Abstract
Guidelines recommend supervised exercise therapy (SET) as first-line treatment for intermittent claudication. However, the use of revascularization is widespread. We addressed the effectiveness of preventing (additional) invasive revascularization after primary SET or revascularization based on lesion and patient characteristics. In this single-center, retrospective, cohort study, 474 patients with intermittent claudication were included. Patients with occlusive disease of the aortoiliac tract and/or common femoral artery (inflow) were primarily considered for revascularization, while patients with more distal disease (outflow) were primarily considered for SET. In total, 232 patients were referred for SET and 242 patients received revascularization. The primary outcome was freedom from (additional) intervention, analyzed by Kaplan–Meier estimates. Secondary outcomes were survival, critical ischemia, freedom from target lesion revascularization (TLR), and an increase in maximum walking distance. In the SET-first strategy, 71% of patients had significant outflow lesions. Freedom from intervention was 0.90 ± 0.02 at 1-year and 0.82 ± 0.03 at 2-year follow-up. In the primary revascularization group, 90% of patients had inflow lesions. Freedom from additional intervention was 0.78 ± 0.03 at 1-year and only 0.65 ± 0.04 at 2-year follow-up, despite freedom from TLR of 0.91 ± 0.02 and 0.85 ± 0.03 at 1- and 2-year follow-up, respectively. In conclusion, SET was effective in preventing invasive treatment for patients with mainly outflow lesions. In contrast, secondary intervention rates following our strategy of primary revascularization for inflow lesions were unexpectedly high. These findings further support the guideline recommendations of SET as first-line treatment for all patients with intermittent claudication irrespective of level of disease.
Keywords: lower extremity, bypass, endarterectomy, endovascular therapy, supervised exercise therapy, peripheral artery disease (PAD), revascularization
Introduction
Intermittent claudication is the most common symptomatic form of peripheral artery disease (PAD), affecting approximately 20–40 million people worldwide and increasing with the ageing population.1,2 Treatment modalities to increase mobility and limit functional disability in patients with claudication are supervised exercise therapy (SET), endovascular revascularization (EVR) or surgical revascularization, either alone or in combination. European and United States guidelines recommend SET as an (cost-) effective first-line treatment.3–5 However, SET takes time, motivation, and compliance, both for the patient and the physician. Furthermore, SET programs are underutilized due to limited access and reimbursement issues in most countries.6 In contrast, invasive interventions provide instant symptom relief, do not require intrinsic motivation or effort by the patient, and are readily available and remunerated. Given their low procedural morbidity and high procedural success, endovascular therapies thus offer an attractive first-line alternative for the treatment of patients with claudication. As a result, the number of endovascular procedures performed in the United States for claudication increased fourfold between 1999 and 2007.7
Recent meta-analyses of randomized controlled trials (RCTs) showed that SET is equally effective as EVR in improving walking distance in patients with intermittent claudication.8,9 However, since most comparative effectiveness trials have enrolled patients with heterogeneous anatomic disease distribution, the optimal treatment strategy in relation to the localization and extent of the arterial lesions remains controversial. As a result, SET is often deemed sufficient for patients with infrainguinal lesions, since femoropopliteal and below-knee revascularizations carry high secondary intervention rates. In contrast, patients with (focal) aortoiliac and/or common femoral artery lesions are frequently revascularized, given the favorable risk–benefit ratio and excellent patency rates for aortoiliac stenting and common femoral endarterectomy.10 The evidence for any long-term benefit of endovascular treatment over supervised exercise is inconclusive, and the question remains of whether this common clinical practice is justified or just leads to more invasive procedures over time.11,12
To answer this question, we evaluated the real-world results in patients who were treated for intermittent claudication between 2009 and 2014 in a single-center observational cohort study. We determined the freedom from intervention during prolonged follow-up in patients with lifestyle-limiting claudication, based on arterial lesion localization, patient characteristics, and patient preference.
Methods
Study design and setting
A retrospective, observational, single-center cohort study was performed using patients referred to the vascular surgical outpatient clinic of the Erasmus University Medical Center for suspected intermittent claudication between July 2009 and January 2014. Data from all consecutive patients referred for SET and those who underwent revascularization were collected from hospital records. The medical ethics committee of the Erasmus University Medical Center (MEC-2016-289) reviewed and approved the non-interventional character of this study. According to Dutch law, written informed consent for a patient to be enrolled in this study was not required. The study was conducted in compliance with the Declaration of Helsinki.
