Abstract
Background:
Glucose metabolism links closely to cholesterol metabolism. Posttransplant diabetes mellitus (PTDM) adversely affects posttransplant outcomes, but its risk factors in relation to cholesterol metabolism have not been fully delineated. The apolipoprotein B/A1 (Apo B/A1) ratio, which is associated with insulin resistance, has not been evaluated in kidney transplant recipients as a risk factor for PTDM.
Objective:
The objective of this study was to determine whether serum apolipoprotein profiles predict late PTDM, defined as a new onset diabetes occurring greater than 3 months posttransplant.
Design:
Retrospective chart review of a prevalent population of kidney transplant recipients.
Setting:
Large transplant center in Ontario, Canada.
Patients:
We identified 1104 previously nondiabetic adults who received a kidney transplant between January 1, 1998, and December 1, 2015, and were followed at 1 transplant center.
Measurements:
Recipients provided testing for serum apolipoprotein B (Apo B) and apolipoprotein A1 (Apo A1) concentrations from 2010, either at 3 months posttransplant for new transplant recipients or the next clinic visit for prevalent recipients. Late PTDM defined using Canadian Diabetes Association criteria as occurring ≥3 months posttransplant was recorded until May 1, 2016.
Methods:
All analyses were conducted with R, version 3.4.0 (The R Foundation for Statistical Computing). Comparisons were made using Student t test, Fisher exact test or chi-square test, Kaplan-Meier methodology with the logrank test, or Cox proportional hazards analysis as appropriate. Covariates for the multivariate Cox proportional hazards models of PTDM as the outcome variable were selected based on significance of the univariate associations and biological plausibility.
Results:
There were 53 incident late PTDM cases, or 1.71 cases per 100 patient-years. Incident late PTDM differed between the highest and lowest quartiles for Apo B/A1 ratio, 2.47 per 100 patient-years vs 0.88 per 100 patient-years (P = .005 for difference). In multiple Cox regression analysis, first measured serum Apo B/A1 concentration better predicted subsequent PTDM than low-density lipoprotein cholesterol (LDL-C; hazard ratio [HR] = 7.80 per unit increase, P = .039 vs HR = 1.05 per unit increase, P = .774). Non-high-density lipoprotein cholesterol (HDL-C) concentrations also did not predict PTDM (P = .136). By contrast to Apo B, Apo A1 was protective against PTDM in statin users (HR = 0.17 per unit increase, P = .016).
Limitations:
Posttransplant diabetes mellitus cases occurring before apolipoprotein testing was implemented were not included in the analysis.
Conclusions:
Apolipoproteins B and A1 better predict late PTDM than conventional markers of cholesterol metabolism.
Keywords: cardiovascular disease, diabetes, metabolic syndrome, tacrolimus, transplantation
Abrégé
Contexte:
Le métabolisme du glucose est étroitement lié à celui du cholestérol. Le diabète sucré post-transplantation (PTDM—Post-Transplant Diabetes Mellitus) compromet l’état de santé après la greffe, mais le risque qu’il représente sur le métabolisme du cholestérol n’est toujours pas clairement défini. Le taux d’apolipoprotéine B/A1 (Apo B/A1), associé à l’insulinorésistance, n’a toujours pas été évalué en tant que facteur de risque pour le PTDM chez les receveurs d’une greffe rénale.
Objectif:
Cette étude visait à déterminer si les profils sériques de l’apolipoprotéine sont prédicteurs d’un PTDM d’apparition tardive, soit d’un diabète se déclenchant plus de trois mois post-transplantation.
Type d’étude:
Une étude rétrospective des dossiers médicaux d’une population prévalente de receveurs d’une greffe rénale.
Cadre:
Un important centre de transplantation de l’Ontario (Canada).
Sujets:
L’étude porte sur 1 104 adultes non-diabétiques ayant subi une greffe rénale entre le 1er janvier 1998 et le 1er décembre 2015 et ayant été suivis dans un centre de transplantation.
Mesures:
À partir de 2010, les sujets se sont soumis à une épreuve mesurant les concentrations sériques d’Apo B et Apo A1 trois mois post-greffe pour les nouveaux receveurs ou lors de la prochaine consultation en clinique pour les receveurs prévalents. La survenue d’un PTDM d’apparition tardive, soit au minimum trois mois post-greffe selon le critère de l’Association canadienne du diabète, a été enregistrée jusqu’au 1er mai 2016.
Méthodologie:
Toutes les analyses ont été menées avec le logiciel R (R Foundation for Statistical Computing version 3.4.0). Selon le cas, les comparaisons ont été effectuées par le test t de Student, le test de Fisher exact, le test de chi-deux, la méthode de Kaplan-Meier avec le test de Mantel-Haenzel ou l’analyse de régression aléatoire proportionnelle de Cox. Les covariables du modèle multivarié de régression aléatoire proportionnelle de Cox avec le PTDM comme variable résultat ont été choisies en fonction de l’importance des associations univariées et de la plausibilité biologique.
Résultats:
On a répertorié 53 nouveaux cas de PTDM d’apparition tardive, soit 1,71 cas par 100 années-patient. Le nombre de nouveaux cas de PTDM d’apparition tardive différait entre le quartile le plus élevé et le quartile le plus bas pour le taux d’Apo B/A1, avec 2,47 par 100 années-patient et 0,88 par 100 années-patient respectivement (P = 0,005 pour la différence). Selon l’analyse par régression multivariée de Cox, la première mesure de la concentration d’Apo B/A1 s’est avérée un meilleur prédicteur d’un PTDM subséquent que la mesure de LDL-C (RR à 7,80 par augmentation d’une unité pour Apo B/A1, P = 0,039 contre 1,05 par augmentation d’une unité pour LDL-C, P = 0,774). Les taux de cholestérol non HDL n’ont pas non plus prédit un PTDM (P = 0,136). Contrairement à Apo B, Apo A1 protégeait contre le déclenchement d’un PTDM chez les utilisateurs de statines (RR: 0,17 par augmentation d’une unité, P = 0,016).
Limite:
Les cas de PTDM survenus avant que l’épreuve d’apolipoprotéine ne soit mise en œuvre n’ont pas été inclus dans cette analyse.
Conclusion:
Les apolipoprotéines B et A1 ont mieux prédit la survenue du PTDM d’apparition tardive que les marqueurs traditionnels du métabolisme du cholestérol.
What was known before
Conventional predictors of posttransplant diabetes mellitus (PTDM) include age, ethnicity, weight gain, and immunosuppressive medications, among other factors.
What this adds
The apolipoprotein profile is superior to conventional lipid parameters in predicting PTDM. Apolipoprotein B predicted PTDM in nonstatin users while Apo A1 protected against PTDM in statin users. The benefit of statins after transplantation might mediate through Apo B reduction, but this benefit may not be captured through low-density lipoprotein cholesterol reduction.
Introduction
Posttransplant diabetes mellitus (PTDM) associates with increased graft failure and mortality.1,2 Despite numerous well-identified risk factors for PTDM, its incidence remains high.3 Posttransplant diabetes mellitus is generally considered an early posttransplant complication occurring in the first 3 months that entails aggressive efforts such as dietary modification and a rapid reduction in corticosteroid dose. However, late PTDM is also described,4 and management strategies targeting late PTDM will likely additionally improve long-term outcomes. Long-term prevention to reduce the overall PTDM burden mandates further efforts to understand the risk factors behind late PTDM as they occur in kidney transplant recipients (KTR).
