Abstract
Aim:
Following soft tissue ankle injury, patients are often referred for out-patient physiotherapy and present symptoms including pain, reduced range of movement and function, and oedema. In this study, we assess the use of a neuromuscular electrical stimulation (NMES) device as an adjunctive therapy to reduce oedema in patients recovering from grade I and II ankle sprains.
Methods:
This was a single-centre, pilot randomised controlled study, recruiting patients referred to physiotherapy following an ankle sprain. Participants presenting with oedema were randomised to one of two treatment groups: (1) the current standard of care and (2) the current standard of care plus NMES use. Participants were identified in an emergency department and referred to a physiotherapy department for treatment 1 to 5 days following the injury and returned to clinic 7 days later.
Results:
Twenty-two participants completed the study and had full data sets for analysis (11 in each group). Mean volumetric displacement was reduced in the intervention group in comparison to the standard care group (P = .011); however, there were no between-group differences in figure of eight measurements, function or pain scores. The device was well tolerated, with no device-related adverse events recorded.
Conclusions:
In this pilot, randomised controlled trial, NMES was well tolerated by patients following ankle sprain and demonstrated statistically significant improvements in oedema reduction as measured by fluid displacement. No other changes were observed. Further work will need to confirm the clinical significance and effect on longer term recovery post-ankle sprain.
Keywords: neuromuscular electrical stimulation (NMES), ankle sprain, oedema, physiotherapy
Introduction
The incidence of soft tissue ankle injuries is high, with over 2 million ankle sprains treated each year in emergency departments in the UK1,2 and the United States of America (USA).3 Despite the high occurrence and significant socioeconomic cost in addition to the acute debilitating symptoms (pain, swelling, and ecchymosis) of an ankle sprain,4 treatment selection remains inconclusive.5 Current routines suggest that early management including rest, ice, elevation, compression, non-steroidal anti-inflammatory drugs, and exercise advice is effective in promoting the recovery of the ankle.4,6 Commonly, physiotherapy treatment aims are to promote normal movement and loading, and the return to function as quickly as possible. However, the long-term prognosis of acute ankle sprain is poor, with a high proportion of patients reporting persistent residual symptoms and injury recurrence.7,8 This may be as it can be difficult for patients to implement and integrate traditional oedema management strategies (such as icing and limb elevation) into normal work and social activities.
In addition to activation via the bodies’ nervous system, muscles can be contracted by the application of electrical stimulation to the common peroneal nerve. Neuromuscular electrical stimulation (NMES) devices are reported to reduce oedema by compressing venous and lymphatic vessels and inducing an increase in venous return and lymphatic flow;9 however, they are not currently used during routine treatment following an ankle sprain. Preliminary work has found NMES to facilitate a reduction in oedema in other populations,10,11 and therefore, this pilot study aims to assess the feasibility of a larger trial to determine whether NMES (firefly™ manufactured by Firstkind Ltd., High Wycombe, UK) could help to reduce oedema in ankle sprain patients when used in addition to standard care.
The firefly device12 was chosen because it is a small and unobtrusive (149 mm × 42 mm × 11 mm), lightweight (18 g), self-adhesive, disposable, internally powered, NMES device, using technology and stimulation settings which have previously been proven to increase blood flow and reduce oedema.9 It has seven stimulation modes with selectable pulse widths of 70, 100, 140, 200, 280, 400, and 560 µs (±5% + 20 µs). The repetition rate is 1 Hz (±5%), with a maximum charge of 20 µC per pulse. The device is intended to be used for up to 24 h (maximum of 30 h), before being replaced. It produces activation of the extensor muscles and additional stretch of the antagonistic flexor muscles which are thus compressed by the fascial envelopes as they are pulled in a distal direction during dorsiflexion of the ankle joint. The passive motion of the flexor muscle acts as a calf muscle pump, promoting venous return by increasing intramuscular pressure. Subsequently, increased venous return may reduce stasis and oedema.