Participants
Patients with suspected intermittent claudication, not treated for PAD in the last 12 months, were eligible for this study. All patients underwent a standardized vascular workup. A graded treadmill test (3.2 km/h, increasing from a grade of 0% to 10%, maximum 10 minutes) was conducted to assess the pain free walking distance (PFWD) and maximum walking distance (MWD). The ankle–brachial index (ABI) was determined before and after the treadmill test. Vascular imaging was performed by computed tomography angiography (CTA), magnetic resonance angiography (MRA), duplex ultrasound, or angiography – whichever was best applicable. The diagnosis of intermittent claudication was confirmed if the resting ABI was ⩽ 0.9, or with a > 0.15 decrease in ABI after the treadmill test, and/or with one or more significant vascular stenoses (⩾ 50% diameter reduction) at the aortoiliac, femoropopliteal, and/or tibiopedal level.
Patients with intermittent claudication received cardiovascular risk management according to guidelines, including an antiplatelet drug, lipid-lowering therapy with a statin, antihypertensive and glucose-lowering medications (if appropriate), and lifestyle modification advice, including smoking cessation.13 Treatment was based on arterial lesion localization, patient characteristics, and patient preference, according to the 2005 ACC/AHA and 2007 TASC II guidelines for the management of patients with PAD.13,14 Patients with significant inflow lesions in the symptomatic leg, defined as a > 50% stenosis or occlusion in the aortoiliac segment and/or the common femoral artery, were offered primary revascularization therapy. This was unless the patient was considered unfit for revascularization, if major reconstructive surgery was required in patients with mild claudication symptoms, or when the patient preferred SET. Patients with intermittent claudication with only significant outflow lesions, defined as a > 50% stenosis or occlusion in the femoropopliteal and/or crural arteries, were offered primary SET. In cases of severely limited ambulation due to a condition other than intermittent claudication not allowing patients to walk on a treadmill or in cases of limited life expectancy, patients were offered medical treatment only.
Supervised exercise therapy
Patients were referred for a community-based SET program to a local physiotherapist participating in a nationwide network of physiotherapists specialized in SET for intermittent claudication (ClaudicatioNet).15 All the physiotherapists provided SET according to the guideline, which recommends training up to sub-maximum pain with short walking intervals.16 Patients generally started with a frequency of two to three sessions of 30 minutes weekly, with the total number of sessions tailored to the patient’s need. The MWD was measured by physiotherapists at regular intervals during the SET program using the standardized graded treadmill test.
Revascularization
The mode of revascularization was individualized on the basis of arterial anatomy, periprocedural risk, and anticipated benefit. Experienced interventional radiologists or vascular surgeons performed the revascularizations. In general, lesions were treated by balloon angioplasty and selective stent placement. Drug-coated balloons were reserved for re-interventions. No drug-eluting stents were used. Common femoral artery lesions were treated by surgical endarterectomy. Bypass surgery was only reserved for extensive TASC Type D lesions for aortoiliac lesions and Type C and D for femoropopliteal lesions. Infrapopliteal lesions were not revascularized in this population.
Outcome measures
The primary outcome was freedom from (additional) intervention, defined as freedom from revascularization and minor or major amputations during follow-up. Secondary outcomes were survival, freedom from worsening to critical ischemia (Rutherford class 4, 5, 6), freedom from target lesion revascularization (TLR) defined as re-revascularization within 5 mm of the primarily treated lesion, and an increase in treadmill MWD during SET. Mortality data were obtained from the civil registry database.