A candidate risk factor in the epidemiology of type 2 diabetes is the serum apolipoprotein profile.5 Apolipoprotein A1 (Apo A1) and apolipoprotein B (Apo B) are the primary constituents of antiatherogenic high-density lipoprotein cholesterol (HDL-C) and atherogenic lower density lipoproteins (very low-density lipoprotein cholesterol [VLDL-C], intermediate-density lipoprotein cholesterol [IDL-C], low-density lipoprotein cholesterol [LDL-C]). To date, interest in Apo A1 and Apo B has primarily focused on their role as a independent predictors of cardiovascular disease in the general population.6 However, the Apo B/Apo A1 ratio was also found to be correlated with insulin resistance7 and components of the metabolic syndrome.8 The Apolipoprotein B/A1 (Apo B/A1) ratio has also been independently associated with type II diabetes mellitus (DM).9
Although a shift in the serum apolipoprotein profile has been documented after kidney transplantation,10 any impact of the posttransplant apolipoprotein profiles on posttransplant outcomes such as PTDM and cardiovascular disease has not been ascertained. The aim of this study therefore was to investigate if the serum apolipoprotein profile is independently associated with late PTDM. Our hypothesis was that a high posttransplant Apo B/A1 ratio would correlate better with PTDM incidence compared with more conventional lipid parameters, even in the presence of confounding factors such as lipid-lowering medication that are often prescribed to KTR.
Patients and Methods
Study Sample and Follow-up
St. Michael’s Hospital (SMH) is an urban university-affiliated tertiary-care medical center that actively follows more than 1700 prevalent KTR and performs approximately 120 single-organ kidney transplants annually. Clinic visit frequency is typically weekly to month 1 posttransplant, biweekly to month 3, monthly to month 6, quarterly to month 12, and twice annually or annually thereafter. At each clinic visit, trained personnel record anthropometry and resting blood pressure (BP). Ethnicity is recorded based on self-report. Laboratory testing is performed as close to each clinic visit as possible, with additional testing performed between visits according to a separate, more frequent schedule. The fasting lipid profile is obtained every 6 months, and fasting or random glucose tests are performed at least once every 3 months. The estimated glomerular filtration rate (eGFR) is calculated by the Modification of Diet in Renal Disease-7 equation.11 Starting in 2010, nonfasting Apo B and Apo A1 concentrations have been measured in all KTR at least once either at the first visit after this policy was implemented in 2010 or at 3 months posttransplant in the case of new KTR starting in 2010.
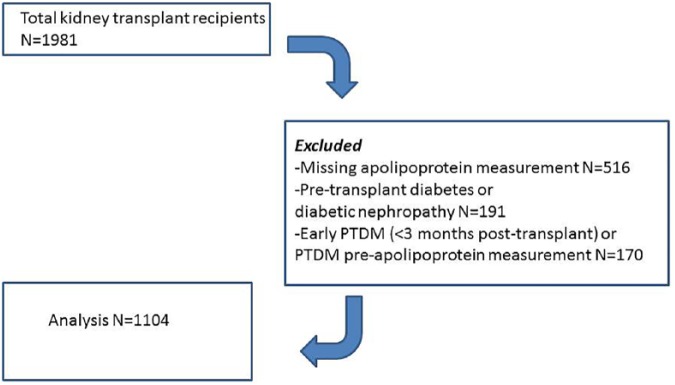
For this study, all KTR followed at SMH and transplanted between January 1, 1998, and December 31, 2015, were identified from the clinical electronic database. We performed a retrospective cohort analysis of the prevalent population of this cohort as of May 1, 2016, to ensure that all patients had a minimum of 3 months potential posttransplant follow-up. The procedure for inclusion and exclusion is outlined in Figure 1. Institutional Research Ethics Board approval (REB10-204c) was obtained for the study. As this study was a retrospective review of clinic data pertaining to a prevalent KTR population, individual informed consent was not obtained. The study was performed in accordance with the 2000 Declaration of Helsinki and the 2008 Declaration of Istanbul.
Figure 1.
Outline of inclusion and exclusion procedure.
Note. PTDM = posttransplant diabetes mellitus.
Data Collection
Occurrence of PTDM was routinely captured by a review of transcriptions from local and citywide hospital databases as part of routine care and supplemented by patient and family physician interviews where necessary. The main outcome measure was incident PTDM, diagnosed and transcribed by a physician using the 2008 Canadian Diabetes Association Guidelines12 while metabolic syndrome status was assessed using National Cholesterol Education Program Adult Treatment Panel III guidelines.13 At least 2 blood glucose readings above diagnostic cut-points or other valid diagnostic tests such as oral glucose tolerance testing were required for the diagnosis of PTDM, which was then validated by dietician and/or endocrinologist consult notes. Patients without measurements for either Apo A1 or Apo B, PTDM within 3 months posttransplant, or known type 2 diabetes that occurred prior to transplant including diabetic nephropathy were excluded. Transplant and pretransplant characteristics were determined at the time of transplantation. All clinical information, laboratory measurement, medication, and physical characteristics data were obtained at the time of apolipoprotein measurement or at the closest posttransplant follow-up date to the date of apolipoprotein measurement. A more restricted 2010-2015 cohort was also identified to assess patients without previous long-term posttransplant follow-up, to exclude any possible impact of subtle preventative measures against PTDM in the older patients.
Our primary outcome of interest was the development of late PTDM, diagnosed 3 months posttransplant or later. Earlier PTDM may be more proportionally attributable to unidentified preexisting disease burden, postoperative stress, and higher immunosuppression rather than traditional risk factors for diabetes, similar to the case with major adverse posttransplant cardiac events.14 The first apolipoprotein measurement, which was used for this analysis, was typically obtained 3 months posttransplant. Temporality between the measurement of apolipoprotein concentrations and PTDM diagnosis was assured by recording PTDM events only after the apolipoprotein measurement. As routine apolipoprotein testing for postoperative KTR began in 2010, patients who developed PTDM prior to 2010 were excluded from analysis. Fasting lipid profile and other laboratory measurements were recorded through a review of the clinical chart utilizing those closest to the date of the apolipoprotein concentration measurement, again preceding any diagnosis of PTDM.
Statistical Analysis
All analyses were conducted with R, version 3.4.0 (The R Foundation for Statistical Computing). All data are reported as mean ± SD, unless otherwise stated. Two-tailed P values below .05 were taken to imply statistical significance. All missing values were handled by exclusion from the relevant analysis without imputation. Comparisons were made using Student t test, Fisher exact test or chi-square test, Kaplan-Meier methodology with the logrank test, or Cox proportional hazards analysis as appropriate. Normality and skewness were assessed visually or with skewness tests for all subgroups, supplemented with the Shapiro-Wilk normality test for small-n subgroups. The selection of covariates for the multivariate Cox proportional hazards models of PTDM as the outcome variable was based on the significance of the univariate associations as well as biological plausibility.15,16
Results
A total of 1981 patients were identified on initial screening. There were 516 patients excluded from further analysis due to missing apolipoprotein concentration measurements, 191 due to pretransplant diabetes or diabetic nephropathy, and 170 due to a diagnosis of PTDM either in the first 3 months posttransplant or prior to apolipoprotein concentration measurement. Therefore, 1104 patients met inclusion criteria for further analysis (Figure 1). Full primary data were available for more than 95% of all physical and laboratory parameters measured. Patients were censored if lost to follow-up (no laboratory testing or clinic visits for 12 months) on the date of their last clinic visit. Median time to first Apo B/A1 measurement was 1.73 years for the 1998-2015 cohort and 3.3 months for the 2010-2015 cohort.