Methods
Trial design
This was a single-centre, pilot randomised controlled study, recruiting patients from a UK district general hospital’s accident and emergency (A&E) department who were referred to physiotherapy following an ankle sprain (registration number: FKD-ffANK-001/NCT02307955, ethical approval: Research Ethics Committee [REC] Reference: 14/EE1018). Following written informed consent, participants (n = 24) presenting with oedema were randomised to one of two treatment groups: (1) the current standard of care and (2) the current standard of care plus NMES use. Participants were identified in an emergency department and referred to a physiotherapy department for treatment 1 to 5 days following the injury and the participants returned to clinic 7 days after their first visit.
Participants
Twenty-four participants were recruited from a UK district general hospital’s A&E department between September 10, 2014 and December 3, 2015 following diagnosis of an ankle sprain and referral to physiotherapy. It is recommended to have 12 participants per group during a pilot trial when there is no prior information to base a sample size on.13 In addition, having 12 participants within each group allows a clinical trial to provide a reliable answer to the question addressed.13
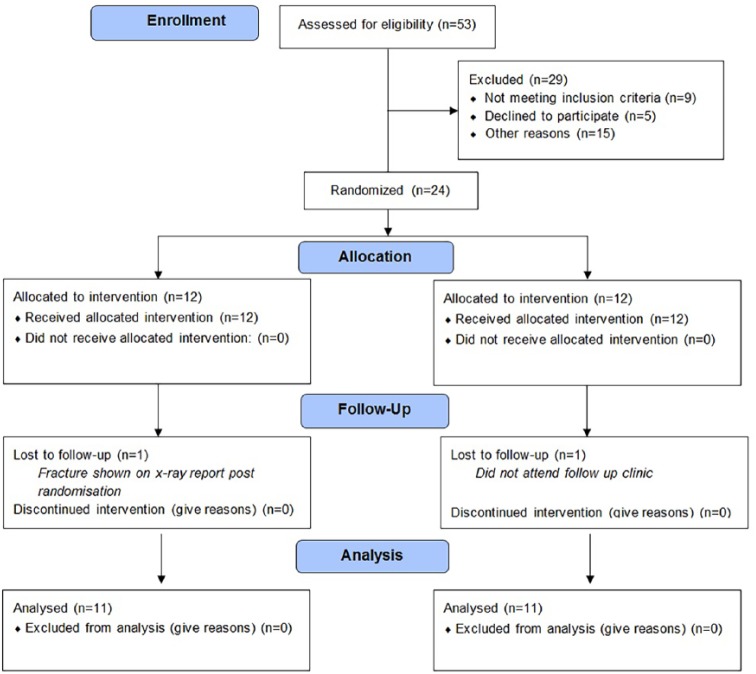
Fifty-three patients were screened for eligibility, with 29 participants not fulfilling the inclusion criteria. Reasons included appointment not arranged within study time (8), patient’s not attending their appointment (7), inability to contact patient (5), declined participation (5), minimal swelling at physiotherapy appointment (4), fracture shown during x-ray (2), sensory loss (1), other foot injuries (1), and a body mass index (BMI) outside of the eligibility guidelines (1).
When hospital administration staff contacted patients to arrange their physiotherapy appointment, the trial was introduced to them and patients gave consent for the research team to contact them. Following the telephone call, the participant information sheet was emailed to them and then written consent was provided on arrival to the physiotherapy department before their initial physiotherapy session. Participants had to be over 18 years old, demonstrating a grade I or II clinically diagnosed ankle sprain (grade III was excluded), with no evidence of a fracture. The ankle sprains were graded following guidelines from the American Academy of Orthopaedic Surgeons14 (Appendix 1). The exclusion criteria also prevented chronically obese (BMI index >40 kg/m2) or pregnant individuals from taking part. Patients with a pacemaker or history of deep or superficial vein thrombosis were also excluded. Following enrolment into the study, participants were then allocated randomly into one of two treatment groups. The method of concealed randomisation utilised within the study was the sealed envelope system, generated by the study sponsor.15
Interventions
Patients were treated and assessed by one of two senior physiotherapists who were independent to the study design and analysis of results. Participants in the first group were provided with the current standard of care which included patient education, manual therapy when indicated, and personalised exercise prescription. The second group of participants was provided with the current standard of care with the addition of NMES. Participants were trained on how to apply NMES to their leg in accordance with the manufacturer’s instructions for use (head of the device to the side of the knee over the top of the fibula, with the tail wrapping to the rear of the leg below the crease of the knee).16 Participants were instructed to wear the device on their injured leg during waking hours each day. The device was worn for a minimum of eight and a maximum of 16 h per day, with NMES usage and any adverse events (such as pain or skin irritation) recorded in patient diaries. Participants returned to the physiotherapy department 7 days later for a follow-up assessment. Due to the nature of the study, the participants and physiotherapists were not blinded to the study.