Statistical methods
Baseline characteristics and outcomes were described as counts and percentages for dichotomous variables, and means and SDs or medians and IQRs for continuous variables. No statistical comparison between the SET and revascularization groups was made because treatment was based on patient and lesion characteristics. Intervention-free survival was assessed using Kaplan–Meier analysis. The start of follow-up was set at the date of referral for the SET group and at the date of endovascular or surgical treatment for the revascularization group. For analysis, patients did not cross over in case the initially planned treatment was not received. Clinical follow-up time was defined as the time until the last outpatient clinic visit or telephone contact. Patients without a secondary invasive intervention were censored at the last clinical follow-up date. Differences between groups were compared using the chi-squared test for categorical data (Fisher’s exact tests when appropriate), and continuous variables with Student’s t-test as a parametric test or Mann–Whitney U-test as a non-parametric test. Statistical analysis was performed using IBM SPSS Statistics 24 (IBM Corp., Armonk, NY, USA).
Results
Participants
During a median clinical follow-up of 2.4 years (IQR 0.8–4.1 years), 474 patients were treated for intermittent claudication. A total number of 232 patients were referred to a physiotherapist for community-based SET. These were patients with mainly outflow lesions (165/220; 71%; 12 patients could not be classified because of no imaging). Of the 232 patients, 192 (83%) actually started the SET program. Reasons for not starting the SET program were primarily financial concerns or lack of patient motivation. The 242 patients primarily treated by revascularization had mainly inflow lesions (217/242; 90%). Treatment in this group was either endovascular (186/242; 77%) or surgical revascularization (56/242; 23%). Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of patients with intermittent claudication referred for SET or treated by revascularization.
| Variable | SET |
Revascularization |
||
|---|---|---|---|---|
| n = 232 | n = 242 | |||
| Demographic data | ||||
| Age, years, mean (± SD) | 66 | (± 10.5) | 63 | (± 9.5) |
| Sex, female | 89 | 38.4% | 86 | 35.5% |
| Cardiovascular risk factors | ||||
| Hypertension | 182 | 78.4% | 177 | 73.1% |
| Hypercholesterolemia | 195 | 84.1% | 190 | 78.5% |
| Diabetes | 79 | 34.1% | 61 | 25.2% |
| Smoking | ||||
| Current | 107 | 47.6% | 137 | 57.8% |
| Former | 64 | 28.4% | 71 | 30.0% |
| Obesity | 38 | 16.4% | 32 | 13.2% |
| Chronic kidney disease | 55 | 23.7% | 53 | 21.9% |
| Comorbidities | ||||
| Cerebrovascular disease | 50 | 21.6% | 33 | 13.6% |
| Cardiac disease | 78 | 33.6% | 72 | 29.8% |
| Prior lower limb revascularization | 33 | 14.2% | 52 | 21.5% |
| Pulmonary disease | 46 | 19.8% | 60 | 24.8% |
| Neurological disease | 30 | 12.9% | 26 | 10.7% |
| Musculoskeletal disease | 54 | 23.2% | 31 | 12.8% |
| Lower limb osteoarthritis | 24 | 10.3% | 8 | 3.3% |
| Arthritis | 6 | 2.6% | 1 | 0.4% |
| Non-specific low back pain | 10 | 4.3% | 14 | 5.8% |
| Other | 14 | 6.0% | 8 | 3.3% |
| History of malignancy | 25 | 10.8% | 11 | 4.5% |
| PAD | ||||
| Duration of claudication, months, median (IQR) | 12 | (6–24) | 12 | (3–24) |
| Ankle–brachial index, median (IQR)a | ||||
| At rest | 0.72 | (0.60–0.86) | 0.71 | (0.59–0.85) |
| After exercise | 0.54 | (0.34–0.73) | 0.56 | (0.35–0.75) |
| Walking distance, m, median (IQR) | ||||
| Maximum | 260 | (140–488) | 220 | (120–380) |
| Pain free | 90 | (50–150) | 80 | (50–120) |
| Imaging | ||||
| No | 12 | 5.2% | 0 | 0% |
| Duplex | 15 | 6.5% | 3 | 1.2% |
| MRA | 22 | 9.5% | 26 | 10.7% |
| CTA | 179 | 77.2% | 212 | 87.6% |
| Angiography | 4 | 1.7% | 1 | 0.4% |
| Arterial lesion levelb | ||||
| Inflowc | 55 | 24% | 217 | 90% |
| Outflowd | 165 | 71% | 25 | 10% |
No statistical comparison between the SET and revascularization groups was made because treatment was based on patient and lesion characteristics.
Data represent the number of patients (n, %) unless indicated otherwise.