Demographic and other characteristics organized by quartiles of the Apo B/A1 ratio are provided in Table 1. Each quartile provided an approximately equal 775 patient-years of follow-up. A higher Apo B/A1 ratio correlated significantly with male sex, as did a higher waist and hip circumference, and polycystic kidney disease. Other notable associations with higher Apo B/A1 ratios included greater use of cyclosporine, higher serum C-reactive protein, parathyroid hormone, and uric acid concentrations, higher urine albumin-to-creatinine ratios, and decreased kidney allograft function (Table 1). More than 98% of all patients received prednisone. All measured lipid parameters demonstrated normality of distribution. Demographics for the restricted 2010-2015 cohort are provided in Supplementary Table 1.
Table 1.
Patient and Transplant Characteristics Across Apolipoprotein B/A1 Quartiles.
| Q1, N = 276 | Q2, N = 276 | Q3, N = 276 | Q4, N = 276 | R 2 | P value | |
|---|---|---|---|---|---|---|
| Characteristic | ||||||
| Patient count, follow-up (p-y) | 276, 797.4 | 276, 792.9 | 276, 741.2 | 276, 767.9 | — | — |
| Apolipoprotein B/A1 ratio | 0.356 ± 0.06 | 0.485 ± 0.03 | 0.603 ± 0.04 | 0.834 ± 0.16 | — | — |
| Apolipoprotein A1 (g/L) | 1.739 ± 0.30 | 1.581 ± 0.24 | 1.452 ± 0.23 | 1.318 ± 0.22 | 0.293 | <.001 |
| Apolipoprotein B (g/L) | 0.615 ± 0.12 | 0.766 ± 0.12 | 0.874 ± 0.14 | 1.089 ± 0.23 | 0.642 | <.001 |
| Demography/allograft data | ||||||
| Age at bloodwork (years) | 54.98 ± 12.9 | 55.40 ± 13.3 | 54.66 ± 13.6 | 53.64 ± 13.4 | 0.000 | .666 |
| Age at transplant (years) | 51.46 ± 12.9 | 51.42 ± 13.5 | 51.06 ± 13.9 | 49.22 ± 13.7 | 0.001 | .261 |
| >2 transplants | 17, 6.1% | 15, 5.4% | 12, 4.3% | 18, 6.5% | — | .977 |
| Live donor | 130, 47.1% | 122, 44.2% | 133, 48.2% | 137, 49.6% | — | .445 |
| Male | 146, 52.9% | 156, 56.5% | 178, 64.5% | 188, 68.1% | — | <.001 |
| White Ethnicity | 123, 44.6% | 114, 41.3% | 125, 45.3% | 124, 44.9% | — | .144 |
| Smoking | 18, 6.52% | 10, 3.62% | 17, 6.16% | 19, 6.88% | — | .125 |
| ESRD cause | ||||||
| Hypertension | 25, 9.1% | 39, 14.1% | 34, 12.3% | 35, 12.7% | 0.44 | .337 |
| Glomerulonephritis | 95, 34.4% | 95, 34.4% | 94, 34.1% | 108, 39.1% | 0.29 | .365 |
| Polycystic kidney disease | 35, 12.7% | 35, 12.7% | 43, 15.6% | 47, 17.0% | 0.96 | .020 |
| Clinical characteristics | ||||||
| Height (cm) | 166.0 ± 22.3 | 168.4 ± 24.1 | 170.3 ± 20.4 | 169.5 ± 34.9 | 0.02 | <.001 |
| Weight (kg) | 69.5 ± 16.0 | 73.1 ± 16.3 | 78.1 ± 17.1 | 76.9 ± 16.8 | 0.03 | <.001 |
| Body mass index (kg/m2) | 25.1 ± 4.5 | 25.7 ± 4.80 | 26.9 ± 5.01 | 26.8 ± 4.73 | 0.02 | <.001 |
| Systolic BP (mm Hg) | 128.3 ± 15.4 | 128.3 ± 14.0 | 127.5 ± 15.9 | 130.0 ± 15.8 | 0.00 | .213 |
| Diastolic BP (mm Hg) | 80.3 ± 9.5 | 81.2 ± 9.8 | 80.5 ± 9.3 | 83.1 ± 10.4 | 0.01 | .003 |
| Waist circumference (cm) | 89.5 ± 16.9 | 91.2 ± 18.5 | 96.4 ± 16.7 | 96.5 ± 20.5 | 0.03 | <.001 |
| Hip circumference (cm) | 95.8 ± 15.1 | 97.0 ± 17.0 | 100.2 ± 15.2 | 100.2 ± 20.0 | 0.02 | <.001 |
| Waist-to-hip ratio | 0.9 ± 0.11 | 0.94 ± 0.14 | 0.96 ± 0.11 | 0.96 ± 0.17 | 0.03 | <.001 |
| Laboratory measurements | ||||||
| Fasting blood glucose | 5.4 ± 1.7 | 5.6 ± 1.6 | 5.8 ± 2.3 | 5.8 ± 1.6 | 0.01 | .004 |
| Random blood glucose | 5.8 ± 1.9 | 5.9 ± 2.0 | 6.4 ± 2.9 | 6.3 ± 2.2 | 0.01 | .002 |
| HbA1c (%) | 5.6 ± 0.8 | 5.7 ± 0.8 | 5.7 ± 1.1 | 5.9 ± 1.1 | 0.02 | <.001 |
| Total cholesterol | 4.2 ± 1.0 | 4.5 ± 1.2 | 4.6 ± 1.1 | 5.2 ± 1.4 | 0.17 | <.001 |
| HDL-cholesterol | 1.6 ± 0.5 | 1.4 ± 0.4 | 1.2 ± 0.4 | 1.1 ± 0.3 | 0.21 | <.001 |
| LDL-cholesterol | 2.0 ± 0.8 | 2.4 ± 1.0 | 2.6 ± 1.1 | 3.1 ± 1.5 | 0.28 | <.001 |
| HDL/LDL ratio | 1.3 ± 0.5 | 1.7 ± 0.7 | 2.2 ± 0.9 | 3.0 ± 1.4 | 0.60 | <.001 |
