google Scholar]
- Wang J.; Gu X.; Ma H.; Peng Q.; Huang X.; Zheng X.; Sung S. H. P.; Shan G.; Lam J. W. Y.; Shuai Z.; Tang B. Z. A Facile Strategy for Realizing Room Temperature Phosphorescence and Single Molecule White Light Emission. Nat. Commun. 2018, 9, 2963. 10.1038/s41467-018-05298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S.; Thilagar P. Organic White-Light Emitting Materials. Dyes Pigm. 2014, 110, 2–27. 10.1016/j.dyepig.2014.05.031. [DOI] [Google Scholar]
- Praveen V. K.; Ranjith C.; Armaroli N. White-Light-Emitting Supramolecular Gels. Angew. Chem., Int. Ed. 2014, 53, 365–368. 10.1002/anie.201306787. [DOI] [PubMed] [Google Scholar]
- Li D.; Wang J.; Ma X. White-Light-Emitting Materials Constructed from Supramolecular Approaches. Adv. Opt. Mater. 2018, 6, 1800273. 10.1002/adom.201800273. [DOI] [Google Scholar]
- Abbel R.; Grenier C.; Pouderoijen M. J.; Stouwdam J. W.; Leclère P. E. L. G.; Sijbesma R. P.; Meijer E. W.; Schenning A. P. H. J. White-Light Emitting Hydrogen-Bonded Supramolecular Copolymers Based on π-Conjugated Oligomers. J. Am. Chem. Soc. 2009, 131, 833–843. 10.1021/ja807996y. [DOI] [PubMed] [Google Scholar]
- Molla M. R.; Gehrig D.; Roy L.; Kamm V.; Paul A.; Laquai F.; Ghosh S. Self-Assembly of Carboxylic Acid Appended Naphthalene Diimide Derivatives with Tunable Luminescent Color and Electrical Conductivity. Chem. - Eur. J. 2014, 20, 760–771. 10.1002/chem.201303379. [DOI] [PubMed] [Google Scholar]
- Liang A.; Dong S.; Zhu X.; Huang F.; Cao Y. White Light-Emitting Diodes Based on an All-Phosphorescent Supramolecular Polymer. Polym. Chem. 2015, 6, 6202–6207. 10.1039/C5PY00832H. [DOI] [Google Scholar]
- Zhang M.; Yin S.; Zhang J.; Zhou Z.; Saha M. L.; Lu C.; Stang P. J. Metallacycle-Cored Supramolecular Assemblies with Tunable Fluorescence Including White-Light Emission. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 3044–3049. 10.1073/pnas.1702510114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbel R.; van der Weegen R.; Pisula W.; Surin M.; Leclère P.; Lazzaroni R.; Meijer E. W.; Schenning A. P. H. J. Multicolour Self-Assembled Fluorene Co-Oligomers: From Molecules to the Solid State via White-Light-Emitting Organogels. Chem. - Eur. J. 2009, 15, 9737–9746. 10.1002/chem.200900620. [DOI] [PubMed] [Google Scholar]
- Vijayakumar C.; Praveen V. K.; Ajayaghosh A. RGB Emission through Controlled Donor Self-Assembly and Modulation of Excitation Energy Transfer: A Novel Strategy to White-Light-Emitting Organogels. Adv. Mater. 2009, 21, 2059–2063. 10.1002/adma.200802932. [DOI] [Google Scholar]
- Giansante C.; Raffy G.; Schäfer C.; Rahma H.; Kao M.-T.; Olive A. G. L.; Del Guerzo A. White-Light-Emitting Self-Assembled NanoFibers and Their Evidence by Microspectroscopy of Individual Objects. J. Am. Chem. Soc. 2011, 133, 316–325. 10.1021/ja106807u. [DOI] [PubMed] [Google Scholar]
- Cao X.; Wu Y.; Liu K.; Yu X.; Wu B.; Wu H.; Gong Z.; Yi T. Iridium Complex Triggered White-Light-Emitting Gel and Its Response to Cysteine. J. Mater. Chem. 2012, 22, 2650–2657. 10.1039/C2JM13826C. [DOI] [Google Scholar]
- Bairi P.; Roy B.; Chakraborty P.; Nandi A. K. Co-Assembled White-Light-Emitting Hydrogel of Melamine. ACS Appl. Mater. Interfaces 2013, 5, 5478–5485. 10.1021/am4013566. [DOI] [PubMed] [Google Scholar]