Missing data: < 5%, except for duration of claudication (30% in primary SET group and 14% in primary revascularization group), PFWD at baseline (14% and 10%), inflow and outflow level (5% in primary SET group).
Minimum value for right and left legs.
The predominant lesion of the symptomatic leg was scored for the SET group and the treated lesion was scored in the primary revascularization group.
Inflow lesions were defined as one or more significant stenoses in the aortoiliac segment and/or the common femoral artery.
Outflow lesions were defined as one or more significant stenoses in the superficial femoral artery, popliteal artery or infrapopliteal arteries.
CTA, computed tomography angiography; MRA, magnetic resonance angiography; PAD, peripheral artery disease; PFWD, pain free walking distance; SET, supervised exercise therapy.
Outcome data
Supervised exercise therapy
In the SET group, 48/232 (21%) of the patients underwent subsequent endovascular (12%) or surgical revascularization (9%) during a median clinical follow-up of 2.9 years (IQR 1.9–4.4 years). The Kaplan–Meier estimates for the primary endpoint of freedom from intervention were 0.90 ± 0.02 at 1 year and 0.82 ± 0.03 at 2 years during clinical follow-up (Figure 1 and Table 2). Sensitivity analysis in 192 patients who actually started the SET program showed similar results for the primary endpoint.
Figure 1.
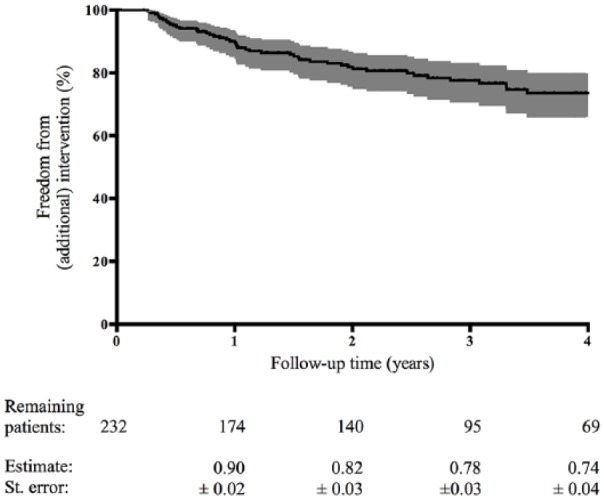
The Kaplan–Meier estimated freedom from intervention after primary SET. The number of remaining patients are shown at various time points.
SET, supervised exercise therapy.
Table 2.
Freedom from intervention following primary SET.
| Inflowa |
Outflowa |
Total |
|
|---|---|---|---|
| n = 55 | n = 165 | n = 232 | |
| 1-year freedom from intervention | 0.83 ± 0.05 | 0.93 ± 0.02 | 0.90 ± 0.02 |
| 2-year freedom from intervention | 0.67 ± 0.07 | 0.87 ± 0.03 | 0.82 ± 0.03 |
12 patients were not classified in the inflow or outflow group because of no imaging.
SET, supervised exercise therapy.
Patients who underwent invasive therapy after SET more often had concomitant pulmonary disease (31% vs 17%; p = 0.026) and significantly more inflow pathology (44% vs 19%; p = 0.001) compared to those who were not revascularized after SET. Furthermore, 15 of these 48 secondary invasively treated patients (31%) subsequently received a third intervention (10 endovascular, five surgical) for either restenosis or disease progression during the study period. During clinical follow-up, nine of 232 patients (4%) progressed to critical limb ischemia and four patients underwent an amputation, resulting in an overall 2% amputation rate. The 5-year Kaplan–Meier estimated survival was 0.81 ± 0.03 in the SET group. In total, 52 patients of the 232 died and 39 patients of the 192 that actually started SET died.
Follow-up measurements of the MWD during the SET program using the standardized graded treadmill test were reported for 129/192 patients who started SET (67%). The treadmill walking test at baseline was limited to a MWD of 530 meters in 10 minutes. To prevent any bias due to this ceiling effect at baseline, six of 129 patients were excluded from the walking distance analysis. The MWD for the remaining 123 patients significantly increased during SET by 560 meters (IQR 253–1100 m) over a median follow-up period of 6 months from 260 meters (IQR 140–488 m) to 892 meters (IQR 500–1540).