Outcomes
The primary outcome measure of the trial was a comparison of ankle oedema reduction between the two treatment groups. Oedema reduction was measured using two methods. The ‘gold standard’ for measuring oedema reduction is fluid displacement17,18 and thus, the participants’ foot was placed into a Baseline® foot set volumetric measuring device (manufactured by Fabrication Enterprises Inc., PO BOX 1500, White Plains, New York), with the volume of water displaced recorded. In addition, a tape measure was used to record figure of eight values, as this method is a time-efficient and reliable alternative often used in clinical practice.19 The functional recovery of the volunteers was also compared using the Foot and Ankle Ability Measure (FAAM) which is a validated, reliable, and responsive measure of physical function for individuals with foot- and ankle-related impairments20 that consist of the 21-item activities of daily living and 8-item sports subscales.
The secondary objectives of the study were to compare patient-reported pain scores, and the feasibility of a future, larger trial. The safety of participants was also monitored through the recordings of adverse events. Balance and proprioception were to be recorded using a single leg stand test; due to pain occurring in the first patients recruited, this objective was removed from the study following consultation with the physiotherapists involved. Pain scores were assessed using the Visual Analogue Scale (VAS), which is a valuable instrument for assessing pain in well-informed patients.21 A 10cm VAS chart was presented to the patient, depicting a scale between 1 and 10, with 0 representing no pain and 10 representing the worst pain imaginable. Participants were asked to record their perceived pain by making a mark across the line. The pain score was recorded as the number between 1 and 10 where the patient marked the scale. Feasibility for a future trial was assessed by (1) recruitment rate, (2) retention rate, (3) compliance to rehabilitation programme, and (4) data collection completion.
Statistical methods
Following the collection of data, results were compared between the two groups of care from the patients’ first physiotherapy clinic to the follow-up time of 7 days later. The mean percentage in reduction in oedema swelling was calculated through fluid displacement. In addition, the mean percentage of change in the figure of eight measurements and the mean percentage of change in pain and ankle functionality were calculated. The means between each group were compared with give an average difference between the two variables. The analysis was undertaken using SPSS Statistics, Version 19 (IBM Corporation, New York, USA). An independent sample t test was used to record the statistical significance between each treatment groups.
Eleven participants (Table 1, Appendix 2) from each group were included within the analysis for the primary outcome. Two participants were excluded from the analysis. The first was ineligible after a fracture was revealed on their x-ray following randomisation. The second excluded participant did not turn up to their follow-up clinic and was unable to be contacted. All patients included within the study presented with a grade II ankle sprain. The results were collected between September 10, 2014 and December 3, 2015 with the follow-up clinic taking place 7 days after. The trial ended when the predefined number of participants had been tested.
Table 1.
Study participants.
| Age (±SD) | Female | Male | Height (±SD) | Weight (±SD) | BMI | |
|---|---|---|---|---|---|---|
| Group 1 (standard of care) | 36 ± 8 years | 7 (64%) | 4 (36%) | 168.00 ± 10 cm | 74 ± 13 kg | 26 ± 4 kg/m2 |
| Group 2 (standard of care and NMES) | 44 ± 10 years | 5 (46%) | 6 (54%) | 174.82 ± 12 cm | 87 ± 13 kg | 29 ± 6 kg/m2 |
Abbreviations: BMI, body mass index; NMES, neuromuscular electrical stimulation; SD, standard deviation.