| Triglycerides | 1.3 ± 0.9 | 1.6 ± 0.8 | 1.7 ± 0.7 | 2.3 ± 1.3 | 0.17 | <.001 |
| Non-HDL cholesterol | 2.5 ± 0.8 | 3.0 ± 1.1 | 2.9 ± 7.6 | 4.1 ± 1.3 | 0.02 | <.001 |
| Total cholesterol/HDL ratio | 2.7 ± 0.8 | 3.3 ± 1.1 | 3.9 ± 0.9 | 5.2 ± 2.9 | 0.36 | <.001 |
| C-reactive protein (mg/L) | 3.2 ± 7.9 | 3.5 ± 5.3 | 4.9 ± 7.8 | 11.2 ± 29.5 | 0.05 | <.001 |
| Parathyroid hormone (pmol/L) | 12.2 ± 14.1 | 12.0 ± 12.8 | 11.9 ± 9.9 | 14.5 ± 16.2 | 0.01 | .023 |
| Vitamin D25 (nmol/L) | 56.5 ± 30.7 | 59.6 ± 30.6 | 55.3 ± 27.8 | 48.0 ± 27.7 | 0.02 | <.001 |
| Uric acid (µmol/L) | 372.1 ± 105.0 | 376.7 ± 99.6 | 400.0 ± 101.0 | 413.5 ± 109.0 | 0.03 | <.001 |
| 24-hour urine protein (g/d) | 0.6 ± 0.7 | 0.5 ± 0.5 | 0.8 ± 1.3 | 0.74 ± 1.2 | 0.00 | .184 |
| Renal function/outcomes | ||||||
| Serum creatinine (µmol/L) | 117.9 ± 53.7 | 119.2 ± 40.6 | 127.4 ± 47.3 | 140.5 ± 78.9 | 0.03 | <.001 |
| Estimated GFR (mL/min/1.73 m2) | 58.3 ± 21.7 | 55.9 ± 18.0 | 53.71 ± 18.1 | 52.5 ± 20.1 | 0.02 | <.001 |
| Urine ACR (mg/mmol) | 10.9 ± 32.9 | 10.9 ± 38.3 | 10.48 ± 29.9 | 22.9 ± 65.7 | 0.01 | <.001 |
| Graft failure | 29, 10.5% | 19, 6.9% | 44, 15.9% | 32, 11.6% | — | .103 |
| Metabolic syndrome | 204, 73.9% | 185, 67.0% | 203, 73.6% | 210, 76.1% | — | .066 |
| Acute rejection | 20, 7.3% | 23, 8.3% | 29, 10.5% | 24, 8.7% | — | .264 |
| Delayed graft function | 14, 5.07% | 9, 3.26% | 12, 4.35% | 15, 5.43% | — | .583 |
| Medications | ||||||
| Statin | 164, 59.4% | 143, 51.8% | 136, 49.3% | 108, 39.1% | — | <.001 |
| Cyclosporine | 30, 10.9% | 37, 13.4% | 38, 13.8% | 46, 16.7% | — | .011 |
| Tacrolimus | 230, 83.3% | 217, 78.6% | 220, 79.7% | 208, 75.4% | — | .028 |
| Mycophenolate mofetil | 170, 61.6% | 169, 61.2% | 172, 62.3% | 174, 63.0% | — | .939 |
| Mycophenolic acid | 60, 21.7% | 63, 22.8% | 73, 26.5% | 58, 21.0% | — | .966 |
Note. R statistic not provided for binary characteristics, as logistic regression was used for these characteristics instead of linear regression. Laboratory measurements with unlisted units are mmol/L. Binary factors expressed in N (%) unless otherwise specified. p-y = patient years; ESRD = end-stage renal disease; BP = Blood pressure; HbA1c = glycated hemoglobin; HDL = high-density lipoprotein; LDL = low-density lipoprotein; GFR = glomerular filtration rate; ACR = albumin-to-creatinine ratio.
Posttransplant Diabetes Mellitus
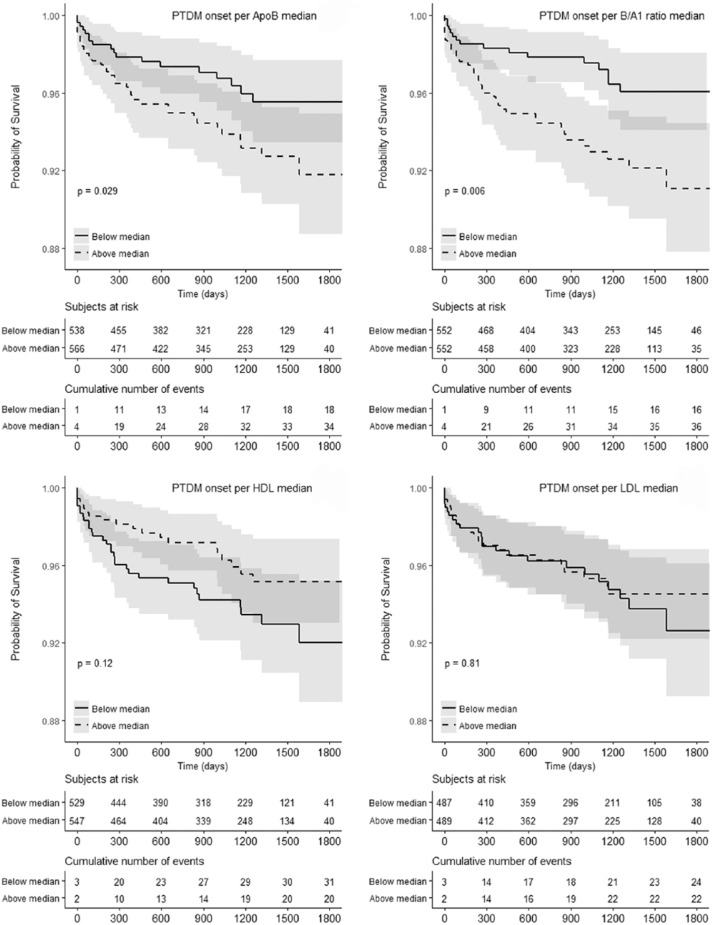
There were 53 incident late PTDM cases in the total cohort over 3100 patient-years follow-up, corresponding to 1.71 cases/100 patient-years, including 29 incident PTDM cases in the 2010-2015 cohort. Median follow-up time after apolipoprotein measurement for the non-PTDM group was 3.07 years. Median time-to-incident PTDM after apolipoprotein measurement was 0.72 years or 262 days. Incidence rates by Apo B/A1 quartile demonstrate progressively increasing PTDM risk with increasing Apo B/A1 ratio (Figure 2). Late PTDM incidence differed between the highest and lowest quartiles for the Apo B/A1 ratio, 2.47 per 100 patient-years vs 0.88 per 100 patient-years (P = .005 for difference). Kaplan-Meier survival analysis of time-to-PTDM diagnosis in the entire cohort (Figure 3) indicates significance of the Apo B and the Apo B1/A ratio higher than the median concentration, but not for above-median serum HDL-C and LDL-C concentrations. Univariate Cox regression analysis of Apo B/A1 ratio on PTDM incidence limited to the 2010-2015 cohort (Table 3) indicates a similar association between Apo B/A1 and PTDM (P = .008, hazard ratio [HR] = 8.246).
Figure 2.
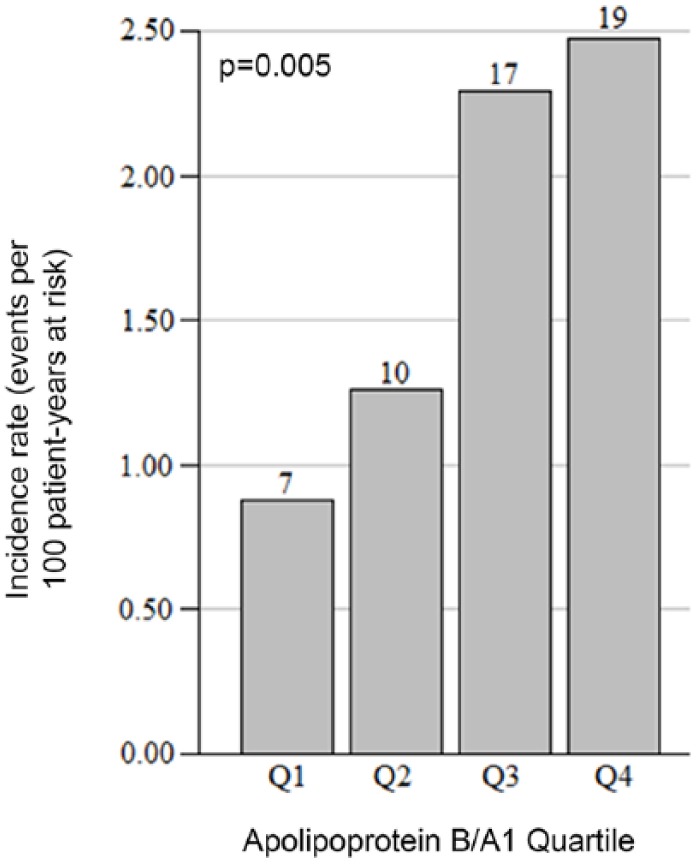
Incidence rate of PTDM per apolipoprotein B/A1 quartile.