Revascularization
In the revascularization group, 73/242 (30%) underwent an additional intervention (22% endovascular, 8% surgical) during a median clinical follow-up of 1.4 years (IQR 0.4–3.3 years). The Kaplan–Meier estimates of freedom from additional intervention after primary revascularization were 0.78 ± 0.03 at 1 year and 0.65 ± 0.04 at 2 years of clinical follow-up (Figure 2 and Table 3).
Figure 2.
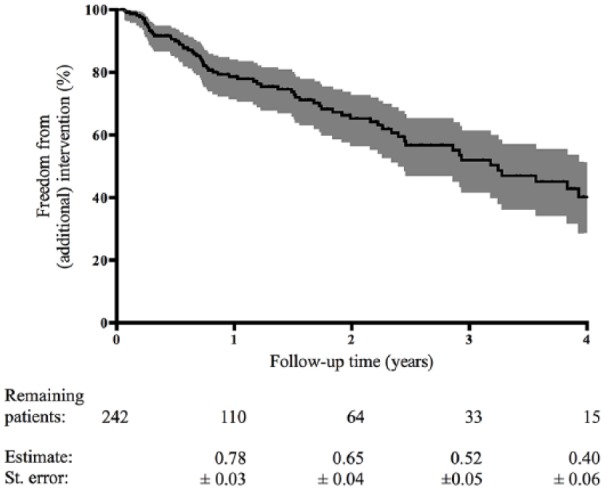
The Kaplan–Meier estimated freedom from additional intervention after primary revascularization. The number of remaining patients are shown at various time points.
Table 3.
Freedom from additional intervention and freedom from TLR following primary revascularization.
| Inflow n = 217 |
Outflow n = 25 |
Total n = 242 |
|
|---|---|---|---|
| 1-year freedom from additional intervention | 0.78 ± 0.03 | 0.75 ± 0.10 | 0.78 ± 0.03 |
| 2-year freedom from additional intervention | 0.64 ± 0.04 | 0.68 ± 0.11 | 0.65 ± 0.04 |
| 1-year freedom from TLR | 0.92 ± 0.02 | 0.86 ± 0.08 | 0.91 ± 0.02 |
| 2-year freedom from TLR | 0.86 ± 0.03 | 0.80 ± 0.10 | 0.85 ± 0.03 |
TLR, target lesion revascularization.
Subgroup analysis for patients revascularized for inflow and outflow lesions were comparable (Table 3). The additional intervention was performed for inflow lesions in 48 patients (66%) and for outflow lesions in 25 patients (34%). Furthermore, 27 of these 73 secondary invasively treated patients (37%) subsequently received a third intervention (23 endovascular, four surgical). The 1-year freedom from TLR in all re-interventions was 0.91 ± 0.02 for the revascularization group (Table 3).
Two patients developed critical ischemia before their primary intervention. During total clinical follow-up, another 6% of patients (15/242) progressed to critical limb ischemia and two patients underwent an amputation, resulting in an overall 1% amputation rate in the revascularization group. The 5-year Kaplan–Meier estimated survival was 0.80 ± 0.03 in the revascularization group. In total, 49 out of the 242 patients died in this group.
Discussion
The role of revascularization in patients with lifestyle-limiting claudication remains controversial. In this study, we show that a SET-first approach for patients with mainly lesions below the common femoral artery resulted in freedom from invasive intervention of 90% after 1 year and 82% after 2 years of follow-up. In contrast, in patients primarily revascularized for lesions of the iliac tract or common femoral artery, the 1-year freedom from additional intervention was 78% and further declined over time.