Results
The results (Table 2) show that oedema was statistically significantly reduced for patients in group 2 (standard of care and NMES) compared with group 1 (standard of care) (P = .011) during the recordings of fluid displacement. In contrast, the figure of eight (in cm) measure of oedema was not significantly better for the NMES treatment group than the standard of care participants. None of the other outcomes produced a statistically significant difference between participants using NMES and those receiving the standard of care. FAAM and rated function did not significantly differentiate. There was also no significant difference found between NMES use and standard of care when assessing pain reduction using VAS; however, both groups experienced a clinically significant reduction in pain from their baseline measurements to their follow-up clinic. Acceptance of the treatment after randomisation at the clinic was high, with all patients managing to fit the device and wearing it for the allocated number of hours (8 h per day within waking hours). No device related adverse events were recorded.
Table 2.
Pre and post intervention measures.
| Group 1 (standard of care) |
Group 2 (standard care and NMES) |
Between group |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Pre mean (±SD) | Post mean (±SD) | Change mean (±SD) | Pre mean (±SD) | Post mean (±SD) | Change mean (±SD) | Difference (±SD) | ||
| Volumetric displacement (% change) | 22 | 1315.9 ± 235.4 | 1322.9 ± 224.2 | –0.8 ± 3.8 | 1483.8 ± 184.1 | 1354.8 ± 163.5 | 8.1 ± 11.2 | 8.9 ± 9.2 | .011 |
| Figure of eight (cm) | 22 | 53.4 ± 4.5 | 52.4 ± 5.0 | 1.8 ± 2.5 | 56.4 ± 3.0 | 55.0 ± 3.0 | 2.5 ± 2.2 | 0.7 ± 4.3 | .25 |
| Foot and Ankle Ability Measure (%) | 22 | 55.8 ± 21.4 | 78.7 ± 12.4 | 22.8 ± 20.9 | 58.4 ± 11.9 | 81.3 ± 18.4 | 22.9 ± 13.3 | 0.1 ± 25 | .50 |
| Pain (VAS score) | 22 | 2.8 ± 2.2 | 1.0 ± 0.8 | 1.8 ± 1.9 | 3.0 ± 1.5 | 1.0 ± 1.1 | 1.9 ± 1.1 | 0.1 ± 2.6 | .41 |
Abbreviations: NMES, neuromuscular electrical stimulation; SD, standard deviation; VAS, Visual Analogue Scale.
Discussion
This study found that using NMES in addition to standard care reduces oedema following a grade I or II ankle sprain and is statistically significant when compared with standard care alone, as measured by volumetric displacement. However, this finding did not have a statistically significant effect on functional recovery, which was also a primary outcome measure of this study. Although the clinical relevance of the oedema reduction may be questioned because no other clinically meaningful changes between the two groups were found, a larger-scale trial with a longer term follow-up is warranted to allow accurate interpretation of the clinical implications of using NMES as an adjunct treatment modality to current standard care following acute ankle sprain.
Reducing oedema post-ankle sprain is proposed to play a vital role in ameliorating the level of pain and dysfunction that patients experience during recovery.22 The study found no significant change in pain and FAAM scores; however, it is possible that this is attributable to the short time frame in which NMES was evaluated. Pain decreased and ankle functionality increased in both treatment groups, demonstrating the value of each care plan; however, the difference between the changes was not significant. These results align with similar studies which have found that a reduction in oedema does not immediately improve self-reported function or pain.23 Interestingly, our volumetric displacement data are inconsistent with a previous study24 whereby no significant differences were found between three treatment groups: (1) NMES, (2) sham NMES, and (3) submotor electrical stimulation (control)) within 5 days following ankle sprain. The authors of this article consider their small sample size with large standard deviations, study design, NMES parameters, and treatment duration to be contributing factors to the apparent ineffectiveness. In our study, physiological effects were evaluated after a period of 7 days, and increasing this time frame may create a longer term clinically meaningful relationship between oedema reduction, pain, and functionality. The ‘dose-response’ relationship between NMES treatment induced strength gains and NMES training intensity has been confirmed in various clinical populations25 and it may be that a greater intensity or duration of electrical stimulation is required to reduce the presence of oedema and its effect on pain and function.
It is important to highlight that NMES was applied independently by patients with no problems and was well tolerated for at least 8 h a day, for seven consecutive days, with no adverse events recorded. NMES still suffers from poor clinical acceptability for rehabilitation,25 and a previously reported complication of NMES is skin irritation,26 of which none was reported within our study. Patient acceptance and tolerance support the feasibility of a future, larger trial, which may produce more clinically relevant results; however as 29 of the 53 patients screened for eligibility did not fulfil the inclusion criteria, recruitment may be a challenge. Despite this, complete data sets were recorded for all patients who attended their follow-up appointment.