- Chen P.; Li Q.; Grindy S.; Holten-Andersen N. White-Light-Emitting Lanthanide Metallogels with Tunable Luminescence and Reversible Stimuli-Responsive Properties. J. Am. Chem. Soc. 2015, 137, 11590–11593. 10.1021/jacs.5b07394. [DOI] [PubMed] [Google Scholar]
- Malakar P.; Modak D.; Prasad E. Pure White Light Emission from Organic Molecules Using Solvent Induced Selective Self-Assembly. Chem. Commun. 2016, 52, 4309–4312. 10.1039/C5CC10112C. [DOI] [PubMed] [Google Scholar]
- Rao K. V.; Datta K. K. R.; Eswaramoorthy M.; George S. J. Highly Pure Solid-State White-Light Emission from Solution-Processable Soft-Hybrids. Adv. Mater. 2013, 25, 1713–1718. 10.1002/adma.201204407. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Görl D.; Würthner F. White-Light Emitting Dye Micelles in Aqueous Solution. Chem. Commun. 2013, 49, 8178–8180. 10.1039/c3cc44875d. [DOI] [PubMed] [Google Scholar]
- Bälter M.; Li S.; Morimoto M.; Tang S.; Hernando J.; Guirado G.; Irie M.; Raymo F. M.; Andréasson J. Emission Color Tuning and White-Light Generation Based on Photochromic Control of Energy Transfer Reactions in Polymer Micelles. Chem. Sci. 2016, 7, 5867–5871. 10.1039/C6SC01623E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Rehm S.; Safont-Sempere M. M.; Würthner F. Vesicular Perylene Dye Nanocapsules as Supramolecular Fluorescent pH Sensor Systems. Nat. Chem. 2009, 1, 623–629. 10.1038/nchem.368. [DOI] [PubMed] [Google Scholar]
- Liu N.; Qi C.-G.; Wang Y.; Liu D.-F.; Yin J.; Zhu Y.-Y.; Wu Z.-Q. Solvent-Induced White-Light Emission of Amphiphilic Rod–Rod Poly(3-triethylene glycol thiophene)-block-poly(phenyl isocyanide) Copolymer. Macromolecules 2013, 46, 7753–7758. 10.1021/ma4016664. [DOI] [Google Scholar]
- Xing P.; Zhao Z.; Hao A.; Zhao Y. Tailoring Luminescence Color Conversion via Affinitive Co-Assembly of Glutamates Appended with Pyrene and Naphthalene Dicarboximide Units. Chem. Commun. 2016, 52, 1246–1249. 10.1039/C5CC08858E. [DOI] [PubMed] [Google Scholar]
- Zhang Q.-W.; Li D.; Li X.; White P. B.; Mecinović J.; Ma X.; Ågren H.; Nolte R. J. M.; Tian H. Multicolor Photoluminescence Including White-Light Emission by a Single Host–Guest Complex. J. Am. Chem. Soc. 2016, 138, 13541–13550. 10.1021/jacs.6b04776. [DOI] [PubMed] [Google Scholar]
- Ni X.-L.; Chen S.; Yang Y.; Tao Z. Facile Cucurbit[8]uril-Based Supramolecular Approach To Fabricate Tunable Luminescent Materials in Aqueous Solution. J. Am. Chem. Soc. 2016, 138, 6177–6183. 10.1021/jacs.6b01223. [DOI] [PubMed] [Google Scholar]
- Santhosh Babu S.; Aimi J.; Ozawa H.; Shirahata N.; Saeki A.; Seki S.; Ajayaghosh A.; Möhwald H.; Nakanishi T. Solvent-Free Luminescent Organic Liquids. Angew. Chem., Int. Ed. 2012, 51, 3391–3395. 10.1002/anie.201108853. [DOI] [PubMed] [Google Scholar]
- Vijayakumar C.; Sugiyasu K.; Takeuchi M. Oligofluorene-Based Electrophoretic Nanoparticles in Aqueous Medium as a Donor Scaffold for Fluorescence Resonance Energy Transfer and White-Light Emission. Chem. Sci. 2011, 2, 291–294. 10.1039/C0SC00343C. [DOI] [Google Scholar]
- Tseng K.-P.; Fang F.-C.; Shyue J.-J.; Wong K.-T.; Raffy G.; Del Guerzo A.; Bassani D. M. Spontaneous Generation of Highly Emissive RGB Organic Nanospheres. Angew. Chem., Int. Ed. 2011, 50, 7032–7036. 10.1002/anie.201101945. [DOI] [PubMed] [Google Scholar]