The only published high-quality RCT in this field during the inclusion period of the current study found no difference in effectiveness between percutaneous transluminal angioplasty (PTA) and SET, irrespective of the level of arterial disease.17 Furthermore, the 2005 ACC/AHA guideline stated that an endovascular procedure was indicated when there was a favorable risk–benefit ratio; for example, in case of focal aortoiliac occlusive disease.13 The 2007 TASC II guideline stated that patients with proximal lesions could be considered for revascularization without initially undergoing extensive medical therapy, including supervised exercise.14 The treatment policy in our institution was therefore based on the favorable risk–benefit ratio and excellent patency rates for aortoiliac stenting and common femoral endarterectomy, as well as on patient characteristics and preferences. We offered a revascularization-first strategy to patients with claudication due to significant aortoiliac and/or common femoral artery lesions (inflow) and a SET-first strategy to those with occlusive disease below the level of the common femoral artery (outflow). The rationale for this observational cohort study was to evaluate the real-world results of this policy. As a result of the treatment selection, 71% of patients in the SET-first cohort had significant outflow lesions only, whereas 90% of patients in the revascularization cohort presented with significant inflow lesions. The main indication to follow the SET-first strategy in patients with inflow lesions was the combination of mild claudication and only major surgical reconstructive options.
The walking distance in the SET cohort increased on average 3.5-fold over the course of 6 months of SET, from 260 to 892 meters. This compares favorably with trial settings. A recent review of randomized trials demonstrated the effectiveness of SET with an average increase in MWD from 382 meters at baseline to 605 and 641 meters at 3 and 6 months, respectively.18 The amputation and mortality rates in our study were low and in line with other reports, confirming the safety of a SET-first approach.19–21
There is a paucity of data on the sustainability of the benefits achieved with SET, since most trials have a maximum follow-up of 1 year.18,19,22,23 Freedom from subsequent intervention after SET (82% at 2 year) is in line with previous studies, which showed intervention-free rates of 89% at 6 months,24 78% at 12 months,20 and 53% at 7 years.19 Approximately one-quarter of the patients with inflow lesions were treated primarily with SET. Also in these patients, we found an intervention-free rate of 67% over the course of 2 years. This was comparable with the patients who had already received revascularization as primary treatment.
As opposed to the low intervention rates in patients with a SET-first strategy in our study, one-third of the patients who were initially revascularized for inflow lesions had a second intervention within 2 years. With TLR rates of only 8% and 14% at 1 and 2 years post-intervention, which compare favorably to those previously reported,25 we conclude that most additional revascularization procedures were performed for downstream lesions in the ipsilateral leg or lesions in the contralateral leg. One of the reasons for the high re-intervention rate may be that PAD is a manifestation of systemic atherosclerotic disease, resulting in multilevel disease in both legs. Revascularization of a singular arterial lesion in one leg may not be effective in the long term due to disease elsewhere in the ipsilateral or contralateral limb, whereas SET treats both lower extremities. Long-term results of a randomized trial, comparing SET and EVR, including only femoropopliteal lesions, also showed the highest re-intervention rate after EVR, with more than half of the re-interventions performed in the contralateral leg.26 Although the setup of this cohort study does not allow for head-to-head comparisons between the two treatment strategies, the improvement in walking distance and the low re-intervention rate during follow-up after SET, combined with the high re-intervention rate of revascularization procedures, underlines the potential of SET to be a valuable treatment option with good and durable results while saving costs.19,27–31 It has recently been shown that pain rating and ischemia intensity are similar for patients with proximal (buttock) claudication due to aortoiliac disease and those with distal (calf) claudication due to femoropopliteal disease.32 Our findings in a real-world setting are in line with the CLEVER trial,33 which addressed the comparative effectiveness of EVR or SET for patients with intermittent claudication with aortoiliac artery PAD. At 18-month follow-up, SET provided similar improvements in functional status and quality of life as EVR. Based on these results, as well as on the 2016 ACC/AHA guidelines,4 we changed our treatment strategy towards a stepped care approach: referral to a supervised exercise program as the initial treatment modality for all patients with intermittent claudication, with revascularization reserved for those with an inadequate response to supervised exercise.
Study limitations
The present study has several limitations. First, there was a variation in length of clinical follow-up at the disadvantage of the revascularization group. Since the re-intervention rate in these patients is already higher at 2 years’ follow-up compared with the SET-first group, the difference will augment with prolonged follow-up. Second, the exact number of training sessions for each patient was not recorded. However, since all patients received a standard SET program according to the guidelines within the nationwide ClaudicatioNet of trained physiotherapists, tailored to the individuals’ needs,16 this reflects the true outcome of SET in real-world practice. Third, we have some missing data on the follow-up measurements of the MWD during the SET program. Nonetheless, the outcome of previous trials that reported on the walking distance after SET are in line with our results, and have shown SET to be an effective treatment for intermittent claudication.18 Notably, the results only pertain to patients with intermittent claudication, not to those with critical limb ischemia.