Limitations of the study
Treatment with NMES was not immediate as the time delay between admittance to A&E and the first physiotherapy visit was up to 5 days after injury, which may have affected results. There were also differences in the demographics and baseline measures of the participants that may have impacted results. Groups were not balanced for injury severity, and there was no control over the manual therapy administered during the intervention period.
Conclusions
In this pilot, randomised controlled trial, NMES was well tolerated by patients following grade II ankle sprain and demonstrated statistically significant improvements in oedema reduction as measured by fluid displacement. No between-group differences in figure of eight measurements, function, or pain scores were observed. Further work is feasible and required to confirm the clinical significance and effect on longer term recovery post-ankle sprain.
Acknowledgments
The authors would like to thank physiotherapists Adrian Hand and Steve Sampson for their assistance in data collection and conducting the trial.
Appendix 1
Ankle sprains guidelines (American Academy of Orthopaedic Surgeons).
| Grade 1 | Grade 2 | Grade 3 |
|---|---|---|
| Minimal tear, minimal pain, slight swelling, unimpaired weight bearing | Incomplete rupture, moderate pain, moderate swelling/bruising, weight bearing is difficult | Complete rupture, severe pain, severe swelling/bruising, loss of function, weight bearing is impossible |
Appendix 2
CONSORT 2010 Flowchart.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by a research grant to Poole Hospitals NHS Foundation Trust made by Firstkind Ltd as part of a grant from the Technology Strategy Board (now Innovate UK), from the Biomedical Catalyst Late Stage funding scheme (ClinicalTrials.gov Identifier: NCT02307955). The sponsor was involved with generating the random allocation sequence using online software Sealed Envelope. The sponsor had no other involvement in the study.
Declaration of conflicting interests:The author(s) declared following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. TWW and RGM are shareholders in Healthdecoded Ltd. Healthdecoded Ltd has performed consultancy activities for Firstkind Ltd (who manufacturer the firefly™ device). There is a potential in the future, for Healthdecoded Ltd to be provided with an option to purchase shares in Sky Medical Technology (the parent company of Firstkind Ltd).
Author Contributions: TWW and RGM planned the trial design; TWW, RGM, and LCB analysed data; and TWW and LCB were the major contributors in manuscript writing. All authors read and approved the final manuscript.
Ethical Approval: Ethical approval: Research Ethics Committee (REC) Reference: 14/EE/1018.
Trial Registration: Registration number: FKD-ffANK-001/NCT02307955; IRAS Project ID: 154509; date of registration: December 4, 2014 (retrospectively registered); protocol: https://clinicaltrials.gov/ct2/show/NCT02307955?term=ankle+firefly&rank=1
ORCID iD: Louise C Burgess  https://orcid.org/0000-0003-1870-9061
https://orcid.org/0000-0003-1870-9061
References
- 1. Cooke MW, Lamb SE, Marsh J, Dale J. A survey of current consultant practice of treatment of severe ankle sprains in emergency departments in the United Kingdom. Emerg Med J. 2003;20:505–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bridgman SA, Clement D, Downing A, Walley G, Phair I, Maffulli N. Population based epidemiology of ankle sprains attending accident and emergency units in the West Midlands of England, and a survey of UK practice for severe ankle sprains. Emerg Med J. 2003;20:508–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiersma AJ, Brou L, Fields SK, Comstock RD, Kerr ZY. Epidemiologic comparison of ankle injuries presenting to US emergency departments versus high school and collegiate athletic training settings. Inj Epidemiol. 2018;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doherty C, Bleakley C, Delahunt E, Holden S. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113–125. [DOI] [PubMed] [Google Scholar]