Note. Rate is expressed in events per 100 patient-years at risk. Case count per quartile is shown over each bar with 276 patients present in each quartile. Significance was assessed using a test-for-trend in proportions. PTDM = posttransplant diabetes mellitus.
Figure 3.
Time-to-PTDM incidence for Apo B, Apo B/A1, HDL-C, and LDL-C.
Note. Kaplan-Meier PTDM-free survival curves for subjects above or below the median for each lipid parameter. Log-rank test was used to assess significance. Apo A1 is not shown (P = .23 in Cox regression). Time is represented in days. PTDM = posttransplant diabetes mellitus; Apo B = apolipoprotein B; Apo B/A1 = apolipoprotein B/A1; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; Apo A1 = apolipoprotein A1.
Table 3.
Univariate Cox Proportional Hazards Regressions for Late PTDM Incidence, 2010-2015.
| Variable | HR (95% CI) | P value |
|---|---|---|
| Apolipoprotein parameters | ||
| Apolipoprotein B/A1 | 8.25 (1.72-39.5) | .008 |
| Apolipoprotein A1 (g/L) | 1.18 (0.37-3.74) | .777 |
| Apolipoprotein B (g/L) | 12.2 (3.13-47.5) | <.001 |
| Clinical characteristics | ||
| Body mass index (kg/m2) | 1.02 (0.95-1.11) | .543 |
| Waist circumference (cm) | 1.02 (0.99-1.05) | .131 |
| Hip circumference (cm) | 1.01 (0.98-1.04) | .711 |
| Waist to hip ratio (cm/cm × 100) | 1.06 (1.01-1.10) | .021 |
| Weight (kg) | 1.00 (0.98-1.03) | .826 |
| Height (cm) | 0.98 (0.95-1.02) | .397 |
| Systolic BP (mm Hg) | 1.00 (0.98-1.03) | .732 |
| Diastolic BP (mm Hg) | 1.01 (0.98-1.05) | .427 |
| Age at transplant (years) | 1.01 (0.98-1.03) | .681 |
| Transplant number | 1.00 (0.24-4.20) | .996 |
| Live donor | 0.54 (0.23-1.27) | .158 |
| Male | 0.95 (0.45-1.99) | .893 |
| White ethnicity | 0.43 (0.18-1.06) | .066 |
| Metabolic Syndrome | 4.42 (1.34-14.6) | .015 |
| Laboratory measurements | ||
| Serum creatinine (µmol/L) | 1.00 (0.99-1.01) | .476 |
| Fasting blood glucose (mmol/L) | 1.17 (1.09-1.25) | <.001 |
| Random blood glucose (mmol/L) | 1.15 (1.08-1.23) | <.001 |
| HbA1c (%) | 1.59 (1.24-2.02) | <.001 |
| eGFR (MDRD, mL/min/1.73 m2) | 1.01 (0.99-1.02) | .590 |
| Total cholesterol (mmol/L) | 1.50 (1.11-2.02) | .009 |
| HDL cholesterol (mmol/L) | 1.60 (0.74-3.47) | .232 |
| LDL cholesterol (mmol/L) | 1.43 (0.96-2.13) | .075 |
| HDL/LDL cholesterol ratio | 1.23 (0.83-1.82) | .299 |
| Triglycerides (mmol/L) | 1.21 (0.96-1.52) | .115 |
| Non-HDL cholesterol (mmol/L) | 1.42 (1.03-1.97) | .034 |
| Total cholesterol/HDL-C ratio | 1.02 (0.91-1.14) | .724 |
| C-reactive protein (mg/L) | 1.01 (1.00-1.02) | .150 |
| Urine ACR (mg/mmol) | 1.00 (0.98-1.02) | .959 |
| Uric Acid (µmol/L) | 1.00 (0.99-1.00) | .133 |
| 24-hour urine protein (g/d) | 0.30 (0.06-1.42) | .129 |
| Medications | ||
| Statin | 0.86 (0.41-1.83) | .696 |
| Prednisone | 1.18 (0.28-4.98) | .821 |
| Cyclosporine | 1.57 (0.54-4.51) | .405 |
| Tacrolimus | 0.66 (0.25-1.72) | .392 |
| Mycophenolic acid | 1.05 (0.451-2.44) | .911 |
Note. PTDM = posttransplant diabetes mellitus; HR = hazard ratio; CI = confidence interval; BP = blood pressure; HbA1c = glycated hemoglobin; eGFR = estimated glomerular filtration rate; MDRD = Modification of Diet in Renal Disease; HDL = high-density lipoprotein; LDL = low-density lipoprotein; ACR = albumin-to-creatinine ratio.
A phenotype characterization of the total cohort to identify differences between late PTDM and non-PTDM subjects is shown in Table 2, and for the restricted cohort in Supplementary Table 2. No significant differences were noted in graft failure, allograft function, or immunosuppression exposure between groups. Univariate Cox proportional hazards analysis for time-to-PTDM diagnosis of the restricted cohort is provided in Table 3, with significant predictors of PTDM including the Apo B/A1 ratio and Apo B concentrations, blood glucose and HbA1c concentrations, waist-to-hip ratio, and serum triglyceride concentrations. The results of multivariate Cox regression analyses in this cohort including other potential indicators of pretransplant dyslipidemia are shown in Table 4. Per-unit increases in serum Apo B (HR = 8.41, P = .007) and Apo B/A1 ratio (HR = 7.80, P = .039) outperformed other conventional lipid parameters, significantly correlating to PTDM incidence.
Table 2.
Differences in Clinical Characteristics Between Incident PTDM and Non-PTDM Cases.
| Variable | PTDM (53) | No PTDM (1051) | P value |
|---|---|---|---|
| Apo A1 (g/L) | 1.5 ± 0.3 | 1.52 ± 0.3 | .328 |
| Apo B (g/L) | 0.9 ± 0.3 | 0.83 ± 0.2 | .015 |
| Apo B/A1 ratio | 0.6 ± 0.2 | 0.57 ± 0.2 | .026 |
| Graft failure | 6, 11.3% | 118, 11.2% | .880 |
| Serum creatinine (µmol/L) | 118.3 ± 41.5 | 126.7 ± 58.3 | .303 |
| eGFR (mL/min/1.73 m2) | 57.5 ± 21.9 | 55.0 ± 19.5 | .372 |
| FBG (mmol/L) | 7.0 ± 2.2 | 5.57 ± 1.8 | <.001 |
| RBG (mmol/L) | 8.1 ± 4.2 | 5.97 ± 2.1 | <.001 |
| Cyclosporine | 9, 17.0% | 142, 13.5% | .547 |
| Tacrolimus | 42, 79.3% | 833, 79.3% | 1.000 |
| Statin | 45, 84.9% | 743, 70.7% | .401 |
Note. Significance assessed using comparison of incidence rates, Student t test, or Fisher exact test, as appropriate. PTDM = posttransplant diabetes mellitus; Apo A1 = apolipoprotein A1; Apo B = apolipoprotein B; Apo B/A1 = apolipoprotein B/A1; eGFR = estimated glomerular filtration rate; FBG = fasting blood glucose; RBG = random blood glucose.
Table 4.