Conclusion
In conclusion, this study shows the durability, in real-world clinical practice, of a SET-first strategy with a low intervention rate in patients with lifestyle-limiting claudication due to lesions mainly below the common femoral artery (outflow). Our strategy of primary revascularization of patients with intermittent claudication with lesions in the aortoiliac and common femoral artery (inflow) was associated with frequent re-interventions despite acceptable TLR rates. Although this study design does not allow for head-to-head comparisons, our results underline that SET is an effective and durable treatment option. In contrast to revascularization of a singular arterial lesion providing temporary symptom relief, SET may improve claudication over time by enhancing muscle strength, efficiency, and performance of both lower extremities.34 A combination of endovascular therapy and SET has recently been shown to be even more effective in improving walking distance than SET only, at least at 1-year follow-up.8 The long-term benefit and cost-effectiveness of such a combined approach deserve further consideration.
Acknowledgments
The authors would like to acknowledge the help of Lidia Bons for data collection.
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HJM Verhagen is a consultant to Medtronic and W L Gore & Associates; JAW Teijink is the founder of ClaudicatioNet.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Elke Bouwens  https://orcid.org/0000-0003-1166-1085
https://orcid.org/0000-0003-1166-1085
References
- 1. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013; 382: 1329–1340. [DOI] [PubMed] [Google Scholar]
- 2. European Stroke Organisation, Tendera M, Aboyans V, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: The Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 2851–2906. [DOI] [PubMed] [Google Scholar]
- 3. National Clinical Guideline Centre (UK). Lower limb peripheral arterial disease: Diagnosis and management. London: Royal College of Physicians: NICE Clinical Guidelines, No. 147, https://www.ncbi.nlm.nih.gov/books/NBK299071/ (2012, accessed 11 November 2018). [PubMed] [Google Scholar]
- 4. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135: e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aboyans V, Ricco JB, Bartelink MEL, et al. ; ESC Scientific Document Group. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763–816. [DOI] [PubMed] [Google Scholar]
- 6. Fakhry F, van de Luijtgaarden KM, Bax L, et al. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg 2012; 56: 1132–1142. [DOI] [PubMed] [Google Scholar]
- 7. Sachs T, Pomposelli F, Hamdan A, et al. Trends in the national outcomes and costs for claudication and limb threatening ischemia: Angioplasty vs bypass graft. J Vasc Surg 2011; 54: 1021–1031.e1. [DOI] [PubMed] [Google Scholar]
- 8. Pandey A, Banerjee S, Ngo C, et al. Comparative efficacy of endovascular revascularization versus supervised exercise training in patients with intermittent claudication: Meta-analysis of randomized controlled trials. JACC Cardiovasc Interv 2017; 10: 712–724. [DOI] [PubMed] [Google Scholar]
- 9. Fakhry F, Fokkenrood HJ, Spronk S, et al. Endovascular revascularisation versus conservative management for intermittent claudication. Cochrane Database Syst Rev 2018; 3: CD010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Indes JE, Pfaff MJ, Farrokhyar F, et al. Clinical outcomes of 5358 patients undergoing direct open bypass or endovascular treatment for aortoiliac occlusive disease: A systematic review and meta-analysis. J Endovasc Ther 2013; 20: 443–455. [DOI] [PubMed] [Google Scholar]
- 11. Malgor RD, Alahdab F, Elraiyah TA, et al. A systematic review of treatment of intermittent claudication in the lower extremities. J Vasc Surg 2015; 61: 54S–73S. [DOI] [PubMed] [Google Scholar]
- 12. Olin JW, White CJ, Armstrong EJ, et al. Peripheral artery disease: Evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol 2016; 67: 1338–1357. [DOI] [PubMed] [Google Scholar]
- 13. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): Executive summary. A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006; 47: 1239–1312. [DOI] [PubMed] [Google Scholar]
- 14. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(Suppl S): S5–67. [DOI] [PubMed] [Google Scholar]