- 5. van Dijk CN. Management of the sprained ankle. Br J Sports Med. 2002;36:83–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooke MW, Marsh JL, Clark M, et al. Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial. Health Technol Assess. 2009;13:iii, ix-x, 1–121. [DOI] [PubMed] [Google Scholar]
- 7. Anandacoomarasamy A, Barnsley L. Long term outcomes of inversion ankle injuries. Br J Sports Med. 2005;39:e14, discussion e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konradsen L, Bech L, Ehrenbjerg M, Nickelsen T. Seven years follow-up after ankle inversion trauma. Scand J Med Sci Sports. 2002;12:129–135. [DOI] [PubMed] [Google Scholar]
- 9. Ingves MV, Power AH. Two cases of transcutaneous electrical nerve stimulation of the common peroneal nerve successfully treating refractory, multifactorial leg edema [published online ahead of print November 20, 2014]. J Investig Med High Impact Case Rep. doi: 10.1177/2324709614559839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wainwright TW, Burgess LC, Middleton RG. A feasibility randomised controlled trial to evaluate the effectiveness of a novel neuromuscular electro-stimulation device in preventing the formation of oedema following total hip replacement surgery. Heliyon. 2018;4:e00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgess LC, Immins T, Swain I, Wainwright TW. Effectiveness of neuromuscular electrical stimulation for reducing oedema: A systematic review. Journal of Rehabilitation Medicine 2019;51:4. [DOI] [PubMed] [Google Scholar]
- 12. Firstkind Limited. Geko: hospital applications device. https://www.gekodevices.com/geko-products/hospital-applications-device/. Up-dated 2019. Accessed February 4, 2019.
- 13. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287–291. [Google Scholar]
- 14. American Academy of Orthopaedic Surgeons. Sprained ankle. https://orthoinfo.aaos.org/en/diseases–conditions/sprained-ankle/. Up-dated 2019. Accessed February 4, 2019.
- 15. Sealed Envelope. Randomisation and online databases for clinical trials. https://www.sealedenvelope.com/.Up-dated 2019. Accessed February 4, 2019.
- 16. Firstkind Limited. Geko: circulation support: instructions for use. http://www.gekodevices.com/media/34381/fk-geko_ifu_1a-r4_a4-v1.pdf. Up-dated 2013. Accessed June 2, 2014.
- 17. Henschke N, Boland RA, Adams RD. Responsiveness of two methods for measuring foot and ankle volume. Foot Ankle Int. 2006;27:826-832. [DOI] [PubMed] [Google Scholar]
- 18. Perrin M, Guex JJ. Edema and leg volume: methods of assessment. Angiology. 2000;51:9–12. [DOI] [PubMed] [Google Scholar]
- 19. Devoogdt N, Cavaggion C, Van der Gucht E, et al. Reliability, validity, and feasibility of water displacement method, figure-of-eight method, and circumference measurements in determination of ankle and foot edema [published online ahead of print January 16, 2019]. Lymphat Res Biol. doi: 10.1089/lrb.2018.0045. [DOI] [PubMed] [Google Scholar]
- 20. Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26:968–983. [DOI] [PubMed] [Google Scholar]
- 21. Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15:S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denegar CR, Saliba E, Saliba SF. Therapeutic Modalities for Musculoskeletal Injuries. 4th ed. Leeds, UK: Human Kinetics; 2009. [Google Scholar]
- 23. Feger MA, Goetschius J, Love H, Saliba SA, Hertel J. Electrical stimulation as a treatment intervention to improve function, edema or pain following acute lateral ankle sprains: a systematic review. Phys Ther Sport. 2015;16:361–369. [DOI] [PubMed] [Google Scholar]
- 24. Man IOW, Morrissey MC, Cywinski JK. Effect of neuromuscular electrical stimulation on ankle swelling in the early period after ankle sprain. Phys Ther. 2007;87:53–65. [DOI] [PubMed] [Google Scholar]
- 25. Maffiuletti NA, Gondin J, Place N, Stevens-Lapsley J, Vivodtzev I, Minetto MA. Clinical use of neuromuscular electrical stimulation for neuromuscular rehabilitation: what are we overlooking. Arch Phys Med Rehabil. 2018;99:806–812. [DOI] [PubMed] [Google Scholar]
- 26. Schuhfried O, Crevenna R, Fialka-Moser V, Paternostro-Sluga T. Non-invasive neuromuscular electrical stimulation in patients with central nervous system lesions: an educational review. J Rehabil Med. 2012;44:99–105. [DOI] [PubMed] [Google Scholar]