Multivariate Cox Proportional Hazards Regressions for Late PTDM Incidence, Using the 2010-2015 Cohort.
| Variable | HR (95% CI) | P | Variable | HR (95% CI) | P |
|---|---|---|---|---|---|
| Apo B/A1 | 7.80 (1.1-55) | .039 | Apo B (g/L) | 8.41 (1.7-40) | .007 |
| BMI (kg/m2) | 0.99 (0.9-1.1) | .859 | BMI (kg/m2) | 0.99 (0.9-1.1) | .841 |
| SBP (mm Hg) | 0.99 (1.0-1.0) | .464 | SBP (mm Hg) | 0.99 (1.0-1.0) | .347 |
| Age (kg/m2) | 1.00 (1.0-1.0) | .981 | Age (kg/m2) | 1.00 (1.0-1.0) | .974 |
| Live donor | 0.47 (0.2-1.4) | .176 | Live donor | 0.43 (0.1-1.3) | .136 |
| White | 0.44 (0.2-1.1) | .083 | White | 0.46 (0.2-1.2) | .097 |
| Tac (vs CsA) | 1.11 (0.3-3.9) | .869 | Tac (vs CsA) | 1.16 (0.3-4.2) | .815 |
| Waist-to-hip | 1.04 (1.0-1.1) | .222 | Waist-to-hip | 1.04 (1.0-1.1) | .142 |
| Metabolic syndrome | 5.34 (1.2-23.0) | .027 | Metabolic syndrome | 5.37 (1.2-24.0) | .026 |
| Non-HDL-C | 1.21 (0.9-1.6) | .136 | LDL-C | 1.05 (0.7-1.5) | .774 |
| BMI (kg/m2) | 0.97 (0.9-1.0) | .392 | BMI (kg/m2) | 0.99 (0.9-1.1) | .717 |
| SBP (mm Hg) | 0.99 (1.0-1.0) | .599 | SBP (mm Hg) | 1.00 (1.0-1.0) | .773 |
| Age (kg/m2) | 1.00 (1.0-1.0) | .893 | Age (kg/m2) | 1.00 (1.0-1.0) | .928 |
| Live donor | 0.70 (0.3-1.3) | .227 | Live donor | 0.61 (0.3-1.2) | .160 |
| White | 0.50 (0.3-1.0) | .036 | White | 0.52 (0.3-1.0) | .054 |
| Tac (vs CsA) | 1.96 (0.8-5.0) | .162 | Tac (vs CsA) | 1.74 (0.7-4.5) | .250 |
| Waist-to-hip | 1.05 (1.0-1.1) | .016 | Waist-to-hip | 1.05 (1.0-1.1) | .265 |
| Metabolic syndrome | 7.46 (1.8-31) | .006 | Metabolic syndrome | 6.60 (1.6-28) | .010 |
Note. Four separate models are shown. Age refers to the age at time of measurement. Binary variables coded as 0 = no, 1 = yes. Multiple regression with Apo A1 not shown, HR (95% CI) = 0.51 (0.2-1.6), P = .248. Metabolic syndrome diagnoses use National Cholesterol Education Program Adult Treatment Panel III criteria. Non-HDL-cholesterol and LDL cholesterol expressed in mmol/L. PTDM = posttransplant diabetes mellitus; HR = hazard ratio; CI = confidence interval; Apo B/A1 = apolipoprotein B/A1; Apo B = apolipoprotein B; Apo A1 = apolipoprotein A1; BMI = body mass index; SBP = systolic blood pressure; Tac = tacrolimus; CsA = cyclosporine A; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol.
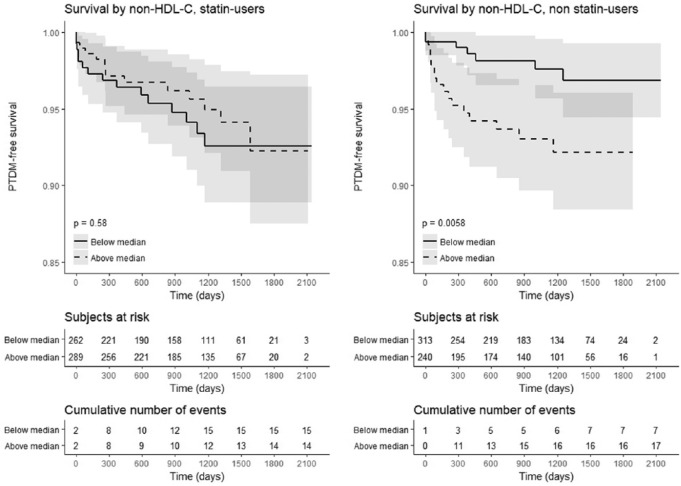
The selective predictive value of Apo B and Apo A1 altered when considering statin use. Table 5 provides 6 stratified survival regression analyses for time-to-PTDM accounting for potential effect modifiers of the apolipoprotein-PTDM relationship. Apolipoprotein B, an obligate protein component of LDL-C, predicted PTDM in nonstatin users while Apo A1, the major protein component of HDL-C, protected against PTDM incidence in statin users (Table 5). Kaplan-Meier analyses of PTDM-free survival (Figure 4) demonstrated a predictive value to non-HDL-C in statin users corroborating this relationship of Apo A1 to PTDM.
Table 5.
Cox Regression Analyses for Apo B and Apo A1, Stratified by Statin Use, High Fasting Blood Glucose and White Ethnicity in the 1998-2015 Cohort.
| Variable |
Cox regression, Apo B |
Cox regression, Apo A1 |
||
|---|---|---|---|---|
| Total n (case count) | Yes | No | Yes | No |
| Statin use Yes: 551 (26), no: 553 (21) |
1.55 (0.34-7.05) P = .571 |
7.19 (2.22-23.3) P = .001 |
0.17 (0.04-0.71) P = .016 |
1.58 (0.45-5.62) P = .476 |
| FBG > 7.0 mmol/L Yes: 73 (14), no: 936 (34) |
11.3 (1.59-79.5) P = .015 |
1.39 (0.35-5.55) P = .639 |
1.64 (0.35-7.80) P = .53 |
0.26 (0.07-0.89) P = .032 |
| White Yes: 441 (13), no: 395 (28) |
0.11 (0.01-1.80) P = .122 |
10.3 (2.78-38.5) P < .001 |
0.2 (0.03-1.16) P = .124 |
0.5 (0.13-1.96) P = .318 |
Note. The stratification is dichotomized. Each group is tested in univariate Cox regression with Apo B and Apo A1, respectively. Apo B = apolipoprotein B; Apo A1 = apolipoprotein A1; FBG = fasting blood glucose.
Figure 4.
Kaplan-Meier analysis of PTDM-free survival by non HDL-C median based on statin use.
Note. PTDM = posttransplant diabetes mellitus; HDL-C = high-density lipoprotein cholesterol.
Discussion
This retrospective study of a large, multiethnic cohort of KTR suggests a predictive value of the posttransplant apolipoprotein profile over more conventional lipid parameters toward late PTDM. Although dyslipidemia and diabetes correlate posttransplantation,17 HDL-C and LDL-C proved less useful than apolipoprotein measurements in this study for this purpose. Metabolic syndrome predicts PTDM18 with the caveat that type 2 diabetes locates within the metabolic syndrome itself.14 The association of a high Apo B/A1 ratio and Apo B concentration with late PTDM identifies a new potential risk factor for diabetes in the presence of chronic kidney disease.