- 15. Lauret GJ, Gijsbers HJ, Hendriks EJ, et al. The ClaudicatioNet concept: Design of a national integrated care network providing active and healthy aging for patients with intermittent claudication. Vasc Health Risk Manag 2012; 8: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The Royal Dutch Society for Physical Therapy (KNGF). Guideline: Intermittent claudication. Available from: https://www.kngf.nl/kennisplatform/richtlijnen/symptomatisch-perifeer-arterieel-vaatlijden (2014, accessed 3 January 2019). [Google Scholar]
- 17. Spronk S, Bosch JL, den Hoed PT, et al. Intermittent claudication: Clinical effectiveness of endovascular revascularization versus supervised hospital-based exercise training—Randomized controlled trial. Radiology 2009; 250: 586–595. [DOI] [PubMed] [Google Scholar]
- 18. Dorenkamp S, Mesters EP, Nijhuis-van der Sanden MW, et al. How well do randomized controlled trials reflect standard care: A comparison between scientific research data and standard care data in patients with intermittent claudication undergoing supervised exercise therapy. PLoS One 2016; 11: e0157921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fakhry F, Rouwet EV, den Hoed PT, et al. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J Surg 2013; 100: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 20. Fakhry F, Spronk S, van der Laan L, et al. Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication: A randomized clinical trial. JAMA 2015; 314: 1936–1944. [DOI] [PubMed] [Google Scholar]
- 21. Gommans LN, Fokkenrood HJ, van Dalen HC, et al. Safety of supervised exercise therapy in patients with intermittent claudication. J Vasc Surg 2015; 61: 512–518.e2. [DOI] [PubMed] [Google Scholar]
- 22. Lane R, Ellis B, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014; (7): CD000990. [DOI] [PubMed] [Google Scholar]
- 23. Ratliff DA, Puttick M, Libertiny G, et al. Supervised exercise training for intermittent claudication: Lasting benefit at three years. Eur J Vasc Endovasc Surg 2007; 34: 322–326. [DOI] [PubMed] [Google Scholar]
- 24. Bendermacher BL, Willigendael EM, Nicolai SP, et al. Supervised exercise therapy for intermittent claudication in a community-based setting is as effective as clinic-based. J Vasc Surg 2007; 45: 1192–1196. [DOI] [PubMed] [Google Scholar]
- 25. Aggarwal V, Waldo SW, Armstrong EJ. Endovascular revascularization for aortoiliac atherosclerotic disease. Vasc Health Risk Manag 2016; 12: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazari FA, Khan JA, Samuel N, et al. Long-term outcomes of a randomized clinical trial of supervised exercise, percutaneous transluminal angioplasty or combined treatment for patients with intermittent claudication due to femoropopliteal disease. Br J Surg 2017; 104: 76–83. [DOI] [PubMed] [Google Scholar]
- 27. Van den Houten MM, Lauret GJ, Fakhry F, et al. Cost-effectiveness of supervised exercise therapy compared with endovascular revascularization for intermittent claudication. Br J Surg 2016; 103: 1616–1625. [DOI] [PubMed] [Google Scholar]
- 28. Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: A randomized controlled trial. J Vasc Surg 2008; 48: 1472–1480. [DOI] [PubMed] [Google Scholar]
- 29. Mahoney EM, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes 2010; 3: 642–651. [DOI] [PubMed] [Google Scholar]
- 30. Sobieszczyk P, Eisenhauer A. Management of patients after endovascular interventions for peripheral artery disease. Circulation 2013; 128: 749–757. [DOI] [PubMed] [Google Scholar]
- 31. Jones DW, Schanzer A, Zhao Y, et al. Growing impact of restenosis on the surgical treatment of peripheral arterial disease. J Am Heart Assoc 2013; 2: e000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fouasson-Chailloux A, Abraham P, Colas-Ribas C, et al. Simultaneous pain intensity rating and quantification of ischemia throughout exercise and recovery in proximal versus distal arterial claudication. Vasc Med 2017; 22: 490–497. [DOI] [PubMed] [Google Scholar]
- 33. Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: The CLEVER study. J Am Coll Cardiol 2015; 65: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: Functional impact and mechanisms of benefits. Circulation 2011; 123: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