- Malinge J.; Allain C.; Brosseau A.; Audebert P. White Fluorescence from Core–Shell Silica Nanoparticles. Angew. Chem., Int. Ed. 2012, 51, 8534–8537. 10.1002/anie.201203374. [DOI] [PubMed] [Google Scholar]
- Nishiyabu R.; Sugino Y.; Kubo Y. White-Light Emitting Boronate Microparticles for Potential Use as Reusable Bright Chemosensors in Water. Chem. Commun. 2013, 49, 9869–9871. 10.1039/c3cc45739g. [DOI] [PubMed] [Google Scholar]
- Das S.; Debnath T.; Basu A.; Ghosh D.; Das A. K.; Baker G. A.; Patra A. Efficient White-Light Generation from Ionically Self-Assembled Triply-Fluorescent Organic Nanoparticles. Chem. - Eur. J. 2016, 22, 8855–8863. 10.1002/chem.201502339. [DOI] [PubMed] [Google Scholar]
- Geng W.-C.; Liu Y.-C.; Wang Y.-Y.; Xu Z.; Zheng Z.; Yang C.-B.; Guo D.-S. A Self-Assembled White-Light-Emitting System in Aqueous Medium Based on a Macrocyclic Amphiphile. Chem. Commun. 2017, 53, 392–395. 10.1039/C6CC09079F. [DOI] [PubMed] [Google Scholar]
- Varghese R.; Wagenknecht H.-A. White-Light-Emitting DNA (WED). Chem. - Eur. J. 2009, 15, 9307–9310. 10.1002/chem.200901147. [DOI] [PubMed] [Google Scholar]
- Inouye M.; Fujimoto K.; Furusyo M.; Nakazumi H. Molecular Recognition Abilities of a New Class of Water-Soluble Cyclophanes Capable of Encompassing a Neutral Cavity. J. Am. Chem. Soc. 1999, 121, 1452–1458. 10.1021/ja9725256. [DOI] [Google Scholar]
- Abe H.; Mawatari Y.; Teraoka H.; Fujimoto K.; Inouye M. Synthesis and Molecular Recognition of Pyrenophanes with Polycationic or Amphiphilic Functionalities: Artificial Plate-Shaped Cavitant Incorporating Arenes and Nucleotides in Water. J. Org. Chem. 2004, 69, 495–504. 10.1021/jo035188u. [DOI] [PubMed] [Google Scholar]
- Maeda H.; Maeda T.; Mizuno K.; Fujimoto K.; Shimizu H.; Inouye M. Alkynylpyrenes as Improved Pyrene-Based Biomolecular Probes with the Advantages of High Fluorescence Quantum Yields and Long Absorption/Emission Wavelengths. Chem. - Eur. J. 2006, 12, 824–831. 10.1002/chem.200500638. [DOI] [PubMed] [Google Scholar]
- Sagara Y.; Kato T. Stimuli-Responsive Luminescent Liquid Crystals: Change of Photoluminescent Colors Triggered by a Shear-Induced Phase Transition. Angew. Chem., Int. Ed. 2008, 47, 5175–5178. 10.1002/anie.200800164. [DOI] [PubMed] [Google Scholar]
- Suneesh C. V.; Gopidas K. R. Long-Lived Photoinduced Charge Separation Due to the Inverted Region Effect in 1,6-Bis(phenylethynyl)pyrene–Phenothiazine Dyad. J. Phys. Chem. C 2010, 114, 18725–18734. 10.1021/jp107606t. [DOI] [Google Scholar]
- Yamane S.; Tanabe K.; Sagara Y.; Kato T. Stimuli-Responsive Photoluminescent Liquid Crystals. Top. Curr. Chem. 2012, 318, 395–406. 10.1007/128_2011_275. [DOI] [PubMed] [Google Scholar]
- Sagara Y.; Komatsu T.; Ueno T.; Hanaoka K.; Kato T.; Nagano T. A Water-Soluble Mechanochromic Luminescent Pyrene Derivative Exhibiting Recovery of the Initial Photoluminescence Color in a High-Humidity Environment. Adv. Funct. Mater. 2013, 23, 5277–5284. 10.1002/adfm.201300180. [DOI] [Google Scholar]
- Sagara Y.; Komatsu T.; Ueno T.; Hanaoka K.; Kato T.; Nagano T. Covalent Attachment of Mechanoresponsive Luminescent Micelles to Glasses and Polymers in Aqueous Conditions. J. Am. Chem. Soc. 2014, 136, 4273–4280. 10.1021/ja412670g. [DOI] [PubMed] [Google Scholar]