Apolipoproteins provide a new potential predictive PTDM risk marker situated outside of established metabolic syndrome definitions.14 Apolipoprotein B/A1 profile abnormalities reflect higher diabetes and cardiovascular risk or mortality in the general population19 and prevalent dialysis patients.20,21 Such associations were previously unclear in KTR. Besides extending these findings to KTR, this study may also provide proximal mechanistic hypotheses of PTDM genesis and ultimately help reduce not only DM but cardiovascular disease located further along the causal pathway from PTDM because PTDM predicts cardiovascular disease.22 Conventional risk markers of lipid turnover may assist in predicting diabetes in the general population but are less useful in KTR due to the presence of multiple confounders, so the apolipoprotein profile may be of greater assistance particularly in late PTDM. This study emphasizes risk factors for late PTDM, which is not the focus of clinical trials in KTR and whose risk factors require better delineation.
Hypertriglyceridemia occurs in type 2 diabetes from increased VLDL triglycerides or defective removal of plasma triglycerides.23 IDL-C and LDL-C are the products of VLDL and chylomicron metabolism, which themselves contain triglycerides.24 A posttransplant state impairs triglyceride clearance.25 Given that Apo B is an obligate component of VLDL-C, IDL-C, and LDL-C, Apo B concentrations may more accurately reflect the hypertriglyceridemia burden of diabetes, and may hence indicate the prediabetic state. Apolipoprotein B overproduction has also been demonstrated to induce insulin resistance.26 This study extends this role of Apo B to the posttransplant milieu after the environmentally murky early posttransplant phase has passed. As dyslipidemia and diabetes cluster as risk factors causing one another,27 posttransplant dyslipidemia better reflects in serum Apo B rather than LDL-C concentrations. Subclinical inflammation is also a component of the insulin resistance syndrome,28 so Apo B may mediate posttransplant insulin resistance. Triglyceride-containing particle count, reflected through the Apo B concentration, may be more important than the triglyceride concentration itself in leading to PTDM.
Treatment of posttransplant dyslipidemia impairs the ascertainment of causal pathways toward important clinical endpoints like PTDM. Statin therapy alters the relationship between Apo B and HDL-C or LDL-C,29 exerting a greater effect on cholesterol and triglyceride than on apolipoprotein concentrations. In this study, statin use was associated with a lower Apo B but not lower triglyceride concentration. The ineffectiveness of LDL-C to predict PTDM in multivariate analysis (Table 4) may reflect the effects of statin therapy, known to reduce posttransplant cardiac events.30 The obligate 1:1 inclusion of Apo B in hepatic-derived VLDL-C, IDL-C, and LDL-C structure31 allows Apo B to serve as a formal count of LDL particles, unlike LDL-C that measures the cholesterol content of LDL particles. LDL-C may predict atherosclerosis only when LDL-C and LDL particle count are concordant.32,33 Therefore, if Apo B and LDL-C are not concordant in KTR, then Apo B and Apo A1 may provide greater predictive value toward posttransplant metabolic outcomes such as PTDM.
Measured HDL-C is a summation of HDL2 and HDL3 concentrations.34 HDL3 less effectively transports apolipoprotein C-II35 while HDL2 more effectively reflects decreases in total HDL-C. Total HDL-C may therefore poorly indicate PTDM risk if HDL structure shifts characterize the posttransplant state. Apolipoprotein A1, representing total HDL-particle count, may consequently also poorly reflect PTDM risk except in the presence of statins, which affects LDL-C more. Clinically, the benefit of statins in metabolic syndrome36 might mediate through Apo B reduction, but this benefit may not be captured through LDL-C reduction.
Although our study presents an association between the posttransplant apolipoprotein profile and PTDM, it is limited by its retrospective, observational nature. The association between apolipoprotein concentrations and PTDM does not imply causality or the direction of the cause-effect relationship. Measurement of apolipoprotein profiles only since 2010 in a 1998-2015 cohort allowed for 3-month posttransplant standardized measurement in only a subgroup of patients, even though incident PTDM was recorded in all patients only after apolipoprotein profiles were measured. PTDM was ascertained through recorded transcription, possibly biasing against detecting more events based on blood glucose measurements particularly when mortality occurs or grafts fail early posttransplant. The small number of late PTDM cases, excluding a much larger number of earlier PTDM cases (170 vs 53), precluded adjusting for more potential confounders in regression analyses. We did not possess sufficiently granular information of immunosuppressive medication or their serum concentration as well as statin dosage for producing meaningful correlations. Greater patient numbers and longer follow-up will be required to better assess these and other relationships among lipid particle components in chronic kidney disease.
In conclusion, the apolipoprotein profile in our cohort was a superior predictor of late PTDM compared with traditional lipid profile parameters. Routine apolipoprotein measurement post kidney transplantation, if validated in other cohorts, may facilitate investigating mechanisms for PTDM, and might ultimately contribute to better monitoring and reducing long-term posttransplant cardiovascular risk.
Supplemental Material
Supplemental material, Apolipoprotein_supplementary_tables_FINAL_UNMARKED_April_2019 for Serum Apolipoprotein B and A1 Concentrations Predict Late-Onset Posttransplant Diabetes Mellitus in Prevalent Adult Kidney Transplant Recipients by Rohit Malyala, Lindita Rapi, Michelle M. Nash and G. V. Ramesh Prasad in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors acknowledge Philip W. Connelly, PhD, Division of Endocrinology and Metabolism, Department of Medicine, University of Toronto, for detailed discussions on cholesterol metabolism.
Footnotes
List of Abbreviations: Apo A1, apolipoprotein A1; Apo B, apolipoprotein B; BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IDL-C, intermediate-density lipoprotein cholesterol; KTR, kidney transplant recipient(s); LDL-C, low-density lipoprotein cholesterol; MDRD, modification of diet in renal diseases; PTDM, posttransplant diabetes mellitus; p-y, patient-years; RBG, random blood glucose; SBP, systolic blood pressure; SD, standard deviation; SMH, St. Michael’s Hospital; TG, triglycerides; VLDL-C, very low-density lipoprotein cholesterol.
Ethics Approval and Consent to Participate: Ethics approval was obtained from the Research Ethics Board at St. Michael’s Hospital. Since the study was a population-based retrospective review, individual consent to participate was not obtained.
Consent for Publication: All authors have reviewed a final version of the manuscript and have consented to publication.
Availability of Data and Materials: Data and materials may be made available to qualified investigators upon written request to the corresponding author. Reasonable requests for data access will be assessed in consultation with the appropriate Research Ethics Board.
Author Contributions: R.M. participated in research design, data collection, data analysis, and writing. L.R. participated in research design and writing. M.M.N. participated in research design and writing. G.V.R.P. coordinated research design, funding, performance of the research, data analysis, and writing.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Kidney Transplant Program, St. Michael’s Hospital, Toronto, ON, Canada.
ORCID iD: G. V. Ramesh Prasad  https://orcid.org/0000-0003-1576-7696
https://orcid.org/0000-0003-1576-7696
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM. Post-transplant diabetes mellitus: increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int. 2001;59(2):732-737. [DOI] [PubMed] [Google Scholar]
- 2. Revanur VK, Jardine AG, Kingsmore DB, Jaques BC, Hamilton DH, Jindal RM. Influence of diabetes mellitus on patient and graft survival in recipients of kidney transplantation. Clin Transplant. 2001;15(2):89-94. [DOI] [PubMed] [Google Scholar]
- 3. Palepu S, Prasad GV. New-onset diabetes mellitus after kidney transplantation: current status and future directions. World J Transplant. 2015;6:445-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Porrini EL, Díaz JM, Moreso F, et al. Clinical evolution of post-transplant diabetes mellitus. Nephrol Dial Transplant. 2016;31(3):495-505. [DOI] [PubMed] [Google Scholar]
- 5. Onat A, Can G, Hergenc G, Yazici M, Karabulut A, Albayrak S. Serum apolipoprotein B predicts dyslipidemia, metabolic syndrome and, in women, hypertension and diabetes, independent of markers of central obesity and inflammation. Int J Obes (Lond). 2007;31(7):1119-1125. [DOI] [PubMed] [Google Scholar]
- 6. McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372(9634):224-233. [DOI] [PubMed] [Google Scholar]
- 7. Sierra-Johnson J, Romero-Corral A, Somers VK, et al. ApoB/apoA-I ratio: an independent predictor of insulin resistance in US non-diabetic subjects. Eur Heart J. 2007;28(21):2637-2643. [DOI] [PubMed] [Google Scholar]
- 8. Sierra-Johnson J, Somers VK, Kuniyoshi FH, et al. Comparison of apolipoprotein-B/apolipoprotein-AI in subjects with versus without the metabolic syndrome. Am J Cardiol. 2006;98(10):1369-1373. [DOI] [PubMed] [Google Scholar]
- 9. Hwang YC, Ahn HY, Kim WJ, Park CY, Park SW. Increased Apo B/A-I ratio independently associated with type 2 diabetes mellitus: cross-sectional study in a Korean population. Diabet Med. 2012;29(9):1165-1170. [DOI] [PubMed] [Google Scholar]
- 10. Cassader M, Ruiu G, Gambino R, et al. Lipoprotein apolipoprotein changes in renal transplant recipients. Lipids. 2002;37(10):967-974. [DOI] [PubMed] [Google Scholar]
- 11. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247-254. [DOI] [PubMed] [Google Scholar]
- 12. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32(suppl 1):S1-S201. [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, National Cholesterol Education Program, The National Cholesterol Guidelines in 2001, Adult Treatment Panel III. Approach to lipoprotein management in 2001 National Cholesterol Guidelines. Am J Cardiol. 2002;90(8A):11i-21i. [DOI] [PubMed] [Google Scholar]
- 14. Prasad GV, Huang M, Silver SA, et al. Metabolic syndrome definitions and components in predicting major adverse cardiovascular events after kidney transplantation. Transpl Int. 2015;28:79-88. [DOI] [PubMed] [Google Scholar]
- 15. Kuypers DR, Claes K, Bammens B, Evenepoel P, Vanrenterghem Y. Early clinical assessment of glucose metabolism in renal allograft recipients: diagnosis and prediction of post-transplant diabetes mellitus (PTDM). Nephrol Dial Transplant. 2008;23(6):2033-2042. [DOI] [PubMed] [Google Scholar]
- 16. Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev. 2016;37(1):37-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choe EY, Wang HJ, Kwon O, et al. HMG CoA reductase inhibitor treatment induces dysglycemia in renal allograft recipients. Transplantation. 2014;97(4):419-425. [DOI] [PubMed] [Google Scholar]
- 18. Israni AK, Snyder JJ, Skeans MA, Kasiske BL, PORT Investigators. Clinical diagnosis of metabolic syndrome: predicting new-onset diabetes, coronary heart disease, and allograft failure late after kidney transplant. Transpl Int. 2012;25:748-757. [DOI] [PubMed] [Google Scholar]
- 19. Lu M, Lu Q, Zhang Y, Tian G. Apo B/Apo A1 is an effective predictor of coronary heart disease risk in overweight and obesity. J Biomed Res. 2011;25(4):266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silbernagel G, Genser B, Drechsler C, et al. HDL cholesterol, apolipoproteins, and cardiovascular risk in hemodialysis patients. J Am Soc Nephrol. 2014;26(2):484-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sato Y, Fujimoto S, Toida T, et al. Apoprotein B/Apoprotein A-1 ratio and mortality among prevalent dialysis patients. Clin J Am Soc Nephrol. 2016;11(5):840-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early diagnosed post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69:588-595. [DOI] [PubMed] [Google Scholar]
- 23. Yoshino G, Hirano T, Kazumi T. Dyslipidemia in diabetes mellitus. Diabetes Res Clin Pract. 1996;33(1):1-14. [DOI] [PubMed] [Google Scholar]
- 24. Levy RI, Bilheimer DW, Eisenberg S. The structure and metabolism of chylomicrons and very low density lipoproteins (VLDL). Biochem Soc Symp. 1971;(33):3-17. [PubMed] [Google Scholar]
- 25. Savdie E, Gibson JC, Crawford GA, Simons LA, Mahony JF. Impaired plasma triglyceride clearance as a feature of both uremic and posttransplant triglyceridemia. Kidney Int. 1980;18(6):774-782. [DOI] [PubMed] [Google Scholar]
- 26. Castro Cabezas M, de Bruin TW, de Valk HW, Shoulders CC, Jansen H, Willem Erkelens D. Impaired fatty acid metabolism in familial combined hyperlipidemia. A mechanism associating hepatic apolipoprotein B overproduction and insulin resistance. J Clin Invest. 1993;92(1):160-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ibels LS, Simons LA, King JO, Williams PF, Neale FC, Stewart JH. Studies on the nature and causes of hyperlipidaemia in uraemia, maintenance dialysis and renal transplantation. Q J Med. 1975;44(176):601-614. [PubMed] [Google Scholar]
- 28. Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome. Circulation. 2000;102(1):42-47. [DOI] [PubMed] [Google Scholar]
- 29. Ballantyne CM, Raichlen JS, Cain VA. Statin therapy alters the relationship between apolipoprotein B and low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol targets in high-risk patients: the MERCURY II (Measuring Effective Reductions in Cholesterol Using Rosuvastatin therapy II) trial. J Am Coll Cardiol. 2008;52(8):626-632. [DOI] [PubMed] [Google Scholar]
- 30. Holdaas H, Fellström B, Jardine AG, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361(9374):2024-2031. [DOI] [PubMed] [Google Scholar]
- 31. Elovson J, Chatterton JE, Bell GT, et al. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J Lipid Res. 1988;29(11):1461-1473. [PubMed] [Google Scholar]
- 32. Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC., Jr. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5(2):105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mora S, Buring JE, Ridker PM. Discordance of LDL cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129(5):553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bakogianni MC, Kalofoutis CA, Skenderi KI, Kalofoutis AT. Clinical evaluation of plasma high-density lipoprotein subfractions (HDL2, HDL3) in non-insulin-dependent diabetics with coronary artery disease. J Diabetes Complications. 2001;15(5):265-269. [DOI] [PubMed] [Google Scholar]
- 35. Kaysen GA, de Sain-van der Velden MG. New insights into lipid metabolism in the nephrotic syndrome. Kidney Int Suppl. 1999;71:S18-S21. [DOI] [PubMed] [Google Scholar]
- 36. Soveri I, Abedini S, Holdaas H, Jardine A, Eriksson N, Fellstrom B. Metabolic syndrome and cardiovascular risk in renal transplant recipients: effects of statin treatment. Clin Transplant. 2009;23:914-920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Apolipoprotein_supplementary_tables_FINAL_UNMARKED_April_2019 for Serum Apolipoprotein B and A1 Concentrations Predict Late-Onset Posttransplant Diabetes Mellitus in Prevalent Adult Kidney Transplant Recipients by Rohit Malyala, Lindita Rapi, Michelle M. Nash and G. V. Ramesh Prasad in Canadian Journal of Kidney Health and Disease