- Sagara Y.; Weder C.; Tamaoki N. Asymmetric Cyclophanes Permit Access to Supercooled Nematic Liquid Crystals with Stimulus-Responsive Luminescence. Chem. Mater. 2017, 29, 6145–6152. 10.1021/acs.chemmater.7b02220. [DOI] [Google Scholar]
- Levitus M.; Garcia-Garibay M. A. Polarized Electronic Spectroscopy and Photophysical Properties of 9,10-Bis(phenylethynyl)anthracene. J. Phys. Chem. A 2000, 104, 8632–8637. 10.1021/jp001483w. [DOI] [Google Scholar]
- Sagara Y.; Weder C.; Tamaoki N. Tuning the Thermo- and Mechanoresponsive Behavior of Luminescent Cyclophanes. RSC Adv. 2016, 6, 80408–80414. 10.1039/C6RA18348D. [DOI] [Google Scholar]
- Sagara Y.; Simon Y. C.; Tamaoki N.; Weder C. A Mechano- and Thermoresponsive Luminescent Cyclophane. Chem. Commun. 2016, 52, 5694–5697. 10.1039/C6CC01614F. [DOI] [PubMed] [Google Scholar]
- Lübtow M.; Helmers I.; Stepanenko V.; Albuquerque R. Q.; Marder T. B.; Fernández G. Self-Assembly of 9,10-Bis(phenylethynyl) Anthracene (BPEA) Derivatives: Influence of π–π and Hydrogen-Bonding Interactions on Aggregate Morphology and Self-Assembly Mechanism. Chem. - Eur. J. 2017, 23, 6198–6205. 10.1002/chem.201605989. [DOI] [PubMed] [Google Scholar]
- Mase K.; Sasaki Y.; Sagara Y.; Tamaoki N.; Weder C.; Yanai N.; Kimizuka N. Stimuli-Responsive Dual-Color Photon Upconversion: A Singlet-to-Triplet Absorption Sensitizer in a Soft Luminescent Cyclophane. Angew. Chem., Int. Ed. 2018, 57, 2806–2810. 10.1002/anie.201712644. [DOI] [PubMed] [Google Scholar]
- Bruns C. J.; Basu S.; Stoddart J. F. Improved Synthesis of 1,5-Dinaphtho[38]crown-10. Tetrahedron Lett. 2010, 51, 983–986. 10.1016/j.tetlet.2009.12.060. [DOI] [Google Scholar]
- Jacquot de Rouville H.-P.; Iehl J.; Bruns C. J.; McGrier P. L.; Frasconi M.; Sarjeant A. A.; Stoddart J. F. A Neutral Naphthalene Diimide [2]Rotaxane. Org. Lett. 2012, 14, 5188–5191. 10.1021/ol3022963. [DOI] [PubMed] [Google Scholar]
- Choudhary U.; Northrop B. H. Rotaxanes and Biofunctionalized Pseudorotaxanes via Thiol-Maleimide Click Chemistry. Org. Lett. 2012, 14, 2082–2085. 10.1021/ol300614z. [DOI] [PubMed] [Google Scholar]
- Hamilton D. G.; Davies J. E.; Prodi L.; Sanders J. K. M. Synthesis, Structure and Photophysics of Neutral π-Associated [2]Catenanes. Chem. - Eur. J. 1998, 4, 608–620. . [DOI] [Google Scholar]
- Cougnon F. B. L.; Jenkins N. A.; Pantoş G. D.; Sanders J. K. M. Templated Dynamic Synthesis of a [3]Catenane. Angew. Chem., Int. Ed. 2012, 51, 1443–1447. 10.1002/anie.201106885. [DOI] [PubMed] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. . [DOI] [PubMed] [Google Scholar]
- Crenshaw B. R.; Weder C. Self-Assessing Photoluminescent Polyurethanes. Macromolecules 2006, 39, 9581–9589. 10.1021/ma061685b. [DOI] [Google Scholar]
- Ayer M. A.; Simon Y. C.; Weder C. Azo-Containing Polymers with Degradation On-Demand Feature. Macromolecules 2016, 49, 2917–2927. 10.1021/acs.macromol.6b00418. [DOI] [Google Scholar]
- Kim T. A.; Robb M. J.; Moore J. S.; White S. R.; Sottos N. R. Mechanical Reactivity of Two Different Spiropyran Mechanophores in Polydimethylsiloxane. Macromolecules 2018, 51, 9177–9183. 10.1021/acs.macromol.8b01919. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


